Abstract
Background
Clinical trials to test safety and efficacy of drugs for patients with spinal muscular atrophy (SMA) are currently underway. Biomarkers that document treatment-induced effects are needed because disease progression in childhood forms of SMA is slow and clinical outcome measures may lack sensitivity to detect meaningful changes in motor function in the period of 1–2 years of follow-up during randomized clinical trials.
Objective
To determine and compare SMN protein and mRNA levels in two cell types (i.e. PBMCs and skin-derived fibroblasts) from patients with SMA types 1–4 and healthy controls in relation to clinical characteristics and SMN2 copy numbers.
Materials and methods
We determined SMN1, SMN2-full length (SMN2-FL), SMN2-delta7 (SMN2-Δ7), GAPDH and 18S mRNA levels and SMN protein levels in blood and fibroblasts from a total of 150 patients with SMA and 293 healthy controls using qPCR and ELISA. We analyzed the association with clinical characteristics including disease severity and duration, and SMN2 copy number.
Results
SMN protein levels in PBMCs and fibroblasts were higher in controls than in patients with SMA (p<0.01). Stratification for SMA type did not show differences in SMN protein (p>0.1) or mRNA levels (p>0.05) in either cell type. SMN2 copy number was associated with SMN protein levels in fibroblasts (p = 0.01), but not in PBMCs (p = 0.06). Protein levels in PBMCs declined with age in patients (p<0.01) and controls (p<0.01)(power 1-beta = 0.7). Ratios of SMN2-Δ7/SMN2-FL showed a broad range, primarily explained by the variation in SMN2-Δ7 levels, even in patients with a comparable SMN2 copy number. Levels of SMN2 mRNA did not correlate with SMN2 copy number, SMA type or age in blood (p = 0.7) or fibroblasts (p = 0.09). Paired analysis between blood and fibroblasts did not show a correlation between the two different tissues with respect to the SMN protein or mRNA levels.
Conclusions
SMN protein levels differ considerably between tissues and activity is age dependent in patients and controls. SMN protein levels in fibroblasts correlate with SMN2 copy number and have potential as a biomarker for disease severity.
Introduction
Hereditary proximal spinal muscular atrophy (SMA) is caused by survival motor neuron (SMN) protein deficiency due to homozygous deletion of the SMN1 gene [1]. A second semi-homologous SMN gene (SMN2) contains a crucial single nucleotide substitution that alters mRNA splicing, resulting in the absence of exon 7 in the large majority of SMN2 mRNA transcripts [1, 2]. Copy number variation of SMN2 is the most important modifier of disease severity [3].
SMN protein is ubiquitously expressed and has generic functions as part of a number of protein complexes in addition to tissue-specific functions, including mRNA processing and splicing [4–6], axonal transport [7, 8] and ubiquitination homeostasis [9, 10]. Quantification of SMN protein and mRNA levels may be useful as a biomarker for SMA severity and to monitor the response to experimental strategies designed to increase SMN protein [11–14] and changes in SMN expression have already been used to study the potential of SMN-inducing drugs as a treatment for SMA [11, 14–19].
Various methods have been developed to (semi-) quantify SMN protein and mRNA levels. Southern and western blotting [20–26], imaging-flow cytometry [27, 28] and simple-cell-immuno-assays [29, 30] were used in studies to investigate SMN levels in lymphoblasts, peripheral blood mononuclear cells (PBMCs) and fibroblasts in small cohorts of SMA patients. qPCR [22, 31, 32] and ELISA [12, 15, 18, 22, 33–35] have shown their applicability in larger studies with patients participating in randomized controlled trials with SMN inducing therapies such as valproic acid and salbutamol [11, 15, 19]. Recently, electrochemiluminescence-based immunoassay (ECLIA or ECL) was introduced for measurements of SMN levels in small amounts of whole blood [32, 36, 37].
Reduced SMN levels have been found in a large variety of tissues in SMA mouse models, including muscle [33, 38], myotubes [39], brain [33, 38, 40], astrocytes [41], spinal cord [33, 36, 38, 40], Schwann cells [42], skin [33] and liver [33]. In humans, similar findings have been reported in a smaller number of tissues that include brain [43], muscle [43], whole blood [32, 36], PBMCs [12, 15, 18, 22, 29, 33, 34], fibroblasts [20, 26, 29] and buccal cells [36, 37]. SMN protein levels have also been investigated in body fluids, most notably in cerebrospinal fluid as an exploratory biomarker in a phase 1 study of intrathecal administration of antisense oligonucleotides [44], but also in urine, plasma and saliva [33, 36, 37]. However, the extent to which tissues differ in SMN mRNA and protein concentrations in humans is still largely unknown [45].
A second unaddressed issue is how aging affects SMN levels. Possible age-dependent changes in levels of SMN have been reported in SMA mice [33]. Previous patient studies have included far more children than adults with SMA and this limitation in age range has precluded a definite conclusion regarding the effect of age on SMN levels [11, 12, 14–18, 22]. We therefore determined SMN protein and mRNA levels in blood and skin-derived fibroblasts from a large cohort of children and adults with SMA and matched healthy controls using ELISA and qPCR methodology.
Materials and Methods
Study population
We performed a cross-sectional, single visit, single-center, nationwide study on SMA in The Netherlands. Inclusion criteria were a genetically confirmed diagnosis of SMA according to the diagnostic criteria defined by the SMA Consortium, i.e. a homozygous deletion of the SMN1 gene, or a hemizygous deletion with an additional pathogenic point mutation in the second SMN1 allele [1, 46, 47]. We used age at onset and acquired motor milestones to define SMA types 1–4 as described previously [46, 48, 49]. Patients with SMA type 1 had an onset of muscle weakness before the age of 6 months and were never able to sit independently. Patients with SMA type 2 had an onset between the age of 6 and 18 months and learned to sit but not to walk independently. Patients with SMA type 3 had an onset after the age of 18 months, learned to walk independently at some stage in life. Onset in patients with SMA type 4 occurred after the age of 30. In case of discrepancy between age at onset and reached motor milestones, the latter determined the final diagnosis. We included 6 adult patients with onset before 6 months of age and who survived infancy but never learned to sit independently. This unusual SMA type 1 phenotype (‘type 1c’) has been reported before [50–53]. Disease duration was calculated as time between the age of first symptoms and date of enrolment. The healthy control group consisted of 293 children and adults without neurological disease or a current infection.
We used Medical Research Council (MRC) sum scores of 38 individual muscle groups to document muscle strength. Each muscle was given a score ranging from 1 to 5 (MRC sum score range 38–190). The Hammersmith Functional Motor Scale Expanded (HFMSE) was used to document motor function [54].
The Medical Ethical Committee of the University Medical Center Utrecht approved the study protocol (protocol number 09–307) and all participants and/or legal representatives gave written informed consent.
SMN copy number analysis
We determined the total number of SMN1 and SMN2 gene copies in patients by Multiplex Ligation-dependent Probe Amplification (MLPA) analysis using SALSA MLPA kits P021-A2 and P060-B2, according to the manufacturer’s protocol (www.mrcholland.com).
PBMCs
Peripheral blood mononuclear cells (PBMCs) were isolated from 5–10 ml Lithium-Heparin-blood samples using Lymfoprep (Axis Shield PoC AS, Oslo, Norway) and complete Lysis-M EDTA-free buffer (Roche Diagnostics GmbH, Mannheim Germany). Isolation was performed within 4 hours of sample collection. PBMC counts from samples ranged from 1.26x107 to 3.3x107 cells.
