Abstract
Mucosal surfaces are the main route of entry for pathogens in all living organisms. In the case of teleost fish, mucosal surfaces cover the vast majority of the animal. As these surfaces are in constant contact with the environment, fish are perpetually exposed to a vast number of pathogens. Despite the potential prevalence and variety of pathogens, mucosal surfaces are primarily populated by commensal non-pathogenic bacteria. Indeed, a fine balance between these two populations of microorganisms is crucial for animal survival. This equilibrium, controlled by the mucosal immune system, maintains homeostasis at mucosal tissues. Teleost fish possess a diffuse mucosa-associated immune system in the intestine, with B cells being one of the main responders. Immunoglobulins produced by these lymphocytes are a critical line of defense against pathogens and also prevent the entrance of commensal bacteria into the epithelium. In this review we will summarize recent literature regarding the role of B-lymphocytes and immunoglobulins in gut immunity in teleost fish, with specific focus on immunoglobulin isotypes and the microorganisms, pathogenic and non-pathogenic that interact with the immune system.
Keywords: Teleost fish, B cells, IgT, Mucosal immunity, Gut, GALT
1. Introduction
It is generally accepted that host mucosal immunity is characterized by tolerance rather than responsiveness (Arrieta and Finlay, 2012; Salinas and Parra, 2015). To maintain the integrity of the body, the host has developed a number of chemical and physical barriers armed with immune potential, which is hypothesized to have been driven by microbial colonization as a contributing factor in the evolution of the adaptive immune system (Lee and Mazmanian, 2010; McFall-Ngai, 2007). Aquatic environments provide an ideal setting for the growth of a variety of microorganisms that are in continuous contact with the mucosal surfaces of the fish body (Gómez et al., 2013). Thus, the intestine is combining two confounding functions, facilitating the absorption of nutrients, while resisting and inhibiting the breach of potential pathogens through its epithelium (Sommer and Bäckhed, 2013). In addition, due to the large microbial community residing in the gut, homeostasis maintenance is critical for the correct immune function of this mucosal tissue. During the last decade, we have witnessed a change in the paradigm, understanding that under normal conditions, the vast majority of microorganisms which are in contact with mucosal surfaces do not pose a threat, but positively contribute to host physiology (Arrieta and Finlay, 2012; Sommer and Bäckhed, 2013).
Together with cartilaginous fish, teleost fish are the earliest living organisms with an adaptive immune system based on B and T cell receptors. Thus, fish not only have most of the elements of the innate immune system of higher vertebrates, but also possess thymus, spleen, major histocompatibility complex class I and II, and T and B cells among the most important components of the adaptive immune system (Sunyer, 2013). Until recently, IgM had been considered the only immunoglobulin class responding to pathogenic challenge in systemic and mucosal compartments of teleost fish (Sunyer, 2013). However, the discovery of a novel immunoglobulin class (IgZ/IgT) in zebrafish (Danilova et al., 2005) and rainbow trout (Hansen et al., 2005) together with the finding that it played a specialized role in gut mucosal immunity (Zhang et al., 2010), triggered a renaissance in the study of teleost mucosal immunology. Later studies demonstrated that IgT plays also a key role in teleost skin and gill mucosal immune responses (Xu et al., 2016; Z. Xu et al., 2013). Such studies revealed that many fundamental mechanisms protecting mucosal surfaces evolved very similarly in both mammals and fish (Sunyer, 2013).
In this review, we aim to explore further the current findings on the role of B lymphocytes in the intestine of teleost fish. First, we will review the anatomy of the intestinal immune system. Later we focus on the available information regarding gut B cells and immunoglobulins of teleost fish. Thereafter, we concentrate our attention on gut mucosal B cells responses to pathogens, followed by responses to probiotics and the microbiota. We later explore the possible parallels between fish and mammalian gut immune responses, and we identify areas in which studies in teleosts may shed light into unresolved paradigms of mammalian gut mucosal immunity. Together, the reviewed and future studies on fish intestinal immunity will provide crucial information for the formulation of fish vaccines and will also aid in the identification of fundamental mechanisms and principles underlying the protection of mucosal surfaces that are common to all jawed vertebrates.
2. The anatomy of the intestinal immune system
2.1. The anatomy of the mucosa-associated lymphoid tissue (MALT)
In order to keep homeostasis throughout evolution, the mucosal barriers have developed an immune system armed with both cellular and humoral defenses, protecting the organisms from the continuous bombardment of microbes and antigens. These mucosa-associated lymphoid tissues (MALT) harbor immune cells and produce humoral mediators, such as cytokines or immunoglobulins, among many others (Rombout et al., 2011, 2014; Salinas et al., 2011). In teleost, four different MALTs have been thus far described depending on their localization: SALT (skin-associated lymphoid tissue), GIALT (gill-associated lymphoid tissue), NALT (nasopharynx-associated lymphoid tissue) and GALT (gut-associated lymphoid tissue) (Gómez et al., 2013; Salinas and Parra, 2015; Salinas et al., 2011; Tacchi et al., 2014).
In higher vertebrates, the mucosal compartments can be divided into inductive and effector sites. The first are involved in the sampling of the antigen and stimulation of cognate naive T and B lymphocytes, while in the latter, the effector cells perform their action (i.e., antibody production) (Brandtzaeg et al., 2008). The inductive sites comprise the so-called organized MALT, such as the Peyer’s patches (PP), mesenteric lymph nodes (MLN) and the isolated lymphoid follicles, in addition to the mucosa-draining lymph nodes. All these structures resemble classical lymph nodes, with T-cell zones intervening between the B-cell follicles, providing the anatomical, physiological and immunological basis for the maturation of antibody responses. On the other hand, the effector sites consist of distinct compartments including the lamina propria (LP) or the intraepithelial lymphocytes (IEL). Since the lymphocytes in LP and IEL are not organized, the tissue that comprises them is commonly termed as diffuse MALT. It is worth pointing out that while considered an effector site, the LP is also vital for the expansion of B cells and their differentiation into plasma cells (Brandtzaeg et al., 2008; Garside et al., 2004; Mason et al., 2008; Rombout et al., 2011; Salinas, 2015).
In teleost, organized lymphoid structures such as PP or MLN are missing, hence, the diffuse MALT may combine both inductive and effector functions, although that remains to be demonstrated (Table 1). Interestingly, the teleost interbranchial lymphoid tissue (ILT) was initially proposed as a possible exception that could represent an organized structure of teleost MALT (Aas et al., 2014; Gómez et al., 2013; Koppang et al., 2010; Salinas et al., 2011), however, this notionwas reexamined and recently the ILT has been classified as a diffuse lymphoid tissue (Dalum et al., 2015). Importantly, it has been established that PP are not absolutely necessary for IgA production in mice (Lorenz and Newberry, 2004; Tsuji et al., 2008) which occurs also extrafollicularly. From that perspective, activation of B cells and mucosal immunoglobulin production in teleosts occurs extrafollicularly in the MALTs, as teleost fish develop pathogen-specific immunoglobulin responses at their mucosal surfaces despite the lack of organized lymphoid structures (Parra et al., 2015; Rombout et al., 2014; Salinas and Parra, 2015; Xu et al., 2016; Z. Xu et al., 2013).
Table 1.
Comparison of main characteristics of intestinal B cells and GALT in teleost and mammals.
| Teleost | Mammals (mice) | |
|---|---|---|
| Organization | Diffuse | Organized (PP, MLN, ILF) and diffuse (IEL, LP) |
| Goblet cells | YES | YES |
| Antigen uptaking cells | M-like cells? (proposed) | M Cells, Dendritic cells |
| Main lymphocyte population | T cells | T cells (D-GALT), B cell (O-GALT) |
| Main site for B cell response | LP | PP |
| % of B cells | 2–12% of LP | CD22 + B cells < 2% of IEL; < 10% of LP; 50–75% of PP |
| Main B cell subset | IgT/Z B cells | IgA-producing B-1 and B-2 B cells |
| Homing receptors | CCR7? (proposed) | α4β7, CCR9 and CCR10 |
| Main Ig in gut mucus | IgM under steady-state conditions. Polymeric IgT after infection | Polymeric IgA |
| Class switch recombination | NO | YES (IgM → IgA) |
| Receptor required for Ig transport into gut lumen | pIgR (transports IgT and IgM) | pIgR (transports IgA and IgM); FcRn (transports IgG) |
| J chain | NO | YES |
| Microbiota coating by Ig | YES (IgT ≫IgM) | YES (IgA ≫IgM or IgG) |
| Main induced pathogen-specific Ig | IgT/Z | IgA |
| Antibody affinity maturation | ? | High |
GALT: gut-associated lymphoid tissue; MLN: mesenteric lymph nodes: ILF: isolated lymphoid follicles; IEL: intraepithelial lymphocytes; D-GALT: diffuse GALT; O-GALT: organized GALT; LP: lamina propria; PP: Peyer’s patches; pIgR: polymeric Ig receptor; FcRn: neonatal Fc receptor.
2.2. Gut-associated lymphoid tissue (GALT)
Teleosts encompass more than 30,000 species, in comparison to approximately 5700 species of mammals and 10,000 species of birds. Naturally, there is a wide range of ecologies and diets within the group that is reflected in the organization of the gastrointestinal system. For example, the stomach develops in 85% of bony fish species, while the rest, including cyprinids, never develop a stomach and lack a region with low pH and predigestion (Balon, 1975). Additionally, depending on the fish species, important differences exist in the length of the intestine and the presence and number of pyloric caeca as well as intestinal loops and valves (Evans et al., 2014). As in mammals, immunological differences can be found along the different segments of the teleost gastrointestinal tract. In nearly all investigated species, the intestine can be subdivided in three segments based on the microscopical anatomy of their mucosa: i) the first part of the intestine (foregut, anterior gut), where enterocytes are considered absorptive cells; ii) the second segment (posterior gut), with enterocytes characterized by large supranuclear vacuoles, irregular microvilli zone and high pinocytotic activity and iii) the hindgut, where the enterocytes were proposed to play mainly osmoregulatory function (Rombout et al., 2011). A number of observations have been made with respect to the uptake and processing of antigens along these segments, indicating a principal role of the second segment in transport of antigens to the local and systemic lymphoid tissues (Rombout and van den Berg, 1985; Rombout et al., 1989, 2011). However, despite these findings, the detailed knowledge about the distribution of various lymphocyte subsets and secretory immunoglobulins in different proportions of the gut is scarce (Salinas and Parra, 2015; Salinas, 2015; Salinas et al., 2011). Regardless of these differences, the structure of GALT can be very similar among various fish species, although important anatomical differences exist in the gut among carnivorous, omnivorous and herbivorous fish which may be reflected also in differences in their GALTs (German and Horn, 2006).
As previously mentioned for MALT, the level of organization in GALT is less complex than in mammals, and lymphoid cells are more diffusely distributed as fish lack organized lymphoid structures. Similar to higher vertebrates, leukocytes can be found in two extrafollicular compartments: the LP, which harbors a variety of immune cells, including macrophages, granulocytes, lymphocytes and plasma cells; and the intraepithelial compartment that contains mostly T cells and very few B lymphocytes (Rombout et al., 2011; Salinas and Parra, 2015; Salinas et al., 2011). Despite being separated only by a thin basement membrane, the LP and the epithelium form very distinct immunological compartments (Mowat and Agace, 2014).
The uptake of antigen in the teleost intestine has been a subject of controversy. It has been proposed that enterocytes can uptake certain types of antigen, such as ferritin (Rombout and van den Berg, 1985). On the other hand, it has been suggested the presence of M cell-like cells, displaying morphological similarities with mammalian M cell (Fuglem et al., 2010). These cells, located in the posterior gut of salmonids, were proposed to represent evolutionary predecessor of mammalian M cells and were shown to take up bovine serum albumin (BSA) (Fuglem et al., 2010). Moreover, as demonstrated in cyprinids and salmonids, large intraepithelial macrophages might also be involved in antigen uptake from the lumen of the intestine (Chen et al., 2015; Rombout and van den Berg, 1985; Temkin and McMillan, 1986). Thus, it is likely that enterocytes, M cell-like cells and large intraepithelial macrophages are implicated in antigen uptake, although more in-depth studies are clearly needed to elucidate the different roles of each cell subtype.
3. Immunoglobulins of teleost fish
3.1. Teleost immunoglobulins
Teleost fish possess three major types of immunoglobulins: IgM and IgD, that have been identified in all teleost fish examined, as in almost all jawed vertebrates (Parra et al., 2013); and IgT (named as IgZ in cyprinids) which was identified in rainbow trout and zebrafish in 2005 (Danilova et al., 2005; Hansen et al., 2005), and has been found to be a specialized mucosal-dedicated immunoglobulin, akin to IgA and IgX in tetrapods and amphibians respectively (Zhang et al., 2010). The teleost igh locus encoding these three isotypes is arranged in a translocon organization (Flajnik and Kasahara, 2010). However, the archetypal genome structure of the teleost igh locus is similar to the mammalian TCRα/δ locus rather than the igh locus (Bengtén and Wilson, 2015; Fillatreau et al., 2013). Moreover, the locus encoding Dτ-Jτ-Cτ cluster (for IgT) is embedded between VH gene segments and the Dμ/δ-Jμ/δ-Cμ-Cδ cluster (for IgM and IgD), while the VH gene segments are shared by all igh genes (IgT, IgM and IgD). However, minor modifications of this archetypal structure of the genomic igh locus occurred in various teleost species (Bengtén and Wilson, 2015; Fillatreau et al., 2013). Importantly, in sharp contrast to tetrapod igh locus that is regulated by an AID-mediated class switch recombination, the genomic structure of teleost igh locus rules out a possible class switch recombination between ighτ/ζ and ighμ, leading to the development of mutually exclusive B-cell lineages expressing IgT or IgM/D (Parra et al., 2013; Zhang et al., 2010). In support of this, B cells uniquely expressing surface IgT or IgM have been described in rainbow trout (Zhang et al., 2010). Similarly, it has been suggested that in zebrafish there are two B cell lineages expressing IgZ or IgM (Page et al., 2014; Schorpp et al., 2006). With regard to light chains, four immunoglobulin light chain isotypes have been reported (κ, λ, σ and σ-cart), although their functional differences remain largely unknown (Bengtén and Wilson, 2015; Fillatreau et al., 2013).
3.1.1. Gene structure of immunoglobulin heavy chain genes in teleost fish
IgM is the most characterized Ig isotype in teleost fish. The ighμ gene is comprised of exons encoding four μ constant (Cμ) and two transmembrane regions, which are evolutionarily conserved between teleost fish and other jawed vertebrates (Bengtén and Wilson, 2015; Fillatreau et al., 2013). The secreted form of ighμ transcript consists of all four Cμ domains and a secretory tail. However, the teleost membrane-bound form of ighμ (mIgM) transcript generally uses only the first three constant domains, which are derived through the alternative splicing of the TM exons to Cμ3 exon. Thus, although four Cμ domains of mIgM are present in other vertebrates, mIgM in teleost fish lack the Cμ4 domain. Moreover, some exceptions have been reported in medaka and Antarctic fish that utilize the first two Cμ domains for mIgM (Magadáan-Mompó et al., 2011; Quiniou et al., 2011). In zebrafish, an additional type of mIgM has been reported with one single Cμ domain and a transmembrane region, generated through alternative splicing (Y. L. Hu et al., 2011).
Similar to IgM, IgD is an ancient immunoglobulin isotype that has been found in most jawed vertebrates, although birds and some mammals are devoid of the ighδ gene (Edholm et al., 2011; Parra et al., 2013; Sun et al., 2011) [i.e. examined birds (Huang et al., 2012; Magor et al., 2013), rabbit (Lanning et al., 2003), opossum (Wang et al., 2009), elephant (Guo et al., 2011)]. The gene for ighδ is located immediately 3′ downstream of the ighμ gene in most tetrapod and teleost fish (Bengtén and Wilson, 2015). However, the structure of the constant region in ighδ (Cδ) shows a remarkable diversity between fish species (Edholm et al., 2011; Sun et al., 2011). The ighδ of teleosts encodes 2–16 constant domains and the transcript of ighδ of all examined teleost fish has been shown to be a chimeric transcript composed of a rearranged VDJ segment followed by Cμ1 and Cδ domains. In catfish and rainbow trout, transcripts encoding the secreted form of ighδ are present, although their gene structure and transcriptional regulation are completely different (Edholm et al., 2011; Ramirez-Gomez et al., 2012). In catfish, two different ighδ genes encode secreted and membrane-bound forms (Bengtén et al., 2006a). The secreted form of ighδ is linked with pseudo ighμ and is located upstream of the region consisting of a functional VH domain, ighμ, and a membrane-bound form of ighδ (Bengtén et al., 2006a). Interestingly, the transcript of the secreted form of ighδ consists of Cδ domains without the VH region or Cμ1, while as described above, the membrane-bound form of ighδ includes the rearranged VDJ and Cμ1 domains combined with the Cδ domains (Edholm et al., 2011). In contrast, a single ighδ gene in rainbow trout is transcribed into both membrane-bound and secreted forms through alternative splicing. Trout secreted ighδ is expressed as Cδ domains associated with a VH and a Cμ1 domain (Ramirez-Gomez et al., 2012).
