Abstract
Objective
To determine the role of the aryl hydrocarbon receptor (AHR) in colitis-associated colorectal tumorigenesis.
Summary Background Data
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in both men and women in United States. Chronic intestinal inflammation increases the risk for the development of CRC. We questioned the involvement of AHR, a transcriptional regulator for intestinal innate immunity and inflammation, in the colitis-associated tumorigenesis.
Methods
We used a mouse model for chemically-induced colorectal tumorigenesis by treatment of azoxymethane (AOM) and sodium dextran sulfate (DSS). We examined the role of AHR using Ahr-deletion mouse model and I3C treatment. Tumor incidence, number and location were visually counted. Tumor multiplicities were evaluated and compared using GraphPad Prism software (version 6, LaJolla, CA).
Results
In Ahr null mice, the tumor incidence was 32% increased and the mean tumor number was approximately 3 time increased compared to WT mice (7 v 2.4, P<0.05). The tumor number was 92% decreased by treatment of I3C in WT mice, while the chemopreventive effect of I3C was not observed in Ahr null mice (P<0.05).
Conclusions
We found that the AHR may play a protective role in colitis-associated colorectal tumorigenesis. This work supports the application of AHR agonists such as I3C as a chemopreventive therapy for CRC in human patient.
MINI-ABSTRACT
The deficiency of AHR led to an increase in the susceptibility to colitis-associated colorectal tumorigenesis in mice. In contrast, the activation of AHR by treatment of indole-3-carbinol (I3C) prevented the development of colitis-associated colorectal tumorigenesis. Therefore, we concluded that AHR may play a protective role in colitis-associated colorectal tumorigenesis.
INTRODUCTION
Inflammatory Bowel Disease (IBD) is a group of diseases characterized by persistent inflammation of the colon and small intestine with two major clinical manifestations: ulcerative colitis (UC) and Crohn's disease (CD) [1]. UC is described as chronic inflammation of the colon and rectum while CD affects the lining of any part of the gastrointestinal tract [2]. Combined, UC and CD afflict nearly 1.4 million people in the United States [3]. Epidemiological studies have reported that patients with IBD have 6 times higher risk for colorectal cancer (CRC) than the general population [4] and 10–15% of all deaths in IBD patients are associated with CRC [5].
Although the pathogenesis of IBD-CRC is not clearly understood, disturbances of the gut mucosa and dysregulation of intestinal immunity are associated with the initiation of the disease [6]. Recent investigations focusing on the aryl hydrocarbon receptor (AHR) have demonstrated its essential role in maintaining intestinal immunity by regulating the homeostasis of innate immune cells [7, 8]. AHR is a member of the basic helix-loop-helix/Per-Arnt-Sim (PAS) superfamily of transcription factors and has been identified as a mediator for environmental pollutants such as 2,3,7,8-tetrachlorodibenzo-p-dioxin and benzo(a)pyrene [9, 10]. Upon binding its ligands, the AHR translocates into the nucleus where it forms a heterodimeric complex with the AHR nuclear translocator (ARNT) protein. This nuclear heterodimer recognizes dioxin-response enhancers found upstream of target genes and the AHR/ARNT-DNA interactions lead to the up-regulation of genes encoding phase I and phase II drug metabolism enzymes (e.g., Cytochrome P450 (Cyp)1a1, Cyp1a2, Cyp1b1 and the Glutathione S-transferase 1a.) [9–11].
Li et al. and Furumatsu et al. have independently reported that the Ahr deficient mouse models displayed severe colitis using a chemically induced experimental model of colitis by treatment with the inflammatory agent dextran sodium sulfate (DSS) [7, 12]. In these mouse models the DSS-induced colitis was ameliorated by treatment with AHR ligands such as Indole-3-carbinol (I3C) [7, 12, 13]. Interestingly, Kawajiri et. al. have reported that the treatment with I3C suppressed tumor development in the cecum and small intestine in Apcmin/+ mice [14]. Therefore we hypothesized that AHR may be implicated in the mechanism of colitis-associated colorectal tumor development. To investigate this hypothesis, we compared the incidence and multiplicity of colorectal tumors between wild-type (WT) and Ahr null (AhrΔ2/Δ2) mice using an experimental model of IBD-CRC by co-treatment with the tumor initiator azoxymethane (AOM) and the inflammatory agent DSS [15]. We then randomly assigned mice to regular purified or purified diet supplemented with the AHR agonist I3C to determine its effect on tumor susceptibility in this model.
METHODS
Animals
C57BL/6J (Wild-type) and Ahr null (AhrΔ2/Δ2) mice were housed in a selective pathogen-free facility on corncob bedding with chow diet and water ad libitum following the protocol established by University of Wisconsin Medical School’s Animal Care and Use Committee. The AhrΔ2/Δ2 mouse has been previously described [16, 17]. The null mice in this study have been backcrossed to the C57BL/6J strain for more than 10 generations and are thus >99.9% C57BL/6J background.
Tumor studies
The two stage colorectal tumor initiation-promotion assay (Fig.1A) consisted of a single dose of 10 mg/kg body weight of azoxymethane (AOM) by intraperitoneal (IP) injection at 8 weeks of age followed by 1% dextran sodium sulfate (DSS) by drinking water on day 7–12 post-injection [15]. Sixteen weeks later, the mice were sacrificed by CO2 euthanasia and the large intestine was harvested. The intestine was longitudinally incised along the main axis. The number, location and size of tumors on the luminal surface of the colon and rectum were recorded. For analysis of spontaneous colon and cecum tumors, 52–56 week old mice were evaluated.
