Abstract
Background
Mast cells (MC) are bone marrow derived haematopoetic cells playing a crucial role not only in immune response but also in the tumor microenvironment with protumorigenic and antitumorigenic functions. The role of MC in primary cutaneous T-cell lymphomas (CTCL), a heterogeneous group of non-Hodgkin lymphomas with initial presentation in the skin, is largely unknown.
Objective
To gain more accurate information about presence, number, distribution and state of activation (degranulated vs. non-degranulated) of MC in CTCL variants and clinical stages.
Materials and Methods
We established a novel computer-aided tissue analysis method on digitized skin sections. Immunohistochemistry with an anti-MC tryptase antibody was performed on 34 biopsies of different CTCL subtypes and on control skin samples. An algorithm for the automatic detection of the epidermis and of cell density based CTCL areas was developed. Cells were stratified as being within the CTCL infiltrate, in P1 (a surrounding area 0–30 μm away from CTCL), or in P2 (30–60 μm away from CTCL) area.
Results
We found high MC counts within CTCL infiltrates and P1 and a decreased MC number in the surrounding dermis P2. Higher MC numbers were found in MF compared to all other CTCL subgroups. Regarding different stages of MF, we found significantly higher mast cell counts in stages IA and IB than in stages IIA and IIB. Regarding MC densities, we found a higher density of MC in MF compared to all other CTCL subgroups. More MC were non-degranulated than degranulated.
Conclusion
Here for the first time an automated method for MC analysis on tissue sections and its use in CTCL is described. Eliminating error from investigator bias, the method allows for precise cell identification and counting. Our results provide new insights on MC distribution in CTCL reappraising their role in the pathophysiology of CTCL.
Introduction
Among the characteristics of cancer is its ability to recruit normal infiltrating and resident cells to generate a specific microenvironment fostering malignant growth.[1]
One of these bystanders is the mast cell (MC) originally discovered by Paul Ehrlich more than 100 years ago and mainly known for its immunological effector function. MC are haematopoetic cells which leave the bone marrow as undifferentiated precursors finding final differentiation in their target tissues under the influence of several microenvironmental growth factors, in particular stem cell factor (SCF), the ligand for the c-kit receptor tyrosine kinase (CD117).[2–6]
Already in 1891 Eugen Westphal, a student of Ehrlich’s, recognized that MC populate the interface between developing tumors and healthy tissues.[7,8]
Since then, MC have been found to accumulate around and within many types of solid cancer. While in parasitic infections and allergies, MC have been investigated for decades, studies on MC in malignant tissues have been somewhat neglected since Westphal and Ehrlich’s discovery. Recent research has revealed inconsistent results, showing a positive as well as a negative relationship between the presence and number of MC and prognosis in human malignancies.[3]
Potential MC effects on tumor growth can be categorized as either direct effects on tumor cells, such as MC cytotoxicity, or as indirect effects, such as immune cell recruitment, tissue remodelling of the neighboring environment and MC-directed angiogenesis.[4]
Angiogenesis is stimulated through release of preformed pro-angiogenic factors from MC granules as vascular endothelial growth factor (VEGF), fibroblastic growth factor-2 (FGF-2), and also through serine proteases as tryptase and chymase.[2,9,10]
Tryptase promotes the proliferation of endothelial cells, vascular tube formation and also dissolves extracellular matrix to provide space for neovascular growth.[2]
Increased numbers of MC have been shown to correlate with tumor progression and poor prognosis in various human malignancies including carcinomas of breast, stomach, rectum, liver, bile ducts, pancreas, prostate, and lung.[11–18]
At the same time other studies found a correlation of high MC counts with good prognosis and improved patient survival in carcinomas of colon, breast, and ovaries and in non-small cell lung cancer.[19–25]
In renal cell cancer patients no correlation between MC numbers and prognosis was found.[26]
Similarly, inconsistent observations have been made in lymphoid neoplasms, with increased numbers of MC correlating with poor prognosis in Hodgkin lymphoma and B-cell non-Hodgkin lymphoma and with favorable outcome in diffuse large B-cell lymphomas.[27–30]
Primary cutaneous lymphomas (PCL) are defined as a diverse group of non-Hodgkin lymphomas (NHL) with primary presentation in the skin and no sign of extracutaneous disease at the time of diagnosis.[30]
The presence and role of MC in PCL is largely unknown. The skin is a common site of extranodal non-Hodgkin lymphoma (NHL), manifestation ranking only behind the gastrointestinal tract.[30]
According to European and US studies, the annual incidence of PCL is estimated to be 1:100,000 with primary cutaneous T-cell lymphomas (CTCL) counting for about 71–77% and primary cutaneous B-cell lymphomas for about 23–29% of PCL.[30,31]
The heterogeneous group of CTCL includes indolent variants, following a chronic course with slow progression over years and decades (e.g. mycosis fungoides (MF) and the CD30+ lymphoproliferative disorders lymphomatoid papulosis (LyP) and primary cutaneous anaplastic large cell lymphoma (C-ALCL)), and aggressive variants (e.g. Sezary syndrome (SS) and extranodal natural killer T-cell lymphoma, nasal type).[30,31]
Although the high density of MC in human skin together with their well known interaction with T-cells provide a rationale for the investigation of MC in CTCL, up to now only one study on MC in primary cutaneous lymphomas has been published demonstrating a protumorigenic role of MC in primary cutanous B- and T-cell lymphomas.[32]
In this and the other studies mentioned above MC numbers were estimated by microscopic counting of several high-power fields by one or more pathologists. The aim of our study was to gain more accurate information about presence, number and distribution of MC in CTCL by establishing and applying a novel computer-aided tissue analysis algorithm.
