Abstract
Campylobacter concisus has been associated with prolonged mild diarrhoea, but investigations regarding the efficacy of antimicrobial treatment have not been reported previously. We initiated a phase 3, single-centre, randomized, double-blinded, placebo-controlled study comparing the efficacy of 500 mg once-daily dose of azithromycin with a 500 mg once-daily dose of placebo for three days, for the treatment of C. concisus diarrhoea in adult patients with a follow-up period of ten days. If symptoms persisted at day ten, the patient was offered cross-over study treatment of three days and another ten-day follow-up period. The primary efficacy endpoint was the clinical response, defined as time to cessation of diarrhoea (<3 stools/day or reversal of accompanying symptoms). Our estimated sample size was 100 patients. We investigated a total of 10,036 diarrheic stool samples from 7,089 adult patients. Five-hundred and eighty-eight C. concisus positive patients were assessed for eligibility, of which 559 were excluded prior to randomization. The three main reasons for exclusion were duration of diarrhoea longer than 21 days (n = 124), previous antibiotic treatment (n = 113), and co-pathogens in stools (n = 87). Therefore, 24 patients completed the trial with either azithromycin (n = 12) or placebo (n = 12). Both groups presented symptoms of mild, prolonged diarrhoea with a mean duration of 18 days (95% CI: 16–19). One person in the azithromycin group and four from the placebo group chose to continue with crossover medication after the initial ten-day period. In the azithromycin group, there was a mean of seven days (95% CI: 5–9) to clinical cure and for the placebo group it was ten days (95% CI: 6–14) (OR—3 (95% CI: -7–1). We observed no differences in all examined outcomes between azithromycin treatment and placebo. However, due to unforeseen recruitment difficulties we did not reach our estimated sample size of 100 patients and statistical power to conclude on an effect of azithromycin treatment was not obtained.
Trial Registration: Clinicaltrials.gov identifier: NCT01531218.
Introduction
Campylobacter jejuni is the most common bacterial cause of human diarrhoeal disease [1]. The infection is usually self-limited and antimicrobial therapy reduces the duration of intestinal symptoms by approximately one day only, when compared to placebo [2]. Consequently, antimicrobial therapy is warranted only for high-risk patients with severe C. jejuni infection, and a macrolide antibiotic (e.g. azithromycin) is generally recommended as the first drug of choice [3]. In recent years, emerging evidence has brought attention to the potential clinical significance of a related species, Campylobacter concisus, originally isolated from patients with periodontal lesions and now considered a part of the normal human oral microbiota [4, 5].
Campylobacter concisus has shown abilities of epithelial adherence and invasion in in-vitro cell-lines [6]. A study of HT-29/B6 cells exposed to C. concisus showed increased production of lactate-dehydrogenase as well as induction of apoptotic leaks and tight junction alterations that may lead to an impaired barrier function, demonstrating a leak-flux mechanism that parallels the clinical manifestation of diarrhoea [7]. Furthermore, in a recent population based study from Denmark C. concisus was more prevalent than C. jejuni in diarrheic stool samples [8].
In two questionnaire studies, adult patients and parents of children infected with C. concisus reported a milder, though more prolonged diarrhoea compared to patients infected with C. jejuni [9, 10]. Half of the adult C. jejuni patients reported receiving antibiotic treatment prescribed by their general practitioner (GP), whereas one-third of C. concisus-positive patients reported treatment with either oral ciprofloxacin or a macrolide [9]. Investigations into the efficacy of antimicrobial treatment of diarrhoea due to C. concisus infection have not previously been performed. Therefore, we initiated a study comparing the efficacy and safety of azithromycin with placebo for 3 days for the treatment of diarrhoea in C. concisus positive adult patients.