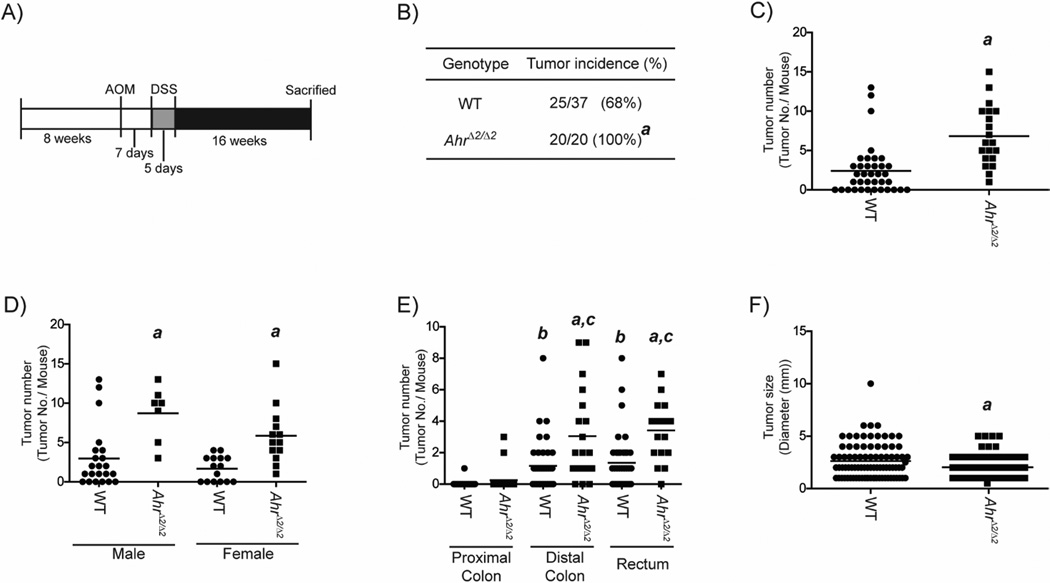
Fig.1. Susceptibility to AOM/DSS-induced colorectal tumorigenesis was increased in AhrΔ2/Δ2 mice.
(A) Schematic protocol for AOM/DSS-induced colorectal tumorigenesis. At 8 weeks of age, AhrΔ2/Δ2 (n=20) and WT mice (n=37) were treated with a single dose of 10 mg/kg body weight of AOM by IP injection. Seven days after the AOM injection, the mice were given 1% DSS by drinking water for 5 days. Sixteen weeks later, the mice were sacrificed for evaluation of colorectal tumorigenesis. (B) Incidence of colorectal tumors in AOM/DSS-treated AhrΔ2/Δ2 and WT mice. (C-E) AOM/DSS-induced colorectal tumor multiplicity in AhrΔ2/Δ2 and WT mice. (C) Total colorectal tumor multiplicity. (D) Gender-based classification. (E) Segmentation-based classification. (F) Size of colorectal tumors in AOM/DSS treated AhrΔ2/Δ2 and WT mice. Bars represent mean values. a; significantly different relative to WT mice (p<0.05), b; significantly different relative to the proximal colon of WT mice (p<0.05), c; significantly different relative to the proximal colon of AhrΔ2/Δ2 (p<0.05).
Chemoprevention treatment by Indole-3-carbinol
Mice were fed with either I3C (0.1% in AIN-76A diet; Test Diet, Richmond, IN) or control diet (AIN-76A; Test Diet, Richmond, IN) from birth continuously until completion of the colorectal tumorigenesis protocol (Fig.1A).
Assessment of DSS-induced colitis
Eight-week old mice were weighed and treated with 1% DSS in drinking water for 5 days followed by single oral gavage administration of 1% DSS solution (3 mL) to normalize the time of last exposure. Twenty-four hours after the final DSS administration, mice were weighed, sacrificed and the colon harvested. Colon length was measured and mucosa tissues were scraped from the distal colon segment. The colon mucosal tissues were used for gene expression analysis.
Gene expression analysis
Total RNA was isolated from colonic mucosal tissues using the Qiagen RNeasy kit (Qiagen, Valencia CA). The isolated RNAs were reverse-transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster city, CA). The mRNA levels were measured with TaqMan Universal PCR Master Mix (Applied Biosystems) and custom-designed probes listed in SDC Methods.
Western blot analysis
Colon and cecum tissue were used for western blot analysis. Western blot analysis is described in SDC Methods.
Measurement of DNA adducts
Mice were treated with AOM and colon mucosa was scraped. DNAs from colon and rectum mucosal cells were isolated. Adducts quantitation was performed by capillary liquid chromatography–electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) as described in SDC Methods. The amount of guanine in each sample was determined by high-performance liquid chromatography-ultraviolet spectrometric analysis.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism version 6.00 for Windows, GraphPad Software (La Jolla California USA), www.graphpad.com. Intergroup comparisons were performed by Fisher exact test or the non-parametric Wilcoxon rank sum test. Differences among groups were considered statistically significant when p value was < 0.05.
RESULTS
Increased susceptibility to colitis-associated colorectal tumorigenesis in AhrΔ2/Δ2 mice
In order to determine whether AHR is involved in the development of tumors associated with colitis, we used a model of colitis-associated colorectal tumorigenesis utilizing treatment with AOM and DSS in AhrΔ2/Δ2 and WT mice [15] (Fig 1A). AhrΔ2/Δ2 mice showed higher colorectal tumor incidence than WT mice (P=0.005) (Fig.1B). The colon tumor multiplicity in AhrΔ2/Δ2 mice was nearly three-fold higher than WT mice (AhrΔ2/Δ2: 6.85 ±0.84; WT: 2.43 ±0.52, P<0.0001) (Fig. 1C). The results of colorectal tumor multiplicity were additionally classified by gender and colorectal segment (Fig.2D–E). The increase of colorectal tumor multiplicity in AhrΔ2/Δ2 mice was independent of sex as the three-fold increase in tumor multiplicity was observed in both male and female mice (male; P<0.01, female; P<0.01) (Fig.1D). In both WT and AhrΔ2/Δ2 mice, most tumors were observed at distal colon and rectum segments (Fig.1E). The tumor multiplicities of distal colon and rectum were significantly higher in AhrΔ2/Δ2 mice compared to WT mice (distal colon; P=0.01, rectum; P<0.01), whereas the proximal colon multiplicity was not significantly different between WT and AhrΔ2/Δ2 mice (P=0.22). We also measured the size of colorectal tumors. Interestingly, the average size of colorectal tumors in AhrΔ2/Δ2 mice was approximately 23% smaller than that in WT mice (AhrΔ2/Δ2: 2.03 ±0.13; WT: 2.65 ±0.18, P=0.03) (Fig. 1F). These findings demonstrated increased susceptibility to tumor formation in AhrΔ2/Δ2 mice compared to WT mice after treatment with a colitis-associated colorectal tumorigenesis model, indicating the presence of AHR is important for protection against colitis-induced neoplastic growth.