IgT/Z is the third immunoglobulin isotype discovered originally in rainbow trout and zebrafish (Danilova et al., 2005; Hansen et al., 2005). These ighτ/ζ genes have been identified in a number of teleost fish species, while no ighτ gene has been described in the igh loci of medaka and catfish (Bengtén et al., 2006b; Magadán-Mompó et al., 2011); while it is possible that those teleost lack IgT/IgZ, it is also conceivable that more completed genome and transcriptome studies may reveal its presence It is important pointing out that unlike ighμ, which encodes four Cμ domains, different numbers of constant regions (Cτ/ζ) of ighτ/ζ have been reported: four Cτ/ζ domains in the majority of analyzed species (Fillatreau et al., 2013); three domains in stickleback, ayu and Antarctic fish (Gambón-Deza et al., 2010; Giacomelli et al., 2015; Kato et al., 2015); and two domains in fugu (Savan et al., 2005). Interestingly, common carp has two separated ighζ loci encoding four (IgZ1) and two (IgZ2) Cζ domains. IgZ1 shows classical feature of teleost ighτ/ζ, while IgZ2 is comprised of a chimeric transcript with Cμ1 and Cζ4 domains (Ryo et al., 2010). Unlike ighμ, which skips the last Cμ domain for a membrane-bound form, both secreted and membrane-bound forms of ighτ/ζ utilize whole Cτ/ζ domains (Bengtén and Wilson, 2015; Fillatreau et al., 2013). As described above, recent functional studies regarding trout IgT have shown that this immunoglobulin plays a specialized role in mucosal surfaces (Zhang et al., 2011). In support, preferential expression of ighζ (IgZ2) in mucosal tissues has been reported in common carp (Ryo et al., 2010). However, regardless of functional similarities with tetrapod mucosal immunoglobulin (e.g. IgX and IgA), IgT/Z is thought to be derived through convergent evolution in teleost fish (Flajnik, 2010; Mashoof et al., 2013).
3.1.2. Protein structure of immunoglobulin isotypes in teleost fish
IgM is the predominant isotype in teleost body fluids. The serum concentration of IgM, which varies among fish species (~0.6–16 mg/ml) fluctuates with various factors (i.e. temperature, water quality, fish size, etc.) (Solem and Stenvik, 2006). In contrast, gut mucus has been reported to contain a significantly lower concentration of IgM (i.e., ~75 μg/ml in trout gut mucus) (Solem and Stenvik, 2006; Zhang et al., 2010). Teleost IgM is generally present as a tetramer (~700–800 kDa) in both serum and mucus (Salinas et al., 2011). This tetrameric IgM shows a variety of interheavy chain disulfide polymerization of the monomeric and/or dimeric subunits (Bromage et al., 2004; Kaattari et al., 1998; Salinas et al., 2011). These various oxidation states have shown to be related to differences in functionality, since increased disulfide polymerizations correlate with a higher affinity and longer half-life of IgM (Ye et al., 2010). IgM plays a key role in systemic immune responses. It is well-known that increased titers of IgM occur in serum after immunization or infection (Solem and Stenvik, 2006; Ye et al., 2013). Thus, upon pathogen infection, IgM titers are mainly detected in serum while the degree of IgM titer induction in mucus is very limited (Salinas et al., 2011). Comparable to mammalian IgM, teleost IgM in serum has been shown to possess effector functions (i.e., complement activation, opsonization, and neutralization) (Ye et al., 2013). However, the characterization of gut mucus IgM effector functions has been poorly analyzed to date, partly due to its instability and easy degradation in the gut mucus (Hatten et al., 2001).
IgD protein has been examined in only two fish species, catfish and rainbow trout. In catfish, two variants of the secreted form of IgD (~130 kDa and ~180 kDa) are present in serum (Edholm et al., 2011), with a total concentration of ~40 μg/ml. As described above, the secreted forms of ighδ transcripts contain no VH segment and Cμ1 domains, the latter considered to be required for association with the IgL chain. Catfish secreted IgD solely consists of Fc region and it has been proposed to function as a pattern recognition molecule (Edholm et al., 2011, 2010). In contrast, akin to IgM, cell surface IgD in catfish associates with IgL chain and CD79a/b (Edholm et al., 2010; Sahoo et al., 2008). In rainbow trout, two monomeric variants with long (~370 and ~400 kDa) and short (~240 kDa) forms of secreted IgD are present, and their concentration in serum is ~2–80 μg/ml (Ramirez-Gomez et al., 2012; Xu et al., 2016). Unlike catfish secreted IgD, trout secreted IgD contains variable regions of heavy and, probably, light chains, allowing for potential trout IgD antigen-specific immune responses (Ramirez-Gomez et al., 2012). However, a recent study by Xu et al. has revealed the absence of pathogen-specific trout IgD responses in both gill mucus and serum, while in the same study pathogen-specific trout IgT and IgM responses were mainly induced in gill mucus and serum, respectively (Xu et al., 2016). Moreover, recent repertoire studies have shown that splenic IgD responses to viral hemorrhagic septicemia virus (VHSV) infection are faint when compared to those of splenic IgM and IgT (Castro et al., 2013). Therefore, the role of teleost IgD in pathogen infection is still largely unknown. It is worthwhile to mention that IgD appears to coat a small proportion of gill microbiota, and thus, it may play a minor role in microbiota homeostasis (Xu et al., 2016). To date, no IgD protein has been determined in gut mucus.
Since antibodies against other teleost IgT/IgZ are still lacking, IgT protein in body fluids has thus far only been analyzed in rainbow trout (Xu et al., 2016; Z. Xu et al., 2013; Zhang et al., 2010). Trout serum IgT is present as a monomer (~180 kDa), while mucosal IgT from the gut, skin and gills is a multimer (~4–5 monomers) where all monomers are held together through non-covalent bonds (Xu et al., 2016; Z. Xu et al., 2013; Zhang et al., 2010). The concentration of IgT is ~7 and ~4 μg/ml in gut mucus and serum respectively, which is much lower than that of IgM (~75 and ~2500 μg/ml, respectively). However, the ratio of IgT/IgM is much higher (~60 fold) in gut mucus when compared to that in serum. Similarly, mucus from skin, nose and gills also show higher values of IgT/IgM than serum (Tacchi et al., 2014; Xu et al., 2016; Z. Xu et al., 2013). Importantly, IgT is the prevalent immunoglobulin coating gut microbiota for immune exclusion of luminal bacteria (Zhang et al., 2010). Subsequent studies have also found that IgT is also the main immunoglobulin coating the microbiota of the skin, gills and nose (Tacchi et al., 2014; Xu et al., 2016; Z. Xu et al., 2013). Main features of the three immunoglobulins here described in rainbow trout are summarized in Table 2.
Table 2.
Main characteristics of immunoglobulins in rainbow trout.
| IgM | IgD | IgT | |
|---|---|---|---|
| Concentration in serum | ~0.6–3.3 mg/ml | ~2–80 μg/ml | ~4 μg |
| Concentration in gut mucus | ~75 μg/ml | Not determined | ~7 μg |
| Structure | Tetramer in serum (~700 kDa) and mucus (~800 kDa) | Long (~370–400 kDa) and short (~240 kDa) monomer | Monomer (~180 kDa) in serum, multimer in mucus (~4–5 monomers) |
| Demonstrated effector functions | Complement activation, opsonization, neutralization, immune exclusion | Not determined | Immune exclusion? |
| Coating of intestinal microbiota | Yes | Not determined | Yes |
| Pathogen-specific responses | Mainly detected in serum | Not determined | Mainly detected in mucus |
3.2. Immunoglobulin transport to gut mucus
Antibodies in gut mucus are mainly present in polymeric forms in tetrapods. Transport of polymeric antibodies into the external mucus lining is mediated by polymeric Ig receptor (pIgR). In mammals, pIgR is expressed in the basolateral surface of mucosal and glandular epithelial cells and is able to transport both IgA and IgM across mucosal epithelial cells to the outer mucus layer. At the apical surface, pIgR is proteolytically cleaved, and subsequently, the extracellular domain of the pIgR (called secretory component (SC)) remains associated with IgA or IgM, creating a complex that is released in the mucus layer (Kaetzel, 2014).
Teleost pIgR has been identified in many fish species (Rombout et al., 2014), such as fugu (Hamuro et al., 2007), carp (Rombout et al., 2008), orange-spotted grouper (Feng et al., 2009), trout (Zhang et al., 2010), salmon (Tadiso et al., 2011) and zebrafish (Kortum et al., 2014). In contrast to the tetrapod pIgR, which contains 4 (Xenopus and birds) or 5 (mammals) Ig-like domains, teleost pIgRs are composed of only two Ig-like domains which correspond to domains 1 and 5 of mammalian pIgR (Rombout et al., 2014). The distribution of pIgR in mucosal tissues has been determined in carp and rainbow trout. In carp, pigr transcripts are detected in gut epithelial cells (Rombout et al., 2008). In addition, in situ binding of carp IgM to gut epithelial cells suggests the presence of pIgR-like molecules in epithelial cells (Rombout et al., 2008). In rainbow trout, although gut epithelial cells have not been examined, anti-trout pIgR antibody revealed that pIgR is distributed in epithelial cells in the skin, nose and gills (Tacchi et al., 2014; Xu et al., 2016; Z. Xu et al., 2013). Thus, it is highly probable that the location of pIgR in the gut will be similar to what is observed in others mucosal tissues in trout. Moreover, IgT and IgM from trout gut, skin and gill mucus was shown to be associated with the SC from trout pIgR (Xu et al., 2016; Z. Xu et al., 2013; Zhang et al., 2010). Similarly, fugu skin mucus contains IgM-SC complexes (Hamuro et al., 2007). The binding of pIgR to teleost IgM has been further demonstrated by detecting the binding of grouper and flounder IgM to recombinant protein of their corresponding pIgRs (Feng et al., 2009; G. Xu et al., 2013). Overall, these observations indicate that, similar to IgA and IgM in mammals, the transportation of polymeric IgT and IgM in teleost fish is mediated by pIgR. Importantly, recent comprehensive analysis of trout gill immunoglobulins by Xu et al. demonstrated the association of secreted trout IgD with SC in gill mucus (Xu et al., 2016), thus representing the first time that IgD is shown to associate with pIgR not only in fish, but also in a vertebrate species (Baker et al., 2010).
In higher vertebrates, a small polypeptide named joining chain (J chain), is required for the multimerization of IgM and IgA and mediates the interaction of pIgR with mucosal immunoglobulins (Castro and Flajnik, 2014). The J chain has been found in most jawed vertebrates, although a gene encoding the J chain has not yet been identified in teleost fish, even though whole genome sequences and extensive transcriptome analysis from a number of teleost fish are available. Therefore, it is believed that the J chain was lost from teleost fish. However, as described above, teleost mucosal immunoglobulins are transported into mucus without the J chain. Although the mechanisms by which teleost immunoglobulins interact with the pIgR remain thus far a mystery, a theoretical model proposed by Kaetzel predicts that without J chain the interaction of IgT to pIgR is mediated by the binding of two domains of pIgR with the constant domain 3 of the IgT heavy chain (Kaetzel, 2014). In a similar vein, although the J chain interacts with IgM in amphibians, IgX multimerization and transport can be mediated without J chain association (Mussmann et al., 1996). Interestingly, some in vitro studies have revealed that the J chain might not be required for IgA polymer formation (Brandtzaeg and Prydz, 1984; Johansen et al., 2000). In support, J chain knockout mice, while they lack biliary and fecal IgA, they possess some IgA dimers in serum, suggesting further that polymerization of IgA might occur in the absence of J chain (Hendrickson et al., 1995). A similar situation occurs with secretory IgM, in which secretion is impaired in J chain knockout mice but it still forms oligomers, and is present in mucosal secretions (Erlandsson et al., 1998; Johansen et al., 2000). However, both immunoglobulins, although present as oligomers when J chain is not expressed, are defective in some functions when compared with J chain-associated immunoglobulins (Castro and Flajnik, 2014; Erlandsson et al., 1998). Thus, a multimerization of certain immunoglobulins and their transport across the epithelium in the absence of a J chain is feasible for mucosal antibodies of teleosts, amphibians and also mammals.
4. B cells of teleost and gut B cells
4.1. B cell development
For a long time, the ontogeny of the fish immune system has been hampered by the absence of reliable tools and thus, it has remained largely restricted to a few species important in aquaculture. This is further pronounced by the diversity in the differentiation and development process, which differ among fish species (Sandström et al., 1997; Solem and Stenvik, 2006; Zapata et al., 2006). Previous ontogenic studies regarding B cells have largely focused on the presence of IgM-expressing and -secreting cells with methods such as immunohistochemistry, flow cytometry, ELISPOT, in situ hybridization or RT-PCR, which have not always provided comparable results (Magnadottir et al., 2005). Additionally, very little is known about the first appearance of IgD+ or IgT/IgZ+ positive cells.
Despite the differences in various species, the ontogeny follows some general rules. The first Ig-producing cells usually appear in the kidney (Hansen and Zapata, 1998; Parra et al., 2013; Zapata et al., 2006). In zebrafish, the first IgM+ B cells appear between the axial vessels of the trunk and also in the kidney around 20 days post fertilization (dpf) (Page et al., 2014), although RAG-1 expression in zebrafish showed that B cell differentiation might start in the pancreas (Danilova and Steiner, 2002). In later ontogenic stages, B cells populate the spleen and finally the MALT. Colonization of the intestine by B cells is poorly understood. Yet, findings in carp, sea bass and spotted wolffish suggest the existence of a remarkable delay between the first appearance of Ig+ cells in the head kidney at 7 dpf and their arrival to the intestine at 6 weeks post fertilization (wpf) (Grøntvedt and Espelid, 2003; Huttenhuis et al., 2006; Picchietti et al., 1997; Romano et al., 1997). A further study investigating the expression of igm, igz1 and igz2 transcripts, confirmed these findings and identified the IgM-expressing cells in carp intestine at 4 weeks post hatch (wph), while IgZ2 was first detected after 16 weeks (Ryo et al., 2010). Similarly, in situ hybridization in mandarin fish (Siniperca chuatsi) revealed the presence of Ig transcripts in HK (20 days post hatch (dph)) and spleen (26 dph), while it was markedly postponed in the intestine and gills (approx. 90 dph) (Tian et al., 2009). Notably, the ontogeny of B cells seems to be correlated with the ecological strategies adopted by freshwater versus marine fish, and thus, the Ig-producing cells appear earlier in freshwater species than in marine fish (Salinas et al., 2011; Solem and Stenvik, 2006; Zapata et al., 2006).
4.2. Teleost B cell populations
As discussed above, teleost express three different Ig heavy chains IgM, IgT/Z and IgD. Despite the presence of activation-induced cytidine deaminase (AID), which facilitates the class switch recombination in higher vertebrates, the structure of the heavy chain locus does not allow this event in fish, that is, the expression of IgM impedes the generation of IgT/IgZ transcripts, and vice versa (Danilova et al., 2005; Hansen et al., 2005). Based on surface Ig expression, three major the teleost B cell populations have been identified thus far: i) the most abundant population composed of IgM+/IgD+ B cells; ii) a population of IgM-/IgD+ B cells described thus far only in channel catfish and European rainbow trout (Castro et al., 2014; Edholm et al., 2010); iii) a population of B cells solely expressing surface IgT/Z found in the zebrafish and rainbow trout (Page et al., 2014; Schorpp et al., 2006; Zhang et al., 2010). IgM+ B cells comprise the majority of B cells in systemic lymphoid organs such as head kidney or spleen, and represent also the majority of B cells in blood and the peritoneal cavity; in contrast IgT+ B cells outnumber the IgM+ cells in gut, skin, gill and nose MALTs (Tacchi et al., 2014; Xu et al., 2016; Z. Xu et al., 2013; Zhang et al., 2010).
4.3. Gut B cells
Thus far, a total of two B cell subsets have been described as resident in the intestine of teleost fish, IgT+ B and IgM+ B cell (Parra et al., 2015; Salinas et al., 2011). Although primarily located in the LP of both anterior and posterior intestine, these cells can infiltrate the epithelium after parasitic infection (Zhang et al., 2010), or vaccination (Ballesteros et al., 2013). Compared with the systemic lymphoid organs, such as spleen and head kidney, the percentage of B cells in GALT is lower, accounting for about 2–12% of isolated leukocytes in rainbow trout (Zhang et al., 2010), common carp (Rombout et al., 1998), or sea bass (dos Santos et al., 2000, 1997). In rainbow trout, ~54% of the B cells in the gut of naïve fish are IgT+ while the rest are IgM+ (Zhang et al., 2010). In catfish, where IgT has not been identified thus far, B cells were found in all segments of the intestine (Hébert et al., 2002). However, while the presence of IgD+/IgM−B cells was reported in peripheral blood leukocytes of this species (Edholm et al., 2010), no IgD single positive cells nor secreted IgD has been thus far reported for the intestine or gut secretions.