We tested three protein extraction buffers using PBMCs that were pelleted and re-suspended in 100 μL of each buffer. Comparison between Complete Lysis-M EDTA-free buffer, RIPA buffer and ENZO lysis buffer showed comparable inter-well variability (Complete Lysis-M EDTA-free buffer: mean 6.0% CV; RIPA buffer: 5.5% CV; ENZO lysis buffer mean 5.9% CV). Mean inter-plate variability was 35% (range 8.7–90%). We used Complete Lysis-M EDTA-free buffer (Roche Diagnostics GmbH, Mannheim Germany) for all samples. Lysates were stored at -80°C in aliquots of 100–200 μl.
Fibroblasts
Patient-derived fibroblasts were generated from explants of 3 mm dermal biopsies. After 1–2 weeks, fibroblast outgrowths from the explants were passaged with trypsin and frozen. Fibroblasts were cultured in standard fibroblast medium (Dulbecco’s modified eagle medium containing 10% fetal bovine serum and 0.5% penicillin and streptomycin), and lysed with Complete Lysis-M EDTA-free buffer (Roche Diagnostics GmbH, Mannheim Germany). Lysates were stored at -80°C.
Measurements of SMN protein concentrations
We determined total soluble protein concentrations of the samples in triplo using protein assay with Bicinchoninic Acid (#23227, Pierce BCA Protein Assay Kit; Thermo Scientific, Rockford, IL) and generated standard curves using dilutions (0.1–3.0 mg/ml) of bovine serum albumin (BSA) (A7906-500G, Sigma Alderich Chemie, Steinheim, Germany).
We normalized samples to 1 gram total soluble protein from BCA-analysis. SMN protein levels in PBMCs and fibroblasts were quantified using the standardized SMN ELISA (2012, #ADI-900-209, Enzo Life Sciences, Farmingdale, NY) [33, 34] and expressed as nanogram per 1 gram of total protein.
Quantitative polymerase chain reaction of SMN transcripts
We used PAXgene blood RNA tubes (BD Biosciences, San Jose, CA, USA) for storage and stabilization of RNA from peripheral blood. RNeasy Mini Kit (Qiagen, Dusseldorf, Germany) was used to extract RNA from blood and fibroblasts.
RNA concentration was determined by absorbance determination and quality was assessed by nanodrop analysis (absorbance of 230, 260 and 280nm). A ratio (260/280) of ±2.0 was accepted as pure. Quality and integrity control of PAXgene samples was performed with an Agilent 2100 bioanalyzer and 90% of samples met the quality criterion of RNA Integrity Number >7 (mean 8.1, median 8.2, range 4.4–9.2). We used Taqman Gold RT-PCR kit (Applied Biosystems, No.N808-0232) for the reverse transcription of 500 ng RNA to cDNA.
We used 2 control primer sets (glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 18S), and three SMN-primer sets on each sample. External standard constructs and primers for SMN1-FL, SMN2-FL, SMN2-Δ7, GAPDH and 18S were designed as reported previously [31]. GAPDH and 18S genes were both used for analysis (median intra-sample variation 0.8 and 1.1% respectively (range 0.1–3.3)) by means of the geometric mean of the two genes [55]. Standard curves were determined with Avogadro’s number. The real-time Taqman PCR reactions were carried out in 1x Taqman universal PCR mastermix (Applied Biosystems, P/N 4326708), 1x Primer-Probe mix (Applied Biosystems), with an input of 10 ng cDNA. qPCR was carried out as described previously [31]. Analysis was performed on Sequence Detection System v2.3 (Applied Biosystems). All samples were normalized against 105 molecules of the reference genes. Outliers in all expression sets per patient were excluded when they failed the Grubb’s test or deviated by >1SD from the sample mean.
Calculated ratios between transcripts of SMN2-FL and SMN2-Δ7 (SMN2-Δ7/SMN2-FL) were used to analyze the dose-effect of the SMN2 gene copy number variation.
Sample size and statistics
A sample size of 324 (allocation 1:2) was needed to reach 90% power to detect a difference in means between SMA patients and controls in SMN protein levels in PBMCs, using a two-group independent t-test with a 0.05 two-sided significance level based upon results from Crawford et al[22]. Post-hoc power analysis of 135 PBMC samples and their correlation with SMN2 copy number, age and SMA type showed a power of 80% using a two-sided ANOVA (alpha 0.01; partial etha2 0.15). Post-hoc power analysis of 87 fibroblast samples and their correlation with SMN2 copy number, age and SMA type showed a power of 87% using a two-sided ANOVA (alpha 0.01; partial etha2 0.43).
Normality was tested with Kolmorogov-Smirnov and Shapiro-Wilk tests. Mean, medians and SD for continuous variables and proportions for categorical variables were calculated. Correlation matrixes were analyzed using the Spearman’s rho. Univariate and multivariate tests including dichotomous data were performed using logistic regression. Multivariate analyses were checked and corrected for co-linearity. Comparison of data between SMA types and between patients and controls was performed using Kruskal-Wallis (KW) test or Chi-square analysis. Multivariate analysis was performed with linear regression including bootstrapping analysis. P-values ≤0.05 were considered significant.
We used SPSS (IBM SPSS Statistics version 19, Inc., Chicago, IL) for statistical analysis.
Results
Clinical characteristics
We included 150 patients with SMA type 1–4 and 293 healthy controls. Clinical characteristics are summarized in Tables 1 and 2. SMN2 copy numbers correlated with SMA type (Chi2 p<0.001). Age and disease duration differed between SMA types, and SMN2 copy numbers (KW p<0.01). Three patients used a stable dose of valproate at the time of this study. One patient had discontinued use of valproate more than one year before inclusion. None of the other patients were on other potentially SMN-inducing therapies (e.g. salbutamol).
Table 1. Baseline characteristics of patients in PBMC study.
| Type 1a (n = 18) | Type 2 (n = 60) | Type 3ab (n = 26) | Type 3b (n = 26) | Type 4 (n = 5) | Controls (n = 229) | |
|---|---|---|---|---|---|---|
| Gender (n) (F:M) | 7:11 | 36:24 | 15:11 | 11:15 | 4:1 | 115:114 |
| Mean age at inclusion in years (range) | 10.6 (0.3–49.7) | 19.6 (1–66.7) | 36.8 (2.4–65.7) | 38.8 (14–75) | 51.2 (41–68.8) | 32.7 (0.3–86) |
| Mean disease duration in years (range) | 11.1 (0.1–48.4) | 18.2 (0.3–64.8) | 33.6 (1.2–62.2) | 29.5 (2–71.4) | 14.3 (7.5–24.2) | NA |
| Mean HFMSE (range) | 0 (0–1) | 8 (0–35) | 17 (0–44) | 36 (4–66) | 48 (43–53) | ND |
| Mean MRC sum score (range) | 51 (34–62) | 89 (43–140) | 104 (56–160) | 146 (100–167) | 147 (121–162) | ND |
| SMN2 copy number (n) | ||||||
| 2 | 4 | 3 | 0 | 0 | 0 | ND |
| 3 | 13 | 52 | 14 | 3 | 0 | ND |
| 4 | 1 | 5 | 10 | 21 | 4 | ND |
| 5 | 0 | 0 | 0 | 2 | 0 | ND |
PBMC = Peripheral blood mononuclear cell; F = female; M = male; SMN = survival motor neuron; HFMSE: Hammersmith Functional Motor Scale Expanded; MRC = Medical Research Council; ND = not determined; NA = not applicable
a = Six patients with SMA type 1 had survived infancy at time of inclusion
b = One patient had a heterozygous SMN1-deletion and a pathogenic point mutation resulting in stop codon in exon 4
Table 2. Baseline characteristics of patients in fibroblast study.