Materials and Methods
Archival formalin-fixed, paraffin-embedded tissue samples were retrieved from 34 patients with different CTCL variants of which the majority were patients with different stages of MF (for patient characteristics see Table 1).
Table 1. Demographic data of 34 primary cutaneous T-cell lymphoma cases.
| Type of lymphoma | n | Sex distribution male/female | Age distribution median | (years) range |
|---|---|---|---|---|
| CTCL | 34 | 24/10 | 60,5 | (13–85) |
| Mycosis fungoides | 25 | 20/5 | 59 | (13–85) |
| MF IA | 16 | 14/2 | 61 | (13–82) |
| MF IB | 1 | 0/1 | 65 | |
| MF IIA | 1 | 1/0 | 54 | |
| MF IIB | 7 | 5/2 | 69 | (23–85) |
| Sezary syndrome | 2 | 0/2 | n/a | (68,85) |
| Lymphomatoid papulosis | 3 | 2/1 | 62 | (53–79) |
| Primary cutaneous CD4+ small/medium pleomorphic T-cell lymphoma | 3 | 2/1 | 58 | (51–72) |
| CD30+ primary cutaneous anaplastic large-cell lymphoma | 1 | 0/1 | 28 |
n = number of patients; m = male, f = female; CTCL = primary cutaneous T-cell lymphoma; MF = Mycosis fungoides.
Only archival samples and historical data were analyzed anonymously precluding informed consent. All experiments were done in accordance with the local regulations and requirements regarding research on archival human tissues.
CTCL diagnosis was made according to the WHO-EORTC classification for cutaneous lymphomas.[30]
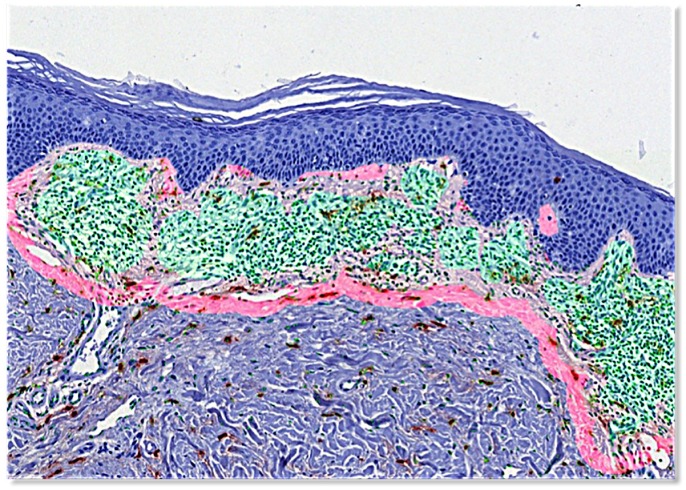
As controls we used biopsies from patients with inflammatory skin diseases (lichen planus, psoriasis, eczema) and normal skin. On all these biopsies we performed immunohistochemistry (Dako REAL Detection Systems, Dako, Denmark) utilizing a mouse monoclonal anti-mast cell tryptase antibody (Anti-Mast Cell Tryptase antibody, AA1, prediluted, ab74506, Abcam, Cambridge, UK) and appropriate alkaline phosphatase labeled second step reagents (Dako) resulting in a combination with 3-amino-9-ethylcarbazole (AEC) as substrate in MC stained in red. Counterstaining was done with hematoxylin (Fig 1a and 1b).
Figs 1a and b. Immunohistochemistry staining with a monoclonal anti-mast cell tryptase antibody.