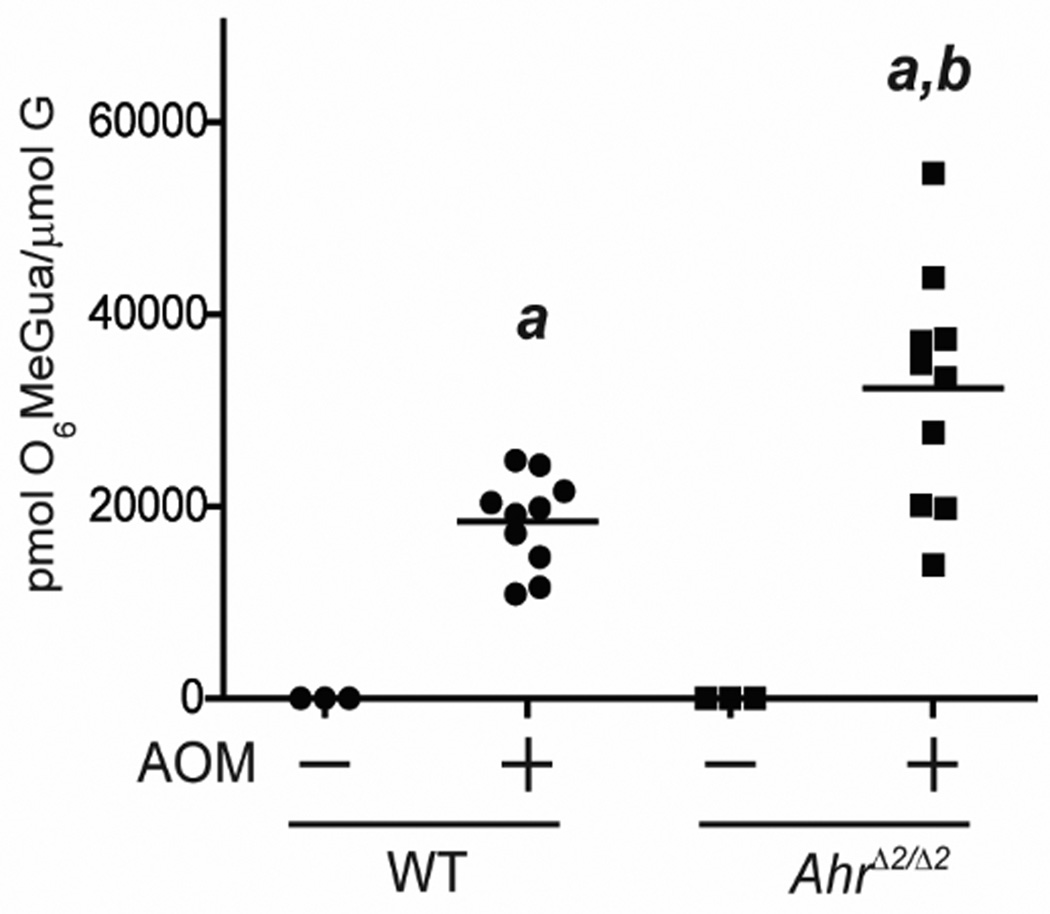
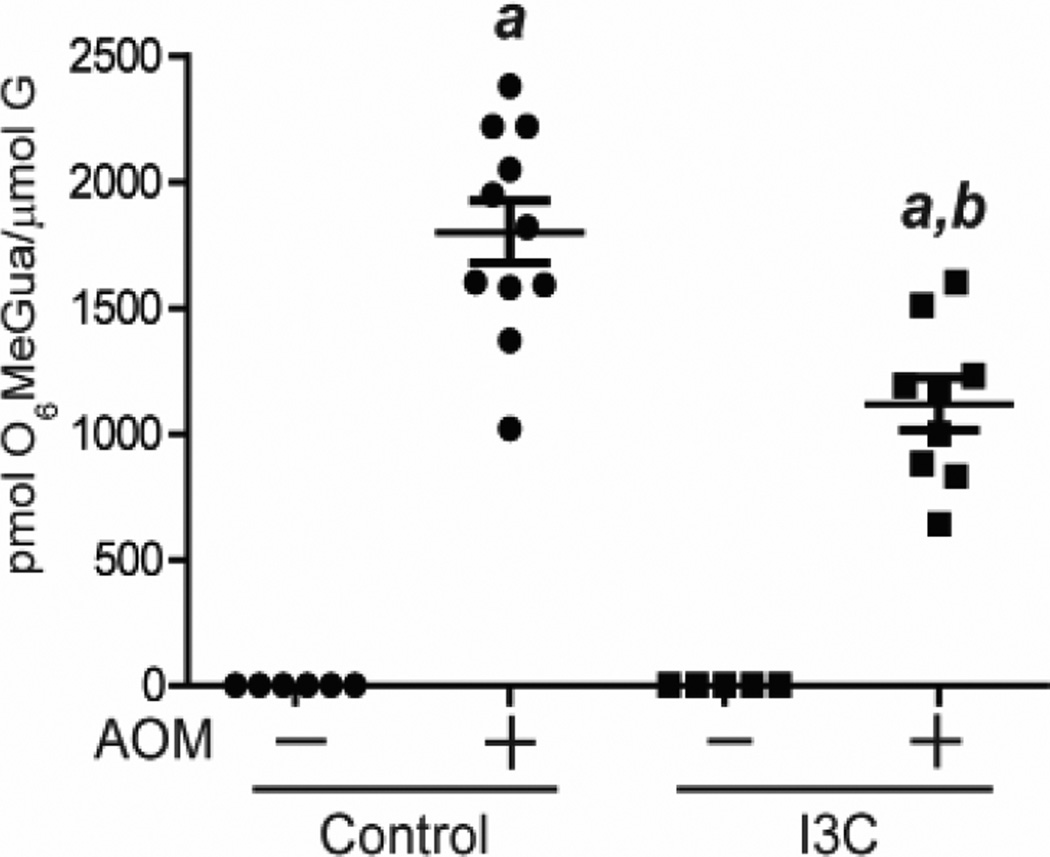
Fig.2. AhrΔ2/Δ2 mice showed higher levels of AOM-induced DNA adduct than WT mice.
