Abstract
Objective
To study whether the circadian variation of plasminogen-activator-inhibitor-1 (PAI-1) levels, with high morning levels, is associated with poor outcome of children with meningococcal sepsis presenting in the morning hours.
Design
Retrospective analysis of prospectively collected clinical and laboratory data.
Setting
Single center study at Erasmus MC-Sophia Children’s Hospital, Rotterdam, the Netherlands.
Subjects
184 patients aged 3 weeks to 18 years with meningococcal sepsis. In 36 of these children, PAI-1 levels at admission to the PICU were measured in plasma by ELISA.
Interventions
None.
Measurements and main results
Circadian variation was studied by dividing one day in blocks of 6 hours. Patients admitted between 6:00 am and 12:00 am had increased illness severity scores and higher PAI-1 levels (n = 9, median 6912 ng/mL, IQR 5808–15600) compared to patients admitted at night (P = 0.019, n = 9, median 3546 ng/mL, IQR 1668–6118) or in the afternoon (P = 0.007, n = 7, median 4224 ng/mL, IQR 1804–5790). In 184 patients, analysis of circadian variation in relation to outcome showed more deaths, amputations and need for skin grafts in patients admitted to the PICU between 6:00 am and 12:00 am than patients admitted during the rest of the day (P = 0.009).
Conclusions
Circadian variation of PAI-1 levels is present in children with meningococcal sepsis and is associated with illness severity, with a peak level in the morning. Whether circadian variation is an independent risk factor for morbidity and mortality in meningococcal sepsis needs to be explored in future studies.
Introduction
Meningococcal endotoxins and the subsequent inflammatory host response induce excessive coagulation and downregulation of fibrinolysis in meningococcal sepsis. Hence, the delicate balance between coagulation and anti-coagulation shifts towards thrombosis and widespread deposition of fibrin throughout the microcirculation, with thromboembolism contributing to the need to amputate extremities, multiple organ dysfunction, and eventually death. [1]
In meningococcal sepsis, plasminogen-activator-inhibitor-1 (PAI-1) levels are increased and result in inhibited fibrinolysis and impaired anticoagulant mechanism, since PAI-1 neutralizes activated protein C [2], leading to severe disseminated intravascular coagulation (DIC). [3, 4] The PAI1 4G/5G polymorphism is associated with PAI-1 levels and with outcome. The highest risk for mortality is present in 4G/4G homozygous individuals, who produce the highest levels of PAI-1. [3]
The genotype is one of the multiple mechanisms which influences PAI-1 levels. Physiologically, levels are also subject to circadian variation, causing a PAI-1 peak in the morning. [5, 6] In meningococcal sepsis, clustering of fatal cases in the morning hours has been reported repeatedly. These findings are generally interpreted as the result of delayed detection of symptoms and subsequent health care seeking behavior, as signs of severe illness may easily be overlooked in the night or early morning hours. [7, 8] We hypothesize that the circadian variation of PAI-1, with high morning levels, is associated with excess mortality of cases presenting in the morning hours, possibly due to aggravation of multiple organ failure secondary to more severe DIC.
Here, we aim to study the circadian variation of PAI-1 levels in children with meningococcal sepsis in relation to outcome.
Materials and Methods
We retrospectively analyzed clinical and laboratory data of a cohort of 184 patients aged 3 weeks to 18 years with meningococcal sepsis, who were enrolled in Rotterdam based meningococcal studies from 1988 to 2005. [3, 9] All patients fulfilled internationally agreed criteria for sepsis. [10] Meningococcal sepsis was diagnosed clinically (n = 28) and/or by positive culture or PCR from sterile sites (n = 156). In 36 of these children, PAI-1 levels at admission to the PICU were measured in plasma by ELISA as described before. [3, 9] Blood samples were taken on admission to the PICU, processed on ice and stored at -80 degree Celsius until analysis. [11] All studies were approved by the ethical committee of Erasmus MC, and written informed consent was obtained from parents or legal guardians.