Teleost Ig is secreted primarily by the plasmablast and plasma cell-like cells, which are located mainly within the head kidney (Zwollo et al., 2010, 2005). Characterization of these populations, as well as that of early stages of B-cell development, has been performed using specific markers, including the Pax5, HCmu and Rag1 (Zwollo et al., 2010, 2008). The presence of plasma cell-like cells in the intestine has been confirmed in a number of species. In earlier studies in common carp, the staining of gut intraepithelial leukocytes for membrane and cytoplasmic immunoglobulin implied that the majority of Ig+ cells had a rim of Ig+ cytoplasm and a minor amount of membrane Ig, suggesting that these are small plasma cell-like cells (Rombout et al., 1993). Similarly, plasma cell-like cells were identified in the intestine of carp and rainbow trout following vaccination (Davidson et al., 1993; Joosten et al., 1997). However, these detailed analyses were performed only with IgM+ B cells and little is known about the existence of gut IgT/Z+ B plasma cell-like cells. In that regard, head kidney IgT+ cells in trout have been shown to increase in size significantly upon stimulation with a vibrio bacterin. Moreover, the capacity of these large IgT+ B cells to produce IgT is significantly superior to that of IgT+ B lymphocytes, thus supporting the notion that IgT+ plasmablast and/or plasma cell-like cells do exist (Zhang et al., 2010). In addition, the same study showed immunohistochemistry images in which IgT+ cells seemed to be secreting IgT (Fig. 1). Whether these cells represent plasma cell-like cells will require further studies. It has been suggested however that teleost intestine has a limited number of classical plasma cells and that they are not easily detectable in the mucosal tissues (Rombout et al., 2014; Salinas, 2015).
Fig. 1.
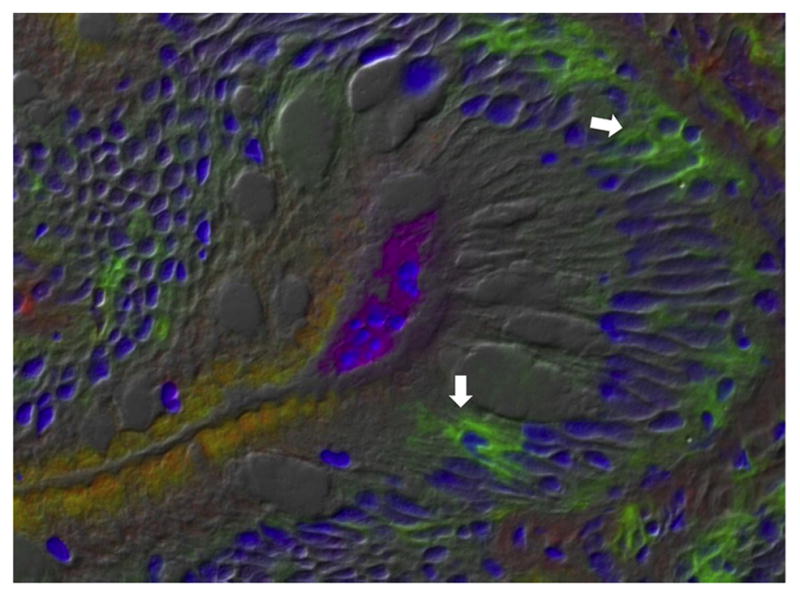
IgT+ cells secreting IgT. Differential interference contrast image showing a trout gut cryosection from a fish infected with the parasite Ceratonova shasta. Arrows point to IgT+ cells (green) that appear to be secreting IgT. A parasite (magenta) can be observed within the luminal area. Nuclei (blue) are stained with DAPI. Image was taken from (Zhang et al., 2010) and reproduced with permission of Nature Immunology.
The homing mechanisms of B cells and plasmablasts to the gut remain largely unknown. It has been proposed that the lack of organized inductive sites may imply a more simple mechanism of function for teleost MALT (Salinas, 2015). Some light was shed on that matter with the demonstration that CCL25/CCR9 signaling is required for IEL homing to the hindgut epithelium of sea bass (Galindo-Villegas et al., 2013). Similarly, in rainbow trout, several chemokines such as CK9 and CK10 has been proposed to be involved in the recruitment of B cells to the intestine following immunization (Ballesteros et al., 2014). However, the existence of the CCL28/CCR10 axis, which mediates the homing of plasmablasts to the intestine of mammals (S. Hu et al., 2011), remains to be determined in teleosts. The recent identification of fish orthologues of CCR10 (Bird and Tafalla, 2015), and the description of CCL28 in salmon and zebrafish provides some promising advances towards a better understanding of B cell homing (Leong et al., 2010; Nomiyama et al., 2013). A better understanding of B cell homing mechanisms in the gut is likely to contribute to a more targeted approach for the rational design of mucosal vaccines and the maintenance of immune memory.
5. Mucosal B cell responses in gut
Responses to parasites, viruses or bacteria mediated by B cells in the gut have been described in several fish species (Table 3) (Fig. 2). The importance of B cells is recognized by their ability to become antibody-secreting cells (ASC), producing immunoglobulins that will target potential pathogens. In addition, B cells of teleost fish have been reported to exhibit additional immunological functions such as phagocytosis and the ability to kill engulfed bacteria (Øverland et al., 2010; Li et al., 2006; Nagasawa et al., 2014; Rønneseth et al., 2015; Zhang et al., 2010). Since mammalian phagocytic B-1 B cells of mammals have a high capacity to present particulate antigen to T cells, the possibility exists that phagocytic B cells of fish play a similar role. In that regard, it has been hypothesized that fish B cells may also play a role similar to that of dendritic cells and thus, act as a bridge between innate and adaptive immune responses (Parra et al., 2015; Zhu et al., 2014). However, phagocytic and microbicidal capacities have only been reported for fish B cells of systemic lymphoid organs, and whether B cells from mucosal sites have a similar potential remains to be determined (Salinas et al., 2011). As previously indicated, in addition to playing a role against pathogens, mucosal immunoglobulins coat a major portion of the gut microbiota, thus playing a key role in immune exclusion and microbial homeostasis (Cerutti and Rescigno, 2008; Zhang et al., 2010). As previously mentioned, the compartmentalization of IgT and IgM responses in mucosal and systemic sites in fish was first described in the rainbow trout intestine (Zhang et al., 2010). However, whether this role is conserved in other teleosts remains unclear, mainly due to the lack of tools (i.e., antibody reagents) to determine the presence and function of IgT/Z. As a result, most of the immune responses analyzed in intestine regarding B cells and immunoglobulins are limited to IgM, while very few studies have focused on IgT, and none on IgD. Most of the examined immune responses in intestine, and in mucosal tissues in general, have been obtained after mucosal vaccination with different pathogens (Munang’andu and Evensen, 2015; Mutoloki et al., 2015). It is important to point out that the immune response in the gut will present great variability among the teleosts, depending on the pathogen, fish species, region of the intestine selected for study, and even the microbiota in the environment, or diet.
Table 3.
Teleost intestinal and systemic B cells and antibody responses after infection or mucosal vaccination. IHC: Immunohistochemistry; i.p.: intraperitoneal.
| Pathogen | Host (route administration) | B Cell regulation | Tissue analyzed | Procedure | Reference |
|---|---|---|---|---|---|
| Parasite | |||||
| Enteromyxum scophthalmi | Turbot | Upregulation of Ig-related genes expression | Pyloric caeca | RNA-seq | Robledo et al., 2014 |
| Increment of IgM+ B cells | Anterior intestine | IHC | Bermúdez et al., 2006 | ||
| Enteromyxum leei | Gilthead sea bream | Upregulation of IgM expression | Posterior intestine | RT-qPCR | Estensoro et al., 2012 |
| Increment of IgM+ B cells | Posterior intestine | IHC | Estensoro et al., 2012 | ||
| Upregulation of IgM and Ig-related genes expression | Posterior intestine | Microarray | Calduch-Giner et al., 2012 | ||
| Increment of IgM+ B cells | Posterior and anterior intestine | IHC | Estensoro et al., 2014 | ||
| Ceratonova shasta | Rainbow trout | Increment of IgT+ B cells | Midgut | IHC | Zhang et al., 2010 |
| Specific IgT in gut mucus | Gut mucus | ELISA | Zhang et al., 2010 | ||
| Chinook salmon | Increment of Ig+ cells | Midgut and anterior gut | IHC | Bjork et al., 2014 | |
| Bacteria | |||||
| Yersinia ruckeri | Rainbow trout (immersion) | Upregulation of IgM and IgT expression | Midgut | RT-qPCR | Evenhuis and Cleveland, 2012 |
| Aeromonas hydrophila | Carp (oral) | Specific IgM | Gut mucus | ELISA | Tu et al., 2010 |
| Seum | ELISA | ||||
| Aeromonas salmonicida | Rainbow trout (oral intubation) | Antibody-secreting cells | Intestine | ELISPOT | Davidson et al., 1993 |
| Flavobacterium psychrophilum | Rainbow trout (i.p.) | Upregulation of IgM expression | Midgut | RT-qPCR | Evenhuis and Cleveland, 2012 |
| Piscirickettsia salmonis | Atlantic salmon (oral boost) | Specific IgM | Serum | ELISA | Tobar et al., 2015 |
| Vibrio anguillarum | Turbot (oral) | Increment mIgM and sIgM expression | Hindgut | RT-qPCR | Gao et al., 2014 |
| African catfish (oral) | Specific IgM | Bile | ELISA | Vervarcke et al., 2005 | |
| Virus | |||||
| IPNV | Rainbow trout (antigen oral) | Increment IgT+ and IgM+ cells | Pyloric caeca | IHC | Ballesteros et al., 2013 |
| Rainbow trout (antigen oral) | Upregulation IgM and IgT expression | Pyloric caeca | RT-qPCR | Ballesteros et al., 2013 | |
| Rainbow trout (infection oral) | Increment IgM+ cells | Pyloric caeca | IHC | Ballesteros et al., 2014 | |
| CHNV | Carp (oral) | Specific Ig response | Serum | ELISA | Sato and Okamoto, 2010 |
| ISAV | Atlantic salmon (oral boost) | Specific IgM | Serum | ELISA | Tobar et al., 2015 |
Fig. 2.
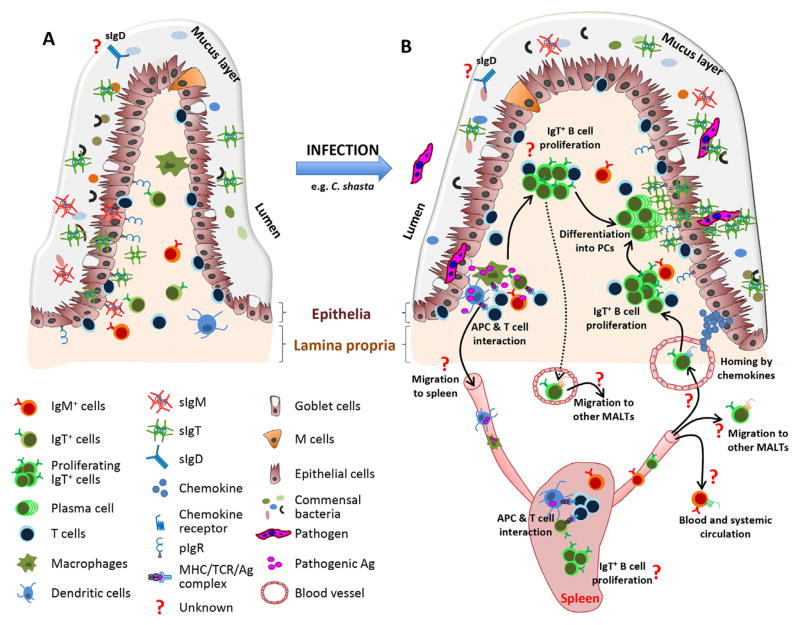
Induction of IgT in the teleost GALT. (A) Scheme of a typical intestinal villus structure in naïve and healthy teleost fish. The number of IgT+ and IgM+ B cells in healthy fish is low and they are typically located in the lamina propria. IgM and IgT are produced by IgM- and IgT-secreting B cells, respectively, and transported from the epithelium into the luminal mucus layer via pIgR. The secreted IgT coats the majority of microbiota in the gut lumen while IgM also contributes to the coating although to a lesser degree. Whether IgD contributes to the recognition of gut microbiota remains to be evaluated. (B) Recent findings based on the rainbow trout model have suggested several models for the induction of mucosal IgT immune responses upon pathogen infection. In the lamina, propria antigen from the pathogen can be taken up by the antigen-presenting cells (APCs) and presented to CD4+ T cells. IgT+ B cells which are activated by Ag-specific CD4+ T cells start to proliferate locally and differentiate into plasmablasts or plasma cell-like cells (PCs). These differentiated IgT-secreting cells can produce large amounts of antigen-specific IgT. The possibility exists that upon antigen uptake, loaded gut APCs (i.e., B cells, dendritic cells, macrophages) may migrate into the spleen or head kidney where they will present antigen to either naive or memory CD4+ T cells. Thereafter, the resulting activated IgT+ B cells may then home into the gut. Alternatively, antigens from the parasite may travel from the gut into the lymphoid tissues through the vascular system, where they may be taken up by systemic APCs to initiate an immune response. If immune responses develop in the spleen or head kidney, we hypothesize that IgT+ B cell proliferation may occur in those lymphoid organs. Alternatively, activated IgT+ B cells would be generated in the spleen or head kidney, where they are imprinted to home into the gut, where they may then proliferate. On the other hand, the systemic activated IgT+ B cells may also migrate to other teleost MALT, in addition to the GALT. Conversely, the possibility exists that activated IgT+ B cells generated in the gut may traffic to other GALTs. In these different scenarios, homing of IgT+ B cells to these different tissues is likely to be mediated by the same or different chemokine/chemokine receptor pairs. Our results suggest that IgT+ B cells may express a specific chemokine receptor required for their homing into the gut and perhaps other MALTs; whereas IgM+ B cells may express a different chemokine receptor from that of IgT+ B cells, and thus, they may not have the ability to home to MALTs upon pathogenic challenge.
5.1. Responses to parasites
Although most parasite infections do not lead to death, the impact of these pathogens over the animal’s welfare and its impact on economic value is enormous. For example, protistan and metazoan parasites produced losses of more than $700 million in the salmon industry worldwide during the last years (Shinn et al., 2015). A better understanding of the immune response and a better design of prophylactic measures against various parasites are needed in the aquaculture sector. Several phyla of parasites are capable of infecting the teleost intestine (Alvarez-Pellitero, 2008), and here we will focus our attention on the most important for the aquaculture industry.
Parasites from the phylum Myxozoa are capable of infecting all mucosal surfaces in many fish species (see review Gómez et al., 2014). Among the different enteric species, Enteromyxum spp. is the most infective and Ceratonova shasta (C. shasta) is one of the most extensively studied.
Enteromyxum leei (E.leei) is an enteric parasite that produces important outbreaks in Mediterranean aquaculture. Humoral specific responses mediated by antibodies together with the presence of B cells and the expression of immune-related genes have been described in several studies concerning E. leei infection. Thus, in gilthead seabream (Sparus aurata) IgM expression increased over time after parasite infection in the posterior intestine, correlating with an increase in the number of IgM+ cells in the same area of the intestine after more than four months of infection (Estensoro et al., 2012). Interestingly, authors reported an increase of IgM+ plasma cells in the seabream posterior intestine after infection, based on morphological observation. However, to date, no functional studies have been published regarding the presence of bona fide plasma cell-like cells in E. leei infected fish. Similar upregulation of IgM gene expression after E. leei infection was observed by Calduch-Giner et al. (Calduch-Giner et al., 2012). In both studies, the introduction of a diet with vegetable oils instead of a standard diet with fish oils, lead to a boost in IgM upregulation after parasite infection, indicating the importance of diet in determining gut immune responses. Different commensal communities that accompany the two diets could explain these changes in IgM expression. In a recent publication, an increase of IgM+ B cells in the posterior intestine, and to a lesser degree in the anterior intestine after E. leei anal infection was also described, albeit the number of B cells increased 40 days post-infection (Estensoro et al., 2014). Thus, from these three studies we can conclude that immune responses to a natural infection in the intestine is delayed up to 3 month post infection, while a direct delivery of the parasite into the intestine produces a much faster response at the mucosal site, reducing by almost one third the time required to enhance IgM+ cell numbers. Notably, in the later study, authors observed an increase in lymphocyte-like cells that were IgM− in the LP of infected fish (Estensoro et al., 2014). Therefore, it is conceivable that these cells are IgT+ B cells and that they expand or infiltrate after parasite infection, as previously described in trout (Zhang et al., 2010). Alternatively, those cells could be also T-lymphocytes. It is worth noting that to our knowledge, no specific antibody responses against E. leei have been described in teleosts, although the involvement of IgT in such responses remains to be evaluated. Of further note, E. leei infects other fish species, such as tiger puffer (Takifugu rubripes) and Japanese flounder (Paralichthys olivaceus), but little is known about the importance and regulation of B cells in these species after infection.
