Abstract
Recent evidence has demonstrated that a variety of pathogens target cellular lipid metabolism for their replication. Lipid droplets are a major contributor to lipid homeostasis and contain neutral fats but are also recognized as dynamic organelles involved in signal transduction, membrane trafficking and modulation of immune and inflammatory responses. Rotaviruses co-opt lipid droplets for their replication. Rotavirus viroplasms, sites of viral RNA replication and immature particle assembly, form complexes with cellular lipid droplets early in infection. Chemical compounds blocking fatty acid synthesis or interfering with lipid droplet homeostasis decrease viroplasm formation and the yield of infectious viral progeny. Lipid droplets are vital for the replication of rotaviruses as well as various members of the Flaviviridae family and several intracellular bacteria. Chemical compounds decreasing intracellular triglyceride content reduced rotavirus replication in an animal model and should be considered as potential therapeutic agents against disease caused by rotaviruses, flaviviruses and intracellular bacteria.
Introduction
Rotaviruses (RVs) remain an important cause of severe dehydrating diarrhea in infants and children under 5 years of age, and still accounting for 200,000 deaths worldwide in 2011, even after the introduction of universal rotavirus vaccination programs in many countries [1].
Rotavirus virions are non-enveloped particles composed of three concentric, icosahedral protein layers; the viral cores contain the genome of 11 segments of double-stranded (ds) RNA and enzymes of the replication complex. After entry into the host cell, the outer layer of the infectious triple-layered particle (TLP) is removed in endocytic vesicles. The resulting double-layered particle (DLP) is a molecular machine, which actively transcribes mRNAs from the genomic dsRNA and then extrudes them into the cytoplasm. The mRNAs are translated into six structural viral proteins (VP1, VP2, VP3, VP4, VP6, VP7) and six non-structural proteins (NSP1, NSP2, NSP3, NSP4, NSP5, NSP6), or serve as templates for dsRNA synthesis in progeny virus. The early stages of double-layered particle morphogenesis and viral RNA replication occur in discrete cytoplasmic inclusion bodies called viroplasms. DLPs interact with membranes of the endoplasmic reticulum (bound via NSP4) and acquire the outer capsid layer proteins in the cytoplasm to mature into infectious virions before being released by cell lysis or budding [2].
Rotavirus viroplasms associate with components of lipid droplets
At least 7 viral proteins (NSP2/5/6 and VP1/2/3/6) have been detected in viroplasms, but co-expression of NSP2 and NSP5 are essential for viroplasm formation. Silencing the expression of NSP2 or NSP5 by RNA interference [3,4] or intrabodies [5], or the use of specific rotavirus ts mutants at the non-permissive temperature [6] prevents viroplasm formation and virion production. In cultured uninfected cells, co-expression of NSP2 with NSP5 forms viroplasm-like structures (VLS) in the absence of other rotaviral proteins, but expression of NSP2 or NSP5 alone is insufficient to form VLS [7].
In addition to requiring the viral NSP2 and NSP5 proteins, viroplasms associate with cellular lipid droplet (LD) components, with the numbers of viroplasm-LD complexes increasing during the replication cycle [8]. Lipid droplets are spherical intracellular organelles containing triacylglycerols (TAG) and sterol esters in the core, which is surrounded by a phospholipid monolayer [9]. More than 200 mammalian proteins are associated with lipid droplets [10]; most prominently, members of the PERILIPIN family of proteins (PLIN1-PLIN5) [11]. Lipid droplets are present in all eukaryotic cells and play various roles beyond neutral lipid storage. Traditionally, lipid droplets were viewed as passive storage depots for excess fat from which neutral lipids are rapidly consumed when carbon sources are depleted and additional energy supplies are required [12,13]. However, lipid droplets are increasingly recognized as dynamic organelles actively involved in diverse cellular processes including lipid homeostasis, signal transduction and membrane trafficking [14-18]. The processes that govern the formation, composition, different functions of lipid droplets have major significance in both basic biology and in the development of metabolic and infectious diseases [9,15].