Circadian variation was studied by dividing one day in blocks of 6 hours. Illness severity was indicated by the probability of death based on the BEP score (P (death BEP)) [12], DIC score [13], and Pediatric Risk of Mortality (PRISM) [14]. Quantitative variables are presented either as mean (±SD) when normally distributed or as median (IQR). For normally distributed variables, t-tests were used to compare two groups, while for non-normal variables the Mann-Whitney U test was used. To compare PAI-1 levels and illness severity between four time periods, we used a One-Way ANOVA when the depending variable was normally distributed and the Kruskal-Wallis test when the depending variable was non-normally distributed. The correlation between PAI-1 and illness severity was studied by Pearson’s (for normally distributed variables) or Spearman’s (for non-normally distributed variables) correlation. Chi squared tests or Fisher’s exact tests-in case of small sample size-were used to assess the association between two categorical variables. Data were analyzed using SPSS version 21.
Results
Baseline characteristics of the total cohort of 184 patients and the 36 children in whom PAI-1 levels were measured (PAI-1 cohort) are presented in Table 1. Both groups had similar demographics, but illness severity in the PAI-1 cohort was higher as reflected by higher P (death BEP) and higher DIC score at admission.
Table 1. Baseline characteristics of all patients (n = 184) and the PAI-1 cohort (n = 36).
| All patients (n = 184) | PAI-1 cohort (n = 36) | P | |
|---|---|---|---|
| Age (median, IQR) | 3y (18m-8y) | 2y (12m-9y) | ns |
| Sex (% male) | 59% | 56% | ns |
| P (death BEP) (median, IQR) | 0.05 (0.03–0.12) | 0.09 (0.04–0.23) | <0.01 |
| DIC score at admission (mean, ±SD) | 4.7 (1.9) | 5.6 (2.0) | <0.05 |
Abbreviations: P (death BEP) = Probability of death based on the BEP score, DIC = Disseminated intravascular coagulation, IQR = Interquartile range, SD = Standard deviation, m = month(s), y = year(s).
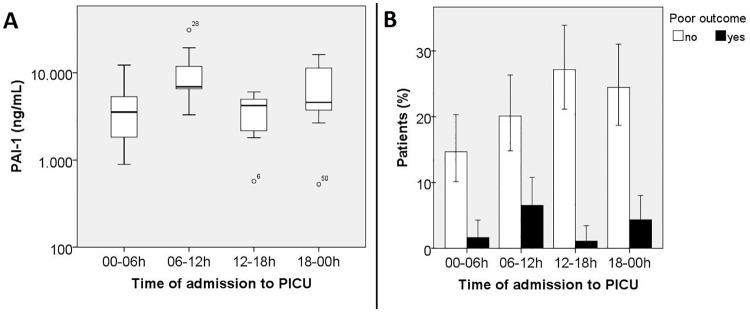
Patients admitted between 6:00 am and 12:00 am had higher PAI-1 levels (n = 9, median 6912 ng/mL, IQR 5808–15600) than patients admitted at night (P = 0.019, n = 9, median 3546 ng/mL, IQR 1668–6118) or in the afternoon (P = 0.007, n = 7, median 4224 ng/mL, IQR 1804–5790). (Fig 1A) The distribution of PAI1 4G/5G genotype (P = 0.71) and allele frequency (P = 0.72) did not differ between four time periods.
Fig 1. Time of admission to PICU in relation to plasminogen-activator-inhibitor-1 levels and outcome.