Levels of O6-methyl-guanine in colonic DNA of AhrΔ2/Δ2 and WT mice. At 8 weeks of age, AhrΔ2/Δ2 and WT mice were administered a single IP injection of PBS or 10 mg/kg body weight of AOM. Twelve hours after the injection, mice were sacrificed.. a; significantly different relative to the no AOM treated mice (p<0.05), b; significantly different compared to the AOM-treated WT mice (p<0.05). Bars represent mean values.
Kawajiri et. al. have previously reported that Ahr deletion mouse model displayed significant spontaneous cecal and colon tumor development [14]. The report demonstrated that all Ahr null mice (AhrΔ1/Δ1, corresponding to loss of exon 1 in the Ahr gene) bore spontaneous colon or cecal tumors by 11 weeks of age. However, spontaneous cecal and colon tumor development was infrequent in our Ahr null mouse model (AhrΔ2/Δ2, corresponding to loss of exon 2 in the Ahr gene). Although the tumor incidence in AhrΔ2/Δ2 was approximately three times higher than in WT mice, the incidences were not significantly different between the two groups (Supl.1A). The levels of colon tumor multiplicity, β-catenin protein and c-myc mRNA were also not statistically different between two groups (Supl.1B–D). These findings suggested that the colorectal tumor formation was induced by AOM and DSS in our mouse models.
Loss of AHR increased AOM- and DSS-induced tumorigenic effects
AOM is a potent colorectal tumor initiator [15]. To compare the AOM-induced colorectal tumor initiation activity between AhrΔ2/Δ2 and WT mice, we measured O6-methyl-guanine levels, an established biomarker of DNA adduct formation, in AhrΔ2/Δ2 and WT mice (Fig.2) [18]. The amount of O6-methyl-guanine was undetectable in untreated animals but was considerably increased by AOM treatment in both AhrΔ2/Δ2 and WT mice (AhrΔ2/Δ2: P=0.01; WT: P=0.01,). The level of O6-methyl-guanine was 75% higher in AOM-treated AhrΔ2/Δ2 mice compared to AOM-treated WT mice (AhrΔ2/Δ2 (AOM): 32,270 ±3,885; WT (AOM): 18,433±1,526, P=0.02). These findings demonstrated increased susceptibility to DNA alteration after treatment with a tumor initiator in AhrΔ2/Δ2 mice.
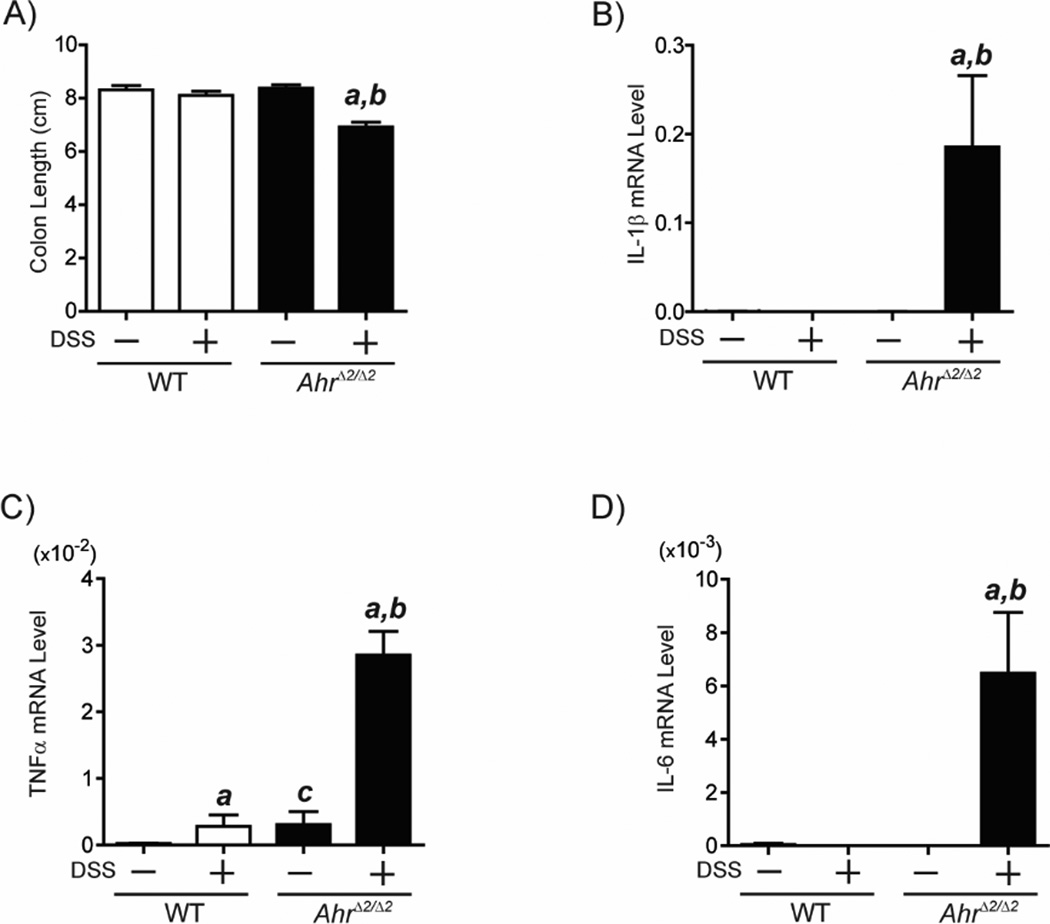
In the two stage colorectal tumor formation protocol of AOM and DSS, DSS-induced intestinal inflammation is involved in the process of colorectal tumor promotion [15, 19]. To investigate the effect of Ahr deletion on the susceptibility of DSS-induced inflammation, we assessed the colon length as an outcome of DSS-induced acute colitis (reduced colon length) in AhrΔ2/Δ2 and WT mice. After oral administration of 1% DSS for 5 days, AhrΔ2/Δ2 mice developed foreshortened colon length compared to untreated AhrΔ2/Δ2 mice, while the colon length was not significantly changed by DSS treatment in WT mice (Fig. 3A). Subsequently, we measured mRNA levels of pro-inflammatory cytokines IL-1β, TNFα and IL-6, inflammatory mediators of acute DSS colitis [20], in colonic mucosa of AhrΔ2/Δ2 and WT mice (Fig. 3B–D). In the colon of Ahr null mice, IL-1β, TNFα and IL-6 mRNA levels were markedly increased by DSS treatment. In contrast, expression of IL-1β and IL-6 was not significantly induced by DSS treatment in WT mice, although an increase in TNFα mRNA was observed. These results reveal the importance of AHR in regulating production of inflammatory mediators integral to the pathophysiology of colitis and colitis-associated CRC.