Skin sections were scanned using TissueFAXS 4.2 and analyzed using StrataQuest 5.0 (both TissueGnostics Gmbh, Vienna, Austria). Images were acquired with 20x magnification at 0.275 μm/pixel and further analysed by adaptive guided segmentation (AGS) as described previously.[33,34]
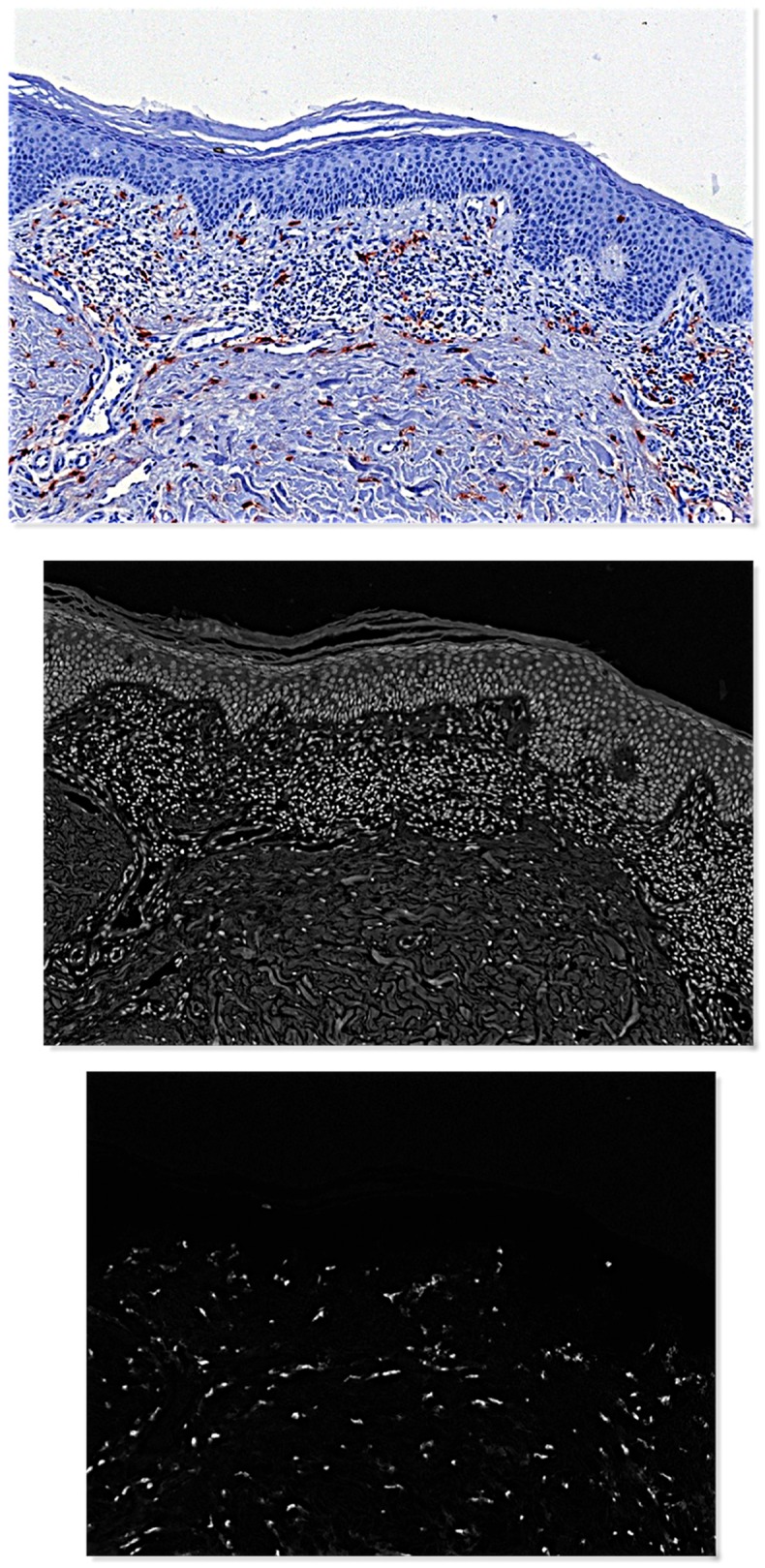
The same principles were applied to extract dermis and epidermis masks after color deconvolution to generate hematoxylin (H) and tryptase (T) optical density images (Fig 2a, 2b and 2c). Within the dermis masks, cells were detected by nuclear segmentation applied on H images. Local cell densities were estimated using the Parzen window method.[34]
Figs 2a–c. Color deconvolution to seperate the hematoxylin stained nuclei from tryptase positive mast cells.
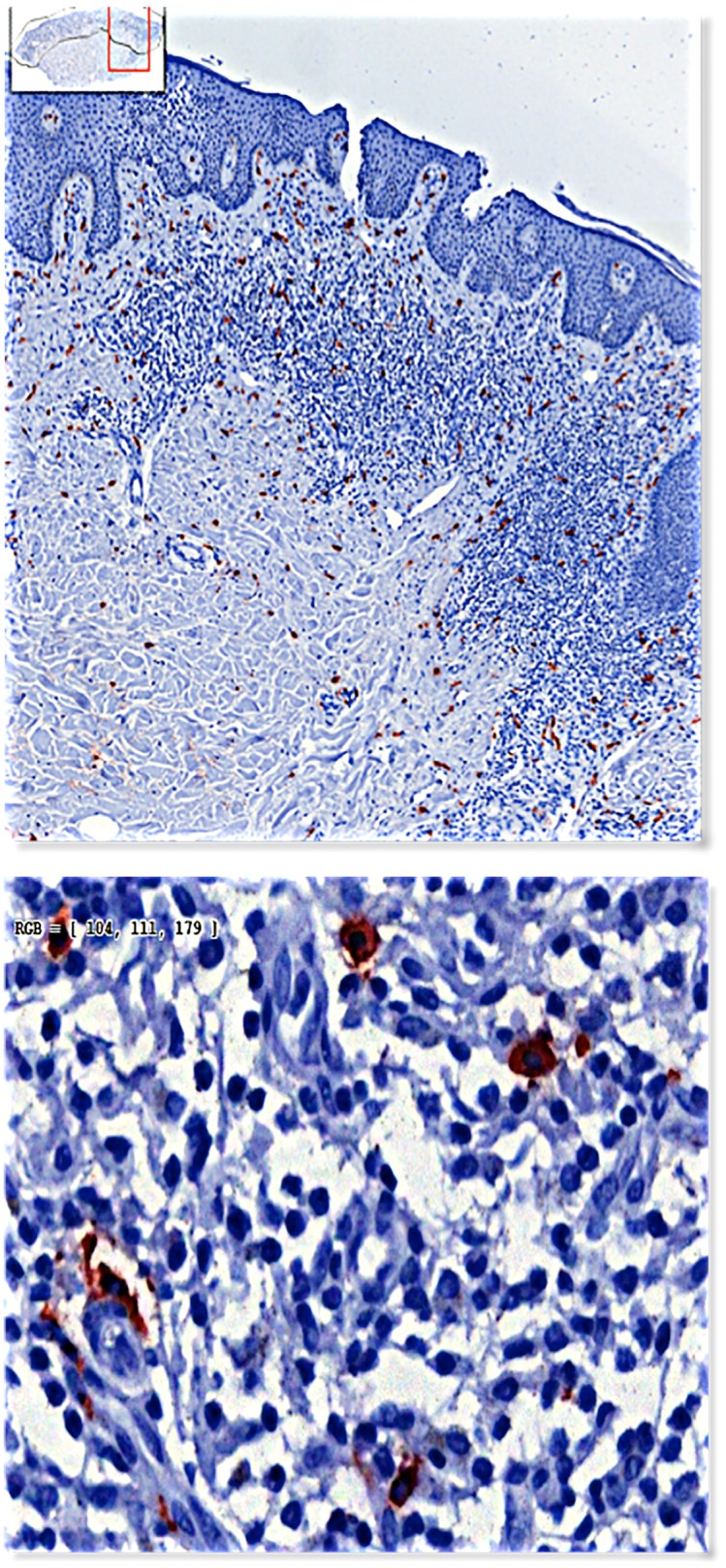
Through another AGS step CTCL areas were extracted automatically, visually corrected as needed, and by distance transformation two proximity areas (P1 = 0 to 30 μm and P2 = 30 to 60 μm outside CTCL areas) were defined (Fig 3). MC were identified on T images by local clustering. Single large compact blobs were defined as non-degranulated MC, while clusters with fragmented blobs were counted as degranulated MC (S1 Fig). Finally, cell counts and densities of all cells, all MC, degranulated and non-degranulated MC were extracted for each of the defined areas (CTCL, P1 and P2).
Fig 3. Regions of interest.
Lymphoma infiltrate: green. P1 (0 to 30 micron from lymphoma infiltrate): light pink. P2 (30 and 60 micron from lymphoma infiltrate): pink.
Statistical analysis
Absolute and relative MC densities are given as MC/mm2 and as percent of total cells in CTCL areas, respectively. Degranulated MC are given as percent of all MC. Given the limited sample size, non-parametric methods were applied for statistical testing. For descriptive statistics median and range were calculated. For paired group comparisons the Wilcoxon matched-pairs signed rank test was used where appropriate and for unpaired group comparisons the Mann-Whitney U test was used. Both are non-parametric tests. P values of less than 0.05 were considered significant.
Results
MC counts are increased in the area immediately surrounding CTCL infiltrates and in early stages compared to later stages of MF
Analysis of MC distribution in the predefined regions (CTCL area, P1, P2) revealed that MC numbers were identical in P1 (median 229.66 MC/mm2; range 24.27–543.21 MC/mm2) and CTCL areas and dropped significantly from P1 to P2 (median 169.20 MC/mm2; range 13.09–444.71 MC/mm2; p = 0.001)
Comparing the number and distribution of mast cells in CTCL areas (n = 34, median 219.55 MC/mm2; range 10.28–447.63 MC/mm2) and in dermis from normal donors (n = 3, median 114.06 MC/mm2; range 97.52–147.82 MC/mm2) we found a non-significant trend towards higher MC counts in CTCL (p = 0,248). Conversely, higher MC counts were found in samples of inflammatory skin diseases (median 391,27 MC/mm2; range 361,28–1332,45 MC/mm2) than in CTCL (see Table 2). Common to all variants of CTCL represented by more than one case is the wide range of MC counts reducing the statistical power to detect significant differences in limited samples.
Table 2. Mast cell counts per mm2 in different CTCL subtypes.
| Type of lymphoma | MC/mm2 median (range) | ||||
|---|---|---|---|---|---|
| n | Tumor | P1 | P2 | Dermis | |
| CTCL | 34 | 220 (10–448) | 230 (24–543) | 169 (13–445) | |
| Mycosis fungoides | 25 | 281 (53–448) | 242 (56–543) | 192 (43–445) | |
| MF IA | 16 | 308 (53–448) | 543 | 185 (69–319) | |
| MF IB | 1 | 402 | 421 | 445 | |
| MF IIA | 1 | 291 | 189 (56–377) | 391 | |
| MF IIB | 17 | 107 (61–337) | (44,261) | 137 (43–284) | |
| Sezary syndrome | 2 | (10,338) | 122 (52–274) | (13,209) | |
| LyP | 3 | 135 (44–241) | 181 (24–203) | 99 (16–221) | |
| SM | 3 | 104 (25–168) | 31 | 125 (16–160) | |
| C-ALCL | 1 | 35 | 25 | ||
| Normal Skin | 3 | 114 (98–148) | |||
| ISD | 3 | 391 (361–1332) |
MC = mast cells; CTCL = primary cutaneous T-cell lyphomas; MF = Mycosis fungoides; LyP = lymphomatoid papulosis; SM = primary cutaneous CD4+ small/medium sized pleomorphic T-cell lymphoma; C-ALCL = primary cutaneous anaplastic large cell lymhpoma; ISD = inflammatory skin diseases.
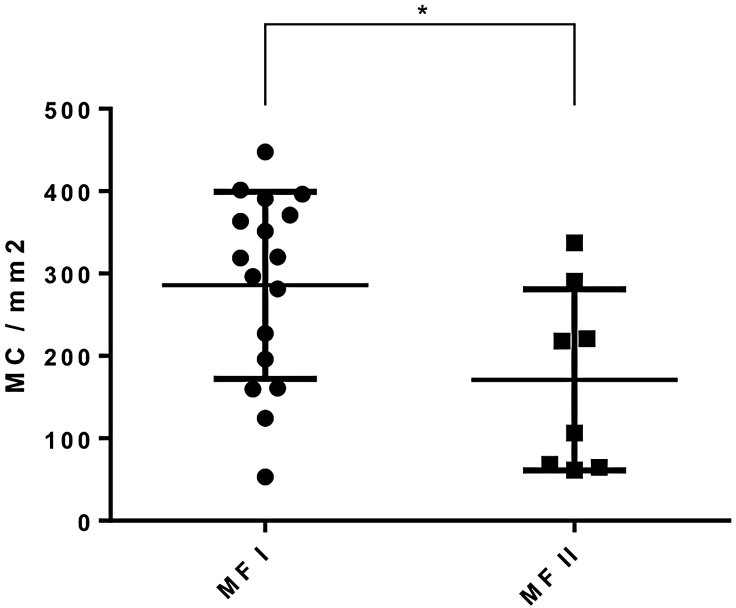
Looking at different stages of MF we found significantly higher mast cell counts in the CTCL areas in combined early stages IA and IB (median 354.58 MC/ mm2; range 53.1–447.63 MC/mm2, n = 17) than in combined advanced stages IIA and IIB (median 198.52 MC/mm2; range 61.39–337.09 MC/mm2, n = 18, p = 0.031) (see Fig 4).
Fig 4. Mast cell count per mm2 as measured by tissueFAXS system showing the difference between combined early MF stages IA and IB and combined advanced stages MF IIA and IIB.
Significantly higher mast cell counts were found in stage I disease.
Relative MC density is increased in MF compared to all other CTCL variants
We were not only interested in MC counts/mm2 but also in MC density in relation to tumor cell density (% MC of total cells) in CTCL clusters and found a higher MC density in MF (median 2,5%; range 0,45–6,74% MC of total cells) compared to all other CTCL subgroups (see Table 3).
Table 3. Mast cell density in relation to tumor cell density in CTCL variants.
| Type of lymphoma | Mast cell density (Mast cells/total cells) |
|---|---|
| Median (range) | |
| CTCL | 2,22 (0,15–6,74) |
| Mycosis fungoides | 2,53 (0,45–6,74) |
| MF IA | 3,54 (0,45–6,74) |
| MF IB | 3,65 |
| MF IIA | 3,03 |
| MF IIB | 1,72 (0,69–3,29) |
| Sezary syndrome | (0,15;4,05) |
| LyP | 1,45 (0,48–2,76) |
| SM | 1,10 (0,22–1,58) |
| C-ALCL | 0,37 |
CTCL = primary cutaneous T-cell lymphomas; MF = Mycosis fungoides; LyP = lymphomatoid papulosis; SM = primary cutaneous CD4+ small/medium sized pleomorphic T-cell lymphoma C-ALCL = primary cutaneous anaplastic large cell lymphoma;
Regarding MF subgroups, highest MC densities were found in stage I disease (median 3,54%; range 0,45–6,74% MC of total cells) (p = 0.026)
Degranulation of MC in CTCL
To determine the state of activation of MC we classified MC into 2 groups, degranulated and non degranulated and determined the percentage of each group in all patients and controls. Regarding all CTCL, in the tumor area 41.9% of all MC were degranulated without a significant difference regarding P1 (39,0%; p = 0.368) and P2 (39.8%; p = 0.520). Regarding MF subgroups, a trend towards higher percentages of degranulated MC was found in more advanced stages MF IIA+IIB (median 47,9%; range 28,4–55,8%) than in MF IA+IB (median 37,9%; range 15,0–51,8%; p = 0,100).
In normal skin samples the percentage of degranulated MC was 45.2%. (MC degranulation in CTCL and subtypes see Table 4)
Table 4. Percentage of degranulated MC in CTCL variants.
| Type of lymphoma | Degranulated MC % median (range) | |||
|---|---|---|---|---|
| Tumor | P1 | P2 | Dermis | |
| CTCL | 41,9 (0–55,8) | 39,0 (4,2–57,5) | 39,8 (0–70,0) | |
| Mycosis fungoides | 42,0 (15,0–55,8) | 39,7 (4,2–57,6) | 40,0 (7,1–70,0) | |
| MF IA | 37,0 (15,0–51,8) | 39,4 (4,2–51,6) | 37,7 (7,1–66,7) | |
| MF IB | 51,0 | 49,4 | 45,7 | |
| MF IIA | 52,4 | 49,6 | 50,0 | |
| MF IIB | 44,0 (28,4–55,8) | 38,8 (20,0–57,6) | 36,4 (25,0–70,0) | |
| Sezary syndrome | n/a (49,2/0,0) | n/a (12,5/43,3) | n/a (0/48,9) | |
| LyP | 38,6 (37,5–48,5) | 41,9 (37,9–45,1) | 40,0 (38,5–45,4) | |
| SM | 42,1 (39,3–43,6) | 34,7 (32,1–38,1) | 39,6 (23,4–46,3) | |
| C-ALCL | 38 | 34,8 | 30,0 | |
| Normal skin | 45,2 (37,1–45,9) |
MC = mast cells; CTCL = primary cutaneous T-cell lyphomas; MF = Mycosis fungoides; LyP = lymphomatoid papulosis; SM = primary cutaneous CD4+ small/medium sized pleomorphic T-cell lymphoma; C-ALCL = primary cutaneous anaplastic large cell lymhpoma;
Discussion
In this study we present for the first time a new computer aided image analysis method for MC quantification in immunohistologically stained tissue sections. We applied the method in CTCL to gain more accurate information about presence, number, distribution and state of activation of MC in CTCL variants and clinical stages. We found increased MC counts in the area immediately surrounding CTCL infiltrates and in early stages of MF compared to later stages. With this new computer aided algorithm it was for the first time possible to determine MC density in relation to tumor cell density in CTCL. MC density was found to be increased in MF compared to all other CTCL variants. Regarding the state of activation, around 40% of all MC were degranulated, irrespective of skin condition and relation to CTCL infiltrates.
Up to now there is only one published study about MC in PCL by Rabenhorst et al., where MC numbers were estimated by microscopic counting of 5 high-power fields by 2 independent observers.[32]
The authors of this study found significantly increased numbers of MC in CTCL and CBCL compared to normal skin and concluded on a protumorigenic role of MC in these diseases. This was confirmed in a mouse model of cutaneous lymphoma where tumor growth in MC deficient transgenic mice was significantly decreased.[32]
Similarly, we found higher numbers of MC in CTCL compared to normal skin samples in this study, although the number of control samples was too small (n = 3) to reach sufficient statistical power. However, primary aim of our study was not the interindividual comparison of CTCL with normal skin samples, since due to the high individual and site specific variability of MC counts in health and disease (including CTCL as shown here) sufficient statistical power is hard to achieve. Instead we rather took the approach of an intraindividual comparison between regions with histopathologically evident infiltration of CTCL with adjacent dermal areas, where each case serves as its own control. Doing so, we found a higher MC count in the lymphoma infiltrate and a narrow peripheral region arbitrarily defined by a 30 μm margin compared to more distant dermis. This supports the results of Rabenhorst et al. who found that MC infiltration was particularly prominent at lymphoma rims.[32]
The unique method of MC quantification used in our study enabled us not only to evaluate MC counts relative to area but for the first time also relative to the number of other nucleated cells within the center of the lymphoma infiltrate, which might be of particular pathophysiological relevance due to potential MC—tumor cell interactions. Similar to MC per area we also found a higher relative density of MC in MF compared to all other CTCL subgroups and within MF in the early stage disease subgroup indicating an inverse relationship between the presence of mast cells and clinical stage in CTCL. This seemingly differs from the protumorigenic role of MC as proposed by Rabenhorst et al. and might be due to different technology used and differences in patient series. However, it cannot be excluded that MC in CTCL might have different roles with protumorigenic and anti-tumorigenic functions depending on disease subtype and stage. Another difference to Rabenhorst’s data relates to MC degranulation where automated image analysis, as imployed here, revealed no difference in degranulation rate, whereas conventional microscopy as used by Rabenhorst indicated a significant increase of extensive degranulation in PCL, a difference most likely accounted for by different technology. Looking at the state of activation, it was emphasized that MC activation and following degranulation play an important role in tumor progression. In a review by Marech et al. it was shown in a study on breast cancer, that degranulated MC were mainly present in the peritumoral tissue whereas the non-degranulated MC were in particular located within the tumor tissue.[10,35]
Patruno and Marech et al. studied MC state of activation and microvessel density in a model of canine cutaneous mast cell tumor, and could show a correlation of degranulated MC with a high microvessel density.[36]
Regarding lymphomas, data about MC distribution in different subtypes of lymphomas is sparse. Increased MC number correlated directly with poor prognosis in multiple myeloma and Hodgkin lymphoma.[27,37]
Similar correlations were noted by a Swedish research group, who observed a worse prognosis for a nodular sclerosing subtype of Hodgkin´s lymphoma exhibiting a high MC number.[27]
On the other hand, MC infiltration was shown to be a favorable prognostic factor in diffuse large B-cell lymphoma.[29]
Thus, looking at the literature, in different solid and haematologic human malignancies contrary results about pro- and antitumorigenic MC functions are reported, which show that the role of MC in cancer is still not clear. There is evidence that tissue resident MC have the potential to strongly shape their tissue microenvironment, influencing tumor behavior by participating and regulating inflammatory and immune reactions.[3]
A different signalling environment may influence changes towards or against tumor progression with the mechanisms that underlie these opposing functional outcomes being unclear.[38]
In CTCL the results of our study might indicate an antitumorigenic role of MC. Although the mechanisms of MC interaction with the tumor biology of CTCL remain elusive, in addition to the mechanisms described above, in a T-cell malignancy interactions of MC and malignant and infiltrating lymphocytes might have to be considered. Under certain conditions MC have been described to interact with regulatory T-cells and overcome T-reg mediated immunosuppression, promote the development of effective antitumor immunity and boost the immune response in the tissue.[38]
It might be hypothesized that similar interactions take place in CTCL. Rabenhorst experiments of CTCL-cell stimulation with MC supernatants point to this direction and warrant further investigation in clinically relevant models. The role of MC mediators in tumor growth is very complex and reaches from promoting angiogenesis through VEGF, FGF-2, tryptase and chymase, generating immunosuppression by releasing Il-10, histamine and TNF-alpha, promoting tumor invasiveness by dissolving extracellular matrix through matrix metalloproteases, to promoting inflammation and inhibiting tumor cell growth and tumor cell apoptosis by releasing cytokines such as Interferon-alpha, TNF-alpha, Il-1, Il-4, Il-6.[2]
In CTCL, Rabenhorst et al. provide the only current availaible data about the presence and role of MC mediators. They could show that MC can induce release of IL-6 and additionally a proliferation of CTCL cells in vitro.[32]
Further studies with larger patient cohorts should be performed to clarify whether MC counts are useful as diagnostic or prognostic marker and even may be a future therapeutic target in CTCL.
Considering the limitations of our study, namely (i) a small patient cohort, limiting analysis of prognostic subgroups and (ii) missing information on clinical outcome that could be correlated with MC counts we still consider that the data presented here prove the feasibility of automated image analysis for the study of MC in tissue sections in general and in CTCL in particular.
Conclusion
This new computer approach not only allows for precise cell identification and quantificaton, but also helps to diminish error from investigator bias inherent to conventional microscopy. By this we introduce a new method for the investigation of MC in the pathology of cancer and other conditions where MC might play a role and at the same time are able to report on novel insights into MC distribution in CTCL. Together with earlier results as described above these data provide an initial basis for further investigation of the role of MC in pathology, disease stage, and prognosis in the various forms of CTCL.
Supporting Information
(TIFF)
Acknowledgments
Radu Rogujanu and Georg Steiner acknowledge financial support from the EC Marie Curie Actions, AIDPATH project (num. 612471)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Georg Steiner is co-owner and founder of TissueGnostics. TissueGnostics supported the project by scanning and by developing software algorithms for the analysis of the slides. Georg Steiner did not influence the data and data interpretation. Writing of the manuscript was done without Georg Steiners influence. Radu Rogujanu is an employee of Tissue Gnostics Gmbh and is paid a salary from TissueGnostics Gmbh. Radu Rogujau and Georg Steiner acknowledge financial support from the EC Marie Curie Actions, AIDPATH project (num. 612471). The funder provided support in the form of salaries for authors [RR], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the 'author contributions' section.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 2.Ribatti D, Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochimica et biophysica acta 2012;1822:2–8. 10.1016/j.bbadis.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 3.Galinsky DS, Nechushtan H. Mast cells and cancer—no longer just basic science. Crit Rev Oncol Hematol 2008;68:115–30. 10.1016/j.critrevonc.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 4.Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: angiogenesis, tissue remodelling and immune-modulation. Biochimica et biophysica acta 2009;1796:19–26. 10.1016/j.bbcan.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proceedings of the National Academy of Sciences of the United States of America 2005;102:11408–13. 10.1073/pnas.0504197102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galli SJ. New insights into "the riddle of the mast cells": microenvironmental regulation of mast cell development and phenotypic heterogeneity. Laboratory investigation; a journal of technical methods and pathology 1990;62:5–33. [PubMed] [Google Scholar]
- 7.Vyas H, Krishnaswamy G. Paul Ehrlich's "Mastzellen"—from aniline dyes to DNA chip arrays: a historical review of developments in mast cell research. Methods in molecular biology (Clifton, NJ) 2006;315:3–11. [PubMed] [Google Scholar]
- 8.Ehrlich P. Beiträge zur Theorie und Praxis der histologischen Färbung. Thesis, Leipzig University 1878.
- 9.Ammendola M, Sacco R, Sammarco G, Luposella M, Patruno R, Gadaleta CD et al. Mast Cell-Targeted Strategies in Cancer Therapy. Transfusion medicine and hemotherapy: offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie 2016;43:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marech I, Ammendola M, Gadaleta C, Zizzo N, Oakley C, Gadaleta CD et al. Possible biological and translational significance of mast cells density in colorectal cancer. World journal of gastroenterology: WJG 2014;20:8910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribatti D, Guidolin D, Marzullo A, Nico B, Annese T, Benagiano V et al. Mast cells and angiogenesis in gastric carcinoma. International journal of experimental pathology 2010;91:350–6. 10.1111/j.1365-2613.2010.00714.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher ER, Paik SM, Rockette H, Jones J, Caplan R, Fisher B. Prognostic significance of eosinophils and mast cells in rectal cancer: findings from the National Surgical Adjuvant Breast and Bowel Project (protocol R-01). Human pathology 1989;20:159–63. [DOI] [PubMed] [Google Scholar]
- 13.Pittoni P, Tripodo C, Piconese S, Mauri G, Parenza M, Rigoni A et al. Mast cell targeting hampers prostate adenocarcinoma development but promotes the occurrence of highly malignant neuroendocrine cancers. Cancer Res 2011;71:5987–97. 10.1158/0008-5472.CAN-11-1637 [DOI] [PubMed] [Google Scholar]
- 14.Strouch MJ, Cheon EC, Salabat MR, Krantz SB, Gounaris E, Melstrom LG et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res 2010;16:2257–65. 10.1158/1078-0432.CCR-09-1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takanami I, Takeuchi K, Naruke M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer 2000;88:2686–92. [PubMed] [Google Scholar]
- 16.Terada T, Matsunaga Y. Increased mast cells in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Journal of hepatology 2000;33:961–6. [DOI] [PubMed] [Google Scholar]
- 17.Marech I, Ammendola M, Sacco R, Capriuolo GS, Patruno R, Rubini R et al. Serum tryptase, mast cells positive to tryptase and microvascular density evaluation in early breast cancer patients: possible translational significance. BMC cancer 2014;14:534 10.1186/1471-2407-14-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ammendola M, Patruno R, Sacco R, Marech I, Sammarco G, Zuccala V et al. Mast cells positive to tryptase and tumour-associated macrophages correlate with angiogenesis in locally advanced colorectal cancer patients undergone to surgery. Expert opinion on therapeutic targets 2016;20:533–40. 10.1517/14728222.2016.1158811 [DOI] [PubMed] [Google Scholar]
- 19.Dabiri S, Huntsman D, Makretsov N, Cheang M, Gilks B, Bajdik C et al. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favorable prognosis. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc 2004;17:690–5. [DOI] [PubMed] [Google Scholar]
- 20.Aaltomaa S, Lipponen P, Papinaho S, Kosma VM. Mast cells in breast cancer. Anticancer Res 1993;13:785–8. [PubMed] [Google Scholar]
- 21.Welsh TJ, Green RH, Richardson D, Waller DA, O'Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol 2005;23:8959–67. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. The Journal of pathology 1999;189:487–95. [DOI] [PubMed] [Google Scholar]
- 23.Tan SY, Fan Y, Luo HS, Shen ZX, Guo Y, Zhao LJ. Prognostic significance of cell infiltrations of immunosurveillance in colorectal cancer. World journal of gastroenterology: WJG 2005;11:1210–4. 10.3748/wjg.v11.i8.1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan JK, Magistris A, Loizzi V, Lin F, Rutgers J, Osann K et al. Mast cell density, angiogenesis, blood clotting, and prognosis in women with advanced ovarian cancer. Gynecologic oncology 2005;99:20–5. 10.1016/j.ygyno.2005.05.042 [DOI] [PubMed] [Google Scholar]
- 25.Fisher ER, Sass R, Watkins G, Johal J, Fisher B. Tissue mast cells in breast cancer. Breast cancer research and treatment 1985;5:285–91. [DOI] [PubMed] [Google Scholar]
- 26.Tuna B, Yorukoglu K, Unlu M, Mungan MU, Kirkali Z. Association of mast cells with microvessel density in renal cell carcinomas. European urology 2006;50:530–4. 10.1016/j.eururo.2005.12.040 [DOI] [PubMed] [Google Scholar]
- 27.Molin D, Edstrom A, Glimelius I, Glimelius B, Nilsson G, Sundstrom C et al. Mast cell infiltration correlates with poor prognosis in Hodgkin's lymphoma. British journal of haematology 2002;119:122–4. [DOI] [PubMed] [Google Scholar]
- 28.Ribatti D, Vacca A, Marzullo A, Nico B, Ria R, Roncali L et al. Angiogenesis and mast cell density with tryptase activity increase simultaneously with pathological progression in B-cell non-Hodgkin's lymphomas. Int J Cancer 2000;85:171–5. [PubMed] [Google Scholar]
- 29.Hedstrom G, Berglund M, Molin D, Fischer M, Nilsson G, Thunberg U et al. Mast cell infiltration is a favourable prognostic factor in diffuse large B-cell lymphoma. British journal of haematology 2007;138:68–71. 10.1111/j.1365-2141.2007.06612.x [DOI] [PubMed] [Google Scholar]
- 30.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768–85. 10.1182/blood-2004-09-3502 [DOI] [PubMed] [Google Scholar]
- 31.Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood 2009;113:5064–73. 10.1182/blood-2008-10-184168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabenhorst A, Schlaak M, Heukamp LC, Forster A, Theurich S, von Bergwelt-Baildon M et al. Mast cells play a protumorigenic role in primary cutaneous lymphoma. Blood 2012;120:2042–54. 10.1182/blood-2012-03-415638 [DOI] [PubMed] [Google Scholar]
- 33.Rogojanu R, Thalhammer T, Thiem U, Heindl A, Mesteri I, Seewald A et al. Quantitative Image Analysis of Epithelial and Stromal Area in Histological Sections of Colorectal Cancer: An Emerging Diagnostic Tool. BioMed research international 2015;2015:569071 10.1155/2015/569071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrlich P. On estimation of a probability density function and mode. Ann Math Statist 1962;33:1065–76. [Google Scholar]
- 35.Mangia A, Malfettone A, Rossi R, Paradiso A, Ranieri G, Simone G et al. Tissue remodelling in breast cancer: human mast cell tryptase as an initiator of myofibroblast differentiation. Histopathology 2011;58:1096–106. 10.1111/j.1365-2559.2011.03842.x [DOI] [PubMed] [Google Scholar]
- 36.Patruno R, Marech I, Zizzo N, Ammendola M, Nardulli P, Gadaleta C et al. c-Kit expression, angiogenesis, and grading in canine mast cell tumour: a unique model to study c-Kit driven human malignancies. BioMed research international 2014;2014:730246 10.1155/2014/730246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribatti D, Vacca A, Nico B, Quondamatteo F, Ria R, Minischetti M et al. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br J Cancer 1999;79:451–5. 10.1038/sj.bjc.6690070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulfone-Paus S, Bahri R. Mast Cells as Regulators of T Cell Responses. Frontiers in immunology 2015;6:394 10.3389/fimmu.2015.00394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.