(A) Patients admitted between 6:00 am and 12:00 am had higher plasminogen-activator-inhibitor-1 levels than patients admitted at night (P = 0.019) or in the afternoon (P = 0.007). (PAI-1 level on y-axis in logarithmic scale. Bar within box represents median, box represents Q1-Q3, whiskers represent 1.5*IQR, dots are outliers) (B) Outcome in patients admitted to the PICU between 6:00 am and 12:00 am was worse than patients admitted during the rest of the day (P = 0.009). (Error bars represent 95% CI)
In concordance with the variation in PAI-1 levels, patients admitted between 6:00 am and 12:00 am had an increased illness severity based on the BEP score (median P (death BEP) 0.26, IQR 0.16–0.44) than patients admitted at night (P = 0.009, median P (death BEP) 0.05, IQR 0.02–0.15), patients admitted in the afternoon (P = 0.001, median P (death BEP) 0.05, IQR 0.04–0.05), or patients admitted in the evening (P = 0.037, median P (death BEP) 0.10, IQR 0.04–0.24). Illness severity reflected by the DIC score did not differ between four time periods (P = 0.13). Moreover, illness severity correlated to PAI-1 levels (correlation between BEP score and PAI-1: r = 0.77, n = 36, P<0.001; correlation between DIC score and PAI-1: r = 0.67, n = 34, P<0.001).
The morning PAI-1 peak level and the morning peak in illness severity in 36 children was not associated with poor outcome, as defined by deaths, amputations and/or need for skin grafts (P = 0.24). To increase power, we analyzed circadian variation in the total group of 184 patients, and found a worse outcome in patients admitted to the PICU between 6:00 am and 12:00 am than patients admitted during the rest of the day (P = 0.009). Of the 184 patients, 49 patients were admitted between 6:00 am and 12:00 am, of whom 12 patients (7%) eventually had a poor outcome (6 deaths, 4 amputations and 2 skin grafts). This in contrast to 135 patients who were admitted during the rest of the day, of whom 13 patients had a poor outcome (00-06h: 2 deaths and 1 amputation (2%); 12-18h: 2 amputations (1%); 18-00h: 4 deaths, 3 amputations and 1 skin graft (4%)). (Fig 1B) Patients admitted between 6:00 am and 12:00 am showed a trend for higher PRISM, BEP score, and DIC score compared to patients admitted during the rest of the day. (PRISM 21.3 (±11.0) vs 16.1 (±10.0), P = 0.06; P (death BEP) 0.06 (IQR 0.02–0.20) vs 0.04 (IQR 0.03–0.10), P = 0.16; DIC 5.2 (±1.6) vs 4.5 (±2.1), P = 0.09)
Data underlying the findings from this study can be found in S1 File.
Discussion
This study provides insight into the circadian variation of PAI-1 levels in children with meningococcal sepsis, and shows a significant PAI-1 peak level in the morning. Because the distribution of PAI1 4G/5G genotype and allele frequency, known to be associated with PAI-1 levels [3], did not differ between time periods, it is likely that PAI-1 levels in meningococcal sepsis are associated with circadian variation. However, numbers are limited and we cannot exclude other factors also influencing PAI-1 levels in these patients. [15]
Illness severity is one of the main factors associated with PAI-1 levels and multiple studies have associated high PAI-1 levels with increased illness severity. [3, 16–19] Also in our cohort, we found a strong correlation between PAI-1 levels and illness severity. Thus, given the correlation between PAI-1 and illness severity, PAI-1 could either be a marker for illness severity or a contributor to illness severity. [20] Future studies should specify the role of circadian variation of PAI-1 levels in meningococcal sepsis severity.
Our results show that patients admitted to the PICU in the morning have a worse outcome than patients admitted during the rest of the day. These results of outcome are in line with a retrospective epidemiological study from Western Norway, where meningococcal sepsis patients, both adults and children, hospitalized between 7:00 am and 11:00 am had a poorer prognosis than those admitted during other hours of the day. [8] Although multiple factors could have influenced increased morning severity, especially a possible delay in detection of symptoms during the night and early morning, in our opinion, morning PAI-1 peak levels might have contributed to this effect.