Material and Methods
Study design
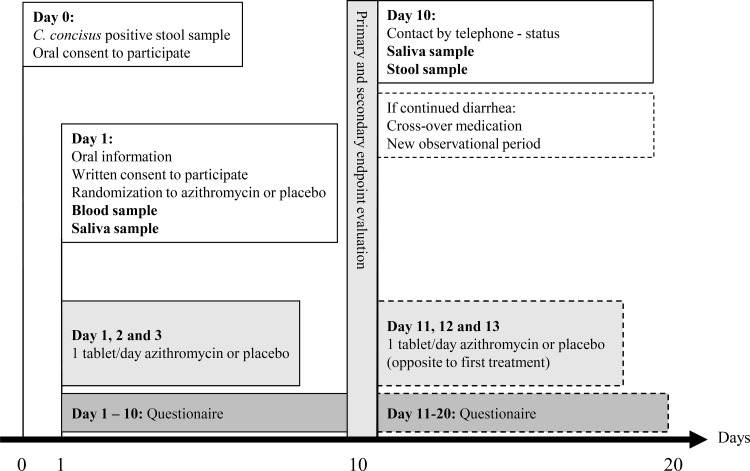
This was an investigator initiated, phase 3, single-centre, randomized, double-blinded, placebo-controlled study comparing the efficacy and safety of 500 mg once-daily dose of azithromycin with a 500 mg once-daily dose of placebo for 3 days for the treatment of diarrhoea in C. concisus positive adult patients (NCT01531218, https://clinicaltrials.gov). Participants were asked to self-report diarrheic symptoms for a follow-up period of initially ten days. If the patient had ongoing diarrheic symptoms (i.e. no effect of either azithromycin or placebo) after the initial ten-day observational period, the patient was offered cross-over treatment, also double-blinded, succeeded by another ten-day follow up period. (Fig 2). The study was conducted in the North Denmark Region, with a population of 578,839 inhabitants.
Fig 2. Upon diagnosis of C. concisus in a stool sample the diarrheic patient was contacted by telephone, through their General Practitioner or physician (if hospitalized), by the principal-investigator (day 0).
If the patient still had diarrhoea the patient was offered to participate in the clinical trial. At day 1 the patient met the principal-investigator in an outpatient setting and was randomized to either azithromycin or placebo 500 mg once-daily dose for three days for the treatment of diarrhoea. Moreover, the patient was asked to deliver a blood sample (CRP and leukocytes) as well as a saliva sample. During the follow-up period of ten days the patient had to fill in a questionnaire on daily basis regarding diarrheic symptoms. At day ten the patient was contacted by telephone by the principal-investigator and if the patient stated ongoing diarrheic symptoms (no effect of study treatment; azithromycin or placebo) the patient was offered another ten-day study period and treatment with cross-over study medication (azithromycin or placebo), also double-blinded. Finally, the patient was asked to deliver a saliva sample as well as a stool sample for an ongoing diagnosis of C. concisus infection.
Bacteriology
Diarrheic stool samples were cultured at the Department of Clinical Microbiology, Aalborg University Hospital combining a previously described filter method with routine diagnostic methods. [11]. Campylobacter concisus was isolated by the filter technique on 5% horse blood agar plates, containing 1% yeast extract (SSI Diagnostica, Hillerød, Denmark) and incubated at 37°C in a microaerobic atmosphere with 3% hydrogen. Final identification was confirmed by MALDI-TOF analysis (BRUKER DALTONIK GmbH, Bremen, Germany) as well as by species-specific real-time PCR based on the cpn60 gene, as described elsewhere [12,13]. Antimicrobial susceptibility testing to azithromycin was performed with use of disk diffusion with 15μg azithromycin using EUCAST-and CLSI potency NEO-SENSITABS™ (Rosco Diagnostica A/S, Taastrup, Denmark) according to the manufacturer’s instructions. Stools from patients eligible for inclusion in the study, were also examined by PCR-based methods for diarrheic Escherichia coli, norovirus, rotavirus, adenovirus, and the protozoes Giardia lamblia, Entamoeba histolytica and Cryptosporidium parvum. Saliva samples were also cultivated by use of the filter method as described above.
Subjects
Inclusion criteria
Study subjects were diarrheic adults ≥18 years, with a C. concisus culture-positive stool sample. Diarrhoea was defined as three or more loose stools per day, or two loose stools a day combined with at least one or more accompanying symptoms such as abdominal pain, nausea, vomiting or fever, persisting for at least 24 hours.