Another important myxozoan parasite with tropism for the intestine is Enteromyxum scophthalmi (E. scophthalmi). Similar to the aforementioned immune response against E. leei, IgM+ B cells increase in the intestine of turbot after E. scophthalmi infection (Bermúdez et al., 2006). Interestingly, in the early stages of the infection Ig+ cells increase in spleen and head kidney, but as the infection progresses this increase is only observed in the intestine, while in spleen and head kidney the number of Ig+ cells decreases. This indicates the importance of B cell responses in the progression of the infection in intestine and also suggests a clear role of Ig+ cells for parasite clearance and animal survival. Although humoral specific responses have not been described in the intestine of E. scophthalmi-infected fish so far, specific antibodies have been observed in serum of long-term infected fish (Sitjà-Bobadilla et al., 2004). In this study, serum from 3 months-long infected fish was analyzed for specific antibodies against E. scophthalmi by ELISA and immunohistochemistry (IHC). It is important to point out that in later studies published by the same group, the authors could not detect any specific titer for IgM in serum from fish infected for less than 2 months (Sitjà-Bobadilla et al., 2006). Thus, these differences may be due to the slow adaptive response that is known to occur in teleosts. However, whether the late increase of mucosal B cells after infection, as observed in E. leei infection (Estensoro et al., 2014), controls the humoral immune response, also in the systemic compartment, is a likely scenario that needs to be studied further. Interestingly, survivor fish (around 4 months post infection) appear to be protected against a secondary infection with E. scophthalmi due to some innate parameters, but mainly by the presence of specific antibodies (Sitjà-Bobadilla et al., 2007). In a recent study in turbot infected with high doses of E. scophthalmi, RNA-seq showed an increase in Ig-related genes in the pyloric caeca region 42 days post oral infection (Robledo et al., 2014), corroborating the late importance of B cells in parasite infection.
Another myxozoan parasite widely studied is C. shasta. Contrary to the previously described Enteromyxum parasites, C. shasta infects freshwater fish and requires the presence of a polychaete worm, which acts as vector, to complete its life cycle (Bartholomew et al., 1997). A large accumulation of IgT+ B cells has been described in the LP of the intestines of infected fish, which is significantly more pronounced in survivor fish 3 months after infection (Zhang et al., 2010). Interestingly, IgM+ cells did not increase in the intestine of infected, or survivor fish. Whether these IgT+ cells accumulations are the result of IgT+ B cell recruitment from the systemic lymphoid tissues (e.g., spleen), or the result of in situ B cell proliferation, remains to be determined. One of the chemokines that could be implicated in B cell trafficking on mucosal sites is CCR7. Significantly, expression of CCR7 in infected fish with C. shasta is upregulated in both IgM+ and IgT+ B cell subsets in the intestine (Ordás et al., 2012). However, this upregulation in CCR7 expression was observed only in splenic IgT+ B cells and not in IgM+ B cells, thus indicating a possible recruitment of IgT+ B cells from spleen to the gut in infected fish. Nonetheless, the fact that IgM+ B cells in the intestine upregulated CCR7 expression may suggest that parasite infection induces CCR7 expression in resident IgM+ B cells. Clearly, more studies arewarranted to understand the role of CCR7 in B cell trafficking in fish. Interestingly, this increase in intestinal B cells after parasite infection has been further supported by using a pan-B cell marker in Chinook salmon (Bjork et al., 2014). Two different strains of Chinook salmon were used in this experiment, one more susceptible and another resistant to C. shasta. Both strains presented aggregations of B cells in the intestine after infection, but surprisingly, in the susceptible strain, these accumulations appeared in an earlier stage of the infection than in the resistant strain. Probably a more effective innate barrier in the resistant strain delayed both parasite entry and parasite load, and, as a consequence, recruitment of B cells to the intestine is delayed too. Apart from this accumulation of cells, a humoral specific response takes part in trout intestines after C. shasta infection. Thus, specific IgT to C. shasta was detected in gut mucus from infected and survivor fish (Zhang et al., 2010) whereas no IgM titers were detected. Conversely, specific IgM titers against the parasite were observed only in serum, but not in gut mucus, from infected and survivor fish.
Several other enteric parasites can affect teleosts, but involvement of B cells in many of these infections is unknown (Alvarez-Pellitero, 2008). Additionally, although the significance of B cells is clear in parasitic infections, the role of these cells and secreted immunoglobulins is still unresolved. Thus, further studies are needed to clarify the role of immunoglobulins and B cells in gut immunity against parasites.
5.2. Responses to bacteria
Although bacterial infections are one of the main problems in the aquaculture industry, very few studies regarding the intestinal response to bacterial pathogens after natural infections can be found in the literature. Additionally, the number of studies performed in the laboratory under controlled conditions is low, prompting the need for more in-depth experiments to determine the role of GALT in bacterial infections.
Enteric redmouth disease (ERM or yersiniosis) is one of the most important diseases of salmonids and is caused by a Gram-negative enterobacterium, Yersinia ruckeri (Y. ruckeri). Its preferential mode of entry is through the gills, but it has also been described to infect the intestine through the mucosal tissue (Ohtani et al., 2014; Tobback et al., 2009, 2010). Despite these findings, few studies have described the immune response against ERM in fish GALT. Alterations in gene expression in the intestine were described after immersion challenge with the Y. ruckeri strain YRNC10-gfp (Evenhuis and Cleveland, 2012). In this study, early responses (up to 10 days post-immersion) were measured by expression analysis of several genes. Among them, IgM and IgT were upregulated, indicating the participation of both immunoglobulins in the innate responses in the intestine after bacterial infection by immersion. Interestingly, in the same study, only IgM, but not IgT, expression varied in the first 10 days after Flavobacterium psychrophilum strain CSF-259-93 infection by intraperitoneal (i.p.) injection. Differences observed in the response against both types of bacteria could be due to the pathogen itself, but also because of the route of infection, since the mucosal route induced an IgT upregulation that could not be produced by the systemic route, where IgM lead the response. This data further confirms that IgT is a dedicated mucosal immunoglobulin in the gut.
Vaccination trials with inactivated Y. ruckeri have been conducted using mucosal routes for administration. Immersion vaccines have been tested with positive results showing protection against a posterior challenge and IgM specific antibodies in serum against the bacteria (Raida et al., 2011). Similarly, oral and anal vaccination against ERM with inactivated Y. ruckeri protects the animal against a subsequent infection with the pathogen (Gravningen et al., 1998; Villumsen et al., 2014). Strikingly, no specific IgM responsewas detected in serum of infected, or survivor fish after the infection, indicating the possibility that protection was not induced by antibodies, or alternatively, that IgT was involved in protective responses, as IgT responses were not measured. A similar result was obtained in a previous experiment, where after oral vaccinated fish were protected against bacteria, while no serum IgM titers were detected (Gravningen et al., 1998). However, immune responses in the intestine were not analyzed in any of these works. Whether specific antibodies in gut mucus, IgM or IgT, were present and conferred protection to the fish is a possibility that requires further investigation.
Using a different approach, a recent publication presented evidence that oral administration of Piscirickettsia salmonis (P. salmonis) vaccine to Salmo salar appeared to increase specific IgM antibodies in serum produced by a previous injection vaccine (Tobar et al., 2015). Notably, oral administration of P. salmonis vaccine, apart from increasing the humoral response in serum against the pathogen, also seemed to extend the time of protection that the injectable vaccine was inducing alone. Thus, the idea of using oral administration as a booster for vaccination could be very profitable for the aquaculture industry as its cost is low. However, the mechanism implicated in the mucosal response after boosting needs to be addressed further to understand and improve this vaccination strategy.
Another important bacterial pathogen that is capable of infecting and entering the teleost intestine is Vibrio anguillarum (V. anguillarum) (Grisez et al., 1996; O’Toole et al., 2004). Involvement of B cells in the immune responses after vibrio infection or vaccination has been described in several studies. Bath challenged turbot (Scophthalmus maximus) with a live vaccine for V. anguillarum presented the highest levels of IgM expression in the intestine 28 days after administration. Interestingly, analysis of secretory and membrane-bound IgM suggested the involvement of B cells at both humoral and cellular levels in V. anguillarum infection in the intestine (Gao et al., 2014). Another vibrio bacterin was tested in African catfish (Clarias gariepinus) by different routes: anal, oral, immersion and i.p. (Vervarcke et al., 2005). Although gut mucosal responses were not measured, specific Ig responses detected in bile reflected the response in the gut. The importance of route and amount of antigen in the B cell response is clearly reflected in this study. Therefore, administration of vaccine by anal intubation was the route that offered the best IgM response in bile and also in skin mucus, whereas oral administration through the mucosal route had the lowest IgM response. These results suggest that a high dose of antigen/bacterin is important for vaccine success, as in anal intubation the amount of antigen that reaches the gut is much higher than by oral administration. Additionally, early studies in carp demonstrated that the second segment of the intestine was the area with higher antigen uptake (Rombout et al., 1989), and Fuglem et al. suggested the presence of M-cells as antigen–sampling cells in the same part of the intestine (Fuglem et al., 2010). Therefore, anal intubation delivers the antigen majorly to that area of the intestine, producing a better immune response than the oral route.
Taken together, it is clear, as initial studies already demonstrated (Johnson and Amend, 1983), that vaccination through intestinal routes (anal and oral) protects the animal against posterior infections by Y. ruckeri and V. anguillarum, and that B cells in GALT might be capable of producing specific antibodies against those bacteria. Surprisingly, the intestinal responses in these processes are still poorly understood, and the role of intestinal B cells (IgM+ and/or IgT+ B cells), although undoubtedly necessary, needs to be delineated.
5.3. Responses to viruses
Our understanding of the immune response in intestinal mucosa after viral infection is still largely unknown. Even in the systemic compartment, our knowledge on the role of B cells and Igs upon viral challenge is still very fragmented. It is well-documented that protection of fish against viruses is not always correlated with the levels of specific antibodies detected in the serum of infected hosts (Kim et al., 2016). However, the importance of mucosal antibodies in viral defense has been tested in very few studies so far. One of these studies examined mucosal responses after infection with infectious hematopoietic necrosis virus (IHNV). The authors observed that mucosal Igs were not important for the protection of the animal, as neutralizing antibodies were not detected in the skin, or gut mucus, while IgM neutralizing antibodies were observed in serum (Cain et al., 1996). On the other hand, innate functions of B cells, such as phagocytosis (Li et al., 2006; Zhang et al., 2010) and cytokine production (Takizawa et al., 2013), has not yet been studied during viral infection in teleosts.
In anothermucosal infection study, recruitment of B cells to the intestine was observed after oral administration of infectious pancreatic necrosis virus (IPNV) VP2 antigen (Ballesteros et al., 2013). IgT+ and IgM+ B cell numbers increased in the pyloric caeca after oral vaccination with alginate encapsulated antigen in trout. The accumulation of cells is corroborated with an increase in IgT and IgM expression in the same segment of the intestine. However, the authors did not find any increase in the expression of secreted IgM in any of the gut segments, indicating that B cell importance might be other than acting as antibody-secreting cells. Authors also described an upregulation of transcription factor blimp-1 in the pyloric caeca after vaccination, suggesting a probable differentiation of B cells into plasma cells (Shaffer et al., 2002). Similar aggregations of IgT+ and IgM+ cells in trout intestine were observed in fish experimentally infected by immersion with IPNV (Ballesteros et al., 2014). Additionally, upregulation of the expression of chemokines implicated in lymphocyte recruitment, such as CCR7, were detected in the intestine after infection, perhaps suggesting a systemic origin of responding B cells present in intestine.
Another experimental oral vaccine based on inactivated crucian carp hematopoietic necrosis virus (CHNV) elicited a potent antiviral response, although IgM responses wereweak and onlymeasured in serum (Sato and Okamoto, 2010). More recently, oral boosting (after injection vaccination) of salmon with a vaccine against infectious salmon anemia virus (ISAV) increased serum-specific IgM in the long term and also increased the time of protection of the previously injected vaccine (Tobar et al., 2015). In the same study, similar results were presented with a bacterial pathogen, promoting the use of oral boosting, and also oral vaccination, as a very good option in aquaculture. The aforementioned studies did not evaluate IgT specific responses, thus, the possibility exists that some of the observed protective responses were due to the induction of pathogen-specific IgT in the gut mucosa.
In conclusion, although B cell responses are induced upon viral infection or vaccination, their specific roles in protection in the gut require to be further studied.
5.4. Responses to probiotic bacteria and microbiota
Pathogenic bacteria are not the only bacteria that elicit immune responses in the intestine, probiotic bacteria appear to enhance immune responses. For that reason, probiotics are being added as a food supplement with the goal to improve fish health. Few studies have evaluated the impact and regulation of Igs after probiotic administration. Most of these studies showed an increase in IgM secretion and/or expression after probiotic administration (Picchietti et al., 2007; Sun et al., 2010). However, a downregulation in IgM has also been reported recently in seabream fed with probiotics (Guzmán-Villanueva et al., 2014). Interestingly, a posterior study, also in Sparus aurata and using the same probiotic encapsulated with alginate, revealed an increase in IgM (Cordero et al., 2015). As mentioned previously, the importance of the dose given to the animal is reflected in these results; the protective effect of the probiotic lead to an increased response, probably due to the increase in the number of bacteria delivered safely to the intestine through encapsulation.
Pathogenic bacteria and probiotics will interact with commensal bacteria in the intestines of teleosts. The balance between these populations is crucial for the maintenance of homeostasis in the intestine. The influence of commensals on mucosal lymphoid tissue development and function, and consequently on disease susceptibility, has been largely demonstrated (Elson and Alexander, 2015; Maynard et al., 2012; Palm et al., 2015). The composition of the microbiota is strongly correlated with a number of factors such as the environment, diet, and genetic backgrounds. Fish intestine harbors approximately 107–108 bacteria per gram, represented by 500 species, consisting mainly of aerobic and facultative anaerobe organisms (Austin, 2006; Gomez and Balcazar, 2008; Lowrey et al., 2015; Pérez et al., 2010). As shown by studies in various species across the animal kingdom, the microbiota is essential for optimal host physiology (Sommer and Bäckhed, 2013; Wesemann, 2015). The conserved nature of these observations was demonstrated by experiments on germ-free zebrafish, which exhibited impaired epithelial renewal and enterocyte morphology, nutrient digestion and innate immune functions (Kanther and Rawls, 2011; Rawls et al., 2004). However, while colonization with normal microbiota has a positive effect on host immune regulatory functions, any disturbance by stress, antibiotic treatment and other factors may contribute to the development of disease (Pérez et al., 2010). Not surprisingly, the manipulation of microbiota has recently received more attention and these new findings have been summarized in a number of extensive reviews (Lazado et al., 2014; Montalban-Arques et al., 2015; Nayak, 2010). Despite recent progress, the influence of microbiota composition on mucosal B lymphocytes of teleost remains largely unknown.
Regarding intestinal B cells in mammals, commensal bacteria are vital for B cell function. Differentiation into IgA plasma cells is regulated by gut microbiota (Uematsu et al., 2008), and a lack of microbiota leads to a drastic decrease in IgA production (Cerutti and Rescigno, 2008). However, the difficulty of generating pathogen-free fish makes investigations on the role of the microbiota on B cells in the teleost intestine challenging. As mentioned previously, only gnotobiotic zebrafish have been studied, which revealed a relationship between microbiota and the innate immune response in the intestine (Rawls et al., 2004). However, no studies concerning the status of B cells in gnotobiotic fish have been published.
Recently, it has been suggested that microbiota plays a pivotal role in shaping the Ig repertoire during a short window of time early in life (Wesemann, 2015). However, these changes take place in secondary structures such as PP, or avian bursa, and involve AID-mediated gene conversion and somatic mutations (Perey et al., 1970; Vajdy et al., 1998). Since these structures and functions are not present in teleosts, it remains to be determined whether the bacteria have a similar importance in shaping the repertoire of the fish. In that regard, one study characterized the zebrafish IgM repertoire during the development and revealed that the IgM+ B cell repertoire undergoes a dramatic restructuring before the fish reaches sexual maturity. Thus, many of the VDJ combinations observed in 2-week old fish disappeared by 1 month of age (Jiang et al., 2011). It should be clarified that the authors used whole fish for their transcript analyses. However, the mechanism underlying these changes in repertoire at different ages, remain to be investigated.
IgT, as stated previously, is the functional homolog of mammalian IgA, and it is implicated in the maintenance of intestinal homeostasis (Zhang et al., 2010). IgT is capable of recognizing and coating bacterial microbiota in the gut lumen, thus, playing a key role in immune exclusion (Zhang et al., 2010). In that regard, it was observed that almost half of the microbiota present in the gut lumen were coated with IgT, while IgM bound to ~25% of the bacteria. Thus, these two immunoglobulins are likely to prevent microbial microbiota and pathogens from gaining access into the intestinal epithelium, although very little is known about the details of this process. Interestingly, very few bacteria were coated with both immunoglobulins, indicating a plausible selective binding of both immunoglobulins to different bacteria strains, or disparate epitopes. However, it is worth pointing out that the gut presents the lowest bacterial diversity among the four MALTs (GIALT, SIALT, GALT, NALT) in trout (Lowrey et al., 2015) and hence, this might be a factor influencing the aforementioned observations regarding immunoglobulin coating of the GALT microbiota. In contrast, the microbiota of both the trout GIALT and NALT present a significantly higher percentage of microbiota coated with more than one immunoglobulin isotype (Tacchi et al., 2014; Xu et al., 2016). Further investigation on this fascinating dissimilar binding to microbiota in the different trout MALTs may shed light on the specific mechanisms used by teleosts to maintain homeostasis in the gut.