| Type 1a (n = 5) | Type 2 (n = 19) | Type 3ab (n = 10) | Type 3b/4 (n = 6) | Controls (n = 47) | |
|---|---|---|---|---|---|
| Gender (n) (F:M) | 3:2 | 11:8 | 7:3 | 1:5 | 26:21 |
| Mean age at inclusion in years (range) | 15.3 (0.4–42.2) | 20.1 (1–66.7) | 34.6 (6–61.9) | 39.1 (14–54.7) | 56.1 (25–77) |
| Mean disease duration in years (range) | 17.5 (0.3–41.2) | 19.8 (2.6–64.8) | 28.9 (4.4–60) | 26.1 (2–39.4) | NA |
| Mean HFMSE (range) | 0 (0) | 8 (0–23) | 19 (0–45) | 43 (14–64) | ND |
| Mean MRC sum score (range) | 37 (34–40) | 94 (52–121) | 123 (59–160) | 151 (141–163) | ND |
| SMN2 copy number (n) | |||||
| 2 | 2 | 0 | 0 | 0 | ND |
| 3 | 4 | 17 | 3 | 0 | ND |
| 4 | 0 | 2 | 5 | 5 | ND |
| 5 | 0 | 0 | 0 | 1 | ND |
F = female; M = male; SMN = survival motor neuron; HFMSE: Hammersmith Functional Motor Scale Expanded; MRC = Medical Research Council; ND = not determined; NA = not applicable
a = Three patients with SMA type 1 had survived infancy at time of inclusion
b = One patient had a heterozygous SMN1-deletion and a pathogenic point mutation resulting in stop codon in exon 4
Sample reproducibility
SMN protein levels in PBMCs and fibroblasts showed sample variability, similar to previous reports [22, 33]. Measurements of total protein used for normalization showed an inter-well variation of 4.2% (range 0–19%) and inter-plate variation of 3.2% (range 0.2–8.7%) with a mean day-by-day variation of 7.3% (range 0.7–21.5%). Analyses of inter-well coefficients of variance (CV) ranged from 0.2–26% (mean 5.3%) for SMN protein normalized for total protein levels. Mean inter-plate variability was 10% (range 0.5–100%; median 6.2%). After one extra freeze-thaw cycle, CV ranged from 1–60% within protein samples and CV between plates increased to 40%. Analyses were therefore only performed once after storage, without any extra freeze-thaw episodes to prevent protein changes due to freeze-thaw effects. Overall time in storage at -80°C varied per protein sample (median = 4 months; range 0–33 months).
CV of mRNA expression levels was good (<5%). Mean inter-well variability in expression levels of SMN1, SMN2-FL, and SMN2-Δ7 was 1.1%, 1.2%, and 0.8% respectively in blood and 1.2%, 0.7%, and 0.6% in fibroblast samples. Mean inter-plate variability was 2.1%, 3.0%, and 2.5% respectively for SMN1, SMN2-FL, and SMN2-Δ7 in both cell types (range 1.6–4.4%).
SMN protein analysis
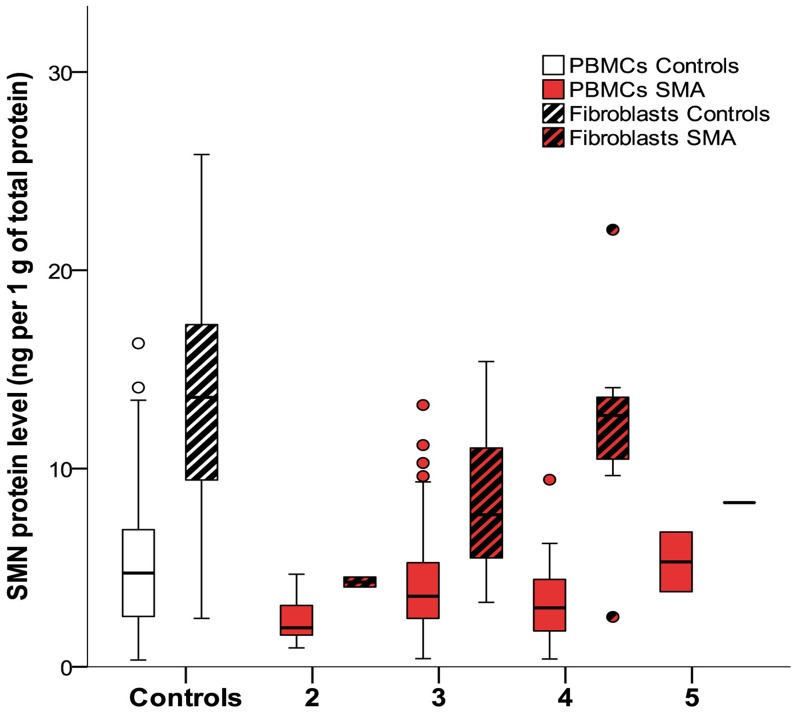
Mean SMN protein levels were higher in controls compared to SMA patients in PBMCs and in fibroblasts (both log regression p<0.01) (Fig 1 and Table 3). There was a trend towards differences in SMN concentrations in PBMCs after stratification for SMN2 copy number (KW p = 0.06). Higher SMN2 copy number was associated with higher levels of SMN protein in fibroblasts (KW p = 0.01) (Fig 1). SMN protein levels did not differ between SMA types (PBMCs KW p = 0.18; fibroblasts KW p = 0.34).
Fig 1. SMN protein levels in PBMCs and fibroblasts from patients and controls and effect of SMN2 copy numbers.
Mean SMN protein levels are higher in controls compared to patients. SMN protein levels in PBMCs did not differ significantly between patients with 2, 3, 4 or 5 SMN2 copies (p = 0.06). Higher SMN2 copy number is associated with higher levels of SMN protein in fibroblasts (p = 0.01). Boxplot elements represent: median (line in the middle), 1st en 3rd quartile (bottom and top of the box), highest case with 1.5 time inter-quartile range (bottom and top whisker) and outliers (dots).
Table 3. Levels of SMN protein in PBMCs and fibroblasts.
| PBMCs | Fibroblasts | |||
|---|---|---|---|---|
| SMA (n = 135) | Controls (n = 229) | SMA (n = 40) | Controls (n = 47) | |
| SMN protein levels* Mean ±SD (range) | 3.7 ± 2.4 (0.4–13.2) | 5.3 ± 3.6 (0.3–18.3) | 8.8 ± 4.3 (2.5–22.1) | 13.4 ± 5.6 (2.4–25.8) |
PBMCs = Peripheral blood mononuclear cells; ND = not determined
* = nanogram per 1 gram total protein
SMA severity reflected by HFMSE score and MRC sum scores did not correlate with SMN protein levels in PBMCs (Spearman’s rho p = 0.15 and p = 0.6 respectively), but did correlate with SMN protein levels in fibroblasts (Spearman’s rho p = 0.004 and p = 0.04).
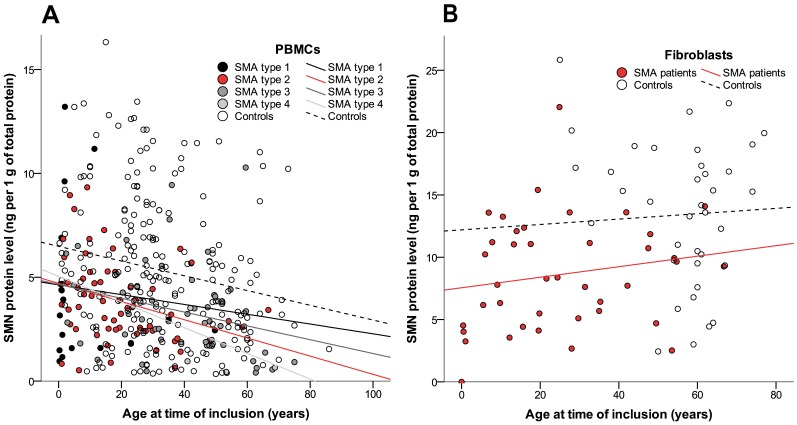
Disease duration and age at time of inclusion correlated inversely with SMN levels in PBMCs (both Spearman’s rho -0.31, p<0.01) (Fig 2A). This correlation between age as well as disease duration, and SMN levels was present in patients and controls (both p<0.01) and persisted when SMA types 2 or 3 were analyzed separately (type 1 Spearman’s rho 0.2, p = 0.4; type 2 Spearman’s rho -0.3, p<0.05; type 3 Spearman’s rho -0.4 p<0.01) (Fig 2A). There was no correlation of SMN levels and age at time of inclusion in fibroblasts (p = 0.43) (Fig 2B).