The first evidence that rotavirus viroplasms associate with components of lipid droplets came from studies demonstrating that viroplasm-associated proteins co-localize with the lipid droplet-associated proteins PLIN1 and PLIN2 and that viroplasm-lipid droplet complexes interact with the lipophilic stain Nile red [8]. In addition, PLIN1 (as a marker for lipid droplets) co-sedimented with NSP5 (as a marker for viroplasms) and dsRNA (as a marker of viral particles) in low-density fractions of ultracentrifugation gradients of rotavirus– infected cell extracts [8]. The close spatial proximity of (Cy3-antibody labeled) NSP5 with PLIN1 was demonstrated by fluorescence resonance energy transfer (FRET) in rotavirus-infected cells co-expressing NSP5-EGFP [8]. Furthermore, co-expression of NSP2 and NSP5 alone was sufficient to detect co-localization of VLS with PLIN1 on lipid droplets [8]. Lipidome analysis demonstrated that the total cellular lipid content increases during RV infection [19,20] and that the lipid increase is consistent with an increase in abundance of lipid droplets that interact with viroplasms [19]. Together, these results confirmed the close association of viroplasms with lipid droplets.
Experimental dissection of viroplasm-lipid droplet complex formation is challenging because the detailed mechanisms of lipogenesis and lipid droplet biogenesis as well as viroplasm formation are unknown. A time course analysis of viroplasm and lipid droplet morphogenesis by confocal microscopy revealed that small viroplasms (identified by NSP5) are detected early during infection prior to the association of viroplasms with lipid droplets [8]. Furthermore, knockdown of NSP5 in rotavirus-infected cells by specific siRNA reduced the production of dsRNA and infectious progeny virus [4,8]. These results suggested that viroplasms recruit and require components of lipid droplets for viral replication. It is possible that the interaction or post-translational modification of either or both NSP2 and NSP5 may induce conformational changes in these viral proteins [21,22] to allow this viral complex to associate with lipid droplets and recruit other viral proteins to form larger viroplasms. Lipid droplets can be considered as platforms for topological organization and assembly of viroplasms, enabling viral early morphogenesis and RNA replication.
Lipogenesis and lipid droplet biogenesis
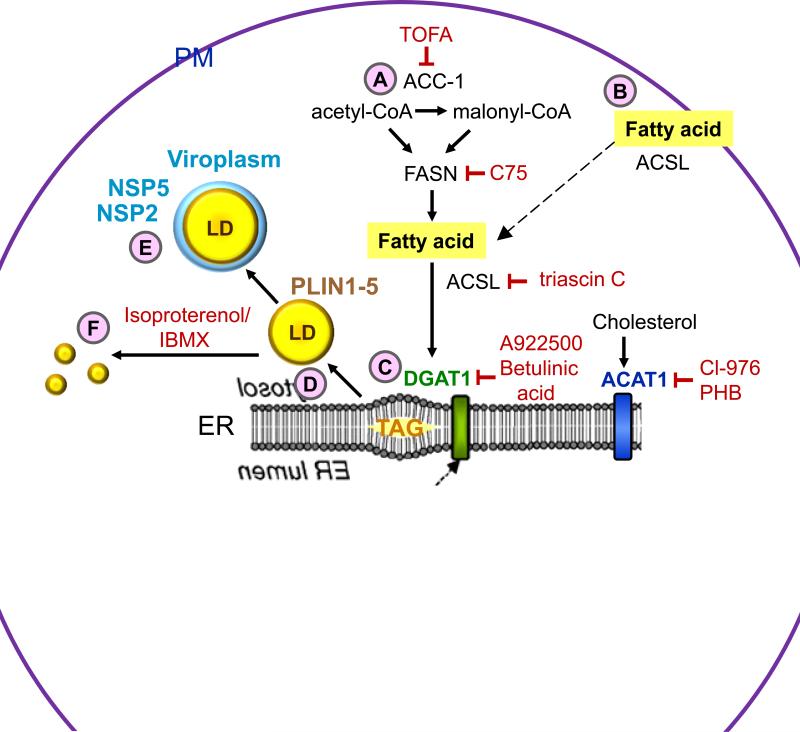
Figure 1 illustrates the current understanding of lipogenesis and lipid droplet biogenesis, and delineates interventions of this process which decrease rotavirus replication. Lipogenesis involves the biogenesis of components of lipid droplets: phospholipids, triacylglycerol and fatty acids. As a first step, acetyl-CoA carboxylase 1 (ACC-1) converts acetyl-CoA into malonyl-CoA (Figure 1-A). In a reiterative process, the fatty acid synthase (FASN) complex catalyzes the reaction of acetyl-CoA with malonyl-CoA resulting in the synthesis of the sixteen carbon fatty acid palmitate. Palmitate is the precursor molecule for synthesis of phospholipids, triacylglycerol and other fatty acids. As an alternative to lipogenesis, cells can import long chain fatty acids from extracellular media (Figure 1-B). Although the mechanism of fatty acid transport through the plasma membrane is not completely understood, it is believed that long chain acyl-CoA synthetase (ACSL) plays a key role in this process [23]. ACSL family members convert long chain fatty acids into hydrophilic acyl-CoAs that can no longer escape the cell. The mechanistic details of lipid droplet biogenesis remain poorly understood [14,24,25], The prevalent model is that lipid droplets form from the neutral lipid within the lipid bilayer of the endoplasmic reticulum (ER) where the enzymes required for the biosynthesis of TAG, diacylglycerol acyltransferases (DGAT1 and DGAT2), and sterol ester synthesis, acyl-coenzyme A (CoA):cholesterol acyltransferases (ACAT1 and ACAT2), reside [14] (Figure 1-C). The accumulation of neutral lipids between the lipid bilayer of the endoplasmic reticulum promotes the cytosolic leaflet to release a lipid droplet into the cytoplasm (Figure 1-D). Subsequently, lipid droplets acquire numerous proteins (PLIN1-PLIN5).