Besides antibody production, a subset of B cells contributes to gut homeostasis through production of a considerable amount of cytokines and chemokines, including the tolerogenic IL-10 (Hamze et al., 2013). Generation of these B cells, which in higher vertebrates restrain excessive immune responses via the release of IL-10, is promoted by the gut microbiota (Rosser et al., 2014). IL-10 producing B cells have been recently described in trout (Takizawa et al., 2013). Whether those IL-10 producing B-lymphocytes contribute to homeostasis in the intestine is still unknown.
6. Rainbow trout as a model for intestinal immune responses
Evolutionarily, teleost fish are the earliest vertebrates with bona fide B cells with compartmentalized immunoglobulin responses in systemic and mucosal sites. Teleosts are capable of mounting an efficient adaptive humoral immune response (i.e., IgM, IgT) that protects the animal against external pathogens. However, the lack of organized structures such as germinal centers or lymph nodes in their MALT may reduce the interaction of B cells with APCs, as evidenced by the relatively slow response speed and absence of affinity maturation in the antibody response to pathogens. Although more “primitive”, immune responses mediated by B cells in teleost intestine are in many respects, similar to those produced in higher vertebrates. PP are the major site for IgA production in the intestine of mammals. However, PP are not indispensable for B cell function in their intestine, as IgA responses are still observed in mice lacking PP (Kang et al., 2002; Pabst, 2012). Importantly, IgT production and function in teleost intestine is always generated extrafollicularly. Thus, results derived from the study of IgT responses in teleost fish could shed light on the understanding of extrafollicular IgA production induced in the intestine of mammals.
Innate functions of teleost B cells have been demonstrated in several species. Thus, phagocytic B cells have been described in rainbow trout, Atlantic salmon, Atlantic cod, ginbuna carp, flounder and lump fish (Øverland et al., 2010; Li et al., 2006; Nagasawa et al., 2014; Rønneseth et al., 2015; Zhang et al., 2010). Also, teleost B cells produce a large quantity of polyreactive antibodies (natural antibodies) implicated in several immune functions (Magnadottir, 2006; Magnadottir et al., 2009). Interestingly, these innate features have been conserved throughout evolution (Parra et al., 2013). Hence, a significant portion of the total IgA present in mouse intestine is polyreactive IgA produced by B-1 B cells, which serves as the first layer of immune defense in the gut mucosa (Suzuki et al., 2010). In addition, this B cell subset has recently been described as phagocytic and, although the function of these cells is as yet unknown, it is likely that they can migrate to the intestine with their phagocytosed cargo and participate in the mucosal immune response (Parra et al., 2013, 2012; Sunyer, 2013). Moreover, it has been hypothesized that these phagocytic B cells may take up microbiota leaking from the gut lumen, which in turn may contribute to the maintenance of gut homeostasis (Sunyer, 2013). Therefore, it is clear that mammalian B-1 B cells and teleost B cells share innate features that are likely to be implicated in intestine immune responses. Notably, B-1 B cells have been recently described in human blood (Griffin et al., 2011), although this finding is controversial and their phagocytic capability has not been studied so far. As such, knowledge resulting from the analysis teleost B cell innate functions should provide insight for elucidation of the role of the mammalian B-1 B cell subset in intestine immunity (Sunyer, 2013).
7. Concluding remarks
Despite findings that have shed some light on the effector functions of teleost B lymphocytes, our understanding of the mechanisms underlying their development and action is far from complete. In many teleost species, the absence of tools limits identification of B cells in the intestine. Also, despite the substantial amount of immunoglobulin in the gut mucus, very little is known about the biology of antibody secreting cells and the spatial analysis of the mucosal area within which B and T cell interactions occur. As in mammals, the teleost GALT harbors an abundant population of T cells able to mount specific adaptive responses towards infection, or vaccination (Koppang et al., 2010; Takizawa et al., 2011). However, in mammals, this interaction occurs mainly in the PP, where antibody class switching takes place, while teleost fish lack these organized structures. Thus, study of extrafollicular T-dependent and independent responses in the intestine of teleosts not only will improve our knowledge for vaccine formulation, but will also serve as a model for mammalian mucosal immunology.
Although compartmentalization of intestinal and systemic responses after parasite infection in fish has been demonstrated (Zhang et al., 2010), the role of intestinal IgT in bacterial or viral infections is still not well characterized. As previously mentioned, IgT has not been found in all analyzed species. Thus, catfish and medaka fish appear to lack IgT, and therefore, IgM may represent the main mucosal immunoglobulin in these species (Magadán-Mompó et al., 2011; Maki and Dickerson, 2003; Peatman et al., 2015). In addition, the existence of an IgM−/IgD+ population described in catfish with an unknown function (Edholm et al., 2010), opens the possibility of the implication of this subset in mucosal responses. Thus, further investigations are needed in these and other fish species to completely determine the role of IgT, IgM and IgD in intestinal immunity.
As described in section 5, aggregations of IgT+ B cells in the fish intestine have been described after pathogen infection. Whether they undergo differentiation and expansion locally, or alternatively, whether these cells are derived from systemic lymphoid organs is still unknown (see Fig. 2B for the different hypotheses raised regarding the induction of gut IgT upon pathogenic challenge). Homing experiments will answer in the future these questions. However, such experiments are difficult to perform in fish due to the limited availability of isogenic strains to work with. In addition, understanding the chemokines and chemokine receptors potentially involved in the migration and traffic of the gut mucosal B cells is critical to elucidate the fish GALT inductive and effector sites and mechanism. In connection with this, it is worth pointing out that evidence of the existence of a common mucosal immune system (CMIS) in fish is still missing, while in mammals this concept has been confirmed and the stimulation of one mucosal site can also lead to specific IgA or IgM responses in other MALTs (Brandtzaeg and Pabst, 2004; Mestecky, 1987). Thus, further studies are required to increase our knowledge on the presence of a CMIS in fish, with the goal to improve mucosal vaccine formulations and delivery methods. Moreover, the answers to the aforementioned questions will play a central role in further exploring the concepts of mucosal and oral tolerance, also critical for the development of effective mucosal vaccines.
As previously stated, understanding the gut IgT inductive and effector sites, as well as the role of gut IgT+ B cells upon vaccination will be vital for the design of vaccines that improve mucosal stimulation and protection. Vaccination is the best alternative to other therapeutic treatments, such as chemicals or antibiotics, which worsen the quality and safety of fish products for human consumption, and have also a detrimental effect on the environment. How the vaccine is administered (i.e., vaccination route) is an important factor to consider for assuring protection and feasibility of the vaccination process. Although intraperitoneal injection is themost effective vaccination route in providing protection, bath and oral immunization routes would seem as better options, since both vaccination strategies require less handling (implying less stress) and induce very few side effects to the vaccinated fish. Recently, a novel mucosal route of vaccination has been described successfully in rainbow trout, the nasal vaccination (LaPatra et al., 2015; Larragoite et al., 2016; Salinas et al., 2015). However, our knowledge regarding B cell mucosal immunity in response to vaccination is still very rudimentary, and thus, significantly more work is required in this area if we want to develop better oral, bath and nasal vaccine strategies that provide better degrees of protection and memory responses. It is important to mention that similar problems and challenges here described regarding mucosal vaccination in teleost, are also found in humans. This is the reason why very few licensed mucosal vaccines exist for mammalian species (Woodrow et al., 2012). Accordingly, many questions regarding B cell responses after mucosal vaccination in mammas remain to be resolved (Chen and Cerutti, 2010).
Another very important field of study is emerging with the realization of the importance of the microbial communities residing in the intestine. However, despite recent progress in this field, the influence of microbiota composition on mucosal B cell responses in teleosts remains largely unexplored. In addition, since organized lymphoid structures and class switch are not present in teleosts, it remains to be determined whether the microbiota has the same influence in shaping the repertoire of the fish. In spite of the generation of gnotobiotic zebrafish several years ago (Kanther and Rawls, 2011), these questions have not yet been addressed. While gnotobiotic zebrafish are likely to address some of these questions, the generation of germ-free fish in species of special interest for aquaculture will also provide important data regarding the interaction of microbiota with the GALT and resident B cells and, in consequence, will improve our potential to obtain better therapeutic tools.
While important structural differences exist in mucosal tissues between fish and mammals, some fundamental aspects of B cell responses against pathogens at mucosal sites seem to be conserved: (1) all vertebrates have compartmentalized responses with a dedicated immunoglobulin for mucosal responses (IgT, IgX or IgA), (2) after mucosal pathogen infection, an accumulation of B cells occurs in the MALT simultaneously with an increase of pathogen-specific immunoglobulin in mucosal secretions, (3) mucosal immunization produces mucosal and systemic antibody responses. Thus, taken together, the growing body of research suggests that the main mechanisms controlling immune responses at mucosal surfaces have been conserved throughout evolution. Further analysis of such mechanisms in teleost fish will probably identify facets shared by all vertebrates and will also allow for the improvement of vaccination strategies urgently required by the aquaculture industry.
Acknowledgments
This work was supported by the US Department of Agriculture Grant USDA-NRI-2013-01107 to J.O.S., the National Science Foundation Grant NSF-IOS-1457282 to J.O.S., the National Institutes of Health Grant 2R01GM085207-05 to J.O.S., a JSPS Postdoctoral Fellowships for Research Abroad to F.T., and by the European Commission under the Work Programme 2012 of the 7th Framework Programme for Research and Technological Development of the European Union (Grant Agreement 311993 TARGETFISH).
Contributor Information
David Parra, Email: david.parra@uab.cat.
J. Oriol Sunyer, Email: sunyer@vet.upenn.edu.
References
- Aas IB, Austbø L, König M, Syed M, Falk K, Hordvik I, Koppang EO. Transcriptional characterization of the T cell population within the salmonid interbranchial lymphoid tissue. J Immunol. 2014;193:3463–3469. doi: 10.4049/jimmunol.1400797. http://dx.doi.org/10.4049/jimmunol.1400797. [DOI] [PubMed] [Google Scholar]
- Alvarez-Pellitero P. Fish immunity and parasite infections: from innate immunity to immunoprophylactic prospects. Vet Immunol Immunopathol. 2008;126:171–198. doi: 10.1016/j.vetimm.2008.07.013. http://dx.doi.org/10.1016/j.vetimm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Arrieta MC, Finlay BB. The commensal microbiota drives immune homeostasis. Front Immunol. 2012;3:33. doi: 10.3389/fimmu.2012.00033. http://dx.doi.org/10.3389/fimmu.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin B. The bacterial microflora of fish, revised. Sci World J. 2006;6:931–945. doi: 10.1100/tsw.2006.181. http://dx.doi.org/10.1100/tsw.2006.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K, Lencer WI, Blumberg RS. Beyond IgA: the mucosal immunoglobulin alphabet. Mucosal Immunol. 2010;3:324–325. http://dx.doi.org/10.1038/mi.2010.15. [Google Scholar]
- Ballesteros NA, Castro R, Abos B, Rodríguez Saint-Jean SS, Pérez-Prieto SI, Tafalla C. The pyloric caeca area is a major site for IgM+ and IgT+ B cell recruitment in response to oral vaccination in rainbow trout. PLoS One. 2013;8:e66118. doi: 10.1371/journal.pone.0066118. http://dx.doi.org/10.1371/journal.pone.0066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros NA, Rodríguez Saint-Jean S, Pérez-Prieto SI, Aquilino C, Tafalla C. Modulation of genes related to the recruitment of immune cells in the digestive tract of trout experimentally infected with infectious pancreatic necrosis virus (IPNV) or orally vaccinated. Dev Comp Immunol. 2014;44:195–205. doi: 10.1016/j.dci.2013.12.009. http://dx.doi.org/10.1016/j.dci.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Balon EK. Terminology of intervals in fish development. J Fish Res Board Can. 1975;32:1663–1670. http://dx.doi.org/10.1139/f75-196. [Google Scholar]
- Bartholomew JL, Whipple MJ, Stevens DG, Fryer JL. The life cycle of Ceratomyxa shasta, a myxosporean parasite of salmonids, requires a freshwater polychaete as an alternate host. J Parasitol. 1997;83:859–868. http://dx.doi.org/10.2307/3284281. [PubMed] [Google Scholar]
- Bengtén E, Clem LW, Miller NW, Warr GW, Wilson M. Channel catfish immunoglobulins: repertoire and expression. Dev Comp Immunol. 2006a;30:77–92. doi: 10.1016/j.dci.2005.06.016. http://dx.doi.org/10.1016/j.dci.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Bengtén E, Quiniou S, Hikima J, Waldbieser G, Warr GW, Miller NW, Wilson M. Structure of the catfish IGH locus: analysis of the region including the single functional IGHM gene. Immunogenetics. 2006b;58:831–844. doi: 10.1007/s00251-006-0139-9. http://dx.doi.org/10.1007/s00251-006-0139-9. [DOI] [PubMed] [Google Scholar]
- Bengtén E, Wilson M. Antibody repertoires in fish. Results Probl Cell Differ. 2015;57:193–234. doi: 10.1007/978-3-319-20819-0_9. http://dx.doi.org/10.1007/978-3-319-20819-0_9. [DOI] [PubMed] [Google Scholar]
- Bermúdez R, Vigliano F, Marcaccini A, Sitjà-Bobadilla A, Quiroga MI, Nieto JM. Response of Ig-positive cells to Enteromyxum scophthalmi (Myxozoa) experimental infection in turbot, Scophthalmus maximus (L.): a histopathological and immunohistochemical study. Fish Shellfish Immunol. 2006;21:501–512. doi: 10.1016/j.fsi.2006.02.006. http://dx.doi.org/10.1016/j.fsi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Bird S, Tafalla C. Teleost chemokines and their receptors. Biol (Basel) 2015;4:756–784. doi: 10.3390/biology4040756. http://dx.doi.org/10.3390/biology4040756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork SJ, Zhang YA, Hurst CN, Alonso-Naveiro ME, Alexander JD, Sunyer JO, Bartholomew JL. Defenses of susceptible and resistant Chinook salmon (Onchorhynchus tshawytscha) against the myxozoan parasite Ceratomyxa shasta. Fish Shellfish Immunol. 2014;37:87–95. doi: 10.1016/j.fsi.2013.12.024. http://dx.doi.org/10.1016/j.fsi.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1:31–37. doi: 10.1038/mi.2007.9. http://dx.doi.org/10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Pabst R. Let’s go mucosal: communication on slippery ground. Trends Immunol. 2004;25:570–577. doi: 10.1016/j.it.2004.09.005. http://dx.doi.org/10.1016/j.it.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984;311:71–73. doi: 10.1038/311071a0. http://dx.doi.org/10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- Bromage ES, Kaattari IM, Zwollo P, Kaattari SL. Plasmablast and plasma cell production and distribution in trout immune tissues. J Immunol. 2004;173:7317–7323. doi: 10.4049/jimmunol.173.12.7317. http://dx.doi.org/10.4049/jimmunol.173.12.7317. [DOI] [PubMed] [Google Scholar]
- Cain KD, LaPatra SE, Baldwin TJ, Shewmaker B, Jones J, Ristow SS. Characterization of mucosal immunity in rainbow trout Oncorhynchus mykiss challenged with infectious hematopoietic necrosis virus: identification of antiviral activity. Dis Aquat Organ. 1996;27:161–172. http://dx.doi.org/10.3354/dao027161. [Google Scholar]
- Calduch-Giner JA, Sitjà-Bobadilla A, Davey GC, Cairns MT, Kaushik S, Pérez-Sánchez J. Dietary vegetable oils do not alter the intestine transcriptome of gilthead sea bream (Sparus aurata), but modulate the transcriptomic response to infection with Enteromyxum leei. BMC Genomics. 2012;13:470. doi: 10.1186/1471-2164-13-470. http://dx.doi.org/10.1186/1471-2164-13-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro CD, Flajnik MF. Putting J chain back on the map: how might its expression define plasma cell development? J Immunol. 2014;193:3248–3255. doi: 10.4049/jimmunol.1400531. http://dx.doi.org/10.4049/jimmunol.1400531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro R, Bromage E, Abós B, Pignatelli J, González Granja A, Luque A, Tafalla C. CCR7 is mainly expressed in teleost gills, where it defines an IgD+IgM-B lymphocyte subset. J Immunol. 2014;192:1257–1266. doi: 10.4049/jimmunol.1302471. http://dx.doi.org/10.4049/jimmunol.1302471. [DOI] [PubMed] [Google Scholar]