Exclusion criteria
Patients were excluded if they presented with diarrheic symptoms for more than 21 days, hypersensitivity to azithromycin or macrolides in general and if pregnant or lactating. They were also excluded if their stool sample was positive for one or more of the following pathogenic enteric bacteria: Salmonella, Shigella, Yersinia, Campylobacter jejuni/coli, Aeromonas, Plesiomonas, Vibrio, Clostridium difficile, or diarrheic Escherichia coli, as well as norovirus, rotavirus and adenovirus, and the protozoes Giardia lamblia, Entamoeba histolytica or Cryptosporidium parvum. Antibiotic therapy within the last 4 weeks, colostomy or ileostomy, severe renal or liver disease, congenital or acquired QT-prolongation or other heart conditions, as well as inflammatory bowel disease (IBD), microscopic colitis or other chronic diarrheal disease were other exclusion criteria. Finally, patients were excluded if they were unable to follow the study protocol (e.g. due to dementia).
Randomization, Double-blinding and Treatment
Patients meeting eligibility criteria were randomly assigned in a one to one ratio to receive either azithromycin or placebo (Lactose Monohydrate) supplied as identical 500 mg capsules approved by the Danish Health and Medicines Authority. A Pharmacist (http://www.glostrup-apotek.dk/) consecutively listed randomisation for patients 1–100. The list was blinded for the Investigator and treatment allocation remained blinded until trial completion. However, in case of acute need for breach of code, the Pharmacist provided round the clock service Azithromycin and matching placebo were taken once daily for three days.
Assessment
The primary efficacy endpoint was clinical response to the azithromycin and placebo regimens, defined as time to cessation of diarrhoea (<3 stools/day or reversal of accompanying symptoms). When a stool sample tested positive for C. concisus, the patient was contacted by telephone by their GP or responsible physician if hospitalized. In a primary interview with the investigator, subjects were asked about their general health, previous medical records, and diarrheic symptoms. At first visit (day 1) the patient met the principal-investigator in an outpatient setting and was asked about the following general symptoms: headache, fatigue, sleeping difficulties, dizziness, itching, sensory disturbances, joint pain, back pain, dyspnoea, angina pectoris, and sweating. Hereafter, the patient was randomized to either azithromycin or placebo 500 mg once-daily dose for 3 days for the treatment of diarrhoea and blood (C-reactive Protein (CRP) and leukocytes) and saliva samples were collected. Subsequently, the participants answered daily questionnaires regarding diarrheic symptoms, number of stools per day, clinical symptoms and possible side effects to the medication for as long as symptoms persisted, or a total follow-up period of ten days. On day ten, the patient was contacted by telephone by the principal-investigator and asked about their current status. If patients stated ongoing diarrheic symptoms (i.e. no effect of either azithromycin or placebo) the patient was offered cross-over study treatment (azithromycin or placebo), also double-blinded, for three days, followed by another ten-day follow-up period. A secondary bacteriological endpoint was eradication of C. concisus in stool and saliva ten days after primary enrolment, defined as culture negative stool and saliva samples on day ten, using the methods described above.
Sample Size Calculation and Statistical Analysis
Statistical analysis was conducted using Stata® software, version 10 (College Station, Texas). The sample size was calculated to achieve appropriate statistical power (β = 0.80), with the acceptable type 1 error (α = 0.05), type 2 error (b = 0.20) and hypothesis that the OR for cure in the azithromycin treatment arm versus placebo was at least three. The required sample with the aforementioned assumptions was 44 subjects per treatment arm. With an expected 10% withdrawal of subjects during the trial, the final sample size was estimated to 50 subjects in each group. Continuous variables were compared using the student t-test, whereas categorical variables were compared using Fisher’s exact test with the statistical significance level set to 0.05 Estimates for clinical efficacy were calculated by linear regression for parametric values (‘regress’ procedure in Stata) and rounded up to the nearest integer (days). Binary values were analysed by logistic regression (‘logit’ procedure in Stata).