Fig.3. AhrΔ2/Δ2 mice displayed higher susceptibility to DSS-induced acute colitis than WT mice.
Male AhrΔ2/Δ2 and WT mice (9 weeks of age) were given 1% DSS by drinking water for 5 days. Twenty-four hours after the treatment, the mice were sacrificed. (A) Colon length. Each group contained more than 13 mice (n=13–23). a; significantly different relative to the control AhrΔ2/Δ2 mice (p<0.05), b; significantly different relative to DSS-treated WT mice (p<0.05). Induction of pro-inflammatory cytokine genes expression in the DSS-treated AhrΔ2/Δ2 mice. Male AhrΔ2/Δ2 and WT mice (9 weeks of age), were given 1% DSS in the drinking water for 5 days. Twenty-four hours after treatment, the mice were sacrificed. Total RNAs were isolated from the distal colon mucosa of AhrΔ2/Δ2 and WT mice treated with or without DSS. The IL-1β (B), TNFα (C) and IL-6 (D) mRNA levels were determined by quantitative real-time RT-PCR and these measured mRNA levels were normalized to β-actin mRNA levels. Each group contained more than 4 mice (n=4–5). a; significantly different relative to control AhrΔ2/Δ2 mice (p<0.05), b; significantly different relative to DSS-treated WT mice (p<0.05), c; significantly different relative to control WT mice (p<0.05), Error bars represent SEM.
Previous studies have reported that DSS treatment may induce gene expression of Ahr and the AHR-driven gene Cyp1a1 (which serves as a highly sensitive and specific surrogate for Ahr activation) in murine colon mucosa [12]. To investigate whether AOM or DSS treatment stimulated Ahr gene expression or activated the AHR pathway, we measured the Ahr and Cyp1a1 mRNA levels in colon of AOM- or DSS-treated WT mice, respectively. Our results indicated that AOM and DSS were not involved in the induction of Ahr gene expression or elevation of AHR activity (Supl.2).
AHR-dependent suppression of colorectal tumor development by I3C
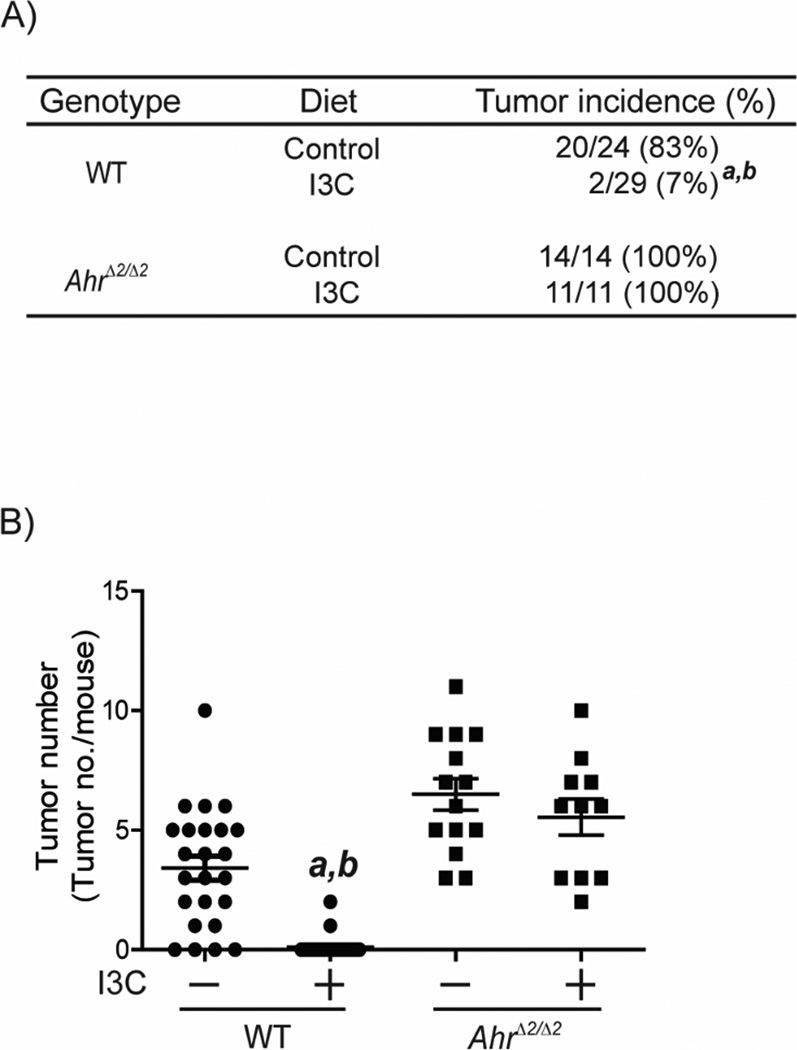
Our results suggested that AHR is required for colorectal protection in colitis-associated colorectal tumorigenesis. That is, the protective function should be enhanced by activation of AHR. To investigate this hypothesis, we fed I3C containing diet to AOM/DSS-treated WT and AhrΔ2/Δ2 mice (Fig.4). The colorectal tumor incidence was 92% decreased in I3C-fed WT mice compared to control-diet fed WT mice (WT (I3C): 7%; WT (control): 83%, P<0.0001) (Fig. 4A). However the colorectal tumor incidence was not influenced by I3C treatment in AhrΔ2/Δ2 mice. The colorectal tumor multiplicity in I3C-diet fed WT mice was 33 fold lower than control diet fed mice (WT (control): 3.42 ±0.51; WT (I3C): 0.10 ±0.07, P<0.0001) (Fig. 4B). In contrast, the decrease of colorectal tumors by I3C diet was not observed in mice AhrΔ2/Δ2 mice (AhrΔ2/Δ2 (control): 6.5 ±0.66; AhrΔ2/Δ2 (I3C): 5.54 ±0.76, P=0.35). Since the I3C diet activates AHR in AHR-dependent manner in intestine of mice (Supl. 3), we concluded that I3C enhanced the function of colorectal protection through AHR.