Fig 2. SMN protein levels in relation to age.
(A) Levels of SMN protein in PBMCs decline with age (p<0.01) in patients and controls (Spearman rho correlation coefficient: patients -0.31; controls -0.21). (B) No correlation between age and SMN protein levels in fibroblasts in patients or controls (p = 0.43).
Paired analysis of SMN levels was possible using PBMCs and fibroblasts from 33 patients with SMA. SMN protein concentrations were higher in fibroblasts than PBMCs (log regression p<0.01). Protein levels in PBMCs and fibroblasts did not correlate (Spearman’s rho p = 0.7).
mRNA expression analysis
Two blood samples from patients with SMA were excluded from analysis due to low quality of RNA (RIN<4), and 8 were excluded because of undetectable mRNA levels of GAPDH and/or 18S.
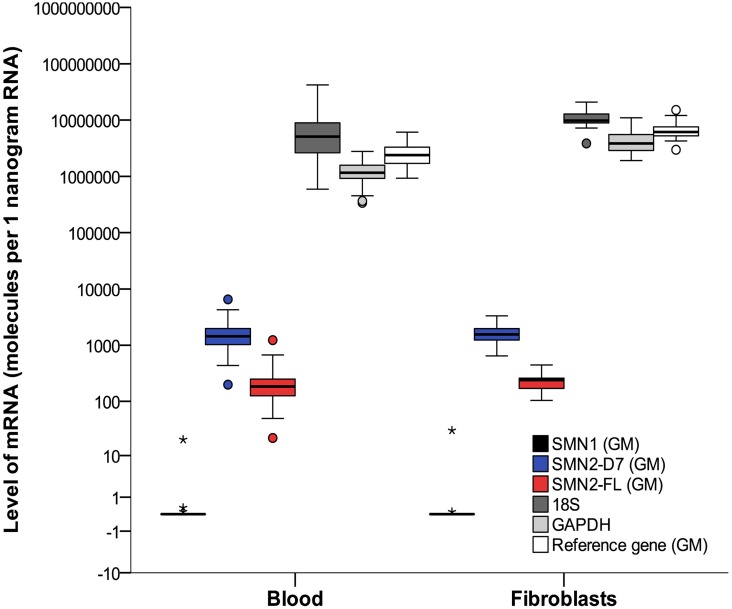
SMN1 mRNA could be detected at low levels in blood and fibroblasts from the one patient with a heterozygous deletion of SMN1 and an additional point mutation in the second allele, but was absent in all other patients (Fig 3).
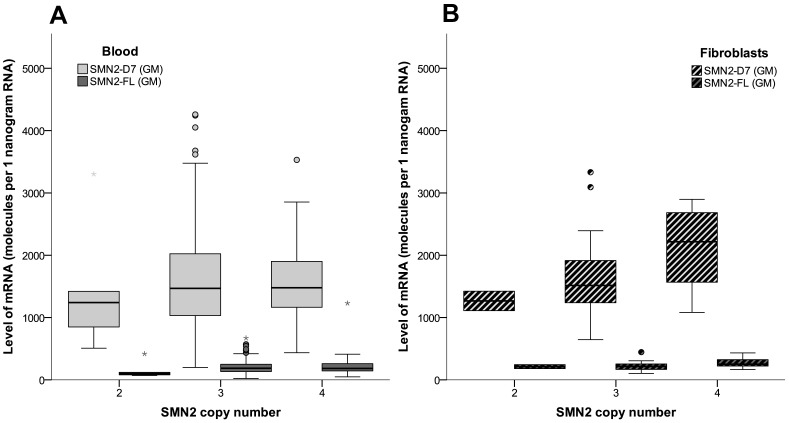
Fig 3. mRNA levels of SMN and reference genes.
Analysis of mRNA was performed in blood and fibroblasts from patients with SMA. Boxplots represent mRNA levels of SMN1, SMN2-FL and SMN2-Δ7 normalized by the geometric mean of the two reference genes (GAPDH and 18S). The reference gene plot (white bar) represents the geometric mean (GM) of GAPDH (light grey bar) and 18S (dark grey bar). For reasons of clarity, individual levels of GAPDH and 18S are presented as well. Levels of SMN2-FL and SMN2-Δ7 did not differ between blood and fibroblasts (p>0.05). One patient had a heterozygote deletion and an additional point mutation of the SMN1 gene, represented by a SMN1-mRNA level of 20 molecules per 1 nanogram of RNA shown by the asterisk (SMN1-levels in 6 controls ranging from 150–350 molecules per 1 nanogram (data not included in this report)). Boxplot elements represent: median (line in the middle), 1st en 3rd quartile (bottom and top of the box), highest case with 1.5 time inter-quartile range (bottom and top whisker) and outliers (dots and asterisks). SMN2-D7 = SMN2-Δ7.
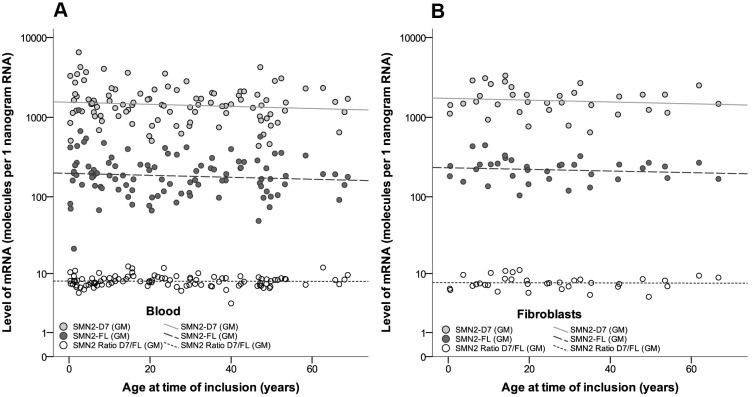
Expression levels of SMN2-FL and SMN2-Δ7 in blood correlated with each other (Spearman’s rho 0.95, p<0.001). There was no effect of gender on expression of SMN2-FL or SMN2-Δ7 (p = 0.3). Levels of SMN2-FL and SMN2-Δ7 did not show a correlation with age at time of inclusion (Fig 4A), SMA type or SMN2 copy number (Fig 5A) in blood (age p = 0.35; SMA type KW p = 0.7; SMN2 copy number KW p = 0.3). Ratios of SMN2-Δ7/SMN2-FL ranged from 4.6 up to 12.5, mostly explained by variation in SMN2-Δ7 transcript levels. Disease severity, reflected by clinical scores (MRC sum score and HFMSE), did not correlate with mRNA expression levels of SMN2-FL or SMN2-Δ7 (p = 0.5 and p = 0.7, respectively).
Fig 4. SMN mRNA transcript levels in blood and fibroblasts from patients with SMA in relation to age.
(A) SMN2 mRNA expression levels in blood from patients with SMA. SMN2-Δ7 levels were significantly higher than SMN2-FL levels. SMN2-FL and SMN2-Δ7 levels in blood did not correlate with age (p = 0.35). (B) SMN2 mRNA expression levels in fibroblasts in patients with SMA. SMN2-Δ7 levels were significantly higher than SMN2-FL levels. Data shown are normalized to geometric mean (= GM) of GAPDH- and 18S-reference genes. SMN2-D7 = SMN2-Δ7.