- Castro R, Jouneau L, Pham HP, Bouchez O, Giudicelli V, Lefranc MP, Quillet E, Benmansour A, Cazals F, Six A, Fillatreau S, Sunyer O, Boudinot P. Teleost fish mount complex clonal IgM and IgT responses in spleen upon systemic viral infection. PLoS Pathog. 2013;9:e1003098. doi: 10.1371/journal.ppat.1003098. http://dx.doi.org/10.1371/journal.ppat.1003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Rescigno M. The biology of intestinal immunoglobulin a responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. http://dx.doi.org/10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010;33:479–491. doi: 10.1016/j.immuni.2010.09.013. http://dx.doi.org/10.1016/j.immuni.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Evensen Ø, Mutoloki S. IPNV antigen uptake and distribution in Atlantic Salmon following oral administration. Viruses. 2015;7:2507–2517. doi: 10.3390/v7052507. http://dx.doi.org/10.3390/v7052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero H, Guardiola FA, Tapia-Paniagua ST, Cuesta A, Meseguer J, Balebona MC, Moriñigo MÁ, Esteban MÁ. Modulation of immunity and gut microbiota after dietary administration of alginate encapsulated Shewanella putrefaciens Pdp11 to gilthead seabream (Sparus aurata L.) Fish Shellfish Immunol. 2015;45:608–618. doi: 10.1016/j.fsi.2015.05.010. http://dx.doi.org/10.1016/j.fsi.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Dalum AS, Austbø L, Bjørgen H, Skjødt K, Hordvik I, Hansen T, Fjelldal PG, Press CM, Griffiths DJ, Koppang EO. The interbranchial lymphoid tissue of Atlantic Salmon (Salmo salar L) extends as a diffuse mucosal lymphoid tissue throughout the trailing edge of the gill filament. J Morphol. 2015;276:1075–1088. doi: 10.1002/jmor.20403. http://dx.doi.org/10.1002/jmor.20403. [DOI] [PubMed] [Google Scholar]
- Danilova N, Bussmann J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat Immunol. 2005;6:295–302. doi: 10.1038/ni1166. http://dx.doi.org/10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- Danilova N, Steiner LA. B cells develop in the zebrafish pancreas. Proc Natl Acad Sci U S A. 2002;99:13711–13716. doi: 10.1073/pnas.212515999. http://dx.doi.org/10.1073/pnas.212515999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson GA, Ellis AE, Secombes CJ. Route of immunization influences the generation of antibody secreting cells in the gut of rainbow trout (Oncorhynchus mykiss) Dev Comp Immunol. 1993;17:373–376. doi: 10.1016/0145-305x(93)90008-e. http://dx.doi.org/10.1016/0145-305X(93)90008-E. [DOI] [PubMed] [Google Scholar]
- dos Santos NM, Romano N, de Sousa M, Ellis AE, Rombout JH. Ontogeny of B and T cells in sea bass (Dicentrarchus labrax, L.) Fish Shellfish Immunol. 2000;10:583–596. doi: 10.1006/fsim.2000.0273. http://dx.doi.org/10.1006/fsim.2000.0273. [DOI] [PubMed] [Google Scholar]
- dos Santos NM, Taverne N, Taverne-Thiele AJ, De Sousa M, Rombout JH. Characterisation of monoclonal antibodies specific for sea bass (Dicentrarchus labrax L) IgM indicates the existence of B cell subpopulations. Fish Shellfish Immunol. 1997;7:175–191. http://dx.doi.org/10.1006/fsim.1996.0073. [Google Scholar]
- Edholm ES, Bengten E, Stafford JL, Sahoo M, Taylor EB, Miller NW, Wilson M. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. J Immunol. 2010;185:4082–4094. doi: 10.4049/jimmunol.1000631. http://dx.doi.org/10.4049/jimmunol.1000631. [DOI] [PubMed] [Google Scholar]
- Edholm ES, Bengten E, Wilson M. Insights into the function of IgD. Dev Comp Immunol. 2011;35:1309–1316. doi: 10.1016/j.dci.2011.03.002. http://dx.doi.org/10.1016/j.dci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Elson CO, Alexander KL. Host-microbiota interactions in the intestine. Dig Dis. 2015;33:131–136. doi: 10.1159/000369534. http://dx.doi.org/10.1159/000369534. [DOI] [PubMed] [Google Scholar]
- Erlandsson L, Andersson K, Sigvardsson M, Lycke N, Leanderson T. Mice with an inactivated joining chain locus have perturbed IgM secretion. Eur J Immunol. 1998;28:2355–2365. doi: 10.1002/(SICI)1521-4141(199808)28:08<2355::AID-IMMU2355>3.0.CO;2-L. http://dx.doi.org/10.1002/(SICI)1521-4141(199808)28:08<2355::AID-IMMU2355>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Estensoro I, Calduch-Giner JA, Kaushik S, Perez-Sanchez J, Sitjà-Bobadilla A. Modulation of the IgM gene expression and IgM immunoreactive cell distribution by the nutritional background in gilthead sea bream (Sparus aurata) challenged with Enteromyxum leei (Myxozoa) Fish Shellfish Immunol. 2012;33:401–410. doi: 10.1016/j.fsi.2012.05.029. http://dx.doi.org/10.1016/j.fsi.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Estensoro I, Mulero I, Redondo MJ, Alvarez-Pellitero P, Mulero V, Sitjà-Bobadilla A. Modulation of leukocytic populations of gilthead sea bream (Sparus aurata) by the intestinal parasite Enteromyxum leei (Myxozoa: Myxosporea) Parasitology. 2014;141:425–440. doi: 10.1017/S0031182013001789. http://dx.doi.org/10.1017/S0031182013001789. [DOI] [PubMed] [Google Scholar]
- Evans DH, Claiborne JB, Currie S. The physiology of fishes. 4. CRC Press; 2014. CRC Marine Biology Series. http://dx.doi.org/10.1007/BF00044133. [Google Scholar]
- Evenhuis JP, Cleveland BM. Modulation of rainbow trout (Oncorhynchus mykiss) intestinal immune gene expression following bacterial challenge. Vet Immunol Immunopathol. 2012;146:8–17. doi: 10.1016/j.vetimm.2012.01.008. http://dx.doi.org/10.1016/j.vetimm.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Feng LN, Lu DQ, Bei JX, Chen JL, Liu Y, Zhang Y, Liu XC, Meng ZN, Wang L, Lin HR. Molecular cloning and functional analysis of polymeric immunoglobulin receptor gene in orange-spotted grouper (Epinephelus coioides) Comp Biochem Physiol B Biochem Mol Biol. 2009;154:282–289. doi: 10.1016/j.cbpb.2009.07.003. http://dx.doi.org/10.1016/j.cbpb.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Six A, Magadan S, Castro R, Sunyer JO, Boudinot P. The astonishing diversity of Ig classes and B cell repertoires in teleost fish. Front Immunol. 2013;4:28. doi: 10.3389/fimmu.2013.00028. http://dx.doi.org/10.3389/fimmu.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF. All GOD’s creatures got dedicated mucosal immunity. Nat Immunol. 2010;11:777–779. doi: 10.1038/ni0910-777. http://dx.doi.org/10.1038/ni0910-777. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 2010;11:47–59. doi: 10.1038/nrg2703. http://dx.doi.org/10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglem B, Jirillo E, Bjerkås I, Kiyono H, Nochi T, Yuki Y, Raida MK, Fischer U, Koppang EO. Antigen-sampling cells in the salmonid intestinal epithelium. Dev Comp Immunol. 2010;34:768–774. doi: 10.1016/j.dci.2010.02.007. http://dx.doi.org/10.1016/j.dci.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Galindo-Villegas J, Mulero I, García-Alcazar A, Muñoz I, Peñalver-Mellado M, Streitenberger S, Scapigliati G, Meseguer J, Mulero V. Recombinant TNFα as oral vaccine adjuvant protects European sea bass against vibriosis: insights into the role of the CCL25/CCR9 axis. Fish Shellfish Immunol. 2013;35:1260–1271. doi: 10.1016/j.fsi.2013.07.046. http://dx.doi.org/10.1016/j.fsi.2013.07.046. [DOI] [PubMed] [Google Scholar]
- Gambón-Deza F, Sánchez-Espinel C, Magadán-Mompó S. Presence of an unique IgT on the IGH locus in three-spined stickleback fish (Gasterosteus aculeatus) and the very recent generation of a repertoire of VH genes. Dev Comp Immunol. 2010;34:114–122. doi: 10.1016/j.dci.2009.08.011. http://dx.doi.org/10.1016/j.dci.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Gao Y, Yi Y, Wu H, Wang Q, Qu J, Zhang Y. Molecular cloning and characterization of secretory and membrane-bound IgM of turbot. Fish Shell-fish Immunol. 2014;40:354–361. doi: 10.1016/j.fsi.2014.07.011. http://dx.doi.org/10.1016/j.fsi.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Garside P, Millington O, Smith KM. The anatomy of mucosal immune responses. Ann N Y Acad Sci. 2004;15:9–15. doi: 10.1196/annals.1309.002. http://dx.doi.org/10.1196/annals.1309.002. [DOI] [PubMed] [Google Scholar]
- German DP, Horn MH. Gut length and mass in herbivorous and carnivorous prickleback fishes (Teleostei: Stichaeidae): ontogenetic, dietary, and phylogenetic effects. Mar Biol. 2006;148:1123–1134. doi: 10.1086/422228. http://dx.doi.org/10.1007/s00227-005-0149-4. [DOI] [PubMed] [Google Scholar]
- Giacomelli S, Buonocore F, Albanese F, Scapigliati G, Gerdol M, Oreste U, Coscia MR. New insights into evolution of IgT genes coming from Antarctic teleosts. Mar Genomics. 2015;24(Pt 1):55–68. doi: 10.1016/j.margen.2015.06.009. http://dx.doi.org/10.1016/j.margen.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Gómez D, Bartholomew J, Sunyer JO. Biology and mucosal immunity to myxozoans. Dev Comp Immunol. 2014;43:243–256. doi: 10.1016/j.dci.2013.08.014. http://dx.doi.org/10.1016/j.dci.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez D, Sunyer JO, Salinas I. The mucosal immune system of fish: the evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013;35:1729–1739. doi: 10.1016/j.fsi.2013.09.032. http://dx.doi.org/10.1016/j.fsi.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez GD, Balcazar JL. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol. 2008;52:145–154. doi: 10.1111/j.1574-695X.2007.00343.x. http://dx.doi.org/10.1111/j.1574-695X.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Gravningen K, Kestin S, Thorarinsson R, Syvertsen C. Oral vaccination against enteric red mouth disease in rainbow trout (Oncorhynchus mykiss Walbaum). The effect of vaccine dose rate on protection against the disease. J Appl Ichthyol. 1998;14:163–166. http://dx.doi.org/10.1111/j.1439-0426.1998.tb00636.x. [Google Scholar]
- Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70- J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. http://dx.doi.org/10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisez L, Chair M, Sorgeloos P, Ollevier F. Mode of infection and spread of Vibrio anguillarum in turbot Scophthalmus maximus larvae after oral challenge through live feed. Dis Aquat Organ. 1996;26:181–187. http://dx.doi.org/10.3354/dao026181. [Google Scholar]
- Grøntvedt RN, Espelid S. Immunoglobulin producing cells in the spotted wolffish (Anarhichas minor Olafsen): localization in adults and during juvenile development. Dev Comp Immunol. 2003;27:569–578. doi: 10.1016/s0145-305x(03)00028-4. http://dx.doi.org/10.1016/S0145-305X(03)00028-4. [DOI] [PubMed] [Google Scholar]
- Guo YC, Bao YH, Wang H, Hu XX, Zhao ZH, Li N, Zhao YF. A preliminary analysis of the immunoglobulin genes in the African elephant (Loxodonta africana) PLoS One. 2011;6:e16889. doi: 10.1371/journal.pone.0016889. http://dx.doi.org/10.1371/journal.pone.0016889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Villanueva LT, Tovar-Ramírez D, Gisbert E, Cordero H, Guardiola FA, Cuesta A, Meseguer J, Ascencio-Valle F, Esteban MA. Dietary administration of β-1,3/1,6-glucan and probiotic strain Shewanella putrefaciens, single or combined, on gilthead seabream growth, immune responses and gene expression. Fish Shellfish Immunol. 2014;39:34–41. doi: 10.1016/j.fsi.2014.04.024. http://dx.doi.org/10.1016/j.fsi.2014.04.024. [DOI] [PubMed] [Google Scholar]
- Hamuro K, Suetake H, Saha NR, Kikuchi K, Suzuki Y. A teleost polymeric Ig receptor exhibiting two Ig-like domains transports tetrameric IgM into the skin. J Immunol. 2007;178:5682–5689. doi: 10.4049/jimmunol.178.9.5682. http://dx.doi.org/10.4049/jimmunol.178.9.5682. [DOI] [PubMed] [Google Scholar]
- Hamze M, Desmetz C, Guglielmi P. B cell-derived cytokines in disease. Eur Cytokine Netw. 2013;24:20–26. doi: 10.1684/ecn.2013.0327. http://dx.doi.org/10.1684/ecn.2013.0327. [DOI] [PubMed] [Google Scholar]
- Hansen JD, Landis ED, Phillips RB. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: implications for a distinctive B cell developmental pathway in teleost fish. Proc Natl Acad Sci U S A. 2005;102:6919–6924. doi: 10.1073/pnas.0500027102. http://dx.doi.org/10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JD, Zapata AG. Lymphocyte development in fish and amphibians. Immunol Rev. 1998;166:199–220. doi: 10.1111/j.1600-065x.1998.tb01264.x. http://dx.doi.org/10.1111/j.1600-065X.1998.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Hatten F, Fredriksen A, Hordvik I, Endresen C. Presence of IgM in cutaneous mucus, but not in gut mucus of Atlantic salmon, Salmo salar. Serum IgM is rapidly degraded when added to gut mucus. Fish Shellfish Immunol. 2001;11:257–268. doi: 10.1006/fsim.2000.0313. http://dx.doi.org/10.1006/fsim.2000.0313. [DOI] [PubMed] [Google Scholar]
- Hébert P, Ainsworth AJ, Boyd B. Histological enzyme and flow cytometric analysis of channel catfish intestinal tract immune cells. Dev Comp Immunol. 2002;26:53–62. doi: 10.1016/s0145-305x(01)00044-1. http://dx.doi.org/10.1016/S0145-305X(01)00044-1. [DOI] [PubMed] [Google Scholar]
- Hendrickson BA, Conner DA, Ladd DJ, Kendall D, Casanova JE, Corthesy B, Max EE, Neutra MR, Seidman CE, Seidman JG. Altered hepatic transport of immunoglobulin A in mice lacking the J chain. J Exp Med. 1995;182:1905–1911. doi: 10.1084/jem.182.6.1905. http://dx.doi.org/10.1084/jem.182.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Yang K, Yang J, Li M, Xiong N. Critical roles of chemokine receptor CCR10 in regulating memory IgA responses in intestines. Proc Natl Acad Sci U S A. 2011;108:E1035–E1044. doi: 10.1073/pnas.1100156108. http://dx.doi.org/10.1073/pnas.1100156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YL, Zhu LY, Xiang LX, Shao JZ. Discovery of an unusual alternative splicing pathway of the immunoglobulin heavy chain in a teleost fish, Danio rerio. Dev Comp Immunol. 2011;35:253–257. doi: 10.1016/j.dci.2010.10.009. http://dx.doi.org/10.1016/j.dci.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Huang T, Zhang M, Wei Z, Wang P, Sun Y, Hu X, Ren L, Meng Q, Zhang R, Guo Y, Hammarstrom L, Li N, Zhao Y. Analysis of immunoglobulin transcripts in the Ostrich Struthio camelus, a primitive avian species. PLoS One. 2012;7:e34346. doi: 10.1371/journal.pone.0034346. http://dx.doi.org/10.1371/journal.pone.0034346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenhuis HT, Romano N, Oosterhoud C, Taverne-Thiele A, Mastrolia L, Muiswinkel W, Rombout JWM. The ontogeny of mucosal immune cells in common carp (Cyprinus carpio L.) Anat Embryol (Berl) 2006;211:19–29. doi: 10.1007/s00429-005-0062-0. http://dx.doi.org/10.1007/s00429-005-0062-0. [DOI] [PubMed] [Google Scholar]
- Jiang N, Weinstein JA, Penland L, White RA, Fisher DS, Quake SR. Determinism and stochasticity during maturation of the zebrafish antibody repertoire. Proc Natl Acad Sci U S A. 2011;108:5348–5353. doi: 10.1073/pnas.1014277108. http://dx.doi.org/10.1073/pnas.1014277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen Braathen, Brandtzaeg Role of J chain in secretory immunoglobulin formation. Scand J Immunol. 2000;52:240–248. doi: 10.1046/j.1365-3083.2000.00790.x. http://dx.doi.org/10.1046/j.1365-3083.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Amend DF. Efficacy of Vibrio anguillarum and Yersinia ruckeri bacterins applied by oral and anal intubation of salmonids. J Fish Dis Oxf. 1983;6:473–476. http://dx.doi.org/10.1111/j.1365-2761.1983.tb00101.x. [Google Scholar]
- Joosten PH, Engelsma MY, van der Zee MD, Rombout JH. Induction of oral tolerance in carp (Cyprinus carpio L.) after feeding protein antigens. Vet Immunol Immunopathol. 1997;60:187–196. doi: 10.1016/s0165-2427(97)00124-4. http://dx.doi.org/10.1016/S0165-2427(97)00124-4. [DOI] [PubMed] [Google Scholar]
- Kaattari S, Evans D, Klemer J. Varied redox forms of teleost IgM: an alternative to isotypic diversity? Immunol Rev. 1998;166:133–142. doi: 10.1111/j.1600-065x.1998.tb01258.x. http://dx.doi.org/10.1111/j.1600-065X.1998.tb01258.x. [DOI] [PubMed] [Google Scholar]
- Kaetzel CS. Coevolution of mucosal immunoglobulins and the polymeric immunoglobulin receptor: evidence that the commensal microbiota provided the driving force. ISRN Immunol. 2014;2014:1–20. http://dx.doi.org/10.1155/2014/541537. [Google Scholar]
- Kang HS, Chin RK, Wang Y, Yu P, Wang J, Newell KA, Fu YX. Signaling via LTbetaR on the lamina propria stromal cells of the gut is required for IgA production. Nat Immunol. 2002;3:576–582. doi: 10.1038/ni795. http://dx.doi.org/10.1038/ni795. [DOI] [PubMed] [Google Scholar]
- Kanther M, Rawls JF. Host-microbe interaction in the developing zebrafish. Curr Opin Immunol. 2011;22:10–19. doi: 10.1016/j.coi.2010.01.006. http://dx.doi.org/10.1016/j.coi.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G, Takano T, Sakai T, Matsuyama T, Sano N, Nakayasu C. Cloning and expression analyses of a unique IgT in ayu Plecoglossus altivelis. Fish Sci. 2015;81:29–36. http://dx.doi.org/10.1007/s12562-014-0820-0. [Google Scholar]
- Kim HJ, Park JS, Choi MC, Kwon SR. Comparison of the efficacy of Poly(IC) immunization with live vaccine and formalin-killed vaccine against viral hemorrhagic septicemia virus (VHSV) in olive flounder (Paralichthys olivaceus) Fish Shellfish Immunol. 2016;48:206–211. doi: 10.1016/j.fsi.2015.11.035. http://dx.doi.org/10.1016/j.fsi.2015.11.035. [DOI] [PubMed] [Google Scholar]