Fig. 4. I3C decreased tumor development induced by AOM/DSS in WT mice.
Mice were fed with I3C or control diet from birth. At 8 weeks of age, WT (Control) (n=24), WT (I3C) (n=29), AhrΔ2/Δ2 (Control) (n=14), AhrΔ2/Δ2 (I3C) (n=11), were treated with a single dose of 10 mg/kg body weight of AOM by IP injection. Seven days after the AOM injection, the mice were given 1% DSS by drinking water for 5 days. Sixteen weeks later, the mice were sacrificed for evaluation of colorectal tumorigenesis. (A) Incidence of colorectal tumors in AOM/DSS-treated AhrΔ2/Δ2 and WT mice. (B) AOM/DSS-induced colorectal tumor multiplicity in AhrΔ2/Δ2 and WT mice. a; significantly different relative to WT mice treated with control diet (P<0.0001), b; significantly different relative to AhrΔ2/Δ2 mice.
I3C prevented AOM-induced tumor initiation and DSS-induced inflammation
To understand the preventive effect of I3C in the colitis-associated colorectal tumorigenesis model, we measured the levels of DNA adduct in AOM-treated WT mice fed with I3C or control diet. The AOM-induced DNA adducts were decreased by nearly 38% in I3C diet fed WT mice compared to control diet fed WT mice (WT (control): 1,798 ±125; WT (I3C): 1,118 ±105, P=0.0006) (Fig.5), while I3C did not influence the DNA adducts in the control (w/o AOM) mice.
Fig.5. I3C treatment decreased AOM-induced DNA adducts.
Levels of O6-methyl-guanine in colonic DNA of WT mice treated with I3C and control diet (n=5–10). At 8 weeks of age, both groups of WT mice were administered a single IP injection of PBS or 10 mg/kg body weight of AOM. Twelve hours after the injection, mice were sacrificed. Bars represent mean values. a; significantly different relative to no AOM treatment group (P<0.0001), b; significantly different relative to WT mice treated with control diet (P<0.0006).
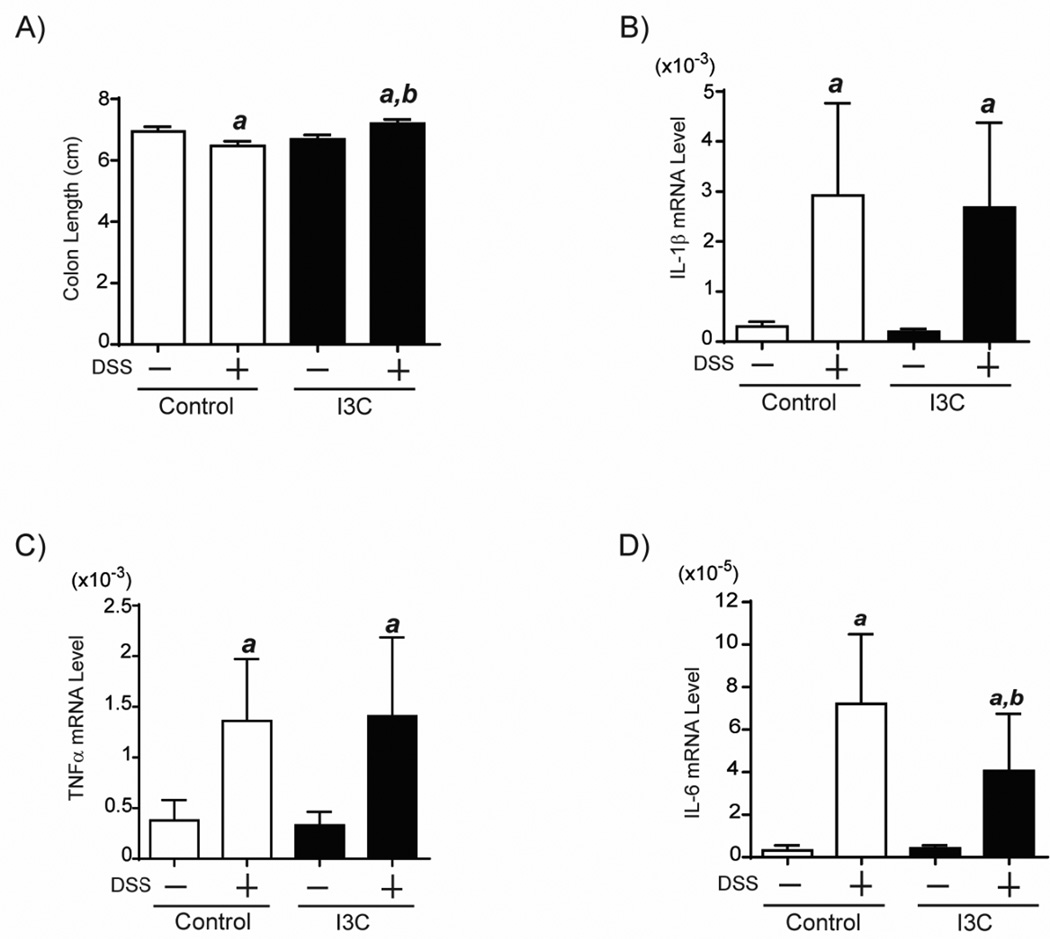
Subsequently, we evaluated the effect of I3C in DSS-induced inflammation (Fig.6). Although the colon length was decreased by DSS in control diet fed mice, the decrease was not observed in I3C-fed mice. (Fig.6B–E). Colon length of WT mice treated with I3C was longer than WT mice on control diet (P=0.002) (Fig.6B). The levels of IL-1β and TNFα were not affected by I3C treatment however levels of IL-6 were significantly reduced in WT-treated with I3C compared to WT mice on control diet (P=0.03). These data suggest that I3C moderately reduced DSS-induced colitis. Collectively, these findings revealed that the chemopreventive effects of I3C in a colitis associated-CRC model functions at the initiation and promotion steps of tumorigenesis.