Fig 5. SMN2 mRNA expression levels in blood and fibroblasts from patients with SMA in relation to SMN2 copy number.
Levels of SMN2-FL and SMN2-Δ7 in relation to SMN2 copy number in blood (Panel A; KW SMN2-FL p = 0.7; KW SMN2-Δ7 p = 0.3) and fibroblasts (Panel B; KW SMN2-FL p = 0.3; KW SMN2-Δ7 p = 0.09) from patients with SMA. Data shown are normalized to the geometric mean (= GM) of GAPDH- and 18S-reference genes. Boxplot elements represent: median (line in the middle), 1st en 3rd quartile (bottom and top of the box), highest case with 1.5 time inter-quartile range (bottom and top whisker) and outliers (dots and asterisks). SMN2-D7 = SMN2-Δ7.
Levels of SMN2-FL and SMN2-Δ7 could be analyzed in fibroblasts from 35 subjects with SMA (Table 4). Levels of SMN2-FL correlated with levels of SMN2-Δ7 (Spearman’s rho 0.74, p<0.001). Ratios of SMN2-Δ7/SMN2-FL ranged from 4.6 to 11. We did not find associations between any of the transcript levels and age (p>0.2; Fig 4B), disease duration (p = 0.4), SMA type (KW p = 0.2), or disease severity reflected by current HFMSE and MRC sum score (p = 0.8 and p = 0.3). Levels of SMN2-FL and SMN2-Δ7 were higher in patients with 4 SMN2 copies compared to 2 or 3 copies, but this was not significant (log regression p = 0.09) (Fig 5B).
Table 4. Levels of SMN mRNA in blood and fibroblasts from patients with SMA.
| Blood | Fibroblasts | |
|---|---|---|
| SMN1* Mean ±SD (range) | 0.4 ± 4.3 (0–20) | 1.1 ± 6.5 (0–29) |
| SMN2-Δ7* Mean ±SD (range) | 1666 ± 1000 (198–6525) | 1745 ± 688 (646–3332) |
| SMN2-FL* Mean ±SD (range) | 219 ± 158 (22–1230) | 231 ± 78 (104–1445) |
ND = not determined
* = Levels presented as molecules per 1 nanogram RNA referenced against the geometric mean of 18S and GAPDH
Paired samples of transcript levels of SMN2 genes and reference genes (GAPDH and 18S) in both blood and fibroblasts were available from 23 patients. Expression levels of SMN2-FL were higher compared to SMN2-Δ7, in blood as well as in fibroblasts (Fig 4A and 4B). Mean levels of SMN2-FL and SMN2-Δ7 did not differ between blood and fibroblasts (log regression p = 0.7; independent t-test p = 0.6) (Table 4). There was no correlation between blood or fibroblast expression levels for the separate transcripts (Spearman’s rho = -0,2; p = 0.50). Correction for age or stratification for SMN2 copy number did not alter results.
Paired analysis of protein and transcript levels in blood was possible in 99 subjects, with 35 samples available for fibroblast analysis. There was no correlation between SMN protein and SMN mRNA expression levels in blood (Spearman’s rho 0.10, p = 0.3 (corrected for age)), nor in fibroblasts (Spearman’s rho 0.10, p = 0.6 (corrected for age)).
Discussion
This is the first comparative study of SMN protein and mRNA levels in PBMCs and fibroblasts in a large cohort of patients with SMA. In addition to the reduced levels of SMN protein and mRNA in patients with SMA, we found an association of SMN2 copy number with SMN protein in fibroblasts only, although we observed a similar trend in PBMCs. There was an age- and disease duration-dependent decline of SMN protein concentrations in PBMCs. Finally, we did not find a correlation of SMN mRNA or protein between blood and fibroblasts, suggesting important expression differences between tissues or cell types.
SMN protein and mRNA levels are obvious biomarker candidates both for disease severity and for efficacy of experimental treatment strategies in SMA. SMN levels have primarily been quantified in blood, first in PBMCs [12, 15, 18, 22, 29], and more recently in whole blood samples [32, 36]. We used the previously described and calibrated SMN-specific qPCR [22, 31, 32] and ELISA [12, 15, 18, 22, 33–35] techniques with minor modifications that previously (and also in our hands) showed good inter- and intra-sample variance. Both techniques offer the advantage of robust high throughput analysis of large numbers of samples in relatively small blood volumes. In contrast to previous studies that often used a single gene as reference (GAPDH [17, 22, 25, 26, 31, 32, 56–58], 18S [29], PKG1 [11, 16, 57], GUSB [17, 29, 57], PPIA [57], HRPLPO [11, 13, 16, 19], Beta-actin [26, 59], MLH1 [60], HPRT [60]), we used the geometric mean of two reference genes (GAPDH and 18S) to quantify SMN mRNA levels. Although this methodological modification complicates comparison between studies, results are less likely to be influenced by random variation in reference gene expression [55, 61]. Ideally, an even larger set of reference genes should be used for reference, but the relatively small blood volumes that can be obtained from the youngest children with SMA obviously complicates this.
Although SMN protein levels have been studied in many (experimental) cell types [12, 15, 18, 20, 22, 26, 29, 32–34, 36–43], there are no comparative studies of SMN expression in tissues that can be easily obtained. Significant differences in SMN protein levels have recently been found in platelets, red blood cells and PBMCs, which underlines the importance to investigate tissue-specific SMN expression [32, 36]. In this study we therefore determined and compared SMN expression in PBMCs and skin-biopsy derived fibroblasts. We found reduced levels of SMN mRNA and protein in both PBMCs and fibroblasts from patients with SMA compared to healthy controls. In line with previous observations [12, 18, 22, 29, 31, 33, 34] there was no association of SMN protein or mRNA levels in blood with SMA type [15, 18, 22, 29, 31–34], although there was a trend towards an association of SMN protein with SMN2 copy number. Despite the significantly smaller sample size of fibroblasts compared to PBMCs, we found a correlation of SMN protein levels with SMN2 copy number in fibroblasts, and also with clinical characteristics such as MRC sum and HFMSE scores. Our data therefore suggest that skin-derived fibroblasts may be a more robust cell type for SMN biomarker studies. The fibroblast study may have been underpowered to show a correlation with SMA type, since this is, although not perfectly, associated with SMN2 copy number.
The lack of correlation of SMN levels between PBMCs and fibroblasts suggests important expression differences between tissues. This may be explained by differences in SMN concentrations required for normal development and function of specific cell types, and may for example be explained by variation in epigenetic modifications in stem cells or germ layers [62]. However, highly related cell types may have significantly different SMN protein levels, as shown by two recent studies using a new electrochemiluminescence (ECL) assay to detect SMN levels in whole blood [32, 36]. In these studies, platelets and red blood cells contributed most to SMN levels in whole blood (both cell types accounted for 40%), whereas SMN levels in PBMCs were relatively low (20% of total SMN) [32, 36].
Optimizing SMN quantification techniques is important for future clinical trials of SMN enhancing therapies, since findings in animal models for SMA suggest improved outcome upon increased peripheral SMN expression [43]. It has been suggested that the recently developed ECL has higher sensitivity to detect relevant differences in SMN expression, for example between patients with varying SMN2 copy numbers or tissues, but this needs to be shown in comparative studies with an adequate sample size. ECL in whole blood may have the advantage of more straightforward sample processing that could reduce variation caused by PBMC processing methods[18, 33, 63, 64], storage conditions[34, 65] and extraction and lysis reagents [22, 33]. We rigorously applied predefined protocols to keep this variation limited. It was also recorded whether patients recently had a viral infection, since this may also cause variation in SMN levels [34, 65–67].