- Koppang EO, Fischer U, Moore L, Tranulis MA, Dijkstra JM, Köllner B, Aune L, Jirillo E, Hordvik I. Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue. J Anat. 2010;217:728–739. doi: 10.1111/j.1469-7580.2010.01305.x. http://dx.doi.org/10.1111/j.1469-7580.2010.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortum AN, Rodriguez-Nunez I, Yang J, Shim J, Runft D, O’Driscoll ML, Haire RN, Cannon JP, Turner PM, Litman RT, Kim CH, Neely MN, Litman GW, Yoder JA. Differential expression and ligand binding indicate alternative functions for zebrafish polymeric immunoglobulin receptor (pIgR) and a family of pIgR-like (PIGRL) proteins. Immunogenetics. 2014;66:267–279. doi: 10.1007/s00251-014-0759-4. http://dx.doi.org/10.1007/s00251-014-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanning DK, Zhai SK, Knight KL. Analysis of the 3′ Cμ region of the rabbit Ig heavy chain locus. Gene. 2003;309:135–144. doi: 10.1016/s0378-1119(03)00500-6. http://dx.doi.org/10.1016/S0378-1119(03)00500-6. [DOI] [PubMed] [Google Scholar]
- LaPatra S, Kao S, Erhardt EB, Salinas I. Evaluation of dual nasal delivery of infectious hematopoietic necrosis virus and enteric red mouth vaccines in rainbow trout (Oncorhynchus mykiss) Vaccine. 2015;33:771–776. doi: 10.1016/j.vaccine.2014.12.055. http://dx.doi.org/10.1016/j.vaccine.2014.12.055. [DOI] [PubMed] [Google Scholar]
- Larragoite ET, Tacchi L, LaPatra SE, Salinas I. An attenuated virus vaccine appears safe to the central nervous system of rainbow trout (Oncorhynchus mykiss) after intranasal delivery. Fish Shellfish Immunol. 2016;49:351–354. doi: 10.1016/j.fsi.2016.01.006. http://dx.doi.org/10.1016/j.fsi.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazado CC, Marlowe C, Caipang A. Mucosal immunity and probiotics in fish. Fish Shellfish Immunol. 2014;39:78–89. doi: 10.1016/j.fsi.2014.04.015. http://dx.doi.org/10.1016/j.fsi.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. http://dx.doi.org/10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong JS, Jantzen SG, von Schalburg KR, Cooper GA, Messmer AM, Liao NY, Munro S, Moore R, Holt RA, Jones SJM, Davidson WS, Koop BF. Salmo salar and Esox lucius full-length cDNA sequences reveal changes in evolutionary pressures on a post-tetraploidization genome. BMC Genomics. 2010;11:279. doi: 10.1186/1471-2164-11-279. http://dx.doi.org/10.1186/1471-2164-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, Lapatra S, Tort L, Sunyer JO. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7:1116–1124. doi: 10.1038/ni1389. http://dx.doi.org/10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- Lorenz RG, Newberry RD. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann N Y Acad Sci. 2004;1029:44–57. doi: 10.1196/annals.1309.006. http://dx.doi.org/10.1196/annals.1309.006. [DOI] [PubMed] [Google Scholar]
- Lowrey L, Woodhams DC, Tacchi L, Salinas I. Topographic mapping of the rainbow trout (Oncorhynchus mykiss) microbiome reveals a diverse bacterial community with antifungal properties in the skin. Appl Environ Microbiol. 2015;81:6915–6925. doi: 10.1128/AEM.01826-15. http://dx.doi.org/10.1128/AEM.01826-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magadán-Mompó S, Sánchez-Espinel C, Gambón-Deza F. Immunoglobulin heavy chains in medaka (Oryzias latipes) BMC Evol Biol. 2011;11:165. doi: 10.1186/1471-2148-11-165. http://dx.doi.org/10.1186/1471-2148-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnadottir B. Innate immunity of fish (overview) Fish Shellfish Immunol. 2006;20:137–151. doi: 10.1016/j.fsi.2004.09.006. http://dx.doi.org/10.1016/j.fsi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Magnadottir B, Gudmundsdottir S, Gudmundsdottir BK, Helgason S. Natural antibodies of cod (Gadus morhua L.): specificity, activity and affinity. Comp Biochem Physiol Part B. 2009;154:309–316. doi: 10.1016/j.cbpb.2009.07.005. http://dx.doi.org/10.1016/j.cbpb.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Magnadottir B, Lange S, Gudmundsdottir S, Dalmo RA. Ontogeny of humoral immune parameters in fish. Fish Shellfish Immunol. 2005;19:429–439. doi: 10.1016/j.fsi.2005.03.010. http://dx.doi.org/10.1016/j.fsi.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Magor KE, Miranzo Navarro D, Barber MRW, Petkau K, Fleming-Canepa X, Blyth GAD, Blaine AH. Defense genes missing from the flight division. Dev Comp Immunol. 2013;41:377–388. doi: 10.1016/j.dci.2013.04.010. http://dx.doi.org/10.1016/j.dci.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JL, Dickerson HW. Systemic and cutaneous mucus antibody responses of channel catfish immunized against the protozoan parasite Ichthyophthirius multifiliis. Clin Diagn Lab Immunol. 2003;10:876–881. doi: 10.1128/CDLI.10.5.876-881.2003. http://dx.doi.org/10.1128/CDLI.10.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashoof S, Goodroe A, Du CC, Eubanks JO, Jacobs N, Steiner JM, Tizard I, Suchodolski JS, Criscitiello MF. Ancient T-independence of mucosal IgX/A: gut microbiota unaffected by larval thymectomy in Xenopus laevis. Mucosal Immunol. 2013;6:358–368. doi: 10.1038/mi.2012.78. http://dx.doi.org/10.1038/mi.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason KL, Huffnagle GB, Noverr MC, Kao JY. Overview of gut immunology. Adv Exp Med Biol. 2008;635:1–14. doi: 10.1007/978-0-387-09550-9_1. http://dx.doi.org/10.1007/978-0-387-09550-9_1. [DOI] [PubMed] [Google Scholar]
- Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. http://dx.doi.org/10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall-Ngai M. Adaptive immunity: care for the community. Nature. 2007;445:153. doi: 10.1038/445153a. http://dx.doi.org/10.1038/445153a. [DOI] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. http://dx.doi.org/10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Montalban-Arques A, De Schryver P, Bossier P, Gorkiewicz G, Mulero V, Gatlin DM, Galindo-Villegas J. Selective manipulation of the gut microbiota improves immune status in vertebrates. Front Immunol. 2015;6:512. doi: 10.3389/fimmu.2015.00512. http://dx.doi.org/10.3389/fimmu.2015.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667–685. doi: 10.1038/nri3738. http://dx.doi.org/10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- Munang’andu HM, Evensen Ø. A review of intra- and extracellular antigen delivery systems for virus vaccines of finfish. J Immunol Res. 2015;2015:960859. doi: 10.1155/2015/960859. http://dx.doi.org/10.1155/2015/960859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussmann R, Du Pasquier L, Hsu E. Is Xenopus IgX an analog of IgA? Eur J Immunol. 1996;26:2823–2830. doi: 10.1002/eji.1830261205. http://dx.doi.org/10.1002/eji.1830261205. [DOI] [PubMed] [Google Scholar]
- Mutoloki S, Munang’andu HM, Evensen Ø. Oral vaccination of fish - antigen preparations, uptake, and immune induction. Front Immunol. 2015;6:519. doi: 10.3389/fimmu.2015.00519. http://dx.doi.org/10.3389/fimmu.2015.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Nakayasu C, Rieger AM, Barreda DR, Somamoto T, Nakao M. Phagocytosis by thrombocytes is a conserved innate immune mechanism in lower vertebrates. Front Immunol. 2014;5:445. doi: 10.3389/fimmu.2014.00445. http://dx.doi.org/10.3389/fimmu.2014.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak SK. Role of gastrointestinal microbiota in fish. Aquac Res. 2010;41:1553–1573. http://dx.doi.org/10.1111/j.1365-2109.2010.02546.x. [Google Scholar]
- Nomiyama H, Osada N, Yoshie O. Systematic classification of vertebrate chemokines based on conserved synteny and evolutionary history. Genes Cells. 2013;18:1–16. doi: 10.1111/gtc.12013. http://dx.doi.org/10.1111/gtc.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole R, Von Hofsten J, Rosqvist R, Olsson PE, Wolf-Watz H. Visualisation of zebrafish infection by GFP-labelled Vibrio anguillarum. Microb Pathog. 2004;37:41–46. doi: 10.1016/j.micpath.2004.03.001. http://dx.doi.org/10.1016/j.micpath.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Villumsen KR, Strøm HK, Raida MK. 3D Visualization of the initial Yersinia ruckeri infection toute in Rainbow trout (Oncorhynchus mykiss) by optical projection tomography. PLoS One. 2014;9:e89672. doi: 10.1371/journal.pone.0089672. http://dx.doi.org/10.1371/journal.pone.0089672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordás MC, Castro R, Dixon B, Sunyer JO, Bjork S, Bartholomew J, Korytar T, Köllner B, Cuesta A, Tafalla C. Identification of a novel CCR7 gene in rainbow trout with differential expression in the context of mucosal or systemic infection. Dev Comp Immunol. 2012;38:302–311. doi: 10.1016/j.dci.2012.07.001. http://dx.doi.org/10.1016/j.dci.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øverland HS, Pettersen EF, Rønneseth A, Wergeland HI. Phagocytosis by B-cells and neutrophils in Atlantic salmon (Salmo salar L.) and Atlantic cod (Gadus morhua L.) Fish Shellfish Immunol. 2010;28:193–204. doi: 10.1016/j.fsi.2009.10.021. http://dx.doi.org/10.1016/j.fsi.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12:821–832. doi: 10.1038/nri3322. http://dx.doi.org/10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- Page DM, Wittamer V, Bertrand JY, Lewis KL, Pratt DN, Schale SE, Mcgue C, Jacobsen BH, Doty A, Pao Y, Chi NC, Magor BG, Traver D, Dc W, Delgado N, Yang H. An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood. 2014;122:e1–11. doi: 10.1182/blood-2012-12-471029. http://dx.doi.org/10.1182/blood-2012-12-471029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, de Zoete MR, Flavell RA. Immune-microbiota interactions in health and disease. Clin Immunol. 2015;159:122–127. doi: 10.1016/j.clim.2015.05.014. http://dx.doi.org/10.1016/j.clim.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra D, Reyes-Lopez FE, Tort L. Mucosal immunity and B cells in teleosts: effect of vaccination and stress. Front Immunol. 2015;6:354. doi: 10.3389/fimmu.2015.00354. http://dx.doi.org/10.3389/fimmu.2015.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra D, Rieger AM, Li J, Zhang YA, Randall LM, Hunter CA, Barreda DR, Sunyer JO. Pivotal advance: peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol. 2012;91:525–536. doi: 10.1189/jlb.0711372. http://dx.doi.org/10.1189/jlb.0711372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra D, Takizawa F, Sunyer JO. Evolution of B cell immunity. Annu Rev Anim Biosci. 2013;1:65–97. doi: 10.1146/annurev-animal-031412-103651. http://dx.doi.org/10.1146/annurev-animal-031412-103651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peatman E, Lange M, Zhao H, Beck BH. Physiology and immunology of mucosal barriers in catfish (Ictalurus spp.) Tissue Barriers. 2015;3:e1068907. doi: 10.1080/21688370.2015.1068907. http://dx.doi.org/10.1080/21688370.2015.1068907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perey DY, Frommel D, Hong R, Good RA. The mammalian homologue of the avian bursa of Fabricius. II Extirpation, lethal x-irradiation, and reconstitution in rabbits Effects on humoral immune responses, immunoglobulins, and lymphoid tissues. Lab Invest. 1970;22:212–227. [PubMed] [Google Scholar]
- Pérez T, Balcázar JL, Ruiz-Zarzuela I, Halaihel N, Vendrell D, de Blas I, Múzquiz JL. Host-microbiota interactions within the fish intestinal ecosystem. Mucosal Immunol. 2010;3:355–360. doi: 10.1038/mi.2010.12. http://dx.doi.org/10.1038/mi.2010.12. [DOI] [PubMed] [Google Scholar]
- Picchietti S, Mazzini M, Taddei AR, Renna R, Fausto AM, Mulero V, Carnevali O, Cresci A, Abelli L. Effects of administration of probiotic strains on GALT of larval gilthead seabream: immunohistochemical and ultrastructural studies. Fish Shellfish Immunol. 2007;22:57–67. doi: 10.1016/j.fsi.2006.03.009. http://dx.doi.org/10.1016/j.fsi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Picchietti S, Terribili FR, Scapigliati MG, Abelli L. Expression of lymphocyte antigenic determinants in developing gut-associated lymphoid tissue of the sea bass Dicentrarchus labrax (L.) Anat Embryol. 1997;196:457–463. doi: 10.1007/s004290050113. [DOI] [PubMed] [Google Scholar]
- Quiniou SMA, Wilson M, Boudinot P. Processing of fish Ig heavy chain transcripts: diverse splicing patterns and unusual nonsense mediated decay. Dev Comp Immunol. 2011;35:949–958. doi: 10.1016/j.dci.2010.12.007. http://dx.doi.org/10.1016/j.dci.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Raida MK, Nylén J, Holten-Andersen L, Buchmann K. Association between plasma antibody response and protection in rainbow trout oncorhynchus mykiss immersion vaccinated against Yersinia ruckeri. PLoS One. 2011;6:e18832. doi: 10.1371/journal.pone.0018832. http://dx.doi.org/10.1371/journal.pone.0018832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Gomez F, Greene W, Rego K, Hansen JD, Costa G, Kataria P, Bromage ES. Discovery and characterization of secretory IgD in rainbow trout: secretory IgD is produced through a novel splicing mechanism. J Immunol. 2012;188:1341–1349. doi: 10.4049/jimmunol.1101938. http://dx.doi.org/10.4049/jimmunol.1101938. [DOI] [PubMed] [Google Scholar]
- Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci U S A. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. http://dx.doi.org/10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo D, Ronza P, Harrison PW, Losada AP, Bermúdez R, Pardo BG, Redondo MJ, Sitjà-bobadilla A, Quiroga MI, Martínez P. RNA-seq analysis reveals significant transcriptome changes in turbot (Scophthalmus maximus) suffering severe enteromyxosis. BMC Genomics. 2014;15:1149. doi: 10.1186/1471-2164-15-1149. http://dx.doi.org/10.1186/1471-2164-15-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano N, Taverne-Thiele JJ, Van Maanen JC, Rombout JHMW. Leucocyte subpopulations in developing carp (Cyprinus carpio L.): immunocytochemical studies. Fish Shellfish Immunol. 1997;7:439–453. http://dx.doi.org/10.1006/fsim.1997.0097. [Google Scholar]
- Rombout JHWM, Abelli L, Picchietti S, Scapigliati G, Kiron V. Teleost intestinal immunology. Fish Shellfish Immunol. 2011;31:616–626. doi: 10.1016/j.fsi.2010.09.001. http://dx.doi.org/10.1016/j.fsi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Rombout JHWM, Joosten PHM, Engelsma MY, Vos AP, Taverne N, Taverne-Thiele JJ. Indications for a distinct putative T cell population in mucosal tissue of carp (Cyprinus carpio L.) Dev Comp Immunol. 1998;22:63–77. doi: 10.1016/s0145-305x(97)00048-7. http://dx.doi.org/10.1016/S0145-305X(97)00048-7. [DOI] [PubMed] [Google Scholar]
- Rombout JHWM, Taverne-Thiele AJ, Villena MI. The gut-associated lymphoid tissue (GALT) of carp (Cyprinus carpio L.): an immunocytochemical analysis. Dev Comp Immunol. 1993;17:55–66. doi: 10.1016/0145-305x(93)90015-i. http://dx.doi.org/10.1016/0145-305X(93)90015-I. [DOI] [PubMed] [Google Scholar]
- Rombout JHWM, van den Berg AA. Uptake and transport of ferritin in the epithelium of carp (Cyprinus Carpio L.) and the possible immunological implications. Cell Biol Int Rep. 1985;9:516. doi: 10.1016/0309-1651(85)90003-7. http://dx.doi.org/10.1016/0309-1651(85)90003-7. [DOI] [PubMed] [Google Scholar]
- Rombout JHWM, van den Berg AA, van den Berg CT, Witte P, Egberts E. Immunological importance of the second gut segment of carp. III Systemic and/ or mucosal immune responses after immunization with soluble or particulate antigen. J Fish Biol. 1989;35:179–186. [Google Scholar]
- Rombout JHWM, van der Tuin SJL, Yang G, Schopman N, Mroczek A, Hermsen T, Taverne-Thiele JJ. Expression of the polymeric Immunoglobulin Receptor (pIgR) in mucosal tissues of common carp (Cyprinus carpio L.) Fish Shellfish Immunol. 2008;24:620–628. doi: 10.1016/j.fsi.2008.01.016. http://dx.doi.org/10.1016/j.fsi.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Rombout JHWM, Yang G, Kiron V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish Shellfish Immunol. 2014;40:634–643. doi: 10.1016/j.fsi.2014.08.020. http://dx.doi.org/10.1016/j.fsi.2014.08.020. [DOI] [PubMed] [Google Scholar]
- Rønneseth A, Ghebretnsae DB, Wergeland HI, Haugland GT. Functional characterization of IgM+ B cells and adaptive immunity in lumpfish (Cyclopterus lumpus L.) Dev Comp Immunol. 2015;52:132–143. doi: 10.1016/j.dci.2015.05.010. http://dx.doi.org/10.1016/j.dci.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, Harris KA, Jones SA, Klein N, Mauri C. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat Med. 2014;20:1334–1339. doi: 10.1038/nm.3680. http://dx.doi.org/10.1038/nm.3680. [DOI] [PubMed] [Google Scholar]
- Ryo S, Wijdeven RHM, Tyagi A, Hermsen T, Kono T, Karunasagar I, Rombout JHWM, Sakai M, Kemenade BMLV, van Savan R. Common carp have two subclasses of bonyfish specific antibody IgZ showing differential expression in response to infection. Dev Comp Immunol. 2010;34:1183–1190. doi: 10.1016/j.dci.2010.06.012. http://dx.doi.org/10.1016/j.dci.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Sahoo M, Edholm ES, Stafford JL, Bengten E, Miller NW, Wilson M. B cell receptor accessory molecules in the channel catfish, Ictalurus punctatus. Dev Comp Immunol. 2008;32:1385–1397. doi: 10.1016/j.dci.2008.05.008. http://dx.doi.org/10.1016/j.dci.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas I. The mucosal immune system of teleost fish. Biol (Basel) 2015;4:525–539. doi: 10.3390/biology4030525. http://dx.doi.org/10.3390/biology4030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas I, LaPatra SE, Erhardt EB. Nasal vaccination of young rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis and enteric red mouth disease. Dev Comp Immunol. 2015;53:105–111. doi: 10.1016/j.dci.2015.05.015. http://dx.doi.org/10.1016/j.dci.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Salinas I, Parra D. Fish mucosal immunity: intestine. Mucosal Heal Aquac. 2015:135–170. http://dx.doi.org/10.1016/B978-0-12-417186-2/00006-6.