Results
Demographics and patient inclusion
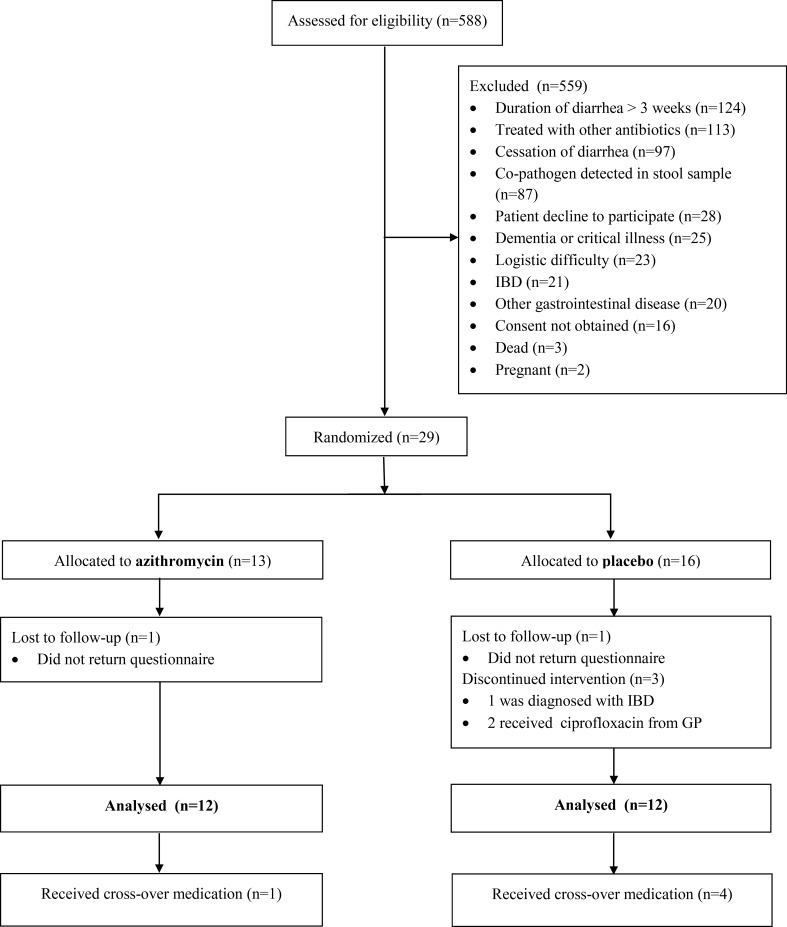
The study was performed from 1. April 2012 till 30. November 2013 and based on routine diarrheic stool samples sent to the Department of Clinical Microbiology at Aalborg University Hospital for cultivation of enteric pathogenic bacteria, from both General Practitioners and Hospital Wards. During the study period, we investigated a total of 10,036 diarrheic stool samples from 7,089 adult patients with a male to female ratio of 3065/4024 and with a mean age of 54 years (95%CI: 53–54). Five hundred and eighty-eight unique adult patients with a male to female ratio of 254/334 and with a mean age of 55 years (95% CI: 53–56) had C. concisus in stools and were assessed for eligibility, but prior to randomization, 559 had to be excluded according to study in- and exclusion criteria (Fig 1). The main reasons for exclusion were duration of diarrhoea for more than 3 weeks (n = 124) and antibiotic treatment prior to inclusion (n = 113). Co-pathogens were detected in 87 subject’s stools; the majority of these were Clostridium difficile. Twenty-one were excluded due to a preceding IBD diagnosis and 20 patients had other gastrointestinal diseases, primarily being microscopic colitis. Due to the high exclusion rate, we did not reach our estimated sample size of 100 patients. In total, the intention-to-treat group comprised of 29 subjects that were randomized to treatment. Two subjects did not return questionnaires and were lost to follow-up and one patient was diagnosed with IBD shortly after inclusion. Two patients did not improve during the study treatment and were prescribed ciprofloxacin for continued diarrheic symptoms by their GP. Baseline characteristics of the intention to treat population was compared to the per protocol population and there was no difference between the two groups (data not shown). The per-protocol population that completed the trial was twenty-four subjects in total, of which five (one azithromycin, four placebo) chose to continue with cross-over treatment after the initial 10-day period (Fig 1).
Fig 1. Flow-chart of subject inclusion.
Patient characteristics
Examining all 24 patients in the per-protocol population revealed a mean age of 44 years (95% CI: 37–52) and a male to female ratio of 9:15. Baseline demographics were similar in both treatment groups (n = 12 in both groups) with a trend toward younger age in the placebo group (p = 0.055) (Table 1). Half of the patients had pre-existing comorbidities including arterial hypertension, depression, migraine and osteoporosis, but none of these were associated with gastrointestinal illness (data not shown). Whilst 6/24 (25%) reported daily fatigue, no other general symptom was reported. Baseline characteristics and clinical presentations of the intention-to-treat population did not differ from the per protocol populations (data not shown).
Table 1. Baseline characteristics and diarrheic symptoms at inclusion for per-protocol population allocated to either azithromycin or placebo.
For continuous variables, 95% confidence intervals are presented in parentheses following mean values.
| Variable | Azithromycin (n = 12) | Placebo (n = 12) | p-value |
|---|---|---|---|
| Sample origin (GP/hospital ward) | 11/1 | 11/1 | 1.00 |
| Sex (male/female) | 6/6 | 3/9 | 0.20 |
| Age, mean years | 50 (39.5–61) | 38 (27–50) | 0.055 |
| BMI (kg/m2) (mean) | 23.9 (21.2–26.6) | 24.2 (21.6–27.0) | 0.58 |
| C-reactive protein (mg/L) (mean) | 7.6 (0.0–16) | 4.9 (1.12–8.71) | 0.27 |
| Leucocytes (109/L) (mean) | 6.9 (5.96–7.9) | 6.48 (5.31–7.6) | 0.25 |
| Duration of diarrhoea, days (mean) | 19 (17–20) | 17 (14–19) | 0.10 |
| Nocturnal diarrhoea (yes/no) | 9/3 | 8/4 | 1.00 |
| Max. stools/day (mean) | 10 (7–13) | 11 (6–15) | 0.62 |
| Consistency of stools (yes/no) | |||
| Watery | 11/1 | 12/0 | 1.00 |
| Mucus in stool | 6/6 | 7/5 | 1.00 |
| Blood in stool | 1/11 | 0/12 | 1.00 |
| Symptoms (yes/no) | |||
| Fever | 2/10 | 4/8 | 0.53 |
| Chills | 2/10 | 4/8 | 0.53 |
| Nausea | 4/8 | 7/5 | 0.41 |
| Vomiting | 1/11 | 3/9 | 0.59 |
| Headache | 2/10 | 8/4 | 0.04 |
| Dizziness | 4/8 | 1/11 | 0.32 |
| Abdominal pain | 8/4 | 9/3 | 1.00 |
| Muscle aches | 2/10 | 2/10 | 1.00 |
| Weight loss (yes/no) | 8/4 | 6/6 | 0.68 |
Clinical presentation
The clinical presentation of the study subjects clearly reflected the symptoms previously reported in gastroenteritis due to C. concisus [9], and were similar in the two groups (Table 1). The overall duration of diarrhoea at the time of inclusion was a median of 18 days (95% CI: 16–19). Seventeen of the 24 (71%) subjects reported nocturnal diarrhoea and half of the patients had mucus in stool, while only one patient reported bloody stools. One-fourth reported having had a fever (temperature >37,5°C) and chills at some time during the course of illness, while only 2 patients (8%) had fever at the time of inclusion. Headache was reported by eight patients in the placebo group, and two in the azithromycin group (p = 0.04). Overall, 17 (71%) patients reported abdominal pain and 14 (58%) reported weight loss, with a fairly even distribution between the two groups. Finally, only four patients (two in each group) had elevated CRP (>10.0 mg/L) at inclusion, whereas all other patients had normal blood parameters.
Primary outcome
The primary outcome was time to cure, defined as time to cessation of diarrhoea (<3 stools/day or reversal of accompanying symptoms). For the azithromycin group 9/12 (75%) subjects reported cessation of diarrhoea after the course of antibiotics. For the placebo group, it was 7/12 (58%) (OR 2.14 (95% CI: 0.38–12.2). One person in the azithromycin group and four from the placebo group chose to continue with crossover medication after the initial 10-day period. In the azithromycin group, there was a mean seven days (95% CI: 5–9) to clinical cure, and for the placebo group it was ten days (95% CI: 6–14) (OR—3 (95% CI: -7–1).