Fig.6. I3C partially reduces DSS-induced inflammation.
WT mice (9 weeks of age) treated with I3C and control diet, were given 1% DSS in the drinking water for 5 days. Twenty-four hours after treatment, the mice were sacrificed. Colon length (A). Additionally, total RNAs were isolated from the distal colon mucosa of WT mice treated with or without DSS. The 1IL-1β (B), TNFα (C) and IL-6 (D) mRNA levels were determined by quantitative real-time RT-PCR and these measured mRNA levels were normalized to GAPDH mRNA levels. Each group contained more than 10 mice. a; significantly different relative to no DSS treated group (p<0.05), b; significantly different relative to WT mice treated with control diet (p<0.05). Error bars represent SEM.
DISCUSSION
Inflammatory bowel disease (IBD) is a well-established risk factor for the development of CRC [21, 22]. While the etiology of this disease is not fully understood, investigations demonstrate that dysregulation of intestinal immunity is a significant contributor to the pathophysiology of IBD [23, 24]. In the last several years, experiments using Ahr deletion mouse models have revealed that AHR is fundamental in mediating intestinal inflammation through regulating both intraepithelial lymphocytes (IELs) and innate lymphoid cells (ILCs) [7, 8]. This relationship led us to hypothesize that aberration in AHR signaling may impact the development of IBD-CRC.
The chemically-induced colorectal tumorigenesis experiment using the AhrΔ2/Δ2 mice revealed that AHR may play a protective role in IBD-associated colorectal tumorigenesis, because loss of AHR increases the susceptibility to AOM/DSS-induced colorectal tumor development (Fig.1) and activation of AHR by treatment of I3C suppressed the colorectal tumor development in an AHR-dependent manner (Fg.4). Moreover, we found that the tumor suppressor effect of AHR was not influenced by gender difference and was more prominent in the distal colon and rectum. Tumor size was not enlarged in AOM/DSS-treated AhrΔ2/Δ2 mice compared to AOM/DSS-treated WT mice and all tumors were adenomas in the two mouse groups (data not shown), indicating that the AHR should be involved in the early stage of colorectal carcinogenesis.
The intestinal innate immunity is a mucosal defense system that assimilates input from antigenic stimulation, including chemical compounds and intestinal bacteria, and orchestrates tolerance as well as repair of cellular damage [23, 24]. Ahr null mice have decreased IELs compared to WT counterparts, decreased epithelial cell turnover, altered tolerance as evidenced by a more indiscriminate immune response to toxicants, as well as diminished intestinal epithelial cell regenerative/reparative capabilities [7]. Therefore, the intestinal epithelial cells (IECs) lacking AHR may be susceptible to harmful stimulation by exposure of toxicants like AOM and DSS.
The mouse model of colitis-associated colorectal tumorigenenesis is composed of AOM-induced tumor initiation and DSS-induced intestinal inflammation [15,]. For the AOM-induced colon tumor initiation, hepatic metabolizing enzyme Cyp2e1 and β-glucuronidase of intestinal flora are required for the generation of the genotoxic metabolite methylazoxymethanol (MAM) [25–28]. It is plausible that the bacterial loads of intestinal flora expressing the β-glucuronidase (e.g. Clostridium, Peptostreptococcus, B. fragilis, Staphylococcus) may be increased in colon of the Ahr null mice [29–31]. Additionally, it is reported that Ahr null mice display a patent ductus venosus that may decrease the AOM metabolism resulting in an increase in AOM toxic effect in these mice. Surprisingly, we found that the activation of AHR by I3C decreased AOM-induced DNA adducts, suggesting that AHR may be involved in tumor initiation process of colitis-associated CRC. Moreover, our findings indicate that I3C may functions as a protector agent against AOM-induced DNA damage. To this regard, it is reported that I3C induces intestinal glutathione-S-transferase (GST), an enzyme involved in the detoxification of xenobiotics and in regulating antioxidant responses [32–34]. It is plausible, that I3C protective effect against DNA damage may be mediated by an increase in the detoxification of AOM, an alkylating agent that provokes DNA damage, resulting in antioxidant responses that confer protection within the colon. However, further investigations must be performed to identify the mechanism of the protective effect of I3C against AOM-induced DNA adducts.
It is reported that intestinal microbiota is also implicated in DSS-induced colitis [20], although the mechanism by which intestinal flora impacts DSS-induced intestinal inflammation is poorly understood. Takamura et al. found that commensal probiotic lactobacillus bulgaricus OLL1181 ameliorated DSS colitis by activation of AHR signaling via a probiotic metabolized dietary component or endogenous AHR ligand [35]. Li et al. also noted that DSS colitis was significantly alleviated when mice were provided with a I3C- supplemented diet [7]. Several reports have demonstrated that treatment with AHR ligands rescue DSS-induced colitis [7, 12, 13]. Therefore, the reduction in bacterially generated AHR ligands in conjunction with loss of the AHR ligand-binding capability in IECs and immune cells in Ahr null mice are likely integral components in the exacerbation of DSS-induced intestinal inflammation. We found that I3C treatment to be associated with decreased levels of IL-6, a major cytokine in the chronic phase the colitis in humans [36]. It is reported that treatment with AHR ligands restores intestinal epithelial cell integrity and regulates the composition of intestinal microflora which can result in a decrease in DSS-induced colitis [7, 8]. Therefore, we suspect that I3C reduces DSS-induced colitis by restoring intestinal immune cells resulting in a decrease in inflammation reflected by reduced levels of IL-6.