Our data show an age dependent decline of SMN protein levels in PBMCs in both patients with SMA and healthy controls. This confirms previous preliminary data from 2 studies including a total of 49 children and adults that suggested an effect of aging on SMN protein levels in PBMCs [34] and whole blood [32]. Meta- analysis of these studies with our results is not possible due to methodological differences, such as variation in laboratory techniques and patient characteristics, including SMA type, age-range, and the inclusion of data that reflect clinical severity. There are several explanations for this observed decline. Reduced SMN expression may be a feature of normal aging. Age-specific differences in SMN expression levels in humans have been reported previously. SMN expression is probably highest in the embryonic period and declines after birth [43, 68, 69]. It is not known whether a continuing decline with age could contribute to the slow deterioration of motor function that has been observed in adult patients[70, 71]. Another explanation may lie in changes in the relative PBMC composition during life [72]. We cannot exclude the possibility that a relative decline of specific mononuclear cells with high SMN expression in the course of life underlies our findings.
The strengths of our study are the size of both patient and control groups, the wide range of age and disease severity and the detailed clinical data, and the novel comparative approach. An apparent weakness of this study is the cross-sectional design that does not allow investigation of the individual rate of decline in SMN protein or expression levels. Future longitudinal studies should attempt to address changes in SMN expression in relation to age in individual patients and explore the added value of the ECL technique, ideally in a comparative study of whole blood, PBMCs and fibroblasts.
Supporting Information
(PDF)
Acknowledgments
We are grateful to the patients with SMA who participated in this study and the support of the Dutch patient organization for neuromuscular diseases (VSN).
The authors wish to thank Dr J. de Graaff, Department of Pediatric Anesthesia at the University Medical Centre Utrecht, and Dr. B.C.M.S. Timmers, Department of Pediatrics at the University Medical Centre Utrecht, for their help with the inclusion of healthy controls.
This study was made possible by the commitment of and referrals by the Dutch SMA study group (N. van Alfen, L. Bok, N. Cobben, I.F.M. de Coo, M. Dousma, B. G.M. van Engelen, J.M. Fock, I.J.M. de Groot, W.G.M. Janssen, M. J. Kampelmacher, R. Koers, I. Kortland, E.T. Kruitwagen, V.J. Langenhorst, P.W.A. Muitjens, J. Nicolai, J.M.F. Niermeijer, E.H. Niks, M. Nuysink, R.G. van Ommen-Koolmees, E.A.J. Peeters, L.T.L. Sie, I. Snoeck, M.W. van Steenbergen, M.J. van Tol-Jager, A.A.P.H. Vaessen-Verberne, A.D. van Velzen, J.J.G.M. Verschuuren, M. Vugts, M.E.J. Wegdam-den Boer, P.J. Wijkstra, M. Wohlgemuth)
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by the Prinses Beatrix Spierfonds (WAR008; https://www.prinsesbeatrixspierfonds.nl/onderzoek/) and Stichting Spieren voor Spieren (https://www.spierenvoorspieren.nl). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–65. [DOI] [PubMed] [Google Scholar]
- 2.Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci U S A. 1999;96(11):6307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wirth B, Garbes L, Riessland M. How genetic modifiers influence the phenotype of spinal muscular atrophy and suggest future therapeutic approaches. Curr Opin Genet Dev. 2013;23(3):330–8. 10.1016/j.gde.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 4.Fallini C, Zhang H, Su Y, Silani V, Singer RH, Rossoll W, et al. The survival of motor neuron (SMN) protein interacts with the mRNA-binding protein HuD and regulates localization of poly(A) mRNA in primary motor neuron axons. J Neurosci. 2011;31(10):3914–25. 10.1523/JNEUROSCI.3631-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao DY, Gish G, Braunschweig U, Li Y, Ni Z, Schmitges FW, et al. SMN and symmetric arginine dimethylation of RNA polymerase II C-terminal domain control termination. Nature. 2016;529(7584):48–53. 10.1038/nature16469 [DOI] [PubMed] [Google Scholar]
- 6.Pellizzoni L, Yong J, Dreyfuss G. Essential role for the SMN complex in the specificity of snRNP assembly. Science. 2002;298(5599):1775–9. 10.1126/science.1074962 [DOI] [PubMed] [Google Scholar]
- 7.Akten B, Kye MJ, Hao le T, Wertz MH, Singh S, Nie D, et al. Interaction of survival of motor neuron (SMN) and HuD proteins with mRNA cpg15 rescues motor neuron axonal deficits. Proc Natl Acad Sci U S A. 2011;108(25):10337–42. 10.1073/pnas.1104928108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossoll W, Jablonka S, Andreassi C, Kroning AK, Karle K, Monani UR, et al. Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons. J Cell Biol. 2003;163(4):801–12. 10.1083/jcb.200304128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wishart TM, Mutsaers CA, Riessland M, Reimer MM, Hunter G, Hannam ML, et al. Dysregulation of ubiquitin homeostasis and beta-catenin signaling promote spinal muscular atrophy. J Clin Invest. 2014;124(4):1821–34. 10.1172/JCI71318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burghes AH, Beattie CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci. 2009;10(8):597–609. 10.1038/nrn2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swoboda KJ, Scott CB, Crawford TO, Simard LR, Reyna SP, Krosschell KJ, et al. SMA CARNI-VAL trial part I: double-blind, randomized, placebo-controlled trial of L-carnitine and valproic acid in spinal muscular atrophy. PLoS One. 2010;5(8):e12140 10.1371/journal.pone.0012140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiziano FD, Lomastro R, Di Pietro L, Barbara Pasanisi M, Fiori S, Angelozzi C, et al. Clinical and molecular cross-sectional study of a cohort of adult type III spinal muscular atrophy patients: clues from a biomarker study. Eur J Hum Genet. 2013;21(6):630–6. 10.1038/ejhg.2012.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kissel JT, Scott CB, Reyna SP, Crawford TO, Simard LR, Krosschell KJ, et al. SMA CARNIVAL TRIAL PART II: a prospective, single-armed trial of L-carnitine and valproic acid in ambulatory children with spinal muscular atrophy. PLoS One. 2011;6(7):e21296 10.1371/journal.pone.0021296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen TH, Chang JG, Yang YH, Mai HH, Liang WC, Wu YC, et al. Randomized, double-blind, placebo-controlled trial of hydroxyurea in spinal muscular atrophy. Neurology. 2010;75(24):2190–7. 10.1212/WNL.0b013e3182020332 [DOI] [PubMed] [Google Scholar]
- 15.Tiziano FD, Lomastro R, Pinto AM, Messina S, D'Amico A, Fiori S, et al. Salbutamol increases survival motor neuron (SMN) transcript levels in leucocytes of spinal muscular atrophy (SMA) patients: relevance for clinical trial design. J Med Genet. 2010;47(12):856–8. 10.1136/jmg.2010.080366 [DOI] [PubMed] [Google Scholar]