Patients were asked to report symptoms on a daily basis, analogous to the questions at inclusion. On day five after treatment allocation, one person in the azithromycin group reported continued headache, and three people abdominal pain. In the placebo group, two people reported nausea, three reported headaches, three suffered from dizziness and seven from abdominal pain. On day 10, no participant from the azithromycin group reported any symptom other than diarrhoea. In the placebo group, two still reported nausea, two complained of headaches, one still suffered from dizziness and five from abdominal pain. We have limited statistical power to show a significant difference between the two groups, but it seems that there was a trend towards faster recovery of symptoms in the azithromycin treatment group, compared to the placebo group.
Secondary outcome
All patients, in both the azithromycin and placebo group, had a negative C. concisus stool test ten days after inclusion. Similarly, while 5/12 subjects in both groups had C. concisus in their saliva at inclusion, and all but one subject in the placebo group tested negative on day 10.
Treatment compliance
Subjects were required to document their intake of the study medication on each day in the questionnaires provided, and asked to bring unused medication, if any, to follow-up visit on day ten. No subjects in the trial reported compliance failure.
Safety
No suspected unexpected serious adverse reactions (SUSAR) or treatment related severe adverse events (SAE) were reported, and no subjects discontinued the study due to adverse effects.
Discussion
This is the first study to assess the clinical efficacy of antibiotic treatment of C. concisus associated diarrhoea. However, due to unforeseen difficulty with recruitment, the sample size does not have statistical power to finally conclude whether patients should be treated with azithromycin or not, leaving the main question unanswered. While there was a trend toward shorter disease duration with azithromycin treatment, the sample size is too small for the results to be conclusive. On the other hand, the study confirms our previous studies that C. concisus is often isolated from human diarrheic stool samples [8, 14], and may lead to prolonged, mild diarrhoea [9, 10].
One-sixth of our previous isolates of C. concisus were resistant to ciprofloxacin, whereas we recorded almost no resistance to azithromycin [14]. The isolates in the present study were all susceptible to macrolides, and azithromycin is generally recommend as the drug of choice if antibiotic treatment is indicated for severe infection due to C. jejuni. However, antibiotic treatment of acute gastroenteritis is usually not recommended in adults without comorbidity [3]. Nevertheless, one-fifth of subjects eligible for enrolment in this study had already been treated with antibiotics by their primary physician, possibly because they presented with more severe diarrhoae. However, it remains unknown whether these patients had any effect of the antimicrobial therapy prescribed, and we were not able to conduct a prospective follow-up, since they were in the out-patient setting. During the study period, two patients in the placebo group were prescribed ciprofloxacin by their GP due to persistent symptoms, and had to be excluded. This is a reflection of the nature of diarrheic infection due to C. concisus, and a major limitation when assessing treatment options, as patients already reported diarrhoea duration for a median of 19 days before enrolment.
In the initiation of the trial, we limited inclusion to patients with duration of diarrhoea for a maximum of three weeks only. However, it is well known that C. concisus infection is associated to prolonged diarrhoea and 21% of eligible participants reported duration of diarrhoea longer than three weeks and had to be excluded. This also reflects the clinical course of C. concisus associated diarrhoea as previously described [9, 10], where patients are symptomatic for weeks, but not affected by general malaise and fever, as seen in C. jejuni infections. Patients will therefore usually only consult their physician late in the course of illness.
Studies have found a high prevalence of C. concisus DNA in mucosal biopsies from patients with inflammatory bowel disease (IBD) [15–18], and we previously reported that 12% of patients with C. concisus associated diarrhoea were diagnosed with microscopic colitis six months after infection. [9]. Consequently, if limitations in the duration of diarrheic symptoms were not included in this study, there could be a potential risk of undiagnosed IBD or microscopic colitis cases. This was exemplified by a subject in the placebo group who was diagnosed with IBD (Crohn's Disease) a few days after inclusion, and therefore consequently excluded.