Scheer and Shea [5] reported a true endogenous circadian rhythm in circulating PAI-1 independent of behavioral and environmental factors. Absolute values of healthy adults had a peak-to-trough amplitude of 1.24 ng/mL, corresponding with an increase from trough to peak of 124%. In our cohort, the lowest median PAI-1 value of a time period was 3546 ng/mL, which increased to a peak value of 6912 ng/mL in the morning, corresponding with an increase of 95%. The extremely high PAI-1 level in meningococcal sepsis compared to patients with meningitis alone or healthy controls has been described before. [3] However, this is the first report describing that circadian variation of PAI-1 levels—and associated circadian variation of illness severity—is also present in meningococcal sepsis patients with extremely high PAI-1 levels.
In conclusion, our data demonstrate an association between circadian variation of PAI-1 levels and illness severity in pediatric meningococcal sepsis patients, with a significant peak level in the morning. Future study in a larger cohort of patients should focus on the question whether the morning PAI-1 peak is an independent risk factor for morbidity and mortality in meningococcal sepsis.
Supporting Information
(SAV)
Acknowledgments
The authors would like to thank the Meningococcal Sepsis Research team for recruitment of patients and Noah N. N. van Dongen, research statistician, for assisting on the statistical analyses.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Research leading to these results has received funding from the European Union’s seventh Framework program under EC-GA no. 279185 (EUCLIDS). Also, research has been supported, in part, by an unrestricted grant from Baxter Bioscience, Vienna, Austria, in 2000. We declare that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zeerleder S, Hack CE, Wuillemin WA. Disseminated intravascular coagulation in sepsis. Chest. 2005;128(4):2864–75. Epub 2005/10/21 10.1378/chest.128.4.2864 [DOI] [PubMed] [Google Scholar]
- 2.Gladson CL, Schleef RR, Binder BR, Loskutoff DJ, Griffin JH. A comparison between activated protein C and des-1-41-light chain-activated protein C in reactions with type 1 plasminogen activator inhibitor. Blood. 1989;74(1):173–81. [PubMed] [Google Scholar]
- 3.Hermans PW, Hibberd ML, Booy R, Daramola O, Hazelzet JA, de Groot R, et al. 4G/5G promoter polymorphism in the plasminogen-activator-inhibitor-1 gene and outcome of meningococcal disease. Meningococcal Research Group. Lancet. 1999;354(9178):556–60. Epub 1999/09/02. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P, Joo GB, Brusletto B, Kierulf P. Plasminogen activator inhibitor 1 and 2, alpha-2-antiplasmin, plasminogen, and endotoxin levels in systemic meningococcal disease. Thromb Res. 1990;57(2):271–8. Epub 1990/01/15. [DOI] [PubMed] [Google Scholar]
- 5.Scheer FA, Shea SA. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood. 2014;123(4):590–3. Epub 2013/11/10. 10.1182/blood-2013-07-517060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Bom JG, Bots ML, Haverkate F, Kluft C, Grobbee DE. The 4G5G polymorphism in the gene for PAI-1 and the circadian oscillation of plasma PAI-1. Blood. 2003;101(5):1841–4. Epub 2002/10/31. 10.1182/blood-2002-07-2181 [DOI] [PubMed] [Google Scholar]
- 7.Halstensen A, Pedersen SH, Haneberg B, Bjorvatn B, Solberg CO. Case fatality of meningococcal disease in western Norway. Scand J Infect Dis. 1987;19(1):35–42. Epub 1987/01/01. [DOI] [PubMed] [Google Scholar]
- 8.Smith I, Bjornevik AT, Augland IM, Berstad A, Wentzel-Larsen T, Halstensen A. Variations in case fatality and fatality risk factors of meningococcal disease in Western Norway, 1985–2002. Epidemiol Infect. 2006;134(1):103–10. Epub 2006/01/18. 10.1017/S0950268805004553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Kleijn ED, de Groot R, Hack CE, Mulder PG, Engl W, Moritz B, et al. Activation of protein C following infusion of protein C concentrate in children with severe meningococcal sepsis and purpura fulminans: a randomized, double-blinded, placebo-controlled, dose-finding study. Crit Care Med. 2003;31(6):1839–47. Epub 2003/06/10. 10.1097/01.CCM.0000072121.61120.D8 [DOI] [PubMed] [Google Scholar]
- 10.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric S. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. Epub 2005/01/08. 10.1097/01.PCC.0000149131.72248.E6 [DOI] [PubMed] [Google Scholar]
- 11.Lewis MR, Callas PW, Jenny NS, Tracy RP. Longitudinal stability of coagulation, fibrinolysis, and inflammation factors in stored plasma samples. Thromb Haemost. 2001;86(6):1495–500. Epub 2002/01/05. [PubMed] [Google Scholar]
- 12.Couto-Alves A, Wright VJ, Perumal K, Binder A, Carrol ED, Emonts M, et al. A new scoring system derived from base excess and platelet count at presentation predicts mortality in paediatric meningococcal sepsis. Crit Care. 2013;17(2):R68 Epub 2013/04/13. 10.1186/cc12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khemani RG, Bart RD, Alonzo TA, Hatzakis G, Hallam D, Newth CJ. Disseminated intravascular coagulation score is associated with mortality for children with shock. Intensive Care Med. 2009;35(2):327–33. Epub 2008/09/20. 10.1007/s00134-008-1280-8 [DOI] [PubMed] [Google Scholar]
- 14.Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16(11):1110–6. Epub 1988/11/01. [DOI] [PubMed] [Google Scholar]
- 15.Henry M, Tregouet DA, Alessi MC, Aillaud MF, Visvikis S, Siest G, et al. Metabolic determinants are much more important than genetic polymorphisms in determining the PAI-1 activity and antigen plasma concentrations: a family study with part of the Stanislas Cohort. Arterioscler Thromb Vasc Biol. 1998;18(1):84–91. Epub 1998/01/28. [DOI] [PubMed] [Google Scholar]
- 16.Kornelisse RF, Hazelzet JA, Savelkoul HF, Hop WC, Suur MH, Borsboom AN, et al. The relationship between plasminogen activator inhibitor-1 and proinflammatory and counterinflammatory mediators in children with meningococcal septic shock. J Infect Dis. 1996;173(5):1148–56. Epub 1996/05/01. [DOI] [PubMed] [Google Scholar]
- 17.Lorente L, Martin MM, Borreguero-Leon JM, Sole-Violan J, Ferreres J, Labarta L, et al. Sustained high plasma plasminogen activator inhibitor-1 levels are associated with severity and mortality in septic patients. Thromb Res. 2014;134(1):182–6. Epub 2014/05/13. 10.1016/j.thromres.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 18.Madoiwa S, Nunomiya S, Ono T, Shintani Y, Ohmori T, Mimuro J, et al. Plasminogen activator inhibitor 1 promotes a poor prognosis in sepsis-induced disseminated intravascular coagulation. Int J Hematol. 2006;84(5):398–405. Epub 2006/12/26. 10.1532/IJH97.05190 [DOI] [PubMed] [Google Scholar]
- 19.Pralong G, Calandra T, Glauser MP, Schellekens J, Verhoef J, Bachmann F, et al. Plasminogen activator inhibitor 1: a new prognostic marker in septic shock. Thromb Haemost. 1989;61(3):459–62. Epub 1989/06/30. [PubMed] [Google Scholar]
- 20.Asakura H, Ontachi Y, Mizutani T, Kato M, Saito M, Kumabashiri I, et al. An enhanced fibrinolysis prevents the development of multiple organ failure in disseminated intravascular coagulation in spite of much activation of blood coagulation. Crit Care Med. 2001;29(6):1164–8. Epub 2001/06/08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.