- Salinas I, Zhang YA, Sunyer JO. Mucosal immunoglobulins and B cells of teleost fish. Dev Comp Immunol. 2011;35:1346–1365. doi: 10.1016/j.dci.2011.11.009. http://dx.doi.org/10.1016/j.dci.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström O, Abrahamsson I, Andersson J, Vetemaa M. Temperature effects on spawning and egg development in Eurasian perch. J Fish Biol. 1997;51:1015–1024. http://dx.doi.org/10.1006/jfbi.1997.0506. [Google Scholar]
- Sato A, Okamoto N. Induction of virus-specific cell-mediated cytotoxic responses of isogeneic ginbuna crucian carp, after oral immunization with inactivated virus. Fish Shellfish Immunol. 2010;29:414–421. doi: 10.1016/j.fsi.2010.04.017. http://dx.doi.org/10.1016/j.fsi.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Savan R, Aman A, Sato K, Yamaguchi R, Sakai M. Discovery of a new class of immunoglobulin heavy chain from fugu. Eur J Immunol. 2005;35:3320–3331. doi: 10.1002/eji.200535248. http://dx.doi.org/10.1002/eji.200535248. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. http://dx.doi.org/10.1016/S1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- Shinn AP, Pratoomyot J, Bron JE, Paladini G, Brooker EE, Brooker AJ. Economic costs of protistan and metazoan parasites to global mariculture. Parasitology. 2015;142:196–270. doi: 10.1017/S0031182014001437. http://dx.doi.org/10.1017/S0031182014001437. [DOI] [PubMed] [Google Scholar]
- Schorpp M, Bialecki M, Diekhoff D, Walderich B, Odenthal J, Maischein HM, Zapata AG, Boehm T. Conserved functions of Ikaros in vertebrate lymphocyte development: genetic evidence for distinct larval and adult phases of T cell development and two lineages of B cells in zebrafish. J Immunol. 2006;177:2463–2476. doi: 10.4049/jimmunol.177.4.2463. 177/4/2463. [DOI] [PubMed] [Google Scholar]
- Sitjà-Bobadilla A, Palenzuela O, Riaza A, Macías MA, Alvarez-Pellitero P. Protective acquired immunity to Enteromyxum scophthalmi (Myxozoa) is related to specific antibodies in Psetta maxima (L.) (Teleostei) Scand J Immunol. 2007;66:26–34. doi: 10.1111/j.1365-3083.2007.01942.x. http://dx.doi.org/10.1111/j.1365-3083.2007.01942.x. [DOI] [PubMed] [Google Scholar]
- Sitjà-Bobadilla A, Redondo MJ, Macias MA, Ferreiro I, Riaza A, Alvarez-Pellitero P. Development of immunohistochemitry and enzyme-linked immunosorbent assays for the detection of circulating antibodies agains Enteromyxum scophtalmi (Myxozoa) in turbot (Scophtalmus maximus L.) Fish Shellfish Immunol. 2004;17:335–345. doi: 10.1016/j.fsi.2004.04.007. http://dx.doi.org/10.1016/j.fsi.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Sitjà-Bobadilla A, Redondo MJ, Bermúdez R, Palenzuela O, Ferreiro I, Riaza A, Quiroga MI, Nieto JM, Alvarez-Pellitero P. Innate and adaptive immune responses of turbot, Scophthalmus maximus (L.), following experimental infection with Enteromyxum scophthalmi (Myxosporea: Myxozoa) Fish Shellfish Immunol. 2006;21:485–500. doi: 10.1016/j.fsi.2006.02.004. http://dx.doi.org/10.1016/j.fsi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Solem ST, Stenvik J. Antibody repertoire development in teleosts – a review with emphasis on salmonids and Gadus morhua L. Dev. Comp Immunol. 2006;30:57–76. doi: 10.1016/j.dci.2005.06.007. http://dx.doi.org/10.1016/j.dci.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Sommer F, Bäckhed F. The gut microbiota–masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. http://dx.doi.org/10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wei Z, Hammarstrom L, Zhao Y. The immunoglobulin δ gene in jawed vertebrates: a comparative overview. Dev Comp Immunol. 2011;35:975–981. doi: 10.1016/j.dci.2010.12.010. http://dx.doi.org/10.1016/j.dci.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Sun YZ, Yang HL, Ma RL, Lin WY. Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immunol. 2010;29:803–809. doi: 10.1016/j.fsi.2010.07.018. http://dx.doi.org/10.1016/j.fsi.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Sunyer JO. Fishing for mammalian paradigms in the teleost immune system. Nat Immunol. 2013;14:320–326. doi: 10.1038/ni.2549. http://dx.doi.org/10.1038/ni.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Maruya M, Kawamoto S, Fagarasan S. Roles of B-1 and B-2 cells in innate and acquired IgA-mediated immunity. Immunol Rev. 2010;237:180–190. doi: 10.1111/j.1600-065X.2010.00941.x. http://dx.doi.org/10.1111/j.1600-065X.2010.00941.x. [DOI] [PubMed] [Google Scholar]
- Tacchi L, Musharrafieh R, Larragoite ET, Crossey K, Erhardt EB, Martin SAM, LaPatra SE, Salinas I. Nasal immunity is an ancient arm of the mucosal immune system of vertebrates. Nat Commun. 2014;5:5205. doi: 10.1038/ncomms6205. http://dx.doi.org/10.1038/ncomms6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadiso TM, Sharma A, Hordvik I. Analysis of polymeric immunoglobulin receptor- and CD300-like molecules from Atlantic salmon. Mol Immunol. 2011;49:462–473. doi: 10.1016/j.molimm.2011.09.013. http://dx.doi.org/10.1016/j.molimm.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Takizawa F, Dijkstra JM, Kotterba P, Korytář T, Kock H, Köllner B, Jaureguiberry B, Nakanishi T, Fischer U. The expression of CD8α discriminates distinct T cell subsets in teleost fish. Dev Comp Immunol. 2011;35:752–763. doi: 10.1016/j.dci.2011.02.008. http://dx.doi.org/10.1016/j.dci.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Takizawa F, Gómez D, Xu Z, Parra D, Zhang YA, Bjork S, Bartholomew J, Wiens GD, Sunyer JO. Identification of regulatory B and T cell subsets in teleost fish. Fish Shellfish Immunol. 2013;34:1679–1680. http://dx.doi.org/10.1016/j.fsi.2013.03.141. [Google Scholar]
- Temkin RJ, McMillan DB. Gut-associated lymphoid tissue (GALT) of the goldfish, Carassius auratus. J Morphol. 1986;190:9–26. doi: 10.1002/jmor.1051900103. http://dx.doi.org/10.1002/jmor.1051900103. [DOI] [PubMed] [Google Scholar]
- Tian JY, Xie HX, Zhang YA, Xu Z, Yao WJ, Nie P. Ontogeny of IgM-producing cells in the mandarin fish Siniperca chuatsi identified by in situ hybridisation. Vet Immunol Immunopathol. 2009;132:146–152. doi: 10.1016/j.vetimm.2009.05.018. http://dx.doi.org/10.1016/j.vetimm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Tobar I, Arancibia S, Torres C, Vera V, Soto P, Carrasco C, Alvarado M, Neira E, Arcos S, Tobar JA. Successive oral immunizations against Piscirickettsia salmonis and infectious salmon anemia virus are required to maintain a long-term protection in farmed salmonids. Front Immunol. 2015;6:244. doi: 10.3389/fimmu.2015.00244. http://dx.doi.org/10.3389/fimmu.2015.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobback E, Decostere A, Hermans K, Ryckaert J, Duchateau L, Haesebrouck F, Chiers K. Route of entry and tissue distribution of Yersinia ruckeri in experimentally infected rainbow trout Oncorhynchus mykiss. Dis Aquat Organ. 2009;84:219–228. doi: 10.3354/dao02057. http://dx.doi.org/10.3354/dao02057. [DOI] [PubMed] [Google Scholar]
- Tobback E, Hermans K, Decostere A, Van Den Broeck W, Haesebrouck F, Chiers K. Interactions of virulent and avirulent Yersinia ruckeri strains with isolated gill arches and intestinal explants of rainbow trout Oncorhynchus mykiss. Dis Aquat Organ. 2010;90:175–179. doi: 10.3354/dao02230. http://dx.doi.org/10.3354/dao02230. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin a generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. http://dx.doi.org/10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Tu FP, Chu WH, Zhuang XY, Lu CP. Effect of oral immunization with Aeromonas hydrophila ghosts on protection against experimental fish infection. Lett Appl Microbiol. 2010;50:13–17. doi: 10.1111/j.1472-765X.2009.02746.x. http://dx.doi.org/10.1111/j.1472-765X.2009.02746.x. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. http://dx.doi.org/10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- Vajdy M, Sethupathi P, Knight KL. Dependence of antibody somatic diversification on gut-associated lymphoid tissue in rabbits. J Immunol. 1998;160:2725–2729. [PubMed] [Google Scholar]
- Vervarcke S, Ollevier F, Kinget R, Michoel A. Mucosal response in African catfish after administration of Vibrio anguillarum O2 antigens via different routes. Fish Shellfish Immunol. 2005;18:125–133. doi: 10.1016/j.fsi.2004.06.004. http://dx.doi.org/10.1016/j.fsi.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Villumsen KR, Neumann L, Ohtani M, Strøm HK, Raida MK. Oral and anal vaccination confers full protection against enteric redmouth disease (ERM) in rainbow trout. PLoS One. 2014;9:e93845. doi: 10.1371/journal.pone.0093845. http://dx.doi.org/10.1371/journal.pone.0093845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Olp JJ, Miller RD. On the genomics of immunoglobulins in the gray, short-tailed opossum Monodelphis domestica. Immunogenetics. 2009;61:581–596. doi: 10.1007/s00251-009-0385-8. http://dx.doi.org/10.1007/s00251-009-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesemann DR. Microbes and B cell development. In: Alt Frederick W., editor. Advances in Immunology. Academic Press; 2015. pp. 155–178. http://dx.doi.org/10.1016/bs.ai.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery. Annu Rev Biomed Eng. 2012;14:17–46. doi: 10.1146/annurev-bioeng-071811-150054. http://dx.doi.org/10.1146/annurev-bioeng-071811-150054. [DOI] [PubMed] [Google Scholar]
- Xu G, Zhan W, Ding B, Sheng X. Molecular cloning and expression analysis of polymeric immunoglobulin receptor in flounder (Paralichthys olivaceus) Fish Shellfish Immunol. 2013;35:653–660. doi: 10.1016/j.fsi.2013.05.024. http://dx.doi.org/10.1016/j.fsi.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Xu Z, Parra D, Gómez D, Salinas I, Zhang YA, Jørgensen LVG, Heinecke RD, Buchmann K, LaPatra S, Sunyer JO. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc Natl Acad Sci U S A. 2013;110:13097–13102. doi: 10.1073/pnas.1304319110. http://dx.doi.org/10.1073/pnas.1304319110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Takizawa F, Parra D, Gómez D, Jørgensen LVG, LaPatra S, Sunyer JO. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat Commun. 2016;7:10728. doi: 10.1038/ncomms10728. http://dx.doi.org/10.1038/ncomms10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Bromage ES, Kaattari SL. The strength of B cell interaction with antigen determines the degree of IgM polymerization. J Immunol. 2010;184:844–850. doi: 10.4049/jimmunol.0902364. http://dx.doi.org/10.4049/jimmunol.0902364. [DOI] [PubMed] [Google Scholar]
- Ye JM, Kaattari IM, Ma CY, Kaattari S. The teleost humoral immune response. Fish Shellfish Immunol. 2013;35:1719–1728. doi: 10.1016/j.fsi.2013.10.015. http://dx.doi.org/10.1016/j.fsi.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Zapata A, Diez B, Cejalvo T, Gutiérrez-De Frías C, Cortés A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006;20:126–136. doi: 10.1016/j.fsi.2004.09.005. http://dx.doi.org/10.1016/j.fsi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Zhang YA, Salinas I, Li J, Parra D, Bjork S, Xu Z, LaPatra SE, Bartholomew J, Sunyer JO. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat Immunol. 2010;11:827–835. doi: 10.1038/ni.1913. http://dx.doi.org/10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YA, Salinas I, Sunyer JO. Recent findings on the structure and function of teleost IgT. Fish Shellfish Immunol. 2011;31:627–634. doi: 10.1016/j.fsi.2011.03.021. http://dx.doi.org/10.1016/j.fsi.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Lin A, Shao T, Nie L, Dong W, Xiang L, Shao J. B cells in teleost fish act as pivotal initiating APCs in priming adaptive immunity: an evolutionary perspective on the origin of the B-1 cell subset and B7 molecules. J Immunol. 2014;192:2699–2714. doi: 10.4049/jimmunol.1301312. http://dx.doi.org/10.4049/jimmunol.1301312. [DOI] [PubMed] [Google Scholar]
- Zwollo P, Cole S, Bromage E, Kaattari S. B cell heterogeneity in the teleost kidney: evidence for a maturation gradient from anterior to posterior kidney. J Immunol. 2005;174:6608–6616. doi: 10.4049/jimmunol.174.11.6608. http://dx.doi.org/10.4049/jimmunol.174.11.6608. [DOI] [PubMed] [Google Scholar]
- Zwollo P, Haines A, Rosato P, Gumulak-Smith J. Molecular and cellular analysis of B-cell populations in the rainbow trout using Pax5 and immunoglobulin markers. Dev Comp Immunol. 2008;32:1482–1496. doi: 10.1016/j.dci.2008.06.008. http://dx.doi.org/10.1016/j.dci.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwollo P, Mott K, Barr M. Comparative analyses of B cell populations in trout kidney and mouse bone marrow: establishing “B cell signatures. Dev Comp Immunol. 2010;34:1291–1299. doi: 10.1016/j.dci.2010.08.003. http://dx.doi.org/10.1016/j.dci.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]