- 16.Swoboda KJ, Scott CB, Reyna SP, Prior TW, LaSalle B, Sorenson SL, et al. Phase II open label study of valproic acid in spinal muscular atrophy. PLoS One. 2009;4(5):e5268 10.1371/journal.pone.0005268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumner CJ, Huynh TN, Markowitz JA, Perhac JS, Hill B, Coovert DD, et al. Valproic acid increases SMN levels in spinal muscular atrophy patient cells. Ann Neurol. 2003;54(5):647–54. 10.1002/ana.10743 [DOI] [PubMed] [Google Scholar]
- 18.Piepers S, Cobben JM, Sodaar P, Jansen MD, Wadman RI, Meester-Delver A, et al. Quantification of SMN protein in leucocytes from spinal muscular atrophy patients: effects of treatment with valproic acid. J Neurol Neurosurg Psychiatry. 2011;82(8):850–2. 10.1136/jnnp.2009.200253 [DOI] [PubMed] [Google Scholar]
- 19.Kissel JT, Elsheikh B, King WM, Freimer M, Scott CB, Kolb SJ, et al. SMA valiant trial: a prospective, double-blind, placebo-controlled trial of valproic acid in ambulatory adults with spinal muscular atrophy. Muscle Nerve. 2014;49(2):187–92. 10.1002/mus.23904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patrizi AL, Tiziano F, Zappata S, Donati MA, Neri G, Brahe C. SMN protein analysis in fibroblast, amniocyte and CVS cultures from spinal muscular atrophy patients and its relevance for diagnosis. Eur J Hum Genet. 1999;7(3):301–9. 10.1038/sj.ejhg.5200286 [DOI] [PubMed] [Google Scholar]
- 21.Martinez TL, Kong L, Wang X, Osborne MA, Crowder ME, Van Meerbeke JP, et al. Survival motor neuron protein in motor neurons determines synaptic integrity in spinal muscular atrophy. J Neurosci. 2012;32(25):8703–15. 10.1523/JNEUROSCI.0204-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford TO, Paushkin SV, Kobayashi DT, Forrest SJ, Joyce CL, Finkel RS, et al. Evaluation of SMN protein, transcript, and copy number in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS One. 2012;7(4):e33572 Epub 2012/05/05. 10.1371/journal.pone.0033572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garbes L, Heesen L, Holker I, Bauer T, Schreml J, Zimmermann K, et al. VPA response in SMA is suppressed by the fatty acid translocase CD36. Hum Mol Genet. 2013;22(2):398–407. [DOI] [PubMed] [Google Scholar]
- 24.Coovert DD, Le TT, McAndrew PE, Strasswimmer J, Crawford TO, Mendell JR, et al. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet. 1997;6(8):1205–14. [DOI] [PubMed] [Google Scholar]
- 25.Brichta L, Holker I, Haug K, Klockgether T, Wirth B. In vivo activation of SMN in spinal muscular atrophy carriers and patients treated with valproate. Ann Neurol. 2006;59(6):970–5. 10.1002/ana.20836 [DOI] [PubMed] [Google Scholar]
- 26.Also-Rallo E, Alias L, Martinez-Hernandez R, Caselles L, Barcelo MJ, Baiget M, et al. Treatment of spinal muscular atrophy cells with drugs that upregulate SMN expression reveals inter- and intra-patient variability. Eur J Hum Genet. 2011;19(10):1059–65. 10.1038/ejhg.2011.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arakawa M, Arakawa R, Tatsumi S, Aoki R, Saito K, Nomoto A. A novel evaluation method of survival motor neuron protein as a biomarker of spinal muscular atrophy by imaging flow cytometry. Biochem Biophys Res Commun. 2014;453(3):368–74. 10.1016/j.bbrc.2014.09.087 [DOI] [PubMed] [Google Scholar]
- 28.Arakawa R, Arakawa M, Kaneko K, Otsuki N, Aoki R, Saito K. Imaging Flow Cytometry Analysis to Identify Differences of Survival Motor Neuron Protein Expression in Patients With Spinal Muscular Atrophy. Pediatr Neurol. 2016;61:70–5. 10.1016/j.pediatrneurol.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 29.Sumner CJ, Kolb SJ, Harmison GG, Jeffries NO, Schadt K, Finkel RS, et al. SMN mRNA and protein levels in peripheral blood: biomarkers for SMA clinical trials. Neurology. 2006;66(7):1067–73. 10.1212/01.wnl.0000201929.56928.13 [DOI] [PubMed] [Google Scholar]
- 30.Kolb SJ, Battle DJ, Dreyfuss G. Molecular functions of the SMN complex. J Child Neurol. 2007;22(8):990–4. 10.1177/0883073807305666 [DOI] [PubMed] [Google Scholar]
- 31.Tiziano FD, Pinto AM, Fiori S, Lomastro R, Messina S, Bruno C, et al. SMN transcript levels in leukocytes of SMA patients determined by absolute real-time PCR. Eur J Hum Genet. 2010;18(1):52–8. 10.1038/ejhg.2009.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czech C, Tang W, Bugawan T, Mano C, Horn C, Iglesias VA, et al. Biomarker for Spinal Muscular Atrophy: Expression of SMN in Peripheral Blood of SMA Patients and Healthy Controls. PLoS One. 2015;10(10):e0139950 10.1371/journal.pone.0139950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi DT, Olson RJ, Sly L, Swanson CJ, Chung B, Naryshkin N, et al. Utility of survival motor neuron ELISA for spinal muscular atrophy clinical and preclinical analyses. PLoS One. 2011;6(8):e24269 10.1371/journal.pone.0024269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi DT, Decker D, Zaworski P, Klott K, McGonigal J, Ghazal N, et al. Evaluation of peripheral blood mononuclear cell processing and analysis for Survival Motor Neuron protein. PLoS One. 2012;7(11):e50763 10.1371/journal.pone.0050763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen thi M, Humphrey E, Lam LT, Fuller HR, Lynch TA, Sewry CA, et al. A two-site ELISA can quantify upregulation of SMN protein by drugs for spinal muscular atrophy. Neurology. 2008;71(22):1757–63. 10.1212/01.wnl.0000313038.34337.b1 [DOI] [PubMed] [Google Scholar]
- 36.Zaworski P, von Herrmann KM, Taylor S, Sunshine SS, McCarthy K, Risher N, et al. SMN Protein Can Be Reliably Measured in Whole Blood with an Electrochemiluminescence (ECL) Immunoassay: Implications for Clinical Trials. PLoS One. 2016;11(3):e0150640 10.1371/journal.pone.0150640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinkellner H, Etzler J, Gmeiner BM, Laccone F. Detection of survival motor neuron protein in buccal cells through electrochemiluminescence-based assay. Assay Drug Dev Technol. 2015;13(3):167–73. 10.1089/adt.2015.635 [DOI] [PubMed] [Google Scholar]
- 38.Chang JG, Hsieh-Li HM, Jong YJ, Wang NM, Tsai CH, Li H. Treatment of spinal muscular atrophy by sodium butyrate. Proc Natl Acad Sci U S A. 2001;98(17):9808–13. 10.1073/pnas.171105098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyer JG, Deguise MO, Murray LM, Yazdani A, De Repentigny Y, Boudreau-Lariviere C, et al. Myogenic program dysregulation is contributory to disease pathogenesis in spinal muscular atrophy. Hum Mol Genet. 2014;23(16):4249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowerman M, Swoboda KJ, Michalski JP, Wang GS, Reeks C, Beauvais A, et al. Glucose metabolism and pancreatic defects in spinal muscular atrophy. Ann Neurol. 2012;72(2):256–68. Epub 2012/08/29. 10.1002/ana.23582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rindt H, Feng Z, Mazzasette C, Glascock JJ, Valdivia D, Pyles N, et al. Astrocytes influence the severity of spinal muscular atrophy. Hum Mol Genet. 2015;24(14):4094–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter G, Aghamaleky Sarvestany A, Roche SL, Symes RC, Gillingwater TH. SMN-dependent intrinsic defects in Schwann cells in mouse models of spinal muscular atrophy. Hum Mol Genet. 2014;23(9):2235–50. [DOI] [PubMed] [Google Scholar]
- 43.Burlet P, Huber C, Bertrandy S, Ludosky MA, Zwaenepoel I, Clermont O, et al. The distribution of SMN protein complex in human fetal tissues and its alteration in spinal muscular atrophy. Hum Mol Genet. 1998;7(12):1927–33. [DOI] [PubMed] [Google Scholar]