We demonstrated that loss of AHR increases susceptibility to AOM/DSS-induced colorectal tumor development; the implication of which is that AHR is requisite for resistance to IBD-associated tumor formation. The clinical importance of this consideration derives from the fact that the AHR system is highly conserved among mammals including humans [9, 10]. Accordingly, genetic or epigenetic gene silencing or environmental inhibition of AHR would be a novel health risk for IBD and IBD- CRC in humans. Additionally we proved that I3C protects against AOM/DSS-induced colorectal tumorigenesis. I3C is a proagonist found in the Brassica family of vegetables. After ingestion, it undergoes condensation reactions within the acidic gastric environment resulting in several metabolites such as 3,3'-diindolylmethane (DIM) and indolo [3,2-b]carbazole (ICZ), both of which have high affinity for AHR [34]. Although the chemopreventive effect of I3C has been recognized before, this is the first study to establish that the I3C protective effect within the colon is dependent on AHR. This work supports the application of I3C as a chemopreventive agent for IBD-CRC in humans and also suggests that the activation of AHR may be used as chemopreventive strategy against CRC.
Supplementary Material
Acknowledgments
Sources of funding: This work was supported by the National Institutes of Health grants K08ES017283, R01ES020900 (GDK), R01ES020668, P30CA077598 and T32CA090217 (SMRK).
Footnotes
Conflict of interest: All authors have no conflict of interest.
REFERENCES
- 1.Robin P, Boushey FAH. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clinics in Colon and Rectal Surgery. 2009;22(4):7. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams SV, et al. Survival after inflammatory bowel disease-associated colorectal cancer in the Colon Cancer Family Registry. World J Gastroenterol. 2013;19(21):3241–3248. doi: 10.3748/wjg.v19.i21.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loftus EV, Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31(1):1–20. doi: 10.1016/s0889-8553(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 4.Lennard-Jones JE, et al. Cancer surveillance in ulcerative colitis. Experience over 15 years. Lancet. 1983;2(8342):149–152. doi: 10.1016/s0140-6736(83)90129-0. [DOI] [PubMed] [Google Scholar]
- 5.Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 2):1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 6.John Triantafillidis GN. Paris Kosmidis, Colorectal Cancer and Inflammatory Bowel Disease: Epidemiology, Risk Factors, Mechanisms of Carcinogenesis and Prevention Strategies. Anticancer Research. 2009;29(7):11. [PubMed] [Google Scholar]
- 7.Li Y, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13(2):144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hankinson O. The aryl hydrocarbon receptor complex. Annual Review of Pharmacology & Toxicology. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Nebert DW, Gonzalez FJ. P450 Genes: Structure, evolution, and regulation. Ann. Rev. Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- 12.Furumatsu K, et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci. 2011;56(9):2532–2544. doi: 10.1007/s10620-011-1643-9. [DOI] [PubMed] [Google Scholar]
- 13.Benson JM, Shepherd DM. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn's disease. Toxicol Sci. 2011;120(1):68–78. doi: 10.1093/toxsci/kfq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawajiri K, et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci U S A. 2009;106(32):13481–13486. doi: 10.1073/pnas.0902132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Robertis M, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pande K, Moran SM, Bradfield CA. Aspects of dioxin toxicity are mediated by interleukin 1-like cytokines. Mol Pharmacol. 2005;67(5):1393–1398. doi: 10.1124/mol.105.010983. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt JV, et al. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93(13):6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong MY, et al. Relationship between DNA adduct levels, repair enzyme, and apoptosis as a function of DNA methylation by azoxymethane. Cell Growth Differ. 1999;10(11):749–758. [PubMed] [Google Scholar]
- 19.Tanaka T, et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94(11):965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattar MC, et al. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4(2):53–61. [PMC free article] [PubMed] [Google Scholar]
- 22.Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18(29):3839–3848. doi: 10.3748/wjg.v18.i29.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11(1):9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- 24.Pott J, Hornef M. Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep. 2012;13(8):684–698. doi: 10.1038/embor.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto H, Takata RH, Komeiji DY. Synthesis of the glucuronic acid conjugate of methylazoxymethanol. Cancer Res. 1979;39(8):3070–3073. [PubMed] [Google Scholar]
- 26.Sohn OS, et al. Metabolism of azoxymethane, methylazoxymethanol and N-nitrosodimethylamine by cytochrome P450IIE1. Carcinogenesis. 1991;12(1):127–131. doi: 10.1093/carcin/12.1.127. [DOI] [PubMed] [Google Scholar]
- 27.Fiala ES, et al. Mechanism of benzylselenocyanate inhibition of azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Res. 1991;51(11):2826–2830. [PubMed] [Google Scholar]
- 28.Takada H, et al. Effect of beta-glucuronidase inhibitor on azoxymethane-induced colonic carcinogenesis in rats. Cancer Res. 1982;42(1):331–334. [PubMed] [Google Scholar]
- 29.Tamura M, Hirakawa K, Itoh K. Comparison of Colonic Bacterial Enzymes in Gnotobiotic Mice Monoassociated with Different Intestinal Bacteria. Microbial Ecology in Health and Disease. 1996;9(6):287–294. [Google Scholar]
- 30.Gadelle D, Raibaud P, Sacquet E. beta-Glucuronidase activities of intestinal bacteria determined both in vitro and in vivo in gnotobiotic rats. Appl Environ Microbiol. 1985;49(3):682–685. doi: 10.1128/aem.49.3.682-685.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arimochi H, et al. Effect of intestinal bacteria on formation of azoxymethane-induced aberrant crypt foci in the rat colon. Biochem Biophys Res Commun. 1997;238(3):753–757. doi: 10.1006/bbrc.1997.7384. [DOI] [PubMed] [Google Scholar]
- 32.Bjeldanes CBaL. EFFECT OF DIETARY INDOLE-3-CARBINOL ON INTESTINAL AND HEPATIC MONOOXYGENASE, GLUTATHIONE S-TRANSFERASE AND EPOXIDE HYDROLASE ACTIVITIES IN THE RAT. Fd Chem. toxic. 1984;22(12) doi: 10.1016/0278-6915(84)90147-9. [DOI] [PubMed] [Google Scholar]
- 33.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 34.Maruthanila VL, PJa MS. Attenuation of carcinogenesis and the mechanism underlying by the influence of Indole-3-carbinol and its metabolite 3,3’-Diindolylmethane: A therapeutic mavel. Advances in Pharmacological Sciences. 2014;2014:7. doi: 10.1155/2014/832161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takamura T, et al. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol Cell Biol. 2011;89(7):817–822. doi: 10.1038/icb.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neurath JMaM. Il-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance. Inflammatory Bowel Diseases. 2007;13(8) doi: 10.1002/ibd.20148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.