Figure 1.
Processes of lipogenesis and lipid droplet biogenesis, and interventions that disrupt viroplasm formation. (A) De novo fatty acid synthesis involves the conversion of acetyl-CoA into malonyl-CoA by acetyl-CoA carboxylase 1 (ACC-1). Fatty acid synthase (FASN) catalyzes the synthesis of the fatty acid palmitate from acetyl-CoA and malonyl-CoA. (B) Long chain acyl-CoA synthetase (ACSL) facilitates extracellular fatty acid uptake and converts fatty acids into their corresponding CoA esters for oxidation or esterification into complex lipids (e.g. triglycerides, phospholipids and cholesterol esters). (C) The ER-localized enzymes diacylglycerol acyltransferases (DGAT1 and DGAT2), and acyl-coenzyme A (CoA):cholesterol acyltransferases (ACAT1 and ACAT2) synthesize triacylglycerol (TAG) from fatty acids and sterol esters from cholesterol, respectively. These products are stored in the lipid bilayer of the ER. (D) Lipid droplets bud from the ER into the cytoplasm and acquire lipid droplet-associated proteins (PLINs 1-5). (E) Rotavirus viroplasms associate with lipid droplets. Inhibitors (shown in red) that block lipid droplet formation or disperse lipid droplets significantly decrease the number and size of viroplasms and the amount of infectious viral progeny. (F) Treatment of cells with isoproterenol and IBMX fragment lipid droplets into smaller microdroplets.
Formation of viroplasm-lipid droplet complexes is vital for rotavirus replication
The formation of complexes between viroplasms and lipid droplets (Figure 1-E) appears to be vital for rotavirus replication as compounds that block lipid droplet formation or disperse lipid droplets significantly decrease the number and size of viroplasms and the amount of infectious progeny produced. Steps in lipogenesis and lipid droplet biogenesis at which intervention affects rotavirus viroplasm formation and replication are also shown in Figure 1. Treatment of rotavirus-infected cells with triascin C, an inhibitor of ASCL3, reduced viroplasm size and number with a corresponding reduction in viral yield [8,26]. Inhibiting key molecules in lipogenesis, ACC-1 with TOFA, or FASN with C75, yielded varying results. Treatment of rotavirus-infected cells with TOFA reduced both the infectivity of progeny virus and viral dsRNA production in a time- and dose-dependent manner. Addition of TOFA at 4 hours prior to infection had the greatest effect on viral infectivity and dsRNA yield but a decrease in both these characteristics was still observed when TOFA was added at 4 hours post infection [27]. Treatment of rotavirus-infected cells with C75 showed only a modest effect, but in combination with TOFA, a synergistic reduction in viral yield was reported [27]. TOFA treatment of rotavirus-infected cells also had a broader effect in that it caused a 2-fold reduction in the production of RV DLPs, but a 20-fold reduction in detectable TLPs [28], suggesting that the blockage of fatty acid synthesis may affect RV replication not only at the steps occurring within viroplasms up to DLP formation but also at the later steps of infectious virus assembly (TLPs). Reduced viral yields were also observed in rotavirus-infected cells treated with inhibitors of DGAT, A922500 or betulinic acid, or ACAT, CI-976 or PHB (for details see ref [26]). Treatment of rotavirus-infected cells with isoproterenol and IBMX, which raise cellular cyclic AMP and disperse lipid droplets into smaller microdroplets in adipocytes [12,29], resulted in reduced number and size of viroplasms, decreased production of viral dsRNA and a 120-200-fold lower yield of infectious progeny [8] (Figure 1-F). In addition, the viability of the drug-treated, rotavirus-infected cells was significantly higher at later time points post infection as compared to non-treated rotavirus-infected cells, suggesting that the reduction in rotavirus-induced cytopathicity is correlated with increased cell viability [8].
Concluding remarks and future directions
Eukaryotic cells regulate astonishingly complex homeostatic networks, yet control over this fine-tuned machinery is co-opted by viruses with expression of just a handful of proteins [17,30,31]. Here we reviewed a striking example of such viral takeover: rotavirus exploitation of lipid metabolism. Co-expression of just two rotavirus proteins, NSP2 and NSP5, is sufficient for the formation of viroplasm-like structures which co-localize with lipid droplets [7,8]. The recognition that rotavirus viroplasms require components of lipid droplets implies a critical role of lipid droplets for rotavirus biology. In this context it should be noted that lipid droplets also are crucially important for the replication of members of the Flaviviridae family [32-34] and of intracellular bacteria such as Chlamydia [35] and Mycobacterium tuberculosis [36].
Clinically, the findings described above may be significant because rotavirus replication occurs in mature enterocytes of the small intestine, the major site of fat absorption in the body. Stem cell-derived human intestinal enteroids (HIE) are a novel, non-transformed cell culture model that is defining new aspects of human intestinal physiology and pathophysiology. HIEs are currently being explored as a rotavirus replication system that more closely mimics the human intestinal epithelium [37]. Infection of HIEs with human rotaviruses demonstrated host range and cell type restriction and virus-induced fluid secretion; in addition, infection of HIEs with human rotaviruses has induced viroplasm and lipid droplet formation [37].
Questions to be answered by rotavirus infection of HIEs are: 1) Do lipid droplets function as a platform for rotavirus viroplasms and viral replication as shown for infected MA104 cells [8]? 2) Which of the viroplasm proteins mediates the interaction with lipid droplets? 3) In addition to interacting with preformed lipid droplets, do rotaviruses actively induce lipid droplet formation during viral replication, and what is the mechanism? 4) Do viral proteins directly interact with lipid droplet proteins or components for the formation of lipid droplets? 5) Are the neutral lipids within the lipid droplet utilized for energy production through beta oxidation in mitochondria or do lipid droplets play different roles during rotavirus infection? 6) Which gene products involved in lipid droplet formation are important for forming complexes with viroplasms? The siRNA approach [38,39] could be used to identify these factors.
Treatment of mice with chemical compounds which decrease the intracellular triglyceride content has led to a reduction of rotavirus shedding 1-3 days post infection compared to rotavirus-infected untreated animals [20]. This initial observation should be explored further to identify potential candidate therapeutic drugs against rotavirus disease. Since lipid droplets are critical for the replication of many microbes, including flaviviruses and intracellular bacteria, lipid droplet biology is wide open for future study [16,31].
Highlights.
Rotavirus viroplasms and lipid droplets associate early during viral replication
Lipid droplets may act as a platform for viral replication and assembly
Inhibition or disruption of lipid droplets reduces rotavirus yield
Rotavirus-infected mice shed less virus upon lipid depletion
Lipid droplet-disrupting compounds may be developed to combat LD-requiring pathogens
Future research on the significance of viroplasm-lipid droplet complexes for rotavirus replication.
- Determining the molecular mechanisms of viroplasm-lipid droplet interaction
- Defining the functions of lipid droplets in the viroplasm-lipid droplet complexes
- Exploring chemical compounds shown to disturb lipid biosynthesis and lipid droplet homeostasis as potential therapeutic agents in animal models of rotavirus infection/disease
- Assessing inhibitors of lipid droplet homeostasis as inhibitors of the replication of other microbes (flaviviruses, intracellular bacteria)
Acknowledgements
We gratefully acknowledge partial support of the rotavirus research from NIH grants R01 AI080656 and P30 DK056338, Wellcome Trust grants WT082031 and RG46760, an Amgen Foundation scholarship and the Addenbrooke's Biomedical Research Centre. Many thanks are due to Drs. Jeanette Criglar and Mary K. Estes for critical comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
*of special interest
**of outstanding interest
- 1.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estes MK, Greenberg HB. Rotaviruses. In: Knipe DM, Howley PH, editors. Fields Virology. 6th ed. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia, PA: 2013. pp. 1347–1401. [Google Scholar]
- 3.Silvestri LS, Taraporewala ZF, Patton JT. Rotavirus replication: plus-sense templates for double-stranded RNA synthesis are made in viroplasms. J Virol. 2004;78:7763–7774. doi: 10.1128/JVI.78.14.7763-7774.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez T, Rojas M, Ayala-Breton C, Lopez S, Arias CF. Reduced expression of the rotavirus NSP5 gene has a pleiotropic effect on virus replication. J Gen Virol. 2005;86:1609–1617. doi: 10.1099/vir.0.80827-0. [DOI] [PubMed] [Google Scholar]
- 5.Vascotto F, Campagna M, Visintin M, Cattaneo A, Burrone OR. Effects of intrabodies specific for rotavirus NSP5 during the virus replicative cycle. J Gen Virol. 2004;85:3285–3290. doi: 10.1099/vir.0.80075-0. [DOI] [PubMed] [Google Scholar]
- 6.Taraporewala ZF, Schuck P, Ramig RF, Silvestri L, Patton JT. Analysis of a temperature-sensitive mutant rotavirus indicates that NSP2 octamers are the functional form of the protein. J Virol. 2002;76:7082–7093. doi: 10.1128/JVI.76.14.7082-7093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabbretti E, Afrikanova I, Vascotto F, Burrone OR. Two non-structural rotavirus proteins, NSP2 and NSP5, form viroplasm-like structures in vivo. J Gen Virol. 1999;80(Pt 2):333–339. doi: 10.1099/0022-1317-80-2-333. [DOI] [PubMed] [Google Scholar]
- 8**.Cheung W, Gill M, Esposito A, Kaminski CF, Courousse N, Chwetzoff S, Trugnan G, Keshavan N, Lever A, Desselberger U. Rotaviruses associate with cellular lipid droplet components to replicate in viroplasms, and compounds disrupting or blocking lipid droplets inhibit viroplasm formation and viral replication. J Virol. 2010;84:6782–6798. doi: 10.1128/JVI.01757-09. [Original observation of the interaction of rotavirus viroplasms with lipid droplets (as shown be confocal microscopy, FRET and equilibrium gradient ultracentrifugation) and of the inhibition of viral replication by chemical compounds interfering with lipid droplet homeostasis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy S, Martin S, Parton RG. Lipid droplet-organelle interactions; sharing the fats. Biochim Biophys Acta. 2009;1791:441–447. doi: 10.1016/j.bbalip.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Hodges BD, Wu CC. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J Lipid Res. 2010;51:262–273. doi: 10.1194/jlr.R003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468–471. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL. The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J Biol Chem. 2006;281:11901–11909. doi: 10.1074/jbc.M600171200. [DOI] [PubMed] [Google Scholar]
- 13.Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18:401–408. doi: 10.1016/j.cellsig.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Ohsaki Y, Suzuki M, Fujimoto T. Open questions in lipid droplet biology. Chem Biol. 2014;21:86–96. doi: 10.1016/j.chembiol.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Farese RV, Jr., Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton NS, Randall G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011;19:368–375. doi: 10.1016/j.tim.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saka HA, Valdivia R. Emerging roles for lipid droplets in immunity and host- pathogen interactions. Annu Rev Cell Dev Biol. 2012;28:411–437. doi: 10.1146/annurev-cellbio-092910-153958. [DOI] [PubMed] [Google Scholar]
- 18.Walther TC, Farese RV., Jr. Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Gaunt ER, Zhang Q, Cheung W, Wakelam MJ, Lever AM, Desselberger U. Lipidome analysis of rotavirus-infected cells confirms the close interaction of lipid droplets with viroplasms. J Gen Virol. 2013;94:1576–1586. doi: 10.1099/vir.0.049635-0. [Analysis of viroplasm-lipid droplet interaction by comprehensive lipidome analysis of the complexes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Kim Y, Chang KO. Inhibitory effects of bile acids and synthetic farnesoid X receptor agonists on rotavirus replication. J Virol. 2011;85:12570–12577. doi: 10.1128/JVI.05839-11. [Observation that bile acids reduced rotavirus replication in cell culture and in mice in correlation with a decrease in cellular triglyceride content.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X, Jayaram H, Kumar M, Ludtke SJ, Estes MK, Prasad BV. Cryoelectron microscopy structures of rotavirus NSP2-NSP5 and NSP2-RNA complexes: implications for genome replication. J Virol. 2006;80:10829–10835. doi: 10.1128/JVI.01347-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Criglar JM, Hu L, Crawford SE, Hyser JM, Broughman JR, Prasad BV, Estes MK. A novel form of rotavirus NSP2 and phosphorylation-dependent NSP2-NSP5 interactions are associated with viroplasm assembly. J Virol. 2014;88:786–798. doi: 10.1128/JVI.03022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nchoutmboube JA, Viktorova EG, Scott AJ, Ford LA, Pei Z, Watkins PA, Ernst RK, Belov GA. Increased long chain acyl-Coa synthetase activity and fatty acid import is linked to membrane synthesis for development of picornavirus replication organelles. PLoS Pathog. 2013;9:e1003401. doi: 10.1371/journal.ppat.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brasaemle DL, Wolins NE. Packaging of fat: an evolving model of lipid droplet assembly and expansion. J Biol Chem. 2012;287:2273–2279. doi: 10.1074/jbc.R111.309088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilfling F, Haas JT, Walther TC, Farese RV., Jr. Lipid droplet biogenesis. Curr Opin Cell Biol. 2014;29:39–45. doi: 10.1016/j.ceb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Kim Y, George D, Prior AM, Prasain K, Hao S, Le DD, Hua DH, Chang KO. Novel triacsin C analogs as potential antivirals against rotavirus infections. Eur J Med Chem. 2012;50:311–318. doi: 10.1016/j.ejmech.2012.02.010. [Exploration of differential anti-rotavirus effects of triacsin C chemical derivatives.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Gaunt ER, Cheung W, Richards JE, Lever A, Desselberger U. Inhibition of rotavirus replication by downregulation of fatty acid synthesis. J Gen Virol. 2013;94:1310–1317. doi: 10.1099/vir.0.050146-0. [Blockage of fatty acid synthesis by TOFA decreases rotavirus replication, even when administered at 4 h after rotavirus infection.] [DOI] [PubMed] [Google Scholar]
- 28.Cheung W, Gaunt ER, Lever A, Desselberger U. Viral Gastroenteritis: Molecular biology, pathogenesis, epidemiology and vaccine development. Vol. 2016. Elsevier Academic Press; Amsterdam: Rotavirus replication: the role of lipid droplets. [Google Scholar]
- 29.Gross DN, Miyoshi H, Hosaka T, Zhang HH, Pino EC, Souza S, Obin M, Greenberg AS, Pilch PF. Dynamics of lipid droplet-associated proteins during hormonally stimulated lipolysis in engineered adipocytes: stabilization and lipid droplet binding of adipocyte differentiation-related protein/adipophilin. Mol Endocrinol. 2006;20:459–466. doi: 10.1210/me.2005-0323. [DOI] [PubMed] [Google Scholar]
- 30.Chukkapalli V, Heaton NS, Randall G. Lipids at the interface of virus-host interactions. Curr Opin Microbiol. 2012;15:512–518. doi: 10.1016/j.mib.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herker E, Ott M. Emerging role of lipid droplets in host/pathogen interactions. J Biol Chem. 2012;287:2280–2287. doi: 10.1074/jbc.R111.300202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Chatel-Chaix L, Bartenschlager R. Dengue virus- and hepatitis C virus-induced replication and assembly compartments: the enemy inside--caught in the web. J Virol. 2014;88:5907–5911. doi: 10.1128/JVI.03404-13. [Crucial role of lipid droplets for the replication of several genera of the Flaviviridae family.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 34.Samsa MM, Mondotte JA, Iglesias NG, Assuncao-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 2009;5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar Y, Cocchiaro J, Valdivia RH. The obligate intracellular pathogen Chlamydia trachomatis targets host lipid droplets. Curr Biol. 2006;16:1646–1651. doi: 10.1016/j.cub.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 36.Daniel J, Maamar H, Deb C, Sirakova TD, Kolattukudy PE. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 2011;7:e1002093. doi: 10.1371/journal.ppat.1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Saxena K, Blutt SE, Ettayebi K, Zeng XL, Broughman JR, Crawford SE, Karandikar UC, Sastri NP, Conner ME, Opekun AR, Graham DY, Qureshi W, Sherman V, Foulke-Abel J, In J, Kovbasnjuk O, Zachos NC, Donowitz M, Estes MK. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J Virol. 2015;90:43–56. doi: 10.1128/JVI.01930-15. [Original observation that human intestinal enteroid cultures contain multiple cell types representing the intestinal epithelium and are superior models for infection with human rotaviruses since they exert host specificity, virus-induced fluid secretion, and viroplasm formation and lipid droplet induction upon infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beller M, Sztalryd C, Southall N, Bell M, Jackle H, Auld DS, Oliver B. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6:e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]