Abstract
Purpose
To describe the trends in pathogens and antibacterial resistance of corneal culture isolates in infectious keratitis during a period of 13 years at Hadassah-Hebrew University Medical Center.
Methods
A Retrospective analysis of bacterial corneal isolates was performed during the months of January 2002 to December 2014 at Hadassah Hebrew University Medical Center. Demographics, microbiological data and antibiotic resistance and sensitivity were collected.
Results
A total of 943 corneal isolates were analyzed during a 13 year period. A total of 415 positive bacterial cultures and 37 positive fungal cultures were recovered, representing 48% of the total cultures. The Annual incidence was 34.78 ± 6.54 cases. The most common isolate was coagulase-negative staphylococcus (32%), which had a significant decrease in trend throughout the study period (APC = -8.1, p = 0.002). Methicillin-resistant Staphylococcus aureus (MRSA) appears to have a decrease trend (APC = -31.2, P = 0.5). There was an increase in the resistance trend of coagulase-negative staphylococci to penicillin (APC = 5.0, P = <0.001). None of the pathogens had developed any resistance to Vancomycin. (P = 0.88).
Conclusions
Coagulase negative staphylococci were the predominant bacteria isolated from patients with keratitis. There was no significant change in the annual incidence of cases of bacterial keratitis seen over the past 13 years. Keratitis caused by MRSA appeared to decrease in contrast to the reported literature.
Introduction
Bacterial keratitis is a significant cause of visual loss. A targeted therapy based on corneal cultures and sensitivity of antibiotics is essential for the effective management of bacterial keratitis[1]. While awaiting cultures, empiric treatment should be started immediately and the antibiotic chosen should be of sufficiently broad spectrum to cover likely pathogens based on local bacterial prevalence and antibiotic susceptibilities.[2] Since regional differences exist in the etiologies of bacterial keratitis[3,4], good local epidemiological data are needed for better empirical treatment of bacterial keratitis.
Many community-based ophthalmologists elect to treat bacterial keratitis with broad-spectrum antibiotics without corneal scraping and susceptibility results.[5] In our institution we routinely culture all cases of suspected bacterial keratitis and start combination therapy with topical fortified cefazolin and gentamicin.
Recent shifts in bacterial etiologies have been reported where an increase in methicillin resistant Staphylococcus aureus has been observed [6,7]. Other trends in antibiotic susceptibility are changing depending on the way bacterial keratitis is treated, especially the recent emergence of resistance to fluoroquinolones among common corneal pathogens. [8–11]
The aim of this study was to describe the epidemiology of bacterial keratitis culture isolates and the trends in etiology and antibacterial resistance during the past 13 years at Hadassah-Hebrew University Medical Center.
Methods
This study conformed to the provisions of the declaration of Helsinki and was approved by the Institutional Review Board of the Hadassah-Hebrew University Medical Center (HMO-0664-13). The Institutional Review Board waived the need for informed consent from all patients who had been cultured for microbial keratitis during the study period, and their culture samples data was included in this study.
Data collection
This is a retrospective study; records were collected prospectively from January 2002 to December 2014. After collecting the sample it was sent immediately to the microbiology department and registered by the IT section recording all corneal samples including culture results, demographics, time of corneal sampling, type of sample, identity of the organism and antibiotic susceptibility. Special care was taken to include samples collected only by corneal scraping and to ensure that only the first isolation of a particular organism was counted for each patient. We encountered 32 cases of mixed infections that were included in the positive culture rate calculations. However, they were excluded out of the trend analysis.
Corneal culture sample
Microbiological examinations were conducted in the Clinical Microbiology Laboratory of the Hadassah-Hebrew University Medical Center. Corneal cultures were taken at the ER/clinic, using a sterilized Kimura spatula, following a diagnosis of suspected microbial corneal ulcer, after no antibiotic treatment was given or following a 12 hours therapeutic window. Topical anesthetic drops were used and special care was taken not to touch the lid margin or lashes to avoid contamination. After collecting the sample it was plated by an Ophthalmology resident directly onto 5% sheep blood agar and chocolate agar (Novamed, Jerusalem) using conventional “C” inocula[12], and two smears were prepared on clean microscope slides for Gram stain and additional staining methods as necessary. All corneal specimens submitted to the laboratory were routinely tested for fungal pathogens as well. This included microscopic evaluation using calcofluor white fluorescent staining and culture on Sabouraud Dextrose Agar and Brain-Heart Infusion Agar. Nevertheless, this report does not include detailed fungal analysis.
Organisms were identified using routine laboratory methods with conventional phenotyping, being largely replaced, from 2013, by Matrix-assisted laser desorption-ionization-time of flight (MALDI-TOF) mass spectrometry (VITEK MS, Biomerieux, France). This technique significantly improved the laboratory’s ability to identify some genera to the species level.[13] Bacterial susceptibility to antibiotics was assessed by the agar disc-diffusion method using CLSI methods and criteria (Clinical Laboratory Standards Institute, USA, as annually updated).
Data analysis
The study was divided into 3 periods for analysis: January 2002 to December 2005, January 2006 to December 2009 and January 2010 to December 2014. To evaluate the effect of time on the day of culture, we recorded the time of the day of the corneal sample and divided into periods following the regular 8 hour work period: from 8:00am to 3:00pm, 3:01pm to 11:00pm and 11:01pm to 7:59am.
Statistics
The statistical analysis was made using SPSS software version 22 (SPSS Inc, Chicago, IL). Descriptive statistics were used to describe the sample. Joinpoint regression analysis program version 4.2.0.2 was used to determine trends. The Joinpoint program selects the best fitting piece-wise continuous log- linear model. The permutation test was performed to determine the minimum number of “joinpoints” necessary to fit the data [14]. For the study period, we utilized trend analysis for selected antibiotic resistance and sensitivity trends. Trends were also assessed for selected pathogens and displayed in tables using the annual percent change (APC) with 95% confidence intervals.
Unpaired t-test was used to be able to evaluate if the introduction of Matrix-assisted laser desorption-ionization-time of flight (MALDI-TOF) mass spectrometry improved the yield of positive culture results.
Binomial proportion testing was used to prove significance in corneal sample culture collection and the yield of positive corneal isolates.
Results
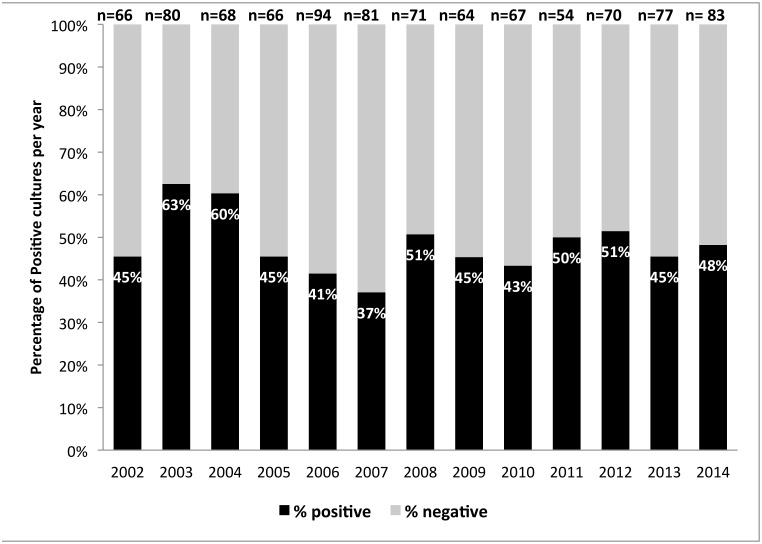
During the 13-year study period (2002–2014) a total of 943 corneal scrapings were sent for cultures. A total of 415 positive bacterial cultures and 37 positive fungal cultures were recovered, representing 48% of the total cultures. Bacterial keratitis represented 92% out of the total positive cultures. 53% of the patients were male. The mean age of patients with positive bacterial cultures was 46.96 ± 25.16 years. The mean number of positive bacterial corneal scrapings per year was 34.78 ± 6.54 cases. Fig 1 (S1 Table) shows the proportions of positive and negative cultures for each of the study years separately. The proportion of positive cultures was essentially stable despite an apparent minor decrease, APC = -1.6 (P = 0.3).
Fig 1. Bacterial keratitis culture distribution per year 2002–2014.
There was no significant difference when comparing the yield of positive culture rate and identification of organisms, before and after the introduction of Matrix-assisted laser desorption-ionization-time of flight (MALDI-TOF) mass spectrometry (VITEK MS, Biomerieux, France) (P = 0.47).
Changes in bacterial trends
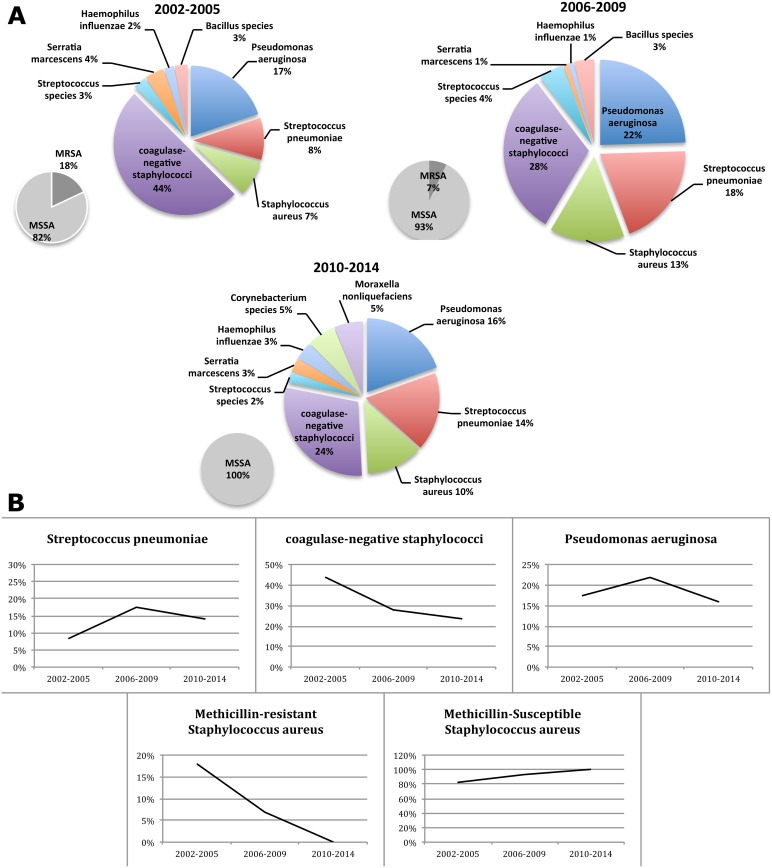
Shifts in the proportions of the main etiological agents of bacterial keratitis were detected during the study (Fig 2A and 2B) (S1 Table). During the period of 2002–2005: Coagulase-negative staphylococci, Pseudomonas aeruginosa, Streptococcus pneumoniae and Staphylococcus aureus comprised 44%, 17%, 8% and 7% respectively of the total positive cultures in the mentioned study period. For the period of 2006–2009: Coagulase-negative staphylococci decreased to 28%, Pseudomonas aeruginosa increased to 22%, Streptococcus pneumoniae increased to 18% and Staphylococcus aureus increased to 13%. For the period of 2010–2014: Coagulase-negative staphylococci remained low at 24%, Pseudomonas aeruginosa dropped to 16%, Streptococcus pneumoniae to 14% and Staphylococcus aureus to 10%. These four groups thus represented 76%, 81% and 64% of all bacterial isolates in the three periods. The drop in laboratory isolation of coagulase negative staphylococci explains the change in trends among the four groups of bacterial cultures. This also emphasizes the need to scrutinize the taxa that had greater proportional (and perhaps numerical) representation in the 2010–2014 periods.
Fig 2.
(A) Shifting proportions of bacterial keratitis isolates by year period 2002–2014. (B) Trends of bacterial keratitis isolates by year period 2002–2014.
Trend analysis
In order to evaluate the changes in positive bacterial corneal scrapings and their resistance to common antibiotics during the study period, we performed trend analysis. This analysis determines the Annual Percent Change (APC) and assigns statistical significance to the slope.
The only statistically significant trend was seen for Coagulase-negative staphylococci with an Annual Percent Change (APC) of -8.1 (P = 0.002). The apparent positive APC trends for Streptococcus pneumoniae, methicillin-sensitive Staphylococcus aureus (MSSA) and Escherichia coli were not significant, being +7.4 (P = 0.2), +7.5 (P = 0.2) and +14.7 (P = 0.1) respectively. Methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, Haemophilus influenzae and Streptococcus species had a non-statistical significant decrease in trend APC of– 31.2 (P = 0.5), -5.3 (P = 0.1), -8.3 (P = 0.1) and -3.5 (P = 0.5) respectively (Table 1) (S1 Table).
Table 1. Trend analysis of bacterial keratitis isolates by year period 2002–2014.
| Common bacterial isolate | 2002–2005 | 2006–2009 | 2010–2014 | APC* | P value |
|---|---|---|---|---|---|
| Grand Total | 155 | 119 | 157 | -1.6 | 0.3 |
| Coagulase-negative staphylococci | 68 | 33 | 37 | -8.1 | 0.002 |
| Pseudomonas aeruginosa | 27 | 26 | 25 | -5.3 | 0.1 |
| Streptococcus pneumoniae | 13 | 21 | 22 | 7.4 | 0.2 |
| Staphylococcus aureus | 11 | 15 | 16 | -0.8 | 0.9 |
| MRSA | 2 | 1 | 0 | -31.2 | 0.5 |
| MSSA | 9 | 14 | 16 | 7.5 | 0.2 |
| Haemophilus influenzae | 4 | 3 | 2 | -8.3 | 0.1 |
| Streptococcus species | 4 | 5 | 3 | -3.5 | 0.5 |
| Escherichia coli | 1 | 1 | 3 | 14.7 | 0.1 |
*APC: Annual Percent Change
Common antibiotic resistance trends
Coagulase-negative Staphylococci showed a statistical significant trend with an increase in the proportion resistant to penicillin in the study period (APC = 5.0, P = <0.001).
The antibiotics analyzed had no statistically significant trend, no vancomycin resistance was observed throughout the 3 study periods for coagulase-negative Staphylococci, Staphylococcus aureus and Streptococcus pneumoniae (Table 2) (S1 Table).
Table 2. Trend analysis of antibiotic resistance for common pathogens.
| 2002–2005 | 2006–2009 | 2010–2014 | APC* | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| % | N | % | N | % | N | ||||
| Pseudomonas aeruginosa | Amikacin | 0% | 0 | 0% | 0 | 0% | 0 | 0.00 | >0.05 |
| Cetazidime | 0% | 0 | 0% | 0 | 0% | 0 | 0.00 | >0.05 | |
| Ciprofloxacin | 0% | 0 | 8% | 2 | 0% | 0 | 0.00 | >0.05 | |
| Gentamicin | 7% | 2 | 12% | 3 | 0% | 0 | -31.20 | 0.4 | |
| Staphylococcus aureus | Chloramphenicol | 18% | 2 | 7% | 1 | 0% | 0 | -31.20 | 0.2 |
| Ciprofloxacin | 0% | 0 | 0% | 0 | 0% | 0 | 0.00 | >0.05 | |
| Gentamicin | 9% | 1 | 7% | 1 | 6% | 1 | 0.00 | >0.05 | |
| Oxacilin/methicilin | 18% | 2 | 7% | 1 | 0% | 0 | -31.20 | 0.2 | |
| Penicilin | 9% | 1 | 13% | 2 | 6% | 1 | 0.00 | >0.05 | |
| Vancomycin | 0% | 0 | 0% | 0 | 0% | 0 | 0.00 | >0.05 | |
| Coagulase-negative staphylococci | Chloramphenicol | 13% | 9 | 27% | 9 | 5% | 2 | -17.10 | 0.3 |
| Ciprofloxacin | 0% | 0 | 6% | 2 | 14% | 5 | 63.10 | 0.2 | |
| Gentamicin | 13% | 9 | 9% | 3 | 16% | 6 | -4.90 | 0.8 | |
| Penicilin | 28% | 19 | 70% | 23 | 76% | 28 | 5.00 | <0.001 | |
| Vancomycin | 0% | 0 | 0% | 0 | 0% | 0 | 0.00 | >0.05 | |
| Streptococcus pneumoniae | Ceftriaxone | 0% | 0 | 0% | 0 | 0% | 0 | 0.00 | >0.05 |
| Chloramphenicol | 0% | 0 | 0% | 0 | 0% | 0 | 0.00 | >0.05 | |
| Penicilin | 8% | 1 | 43% | 9 | 50% | 11 | 35.00 | 0.3 | |
| Vancomycin | 0% | 0 | 0% | 0 | 0% | 0 | 0.00 | >0.05 | |
| Total | Cefazolin | 8% | 12 | 4% | 5 | 4% | 7 | -6.50 | 0.6 |
| Gentamycin | 8% | 12 | 6% | 7 | 6% | 10 | -2.30 | 0.8 | |
| Vancomycin | 0% | 0 | 0% | 0 | 0% | 0 | 0.00 | >0.05 | |
| Ciprofloxacin | 0% | 0 | 3% | 4 | 5% | 8 | 72.90 | 0.2 | |
*APC: Annual Percent Change
Timing of corneal cultures and results
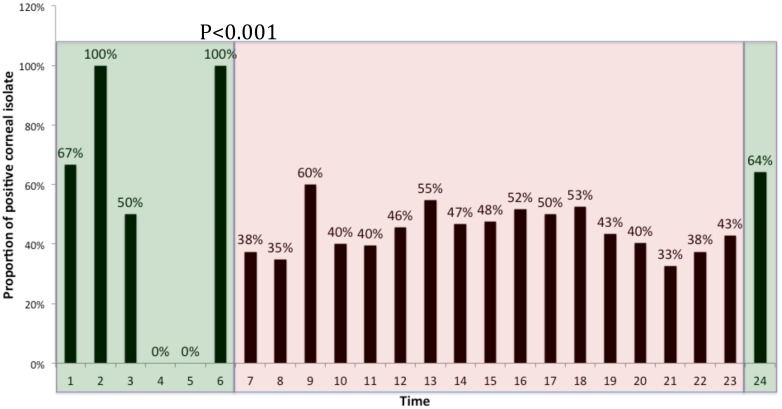
The average hourly yield of positive cultures for bacterial keratitis has been proven to be significantly lower during the night (23:00–06:00) compared to day (07:00–15:00) and evening (16:00–22:00) shifts P<0.01 (Fig 3) (S1 Table).
Fig 3. Proportion of Positives Corneal Isolates in a 24-hour period during 2002–2014.
Discussion
Bacterial keratitis remained the most common cause of infectious keratitis in our hospital. Coagulase negative staphylococci were the predominant bacteria isolated from patients with keratitis. An increased trend in Penicilin resistance to coagulase-negative staphylococci was demonstrated. There was no significant change in the overall number of cases of bacterial keratitis seen over the past 13 years.
This study is the first to bring data on bacterial keratitis culture isolates from a major tertiary medical center in Israel and with a relatively large follow up period compared to most of the studies commonly cited in the literature (Table 3).
Table 3. Literature review summary table.
| Author | Year published | Follow-up (Years) | Location | Number of corneal scrapes | Comments |
|---|---|---|---|---|---|
| Alexandrakis G et al. | 2000 | 9 | Florida | 2920 | Increase in number of S. aureus keratitis in the study period. |
| Decrease in the number of Pseudomonas aeruginosa keratitis in the study period | |||||
| Increasing laboratory resistance of S. Aureus keratitis isolates to fluoroquinolones | |||||
| Lalitha et al. | 2014 | 11 | India | 5912 | Annual number of keratitis cases due to bacteria decrease and the annual number due to fungus increased |
| Lichtinger et al. | 2012 | 11 | Toronto | 1701 | Decrease trend in Gram-positive isolates |
| Most common isolate overall was coagulase-negative Staphylococcus | |||||
| Increase trend toward laboratory resistance to methicillin | |||||
| MRSA was resistant to cefazolin and sensitive to vancomycin in all isolates | |||||
| Sand et al. | 2015 | 4 | Los Angeles | 290 | Coagulase negative Staphylococcus most common gram-positive isolate, Pseudomonas aeruginosa most common gram-negative isolate |
| Ciprofloxacin and levofloxacin was susceptible on all tested pathogens in 73% and 81% respectively | |||||
| Shalchi Z et al. | 2011 | 10 | UK | 476 | Increase in the number of gram-negative isolates in the study period |
| In vitro wide spread gram-negative resistance to chloramphenicol | |||||
| No increase trend found on ciprofloxacin resistance | |||||
| 97.3% of isolates were sensitive to combination of gentamicin and cefuroxime | |||||
| Zhang et al. | 2008 | 4 | China | 279 | Pseudomonas sp. most common organism isolated |
| Ciprofloxacin showed the highest rate of resistance in all isolates | |||||
| The resistance of isolates from patients older than 60Y to ciprofloxacin, levofloxacin and tobramycin was higher that from younger adults (14–59) | |||||
| Hong J et al. | 2013 | 6 | Shanghai | 436 | Most common isolate Streptococcus species |
| MRSA was found in 8.3% of the S. Aureus isolates | |||||
| Increase trend toward laboratory resistance to fluoroquinolones | |||||
| No resistance found for Gram-positive isolates to vancomycin | |||||
| Hernandez-Camarena JC et al. | 2015 | 10 | Mexico city | 616 | Most common isolated pathogen was Staphylococcus epidermidis |
| Non-significant increasing trend on Gram-negative isolates | |||||
| MRSA was present in 45% out of the total S. Aurues isolates | |||||
| Increasing resistance to ceftazidime for Pseudomonas aeruginosa | |||||
| Lavinsky F et al. | 2013 | 3 | Tel Aviv, Israel | 276 | Staphylococcus aureus was the most common isolate found on corneal scrapings |
| Orlans HO et al. | 2011 | 10 | UK | 467 | Most common isolate Staphylococci sp. |
| Increase in the number of Coagulase-negative Staphylococci isolates | |||||
| Increase resistance of non-Pseudomonas Gram-negative isolates to chloramphenicol | |||||
| 93.2% of all isolates were susceptible to ciprofloxacin and 99.5% to either gentamicin or cefuroxime | |||||
| Ng AL-K et al. | 2015 | 10 | Hong Kong | 347 | Most common isolate overall was coagulase-negative Staphylococcus |
| Fluoroquinolone was found susceptible in 93.6% of al Gram-negative and in 100% of all Pseudomonas aeruginosa | |||||
| No emergence of resistant strain during the study period | |||||
| Ibrahim MM et al. | 2011 | 3 | Brazil | 118 | Predominant bacterial pathogen isolated was S. Epidermidis |
| Chang VS et al. | 2015 | 20 | Pittsburgh,USA | 398 | Analyzed only antibiotic susceptibility to MRSA and MSSA keratitis |
| MRSA represented 30.7% of total S. aureus isolates | |||||
| Vancomycin was susceptible to all S. aureus | |||||
| MRSA was found more resistant to second-generation fluoroquinolones than to the fourth-generation fluoroquinolones | |||||
| Increase in resistance to fourth-generation fluoroquinolone was detected during the study period for MSSA and MRSA | |||||
| Politis et al. | 13 | Jerusalem,Israel | 943 | Cultures were recovered in 48% of all cultures | |
| Most common isolate overall was coagulase-negative Staphylococcus | |||||
| Significant decrease trend in cases of coagulase-negative Staphylococcus (APC -8.1) | |||||
| Increase trend of coagulase-negative Staphylococcus resistance to penicillin. | |||||
| Vancomycin was susceptible to all pathogens | |||||
| Inverted correlation between temperature and number of cases of bacterial keratitis |
Bacterial keratitis is a devastating blinding disease. Appropriate therapy is crucial for eradication of the infective organism. [4] Bacterial resistance has been a concern since the 1950´s when Australia and Great Britain first reported penicillin resistance in Staphylococcus aureus.[15] Geographical location and cultural features of study populations are factors that are related to the differences in bacterial resistance patterns between countries.[16] Up to now there has been a dearth of reliable and comprehensive data regarding the etiology of bacterial keratitis in Israel.
In our 13 year study, 943 corneal scrapings were performed, with a mean of 34.78 ± 6.54 positive bacterial cultures per year. There was a stable trend of total bacterial cultures during the study period, contrary to the study by Lichtinger et al. [4] who had found a decreased trend of positive bacterial cultures during his study period, specifically of gram positive bacteria. However, this could reflect the facts that community based ophthalmologists are treating many patients empirically, and successfully, so that most patients are not referred to tertiary care centers.
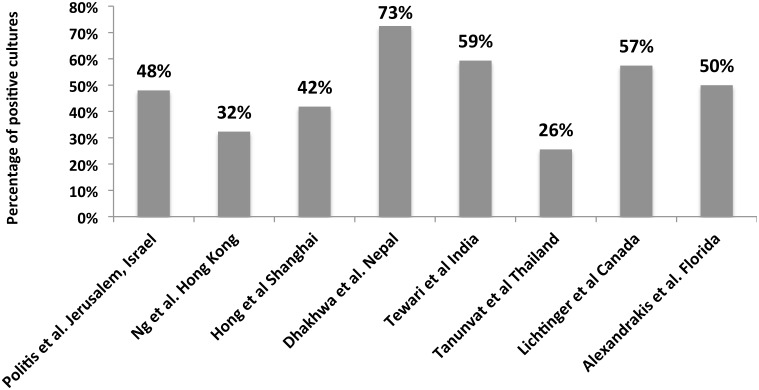
Our positive culture rate was 48%, which is consistent with the range reported in the literature of 36–86%[1,4,7,17–19] (Fig 4). Bacterial isolates represented 92% of the total cultures as seen in other series where bacteria account for 88–91% [4,17] of positive cultures.
Fig 4. Comparison of positive culture isolates in the published literature.
The most common bacterial isolates throughout the study period were coagulase negative staphylococci, in accordance with many studies[4,17,20,21] including an Israeli report by Mezer et al[22]. However, a study by Lavinsky et al[18] showed that Staphylococcus aureus was the most common pathogen. We found a significant decreasing trend in the proportion of coagulase negative staphylococci in corneal cultures during the study period. This may have been related to increases in the relative frequencies of other pathogens like Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pneumonia.
Fungal keratitis represented 8.2% (N = 37) of the total positive cultures, consistent with other reports where the proportions ranged from 5–34%[3,17,23].
Methicillin resistant Staphylococcus aureus has become increasingly important in the management of bacterial keratitis due to the challenges in treatment choices that this represents. Community acquired MRSA infections have become a significant problem in Israel [24]. In our study the percentage of MRSA isolates decreased from 18% in the first study period of 2002–2005 to 0% in the last study period of 2010–2014 with a non-statistical decrease in trend. This differs significantly from frequencies reported in the literature [4,6,7,17,25]. Furthermore, much higher frequencies of MRSA isolates from corneal cultures have been reported in other studies, ranging from 42–45%[6,7]. The choice of treatment for culture confirmed MRSA keratitis has been even more challenging where some studies have found an increase in resistance to fluoroquinolones, thus resulting in a shift to fortified topical vancomycin[26,27]. In our study we found a 0% resistance rate to vancomycin supporting the choice of vancomycin as a preferred treatment for culture confirmed MRSA keratitis. However, despite reports[17] of resistance to vancomycin, this agent remains a suitable option for treatment in our population.
In an attempt to identify factors that might influence the recovery of bacteria from corneal cultures, we analyzed the percentage of positive and negative cultures by time of sample collection. We hypothesized that the time of day and the experience of the individual taking the specimen might be associated with pathogen recovery rates. In our Ophthalmology Department, residents work together with fellows and senior Ophthalmologists during the day in the clinics from 07:00 to 15:00. After 15:00 only the residents are on call, with limited support from senior personnel. The proportion of positive cultures decreased significantly when the sample was taken after 23:00. This phenomenon might be explained by the stressful workload in the evening and the lack of experienced senior ophthalmologist, resulting in suboptimal technique in culture recollection. However, other factors should be considered, such as delays in transportation of specimens to the laboratory and staff availability for processing them.
We recognize that our study had several limitations. Most of the community based empirical treatment of corneal ulcers are based on the fourth generation fluorquinolones [28]. These drugs were not available in our hospital formulary, and therefore were not included in the microbiology laboratory susceptibility analysis during the study period. In addition, our study was conducted in a university referral center serving as a tertiary care hospital; therefore our findings may not be generalizable to other settings or populations. The data presented in our study represents the culture isolates found in a patient population, which is referred by community Ophthalmologists for management in a tertiary referral center, and therefore may include pathogens and susceptibility trends which are different than those found in corneal infections treated in the community.
This study has provided the first trend analysis of bacterial keratitis in Israel. Coagulase negative staphylococci were the predominant bacteria isolated from patients with keratitis, with a stable trend of positive cultures of bacterial keratitis in the past 13 years. Based on this study, we suggest the use of routine culture techniques for each case with a suspected infectious keratitis, with the regular use of antibiotics sensitivity and resistance in deciding the treatment plan. In addition, we recommend using fortified topical vancomycin as part of the regimen as a first-line agent in culture-confirmed MRSA, which is non-sensitive to the regular empiric treatment with cefazolin and gentamicin.
Continued monitoring of the microbial causes and their antibiotic susceptibilities of keratitis should be established. The policy of testing only drugs available in the hospital formulary should be reconsidered. This would allow the accumulation of much needed data regarding community-associated pathogens as well as to provide early warning of the emergence of new resistant pathogens.
Supporting Information
(XLSX)
Data Availability
All relevant data are within the paper and Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Alexandrakis G, Alfonso EC, Miller D (2000) Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. 107: 1497–1502. [DOI] [PubMed] [Google Scholar]
- 2.McDonald EM, Ram FSF, Patel DV, McGhee CNJ (2014) Topical antibiotics for the management of bacterial keratitis: an evidence-based review of high quality randomised controlled trials. Br J Ophthalmol 98: 1470–1477. 10.1136/bjophthalmol-2013-304660 [DOI] [PubMed] [Google Scholar]
- 3.Lalitha P, Prajna NV, Manoharan G, Srinivasan M, Mascarenhas J, Manoranjan D, et al. (2015) Trends in bacterial and fungal keratitis in South India, 2002–2012. Br J Ophthalmol 99: 192–194. 10.1136/bjophthalmol-2014-305000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shifting Trends in Bacterial Keratitis in Toronto (2012) Shifting Trends in Bacterial Keratitis in Toronto. 119: 1785–1790. Available: 10.1016/j.ophtha.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Mills R (2003) Microbial keratitis: what's the preferred initial therapy? View 1: corneal scraping and combination antibiotic therapy is indicated. Br J Ophthalmol 87: 1167–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asbell PA, Sahm DF, Shaw M, Draghi DC, Brown NP (2008) Increasing prevalence of methicillin resistance in serious ocular infections caused by Staphylococcus aureus in the United States: 2000 to 2005. J Cataract Refract Surg 34: 814–818. 10.1016/j.jcrs.2008.01.016 [DOI] [PubMed] [Google Scholar]
- 7.MD S D, MD S R, MD S IA, BA C DS, BA S M, MD H HY, et al. (2015) Microbial Keratitis in Los Angeles: The Doheny Eye Institute and the Los Angeles County Hospital Experience. 122: 918–924. 10.1016/j.ophtha.2014.11.027 [DOI] [PubMed] [Google Scholar]
- 8.Shalchi Z, Gurbaxani A, Baker M, Nash J (2011) Antibiotic resistance in microbial keratitis: ten-year experience of corneal scrapes in the United Kingdom. Ophthalmology 118: 2161–2165. 10.1016/j.ophtha.2011.04.021 [DOI] [PubMed] [Google Scholar]
- 9.Alexandrakis G, Alfonso EC, Miller D (2000) Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 107: 1497–1502. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Liang Y, Deng S, Wang Z, Li R, Abdalla Y, et al. (2008) Distribution of bacterial keratitis and emerging resistance to antibiotics in China from 2001 to 2004. Clin Ophthalmol 2: 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong J, Xu J, Hua J, Sun X (2013) Bacterial keratitis in Shanghai. Ophthalmology 120: 647 10.1016/j.ophtha.2012.10.038 [DOI] [PubMed] [Google Scholar]
- 12.Kaye SB, Rao PG, Smith G, Scott JA, Hoyles S, Morton C, et al. (2003) Simplifying collection of corneal specimens in cases of suspected bacterial keratitis. J Clin Microbiol 41: 3192–3197. 10.1128/JCM.41.7.3192-3197.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark AE, Kaleta EJ, Arora A, Wolk DM (2013) Matrix-assisted laser desorption ionization-time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology. Clin Microbiol Rev 26: 547–603. 10.1128/CMR.00072-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HJ, Fay MP, Feuer EJ, Midthune DN (2000) Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 19: 335–351. [DOI] [PubMed] [Google Scholar]
- 15.PURCELL EM, WRIGHT SS, FINLAND M (1953) Antibiotic combinations and resistance to antibiotics; penicillin-erythromycin and streptomycin-erythromycin combinations in vitro. Proc Soc Exp Biol Med 82: 124–131. [DOI] [PubMed] [Google Scholar]
- 16.Kowalski RP, Karenchak LM, Romanowski EG (2003) Infectious disease: changing antibiotic susceptibility. Ophthalmol Clin North Am 16: 1–9. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Camarena JC, Graue-Hernandez EO, Ortiz-Casas M, Ramirez-Miranda A, Navas A, Pedro-Aguilar L, et al. (2015) Trends in Microbiological and Antibiotic Sensitivity Patterns in Infectious Keratitis: 10-Year Experience in Mexico City. Cornea 34: 778–785. 10.1097/ICO.0000000000000428 [DOI] [PubMed] [Google Scholar]
- 18.Lavinsky F, Avni-Zauberman N, Barequet IS (2013) Clinical characteristics and outcomes of patients admitted with presumed microbial keratitis to a tertiary medical center in Israel. Arq Bras Oftalmol 76: 175–179. [DOI] [PubMed] [Google Scholar]
- 19.Orlans HO, Hornby SJ, Bowler ICJW (2011) In vitro antibiotic susceptibility patterns of bacterial keratitis isolates in Oxford, UK: a 10-year review. Eye (Lond) 25: 489–493. 10.1038/eye.2010.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng AL-K, To KK-W, Choi CC-L, Yuen LH, Yim S-M, Chan KS-K, et al. (2015) Predisposing Factors, Microbial Characteristics, and Clinical Outcome of Microbial Keratitis in a Tertiary Centre in Hong Kong: A 10-Year Experience. J Ophthalmol 2015: 769436 10.1155/2015/769436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim MM, Vanini R, Ibrahim FM, W de P Martins, RT de C Carvalho, R de S Castro, et al. (2011) Epidemiology and medical prediction of microbial keratitis in southeast Brazil. Arq Bras Oftalmol 74: 7–12. [DOI] [PubMed] [Google Scholar]
- 22.Mezer E, Gelfand YA, Lotan R, Tamir A, Miller B (1999) Bacteriological profile of ophthalmic infections in an Israeli hospital. Eur J Ophthalmol 9: 120–124. [DOI] [PubMed] [Google Scholar]
- 23.Liesegang TJ, Forster RK (1980) Spectrum of microbial keratitis in South Florida. Am J Ophthalmol 90: 38–47. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-moein KA, El-Hariri M, Samir A (2012) Methicillin-resistant Staphylococcus aureus: an emerging pathogen of pets in Egypt with a public health burden. Transbound Emerg Dis 59: 331–335. 10.1111/j.1865-1682.2011.01273.x [DOI] [PubMed] [Google Scholar]
- 25.Chang VS, Dhaliwal DK, Raju L, Kowalski RP (2015) Antibiotic Resistance in the Treatment of Staphylococcus aureus Keratitis: a 20-Year Review. Cornea 34: 698–703. 10.1097/ICO.0000000000000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsahn AF, Yildiz EH, Jungkind DL, Abdalla YF, Erdurmus M, Cremona FA, et al. (2010) In vitro susceptibility patterns of methicillin-resistant Staphylococcus aureus and coagulase-negative Staphylococcus corneal isolates to antibiotics. Cornea 29: 1131–1135. 10.1097/ICO.0b013e3181d2ce25 [DOI] [PubMed] [Google Scholar]
- 27.Falcone M, Russo A, Pompeo ME, Vena A, Marruncheddu L, Ciccaglioni A, et al. (2012) Retrospective case-control analysis of patients with staphylococcal infections receiving daptomycin or glycopeptide therapy. Int J Antimicrob Agents 39: 64–68. 10.1016/j.ijantimicag.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 28.Hsu HY, Nacke R, Song JC, Yoo SH, Alfonso EC, Israel HA, et al. (2010) Community opinions in the management of corneal ulcers and ophthalmic antibiotics: a survey of 4 states. Eye Contact Lens 36: 195–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and Supporting Information files.