In the small group of subjects to complete this trial, there was no statistically significant difference in clinical or bacteriological efficacy between the two groups. Not surprisingly, participants presented with a mean duration of diarrhoea of 19 days. Half of the patients had mucus in stools and more than two-thirds had nocturnal diarrhoea, also in accordance with previously findings [9]. Even though half of the patients reported mucus in their stool, the C. concisus infection still appeared localized in the gut, exerting no major systemic response—only one-fourth reported having had a fever and all but four patients presented with normal CRP levels at inclusion. At baseline there was no overall difference between the two small groups, although headache was more prevalent in the placebo group, which also had more females (not significant).
After randomization there was a trend towards faster recovery of symptoms in the azithromycin group compared to the placebo group and while this should interpreted with caution, it could mimic the effects of antibiotic treatment of C. jejuni infections that may shorten the duration of clinical symptoms only slightly [3]. Only one patient in the azithromycin group requested crossover treatment, whereas four patients in the placebo group requested azithromycin after the initial ten-day study period. The patients in the azithromycin group had a two-day shorter time to clinical cure compared to the patients in the placebo group, but this may merely reflect the chronic nature of C. concisus diarrhoea.
Before the study, we had no data describing the duration of C. concisus excretion in stool. Interestingly, all patients had a negative C. concisus stool test ten days after inclusion independently of treatment with azithromycin or placebo, suggesting that infection was cleared by immune cells, rather than antibiotics. There was also a difference in the mean age between the azithromycin and placebo groups (50 vs. 38). Since the infection could be cleared by immune cells rather than antibiotics and the efficiency of the immune system declines with age, age may also have acted as a confounding variable.
A similar trial, conducted earlier in the course of illness would be of great significance in elucidating the effects of azithromycin treatment, but the natural course of C. concisus diarrhoea limits this possibility. Moreover, Danish Ethical regulations prohibit the enrolment of patients from general practice by others than the patient’s own GP, impeding recruitment time for the majority of patients.
We cannot exclude selection bias in the decision for microbiological examination, as stool samples were requested by patients’ GP’s or hospital doctors. However, we included only culture-positive cases of C. concisus, and excluded stool samples with other identified gastroenterological pathogens. Fifteen percent of C. concisus positive patients also had a co-pathogen detected in stools, the majority of these being Clostridium difficile, in agreement with our previous findings [14].
In conclusion, this is the first study to assess clinical efficacy of antibiotic treatment of C. concisus associated diarrhoea. The study confirms our previous findings, that C. concisus is often isolated from human diarrheic stool samples, and may lead to prolonged, mild diarrhoea. We observed a trend towards shorter disease duration with azithromycin treatment and faster symptom recovery.
However, due to unforeseen recruitment difficulties we did not reach our estimated sample size of 100 patients and statistical power to conclude on an effect of azithromycin treatment was not obtained.
Supporting Information
(PDF)
(PDF)
(XLSX)
(PDF)
(PDF)
(PDF)
Acknowledgments
We gratefully acknowledge the assistance of the microbiology staff in the faeces laboratory at Department of Clinical Microbiology, Aalborg Hospital. Moreover, we wish to acknowledge Kirsten Østergaard from the GCP unit of Aalborg/Aarhus, Denmark, and all patients who took part in the trial.
Abbreviations
- IQR
Inter quartile range
- GP
General practitioner
- IBD
Inflammatory bowel disease
- CRP
C-reactive Protein
- OR
Odds ratio
- SUSAR
Suspected unexpected serious adverse reactions
- SAE
severe adverse events
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from The Obel Family Foundation, http://www.obel.com/.
References
- 1.European Centre for Disease Prevention and Control. Annual epidemiological report 2014 –food- and waterborne diseases and zoonoses. Campylobacteriosis, p. 20–24. Stockholm. ECDC;2014
- 2.Ternhag A, Asikainen T, Giesecke J, Ekdahl K. A Meta-Analysis on the Effects of Antibiotic Treatment on Duration of Symptoms Caused by Infection with Campylobacter Species. Clin Infect Dis 2007;44:696–700. 10.1086/509924 [DOI] [PubMed] [Google Scholar]
- 3.Riddle MS, DuPont HL, Connor BA. ACG Clinical Guideline: Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults. Am J Gastroenterol 2016;111:602–22. 10.1038/ajg.2016.126 [DOI] [PubMed] [Google Scholar]
- 4.Tanner ACR, Badger S, Lai C-H, Listgarten MA, Visconti RA, Socransky SS. Wolinella gen. nov., Wolinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodens from humans with periodontal disease. Int J Syst Bacteriol 1981;31:432–45. [Google Scholar]
- 5.Zhang L, Budiman V, Day AS, Mitchell H, Lemberg DA, Riordan SM, et al. Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. J Clin Microbiol 2010;48:2965–7. 10.1128/JCM.02391-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Man SM, Kaakoush NO, Leach ST, Nahidi L, Lu HK, Norman J, et al. Host attachment, inva-sion, and stimulation of proinflammatory cytokines by Campylobacter concisus and other non-Campylobacter jejuni Campylobacter species. J Infect Dis 2010;202:1855–1865. 10.1086/657316 [DOI] [PubMed] [Google Scholar]
- 7.Nielsen HL, Nielsen H, Ejlertsen T, Engberg J, Gunzel D, Zeitz M, et al. Oral and fecal Campylobacter concisus strains perturb barrier function by apoptosis induction in HT-29/B6 intestinal epithelial cells. PLoS One 2011;6:e23858 10.1371/journal.pone.0023858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen HL, Ejlertsen T, Nielsen H. Polycarbonate filtration technique is noninferior to mCCDA for isolation of Campylobacter species from stool samples. Diagn Microbiol Infect Dis. 2015;83:11–2. 10.1016/j.diagmicrobio.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 9.Nielsen HL, Engberg J, Ejlertsen T, Bucker R, Nielsen H. Short-term and medium-term clinical outcomes of Campylobacter concisus infection. Clin Microbiol Infect 2012;18:E459–65. 10.1111/j.1469-0691.2012.03990.x [DOI] [PubMed] [Google Scholar]
- 10.Nielsen HL, Engberg J, Ejlertsen T, Nielsen H. Clinical Manifestations of Campylobacter concisus Infection in Children. Pediatr Infect Dis J 2013;11:1194–98. [DOI] [PubMed] [Google Scholar]
- 11.Engberg J, Holt HM, Lemming L, Lester A, Nielsen HL, Olesen B, et al. Diagnostik af bakterielle mave-tarminfektioner. Danish Society of Clinical Microbiology. 2016. http://dskm.dk/Diagnostik%20af%20bakterielle%20mave-tarminfektioner%202016.pdf Accessed March 2016
- 12.Nielsen HL, Mølvadgaard M, Nielsen H, Kostrzewa M. Identification and Differentiation of Highly Diverse Campylobacter concisus Strains using the MALDI Biotyper. Clin Microbial 2016;5:230. [Google Scholar]
- 13.Chaban B, Musil KM, Himsworth CG, Hill JE. Development of cpn60-based real-time quantitative PCR assays for the detection of 14 Campylobacter species and application to screening of canine fecal samples. Appl Environ Microbiol 2009;75:3055–3061. 10.1128/AEM.00101-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen HL, Ejlertsen T, Engberg J, Nielsen H. High incidence of Campylobacter concisus in gastroenteritis in North Jutland, Denmark: a population-based study. Clin Microbiol Infect 2013;19:445–50. 10.1111/j.1469-0691.2012.03852.x [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Man SM, Day AS, Leach ST, Lemberg DA, Dutt S, et al. Detection and isolation of Campylobacter species other than C. jejuni from children with Crohn's disease. J Clin Microbiol 2009;47:453–455. 10.1128/JCM.01949-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen R, Berry SH, Mukhopadhya I, Thomson JM, Saunders KA, Nicholl CE, et al. The microaerophilic microbiota of de-novo paediatric inflammatory bowel disease: the BISCUIT study. PLoS One 2013;8:e58825 10.1371/journal.pone.0058825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahendran V, Riordan SM, Grimm MC, Tran TA, Major J, Kaakoush NO, et al. Prevalence of Campylobacter species in adult Crohn's disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS One 2011;6:e25417 10.1371/journal.pone.0025417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhya I, Thomson JM, Hansen R, Berry SH, El-Omar EM, Hold GL. Detection of Campylobacter concisus and other Campylobacter species in colonic biopsies from adults with ulcerative colitis. PLoS One 2011;6:e21490 10.1371/journal.pone.0021490 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(XLSX)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.