- 44.Chiriboga CA, Swoboda KJ, Darras BT, Iannaccone ST, Montes J, De Vivo DC, et al. Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology. 2016;86(10):890–7. 10.1212/WNL.0000000000002445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton G, Gillingwater TH. Spinal muscular atrophy: going beyond the motor neuron. Trends Mol Med. 2013;19(1):40–50. 10.1016/j.molmed.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 46.Zerres K, Rudnik-Schoneborn S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol. 1995;52(5):518–23. [DOI] [PubMed] [Google Scholar]
- 47.Munsat TL, Davies KE. International SMA consortium meeting. (26–28 June 1992, Bonn, Germany). Neuromuscul Disord. 1992;2(5–6):423–8. [DOI] [PubMed] [Google Scholar]
- 48.Mercuri E, Bertini E, Iannaccone ST. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol. 2012;11(5):443–52. Epub 2012/04/21. 10.1016/S1474-4422(12)70061-3 [DOI] [PubMed] [Google Scholar]
- 49.Dubowitz V. Very severe spinal muscular atrophy (SMA type 0): an expanding clinical phenotype. Eur J Paediatr Neurol. 1999;3(2):49–51. Epub 2000/03/04. [DOI] [PubMed] [Google Scholar]
- 50.Rudnik-Schoneborn S, Berg C, Zerres K, Betzler C, Grimm T, Eggermann T, et al. Genotype-phenotype studies in infantile spinal muscular atrophy (SMA) type I in Germany: implications for clinical trials and genetic counselling. Clin Genet. 2009;76(2):168–78. Epub 2009/09/29. 10.1111/j.1399-0004.2009.01200.x [DOI] [PubMed] [Google Scholar]
- 51.Petit F, Cuisset JM, Rouaix-Emery N, Cances C, Sablonniere B, Bieth E, et al. Insights into genotype-phenotype correlations in spinal muscular atrophy: a retrospective study of 103 patients. Muscle Nerve. 2011;43(1):26–30. Epub 2010/12/21. 10.1002/mus.21832 [DOI] [PubMed] [Google Scholar]
- 52.Dubowitz V. Chaos in classification of the spinal muscular atrophies of childhood. Neuromuscul Disord. 1991;1(2):77–80. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 53.Cobben JM, Lemmink HH, Snoeck I, Barth PA, van der Lee JH, de Visser M. Survival in SMA type I: a prospective analysis of 34 consecutive cases. Neuromuscul Disord. 2008;18(7):541–4. Epub 2008/06/27. 10.1016/j.nmd.2008.05.008 [DOI] [PubMed] [Google Scholar]
- 54.Glanzman AM, O'Hagen JM, McDermott MP, Martens WB, Flickinger J, Riley S, et al. Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J Child Neurol. 2011;26(12):1499–507. Epub 2011/09/24. 10.1177/0883073811420294 [DOI] [PubMed] [Google Scholar]
- 55.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brahe C, Vitali T, Tiziano FD, Angelozzi C, Pinto AM, Borgo F, et al. Phenylbutyrate increases SMN gene expression in spinal muscular atrophy patients. Eur J Hum Genet. 2005;13(2):256–9. 10.1038/sj.ejhg.5201320 [DOI] [PubMed] [Google Scholar]
- 57.Simard LR, Belanger MC, Morissette S, Wride M, Prior TW, Swoboda KJ. Preclinical validation of a multiplex real-time assay to quantify SMN mRNA in patients with SMA. Neurology. 2007;68(6):451–6. 10.1212/01.wnl.0000252934.70676.ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreassi C, Angelozzi C, Tiziano FD, Vitali T, De Vincenzi E, Boninsegna A, et al. Phenylbutyrate increases SMN expression in vitro: relevance for treatment of spinal muscular atrophy. Eur J Hum Genet. 2004;12(1):59–65. 10.1038/sj.ejhg.5201102 [DOI] [PubMed] [Google Scholar]
- 59.Jong YJ, Chang JG, Lin SP, Yang TY, Wang JC, Chang CP, et al. Analysis of the mRNA transcripts of the survival motor neuron (SMN) gene in the tissue of an SMA fetus and the peripheral blood mononuclear cells of normals, carriers and SMA patients. J Neurol Sci. 2000;173(2):147–53. [DOI] [PubMed] [Google Scholar]
- 60.Vezain M, Saugier-Veber P, Melki J, Toutain A, Bieth E, Husson M, et al. A sensitive assay for measuring SMN mRNA levels in peripheral blood and in muscle samples of patients affected with spinal muscular atrophy. Eur J Hum Genet. 2007;15(10):1054–62. 10.1038/sj.ejhg.5201885 [DOI] [PubMed] [Google Scholar]
- 61.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25(2):169–93. [DOI] [PubMed] [Google Scholar]
- 62.Hauke J, Riessland M, Lunke S, Eyupoglu IY, Blumcke I, El-Osta A, et al. Survival motor neuron gene 2 silencing by DNA methylation correlates with spinal muscular atrophy disease severity and can be bypassed by histone deacetylase inhibition. Hum Mol Genet. 2009;18(2):304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnes MG, Grom AA, Griffin TA, Colbert RA, Thompson SD. Gene Expression Profiles from Peripheral Blood Mononuclear Cells Are Sensitive to Short Processing Delays. Biopreserv Biobank. 2010;8(3):153–62. 10.1089/bio.2010.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torrecilla E, Gonzalez-Munoz M, Lahoz C, Mostaza J. Time-dependent changes in the expression of lymphocyte and monocyte cell adhesion molecules after meals of different composition. Br J Nutr. 2010;104(11):1650–4. 10.1017/S0007114510002710 [DOI] [PubMed] [Google Scholar]
- 65.Hunter G, Roche SL, Somers E, Fuller HR, Gillingwater TH. The influence of storage parameters on measurement of survival motor neuron (SMN) protein levels: implications for pre-clinical studies and clinical trials for spinal muscular atrophy. Neuromuscul Disord. 2014;24(11):973–7. 10.1016/j.nmd.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 66.Kleeberger CA, Lyles RH, Margolick JB, Rinaldo CR, Phair JP, Giorgi JV. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin Diagn Lab Immunol. 1999;6(1):14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mallone R, Mannering SI, Brooks-Worrell BM, Durinovic-Bello I, Cilio CM, Wong FS, et al. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin Exp Immunol. 2011;163(1):33–49. 10.1111/j.1365-2249.2010.04272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabanella F, Carissimi C, Usiello A, Pellizzoni L. The activity of the spinal muscular atrophy protein is regulated during development and cellular differentiation. Hum Mol Genet. 2005;14(23):3629–42. [DOI] [PubMed] [Google Scholar]
- 69.Giavazzi A, Setola V, Simonati A, Battaglia G. Neuronal-specific roles of the survival motor neuron protein: evidence from survival motor neuron expression patterns in the developing human central nervous system. J Neuropathol Exp Neurol. 2006;65(3):267–77. 10.1097/01.jnen.0000205144.54457.a3 [DOI] [PubMed] [Google Scholar]
- 70.Deymeer F, Serdaroglu P, Parman Y, Poda M. Natural history of SMA IIIb: muscle strength decreases in a predictable sequence and magnitude. Neurology. 2008;71(9):644–9. Epub 2008/08/30. 10.1212/01.wnl.0000324623.89105.c4 [DOI] [PubMed] [Google Scholar]
- 71.Piepers S, van den Berg LH, Brugman F, Scheffer H, Ruiterkamp-Versteeg M, van Engelen BG, et al. A natural history study of late onset spinal muscular atrophy types 3b and 4. J Neurol. 2008;255(9):1400–4. Epub 2008/06/26. 10.1007/s00415-008-0929-0 [DOI] [PubMed] [Google Scholar]
- 72.Shahabuddin S, Al-Ayed I, Gad El-Rab MO, Qureshi MI. Age-related changes in blood lymphocyte subsets of Saudi Arabian healthy children. Clin Diagn Lab Immunol. 1998;5(5):632–5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper.