Abstract
Objective
To evaluate long-term results of aortic root procedures combined with ascending aorta replacement for aneurysms, using 4 surgical strategies.
Methods
From January 1995 to January 2011, 957 patients underwent 1 of 4 aortic root procedures: valve preservation (remodeling or modified reimplantation, n = 261); composite biologic graft (n = 297); composite mechanical graft (n = 156); or allograft root (n = 243).
Results
Seven deaths occurred (0.73%), none after valve-preserving procedures, and 13 strokes (1.4%). Composite grafts exhibited higher gradients than allografts or valve preservation, but the latter 2 exhibited more aortic regurgitation (2.7% biologic and 0% mechanical composite grafts vs 24% valve-preserving and 19%allografts at 10 years). Within 2 to 5 years, valve preservation exhibited the least left ventricular hypertrophy, allograft replacement the greatest; however, valve preservation had the highest early risk of reoperation, allograft replacement the lowest. Patients receiving allografts had the highest risk of late reoperation (P<05), and those receiving composite mechanical grafts and valve preservation had the lowest. Composite bioprosthesis patients had the highest risk of late death (57%at 15 years vs 14%-26%for the remaining procedures, P<.0001), because they were substantially older and had more comorbidities (P<.0001).
Conclusions
These 4 aortic root procedures, combined with ascending aorta replacement, provide excellent survival and good durability. Valve-preserving and allograft procedures have the lowest gradients and best ventricular remodeling, but they have more late regurgitation, and likely, less risk of valve-related complications, such as bleeding, hemorrhage, and endocarditis. Despite the early risk of reoperation, we recommend valve-preserving procedures for young patients when possible. Composite bioprostheses are preferable for the elderly.
Keywords: aorta, root, aortic valve, root sparing, valve preservation
Graphical Abstract
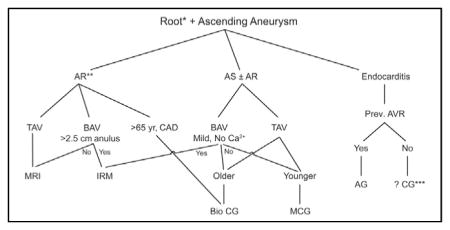
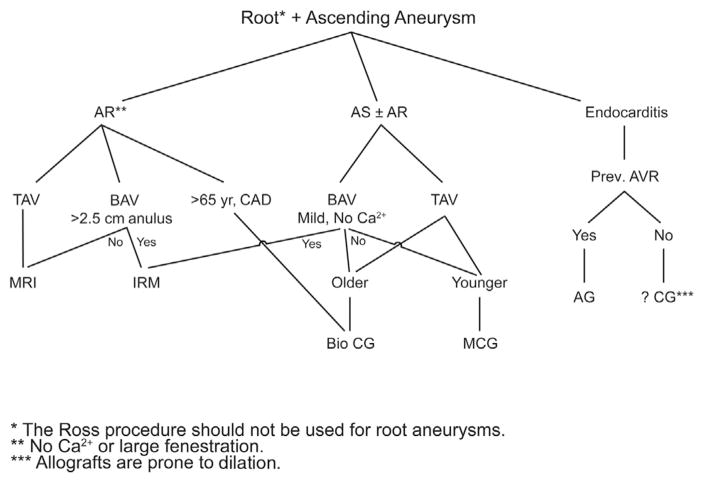
Current Cleveland Clinic treatment algorithm for root and ascending aortic aneurysm.
In the past, a Bentall operation, which incorporates an artificial aortic valve within an ascending aorta tube graft, was the standard treatment for various combined aortic root and ascending aorta pathologies.1 Initially, the valve was mechanical (“composite grafts”), but bioprostheses were eventually introduced,2 as were allografts,3 for combined aortic root and ascending aorta replacement. Over the past 25 years, David and colleagues4 have championed a variety of valve-preserving techniques. It remains uncertain, however, which of these techniques—older or newer—is the right one for the right patient at the right time.5–7
In a previous comparison of appropriate root procedures (modified reimplantation for tricuspid aortic valves and remodeling for bicuspid ones) versus a biologic composite valve, we showed that modified reimplantation exhibited superior durability after 9 to 10 years, compared with remodeling, which showed better durability after 10 to 12 years.5 Furthermore, bicuspid valve repair—40% combined with aortic surgery—carried a 0.47% risk of hospital death and a 0.25% risk of stroke, and long-term durability improved over time with newer techniques, such as higher commissure implantation.8 However, how reparative procedures compare with mechanical and biologic graft root replacement alternatives in the long term remains unclear.6,9
The present study goes beyond our previous reports, to examine our experience over the past 20 years with aortic root procedures combined with ascending aorta replacement. All of the patients in the study were managed with (1) valve preservation (remodeling or modified reimplantation); (2) biologic valve composite grafts; (3) mechanical valve composite grafts; or (4) allograft root and ascending aorta replacement with coronary reimplantation. On the basis of long-term outcomes and surveillance in an era that favors reparative techniques, do mechanical and biologic composite grafts and allografts still have a place? If so, in what kind of patient, at what time?
METHODS
Patients
From January 1995 to January 2011, 957 patients underwent 1 of 4 aortic root procedures for aneurysms of the root and ascending aorta: (1) valve preservation (n = 261; remodeling [n = 56] or reimplantation [n = 205]); (2) composite biologic graft (n = 297); (3) composite mechanical graft (n = 156); or (4) allograft root (n = 243). Patients who underwent emergency surgery, had endocarditis or acute aortic dissection, or did not have an ascending aorta replacement were excluded.
Operative Techniques
The operative techniques have been described before for the root part of the procedure, including a L.G.S.-modified valve reimplantation technique using pledgets, sizing to body surface area, and Hegar’s dilators5,10; an inclusion type of remodeling of the root6; composite mechanical valve implantation, including with a tube graft to the left main coronary artery (which we now use primarily for patients with acute dissection or who have undergone reoperation11,12); standard techniques for biologic implants with coronary buttons; and allograft root implantation by the inclusion or button technique. In 234 patients (24%), circulatory arrest was used for concomitant aortic arch replacement (Table 1).
TABLE 1.
Procedural characteristics stratified by aortic root procedure
| Characteristic | Valve preservation (n = 261)
|
Composite graft
|
Allograft (n = 243)
|
P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Biologic (n = 297)
|
Mechanical (n = 156)
|
||||||||
| n* | No. (%) or mean ± SD | n* | No. (%) or mean ± SD | n* | No. (%) or mean ± SD | n* | No. (%) or mean ± SD | ||
| Approach | |||||||||
| Full sternotomy | 181 | 174 (96) | 178 | 159 (89) | 113 | 105 (93) | 174 | 135 (78) | <.0001 |
| Partial upper J-incision | 181 | 5 (2.8) | 178 | 18 (10) | 113 | 8 (7.1) | 174 | 36 (21) | <.0001 |
| Partial converted to full sternotomy | 181 | 2 (1.1) | 178 | 1 (0.56) | 113 | 0 (0) | 174 | 3 (1.7) | .5 |
| Support | |||||||||
| Circulatory arrest | 261 | 41 (16) | 297 | 89 (30) | 156 | 42 (27) | 243 | 62 (26) | .001 |
| Circulatory arrest time (min) | 41 | 17 ± 9.8 | 89 | 16 ± 8.6 | 42 | 14 ± 5.0 | 62 | 16 ± 6.4 | .2 |
| Aortic clamp time (min) | 261 | 109 ± 28 | 297 | 99 ± 34 | 156 | 95 ± 33 | 243 | 98 ± 31 | <.0001 |
| CPB time (min) | 261 | 134 ± 37 | 297 | 123 ± 45 | 156 | 120 ± 43 | 243 | 120 ± 41 | <.0001 |
| Concomitant procedures | |||||||||
| Mitral valve surgery | 261 | 19 (7.3) | 297 | 20 (6.7) | 156 | 11 (7.1) | 243 | 12 (4.9) | .7 |
| Repair | 261 | 19 (7.3) | 297 | 16 (5.4) | 156 | 6 (3.8) | 243 | 10 (4.1) | .3 |
| Replacement | 261 | 0 (0) | 297 | 4 (1.3) | 156 | 5 (3.2) | 243 | 2 (0.82) | .03 |
| Tricuspid valve repair | 261 | 2 (0.77) | 297 | 7 (2.4) | 156 | 1 (0.64) | 243 | 2 (0.82) | .2 |
| Coronary artery bypass grafting | 261 | 23 (8.8) | 297 | 99 (33) | 156 | 22 (14) | 243 | 41 (17) | <.0001 |
| Atrial fibrillation procedure | 261 | 7 (2.7) | 297 | 23 (7.7) | 156 | 3 (1.9) | 243 | 10 (4.1) | .008 |
SD, Standard deviation; CPB, cardiopulmonary bypass.
Patients with data available.
Data
Data were collected prospectively and entered into our Cardiovascular Information Registry. Use of these data for research was approved by the Cleveland Clinic Institutional Review Board, with requirements for patient consent waived.
Endpoints
Study endpoints were (1) in-hospital postoperative morbidity and mortality; (2) time-related aortic valve function (assessed by gradients and regurgitation on longitudinal echocardiograms); (3) left ventricular reverse remodeling, assessed by left ventricular mass on longitudinal echocardiograms; (4) aortic valve and aorta-related reoperations; and (5) short- and long-term mortality.
Longitudinal echocardiographic data for aortic valve function and left ventricular reverse remodeling obtained at follow-up were extracted from our echocardiogram database to ascertain valve function. However, surveillance echocardiograms were available only in patients whowere followed at Cleveland Clinic. Previously, we have shown that these patients constitute a representative sample, one not confounded by return of patients for aortic problems.13 Few statistically significant differences were found between patients surveilled or not at Cleveland Clinic (data not shown).
A total of 1626 echocardiograms, performed on 718 patients (75%), were available for analysis in the postoperative period (Figure E1). Time-related survival and aortic-related reoperations were obtained from yearly follow-up questionnaires (with phone follow-up if questionnaires were not returned). Follow-up was available for 943 patients (98%). The median follow-up time was 5.3 years (mean, 5.6 ± 4.6 years), with 5351 patient-years of data available for analysis; 25%of patients were followed for>9 years, and 5% for>15 years.
Data Analysis
The following outline of our data analysis is presented in detail in Appendix E1. To reduce bias in comparing outcomes among groups, 4 propensity scores were generated for each patient and forced into models of outcome. The temporal patterns of follow-up echocardiographic measures were estimated using longitudinal data analysis, with risk-adjusted comparisons made by including propensity scores in the models. Risks of reoperation and death were estimated by the Kaplan-Meier method, and a nonproportional hazards model was used to identify risk-adjusted mortality differences.
Presentation
Continuous variables are summarized as mean ± standard deviation, or as equivalent 15th, 50th (median), and 85th percentiles when the distribution of values was skewed. Categoric data are summarized as frequencies and percentages. Uncertainty is expressed by confidence limits equivalent to ±1 standard error (68%). Comparison of groups was done with the Kruskal-Wallis nonparametric test for continuous variables and the χ2 test for categoric data.
RESULTS
Patient Characteristics and Procedural Details
Patients in the valve-preserving and mechanical composite valve groups were the youngest, and those receiving a biologic composite graft were the oldest (Table E1). Patients in the valve-preserving group also exhibited the least aortic valve regurgitation, stenosis, and left ventricular remodeling and dysfunction preoperatively; large aneurysms; and a lower likelihood of having a bicuspid valve, valve calcification, or heart failure. By contrast, patients in the biologic composite graft group were older and more symptomatic, and had greater ventricular hypertrophy than those in the valve-preserving group. The mechanical composite and allograft groups had greater ventricular hypertrophy.
Patients who received a mechanical composite had the largest label size prosthesis: 61% had size 22 or 27 mm, with 19% larger than this; 66% of biologic composite grafts contained a bioprosthesis label size of 25 or 27 mm, with only 4.7% larger than this. Most allografts (57%) were size 21 or 22 mm (these are internally sized) (Table E2).
These differences, differences in indication for operation (including need to address severe aortic regurgitation with modest aortic dilatation as well as modest aortic valve pathology in patients with importantly enlarged roots), and differences over time in the way the aortic root was measured meant that average root dimension was at times not severely enlarged and the aortic regurgitation was at times not severe. For example, the primary indication may have been aortic root size, and yet the ascending aorta may not have been severely enlarged, and vice versa. Hence, the average measured sizes may seem artificially small. In addition, some patients require aortic root and ascending aortic procedures before undergoing aorta operations that are distal to the subclavian artery, and their root and aorta may not have been as enlarged.
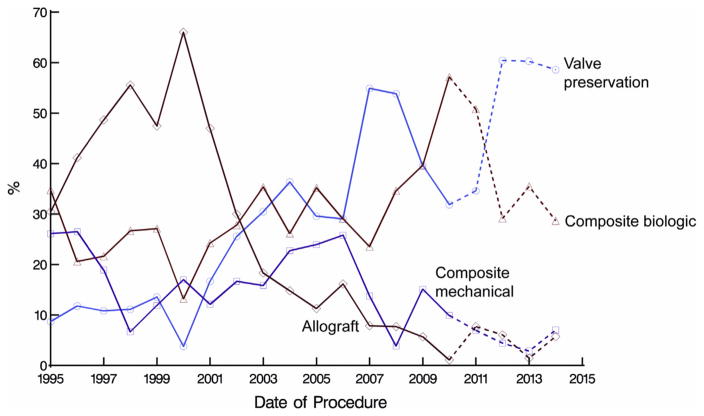
Over the course of the study, use of valve-preserving and biologic composite graft procedures increased; mechanical composite graft procedures increased in the early 2000s, then decreased; and allograft root replacement peaked in about 2000, then declined to a low level (Figure 1). Allograft roots were the most likely to be inserted during a less-invasive procedure; circulatory arrest was least used during valve-preserving procedures; and concomitant coronary artery bypass grafting was most likely to be performed in patients who received composite biologic grafts (Table 1). Although most of these coronary artery bypass grafting procedures were used for coronary artery disease (144 of 178 patients [81%] with coronary stenosis ≥50%), they were used in 5 patients in the valve-sparing group (3 with balloon perfusion catheter–associated coronary dissection), and in 36 in the remaining groups, particularly in elderly patients who received bioprosthetic composite grafts (n = 23) for coronary ostial technical reasons, which occurred in 5 patients who had mechanical composites and 8 who had allografts.
FIGURE 1.
Yearly volume of aortic root procedure use, all combined with ascending aorta replacement. Dashed lines indicate trends for the 4 years since the end of the study period for this article. They demonstrate that biologic composite grafts comprise approximately 30% of current procedures, valve-preserving procedures about 60%, and mechanical composite grafts and allografts approximately 5% each.
In-Hospital Outcomes
Atrial fibrillation (P < .0001) and reoperation for bleeding (P = .06) tended to be more common after composite biologic procedures in these older patients (Table 2). Patients who received allografts had the shortest intensive care unit stay, but those in the valve-preserving group had the shortest overall postoperative hospital stay. Risks of stroke (n = 13; 1.4%) and death (n = 7; 0.73%) were similar among groups.
TABLE 2.
Hospital course and adverse events stratified by aortic root procedure
| Event | Valve preservation (n = 261)
|
Composite graft
|
Allograft (n = 243)
|
P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Biologic (n = 297)
|
Mechanical (n = 156)
|
||||||||
| n* | No. (%) or mean ± SD | n* | No. (%) or mean ± SD | n* | No. (%) or mean ± SD | n* | No. (%) or mean ± SD | ||
| Hospital death | 261 | 0 (0) | 297 | 4 (1.3) | 156 | 1 (0.64) | 243 | 2 (0.82) | .3 |
| Reoperation for valve dysfunction | 255 | 4 (1.6) | 282 | 0 (0) | 141 | 0 (0) | 222 | 1 (0.45) | .07 |
| Atrial fibrillation | 261 | 65 (25) | 297 | 132 (44) | 156 | 43 (28) | 243 | 61 (25) | <.0001 |
| Prolonged ventilation (>24 h) | 225 | 13 (5.8) | 222 | 18 (8.1) | 107 | 8 (7.5) | 85 | 6 (7.1) | .8 |
| Reoperation for bleeding or tamponade | 261 | 7 (2.7) | 297 | 21 (7.1) | 156 | 5 (3.2) | 243 | 10 (4.1) | .06 |
| Permanent stroke | 261 | 3 (1.1) | 297 | 4 (1.3) | 156 | 1 (0.64) | 243 | 5 (2.1) | .7 |
| Perioperative myocardial infarction | 261 | 6 (2.3) | 297 | 0 (0) | 156 | 0 (0) | 243 | 4 (1.6) | .02 |
| Intra-aortic balloon pump | 261 | 5 (1.9) | 297 | 5 (1.7) | 156 | 0 (0) | 243 | 0 (0) | .06 |
| Insertion of ventricular assist device | 261 | 2 (0.77) | 297 | 2 (0.67) | 156 | 0 (0) | 243 | 0 (0) | .4 |
| Deep sternal wound infection | 261 | 0 (0) | 297 | 1 (0.34) | 156 | 0 (0) | 243 | 2 (0.82) | .3 |
| Renal failure requiring dialysis | 261 | 0 (0) | 297 | 1 (0.34) | 156 | 0 (0) | 243 | 1 (0.41) | .7 |
| Length of stay† | |||||||||
| Intensive care unit (h) | 261 | 23/28/70 | 297 | 23/29/80 | 156 | 22/28/72 | 243 | 24/24/48 | <.0001 |
| Postoperative (d) | 261 | 5.1/6.2/10 | 297 | 5.1/6.2/12 | 156 | 5.2/7.1/10 | 243 | 4.8/5.9/9.0 | <.0001 |
| Hospital (d) | 261 | 5.3/6.3/11 | 297 | 5.3/7.2/15 | 156 | 6.1/8.0/12 | 243 | 5.2/6.3/11 | <.0001 |
SD, Standard deviation.
Patients with data available.
15th/50th/85th percentiles.
Valve Function
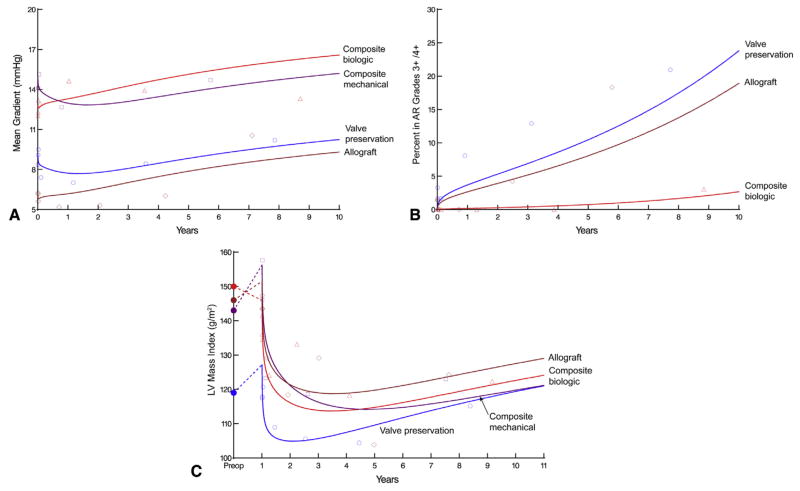
Both valve-preserving and allograft procedures had lower early mean gradients than did composite grafts with either a biologic or mechanical valve (P<.0001; Figure 2, A). Both mechanical and bioprosthetic composite grafts were predominantly sizes 23, 25, or 27 mm, but prosthesis size was associated with higher postoperative gradients (P <.0001 and P = .02, respectively), as was true of allografts (P = .03), for which the internal diameter was predominantly 21 or 22 mm (Table E2). Aortic valve regurgitation increased over time (Figure 2, B), particularly for valve-preserving and allograft procedures, although it was similar in these 2 groups (24% and 19% at 10 years, respectively; P = .2; risk-adjusted P = .08). Severe regurgitation after composite graft replacement with a mechanical valve occurred in only 1 patient, but it reached 2.7%at 10 years with a bioprosthesis. The mode of failure of the biologic composite grafts was predominantly aortic valve stenosis, whereas for valve-preserving and allograft procedures, the mode of failure was predominantly regurgitation.
FIGURE 2.
Temporal trends of longitudinal measures after 4 aortic root procedures for aneurysms. Solid lines represent unadjusted estimates of temporal trend, and symbols represent data grouped (without regard to repeated measurements) within a timeframe to provide a crude verification of model fit. A, Mean aortic valve gradient; (B) Grade 3+/4 + postoperative AR. Only 1 mechanical composite valve developed severe regurgitation. C, LV mass index. AR, Aortic valve regurgitation; LV, left ventricular. Red triangles = composite graft replacement with biologic valve; purple squares = composite graft replacement with mechanical valve; blue circles = valve preservation; and brown diamonds = allograft root and ascending aorta replacement with coronary reimplantation.
Left Heart Reverse Remodeling
Left ventricular reverse remodeling occurred differently among these 4 procedure groups. The pattern of postoperative left ventricular mass index was to decrease during the first 3 years, and increase thereafter. The valve-preservation group had the lowest left ventricular mass index at 3 years, compared with the other groups (P = .001; Figure 2, C). However, the preoperative left ventricular mass index was lowest in this group. Thus, the proportionate decrease in the index in the valve-preservation group was less than that in other groups.
Reoperation
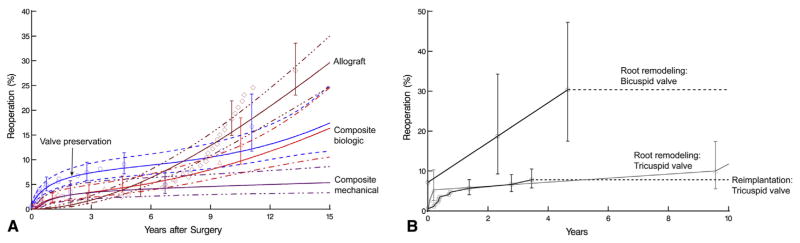
In all, 74 aorta or aortic valve–related reoperations took place, in 68 patients (Table 3). Hospital mortality was 1.5% for the first reoperation and 0% for the second. The probability of reoperation varied by strategy (Figure 3, A). Early reoperation was more common after valve preservation and biologic composite graft procedures (Table 4; Table E3). Allograft root replacement and biologic composite grafts had the highest late risk of reoperation, and mechanical composite grafts and valve preservation had the lowest, suggesting good outcomes for valve preservation once the initial early failures were addressed.
TABLE 3.
Reoperation details
| Reoperation | Valve preservation | Composite graft
|
Allograft | |
|---|---|---|---|---|
| Biologic | Mechanical | |||
| AVR | 7 | 2 | 0 | 15 |
| AVR + proximal aorta replacement | 6 | 7 | 3 | 16 |
| Proximal aorta replacement | 0 | 0 | 1 | 1 |
| Proximal and descending aorta replacement | 4 | 2 | 0 | 1 |
| Descending aorta replacement | 5 | 2 | 2 | 0 |
Total n for table = 74. Proximal is defined as aorta proximal to the left subclavian artery and includes arch operations distal to the previous ascending aorta replacement. AVR, Aortic valve replacement.
FIGURE 3.
Probability of first aortic or aortic valve–related reoperation. Solid lines represent parametric estimates enclosed within 68%confidence bands equivalent to ± 1 SE. Symbols are nonparametric Kaplan-Meier estimates with 68%confidence band. A, Stratified by 4 surgical strategies. B, Stratified by root remodeling for bicuspid valves versus reimplantation or remodeling for tricuspid aortic valves.
TABLE 4.
Propensity-score risk–adjusted P values comparing aortic root procedure groups for risk of reoperation
| Factor | Composite graft
|
Allograft | |
|---|---|---|---|
| Biologic | Mechanical | ||
| Early phase | |||
| Valve preservation | .4* | .05* | .02* |
| Composite biologic | .3* | .05* | |
| Composite mechanical | .3* | ||
| Late phase | |||
| Valve preservation | .02† | .7* | .008† |
| Composite biologic | .02* | .7† | |
| Composite mechanical | .009† | ||
Lower risk of reoperation.
Higher risk of reoperation.
In a subgroup analysis of the risk of reoperation after valve preservation using root remodeling for bicuspid valves versus reimplantation for tricuspid valves (Figure 3, B), reimplantation for tricuspid aortic valves had lower risk (Plog-rank = .02) and remodeling an intermediate risk (Plog-rank = .11).
Death
Patients who underwent composite graft replacement with a biologic valve had the worst survival (P <.0001; Figure 4), which was attributable to differences in patient characteristics rather than operative strategy (propensity-score risk–adjusted P>.2).
FIGURE 4.
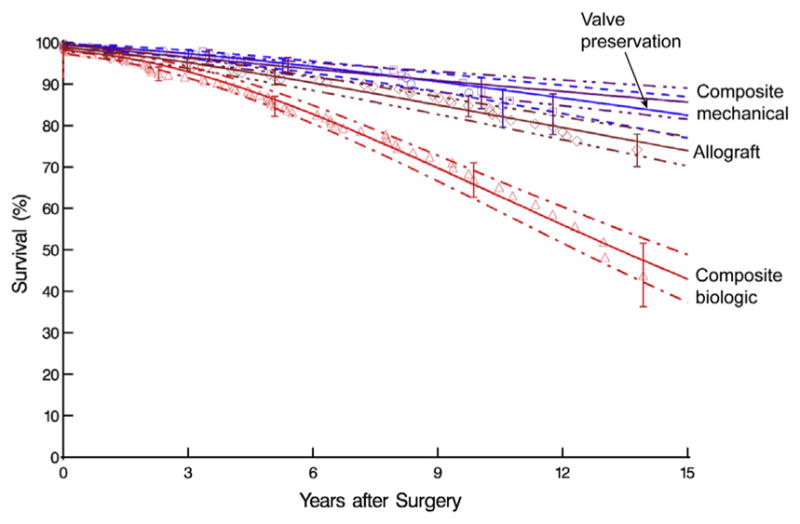
Survival stratified by 4 surgical strategies. Format is as in Figure 3, A.
DISCUSSION
Principal Findings
For patients who have various aortic root pathologies and ascending aortic aneurysm, all of whom would have been considered for a Bentall-type procedure in the past, we see in this large, single-institution series a nearly complete shift. Changes include movement away from mechanical composite grafts to valve-preserving procedures in younger patients; emergence of biologic composite grafts for older patients and those who believe they will be candidates for valve-in-valve transcatheter valve replacement in the future; and a near disappearance of allograft root and ascending aorta replacement.
However, mean aortic gradients were elevated in composite grafts, particularly those of small size, above that of either valve-preserving or allograft procedures, although the latter 2 developed regurgitation over time. Risk of regurgitation was greatest for allografts and least for composite mechanical devices. Risk of late death was related to age and comorbidities, not to strategy for managing the aortic root. All these procedures, either primary or reoperation, were performed with low hospital mortality and low risk of stroke.
Findings in Context
This study shows excellent early results after aortic root surgery combined with ascending aorta replacement, with a 0.73% in-hospital mortality, and no in-hospital deaths after valve-preservation procedures. Other recent reports, including our own, have demonstrated mortality ranging from 0.5% to 3.4% for valve preservation.4,5,14–17 Valve preservation was associated with lower postoperative gradients, resulting in left ventricular reverse remodeling to nearly the upper limit of normal, although this group of patients had the least remodeling preoperatively. Risk of death was also lower over time with valve preservation than with biologic composite grafts, but similar compared with mechanical composite grafts or allograft roots. However, any differences in survival were attributable to differences in patient demographics, particularly age, and to the prevalence of comorbidities.
The downside of valve preservation was an early risk of reoperation, although this risk was specifically related to remodeling of bicuspid valves, whereas reimplantation of tricuspid aortic valves provided excellent results. However, this comparison is likely one of worst case versus best case. Our previous studies have shown that remodeling is associated with a greater risk of failure, particularly for patients who have Marfan syndrome and tricuspid aortic valves.5,14 In addition, failure risk is higher for patients who have bicuspid versus tricuspid aortic valves.5,8 This finding was also reported by David’s group in Toronto.4
The obvious question, based on analysis of these data, is whether our use of mechanical composite grafts should have abated as quickly as it did. Clearly, early mortality is low, as we and others have reported,6,11 as is risk of late death and reoperation. This population is similar to the more-current valve-preservation population,5 with the exception of those patients who have aortic valve stenosis and receive mechanical composite grafts. A few patients with unicuspid or bicuspid valves may undergo repair despite stenosis, because partial fusions can be resected. Nevertheless, our data show that postoperative mechanical composite graft gradients are higher, and left ventricular remodeling is less complete, although we cannot determine whether these differences will result over time in more heart failure from either diastolic or systolic heart dysfunction.
The other obvious issues are the mechanical valve–related complications of embolism, endocarditis, pannus tissue ingrowth, hemorrhage from anticoagulation, high-intensity transient signals that may be related to nitrogen bubbles in solution, and late neurocognitive deficits.5,6,9 These valve-related risks are lower with valve preservation, but the downside of this approach is an early risk of reoperation in patients who have bicuspid valves. Nevertheless, risk of death for reoperations was low for all patients, including those with endocarditis on prior root replacements; a similar low risk was previously reported by Lytle and colleagues.18
At present, this study cannot settle the debate about whether increased use of biologic composite grafts, which comprise approximately 30% of our most recent procedures (Figure 1), is justified on the basis of potential transcatheter valve-in-valve replacement. Clearly, patients with aortic valve stenosis and the elderly are good candidates. Nevertheless, younger patients are increasingly requesting biologic valves in hopes of later receiving a valve-in-valve procedure; long-term surveillance will be needed to evaluate whether this choice is justified.
Additional drawbacks are less left ventricular remodeling, increasing gradients, valve-related complications, and risk of reoperation. The same applies to allograft root replacement, although as a rule, these patients are not good candidates for percutaneous valve-in-valve procedures because of the risk of coronary artery occlusion.19 Thus, with the exception of endocarditis, we continue to advocate for valve preservation and biologic composite grafts as better alternatives to allografts. Furthermore, late allograft dilatation poses a substantial risk when allograft root replacement is used in patients who have aneurysmal disease, and late calcification makes reoperation more challenging.
Limitations
This is not a randomized study, but rather a comparison of outcomes of root procedures based on a selection of procedures deemed appropriate by surgeons, in discussion with their patients at a single institution. Clearly, the underlying pathology and time period also influenced the type of procedure chosen, but long-term appropriateness can be measured only with the passage of time. However, the temporal and procedural heterogeneity allowed us to evaluate the appropriateness of each approach. In addition, we did not fully evaluate valve-related complications, although these seem to be lower for valve preservation and allograft root replacement.
Clinical Implications
Based on our current knowledge, for patients who have aneurysmal disease of the aortic root and ascending aorta, we recommend the following root procedure in combination with ascending aorta replacement, as shown by our treatment algorithm (Figure 5). (1) Valve preservation by modified reimplantation is preferred for tricuspid aortic valves without aortic valve stenosis (to November 2015, we have performed 541 modified reimplantation procedures). (2) Valve preservation by remodeling is the best approach for bicuspid nonstenotic valves, particularly those with larger annuli, but results of modified reimplantation need to be further evaluated. (3) Excellent results can be achieved with composite graft replacement with a mechanical valve for young patients who have stenotic aortic valves; and composite graft replacement with a biologic valve is a reasonable option in elderly patients who have aortic valve stenosis. (4) Allograft root and ascending aorta replacement with coronary reimplantation is best reserved for patients who have endocarditis, and perhaps for elderly patients with a small aortic annulus.
FIGURE 5.
Current Cleveland Clinic treatment algorithm for combined root and ascending aortic aneurysms. AR, Aortic regurgitation; AS, aortic valve stenosis; TAV, tricuspid aortic valve; BAV, bicuspid aortic valve; CAD, coronary artery disease; Ca2+, calcification; Prev., previous; AVR, aortic valve replacement; MRI, modified root-preserving reimplantation10; IRM, inclusion type of remodeling6,8; AG, allograft; ?CG, possibly composite valve graft,12 depending on root abscess presence or active infection; Bio, biologic12; MCG, mechanical composite graft.12
Supplementary Material
Central Message.
Aortic valve-preserving root procedures are recommended for young patients; composite bioprostheses are reasonable for the elderly.
Perspective.
Four aortic root procedures combined with ascending aorta replacement—valve preservation, mechanical or biologic composite grafts, and allografts—provide excellent survival and good durability. Valve-preserving and allograft procedures have the lowest gradients, but more late regurgitation. We recommend valve-preserving procedures for young patients; composite bioprostheses are reasonable for the elderly.
Acknowledgments
This study was funded in part by the Gus P. Karos Family Registry Fund, the David Whitmire Hearst Jr Foundation, the John and Rosemary Brown Endowed Chair in Cardiovascular Medicine (held by Dr Griffin), the Peter and Elizabeth C. Tower and Family Endowed Chair in Cardiothoracic Research (held by Dr Pettersson), the Judith Dion Pyle Endowed Chair in Heart Valve Research (held by Dr Gillinov), the Sheikh Hamdan bin Rashid Al Maktoum Distinguished Chair in Thoracic and Cardiovascular Surgery (held by Dr Sabik), and the Kenneth Gee and Paula Shaw, PhD, Chair in Heart Research (held by Dr Blackstone). These individuals and funding organizations played no role in the collection of data or analysis and interpretation of the data, and had no right to approve or disapprove publication of the finished article.
The nonlinear mixed-effects models were developed with support from National Institutes of Health grant 1R01HL103552-01A1: Ancillary Comparative Effectiveness of Atrial Fibrillation Ablation Surgery.
Footnotes
Conflict of Interest Statement
Dr Svensson is an unpaid member of the executive committee for PARTNER trials I and II, Chairman of the PARTNER Publication Committee, and an unpaid member of the executive committee of the COMMENCE trial, sponsored by Edwards Lifesciences (Irvine, Calif). Dr Roselli has financial relationships with Medtronic, Sorin, St Jude, Edwards Lifesciences, and Terumo. Dr Gillinov is a consultant for Edwards Lifesciences, Medtronic, On-X, Abbott Vascular, and Tendyne, receives research support from and is a speaker for St Jude, and holds equity in Clear Catheter. Dr Navia is a consultant for Edwards Lifesciences, St Jude Medical, and MAQUET, and is a member of the Scientific Board of NaviGate Cardiac Structures. Dr Smedira is a consultant for Medtronic and Edwards Lifesciences. Dr Sabik discloses financial relationships with Medtronic, Sorin, Edwards Lifesciences, and Abbott. All other authors have nothing to disclose with regard to commercial support.
You can watch a Webcast of this AATS meeting presentation by going to: http://webcast.aats.org/2015/Video/Tuesday/04-28-15_4E_0845_Svensson.mp4.
References
- 1.Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax. 1968;23:338–9. doi: 10.1136/thx.23.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Paulis R, Scaffa R, Maselli D, Weltert L, Salica A, Bellisario A. Valsalva graft in the Bentall procedure: from mechanical valve to the BioValsalva, world’s first biological aortic conduit. Surg Technol Int. 2008;17:216–21. [PubMed] [Google Scholar]
- 3.Gulbins H, Kreuzer E, Uhlig A, Reichart B. Homografts in patients with combined disease of the aortic valve and the ascending aorta: an alternative to the classical Bentall procedure. J Heart Valve Dis. 2001;10:650–5. [PubMed] [Google Scholar]
- 4.David TE, Feindel CM, David CM, Manlhiot C. A quarter of a century of experience with aortic valve-sparing operations. J Thorac Cardiovasc Surg. 2014;148:872–9. doi: 10.1016/j.jtcvs.2014.04.048. discussion 9–80. [DOI] [PubMed] [Google Scholar]
- 5.Svensson LG, Batizy LH, Blackstone EH, Gillinov AM, Moon MC, D’Agostino RS, et al. Results of matching valve and root repair to aortic valve and root pathology. J Thorac Cardiovasc Surg. 2011;142:1491–8. doi: 10.1016/j.jtcvs.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Svensson LG, Longoria J, Kimmel WA, Nadolny E. Management of aortic valve disease during aortic surgery. Ann Thorac Surg. 2000;69:778–83. doi: 10.1016/s0003-4975(99)01415-0. discussion 83–4. [DOI] [PubMed] [Google Scholar]
- 7.Blehm A, Schurr P, Sorokin VA, Zianikal I, Kamiya H, Albert A, et al. Comparison of different surgical techniques in 112 consecutive patients with aortic root operations: When should the valve be spared? J Heart Valve Dis. 2014;23:9–16. [PubMed] [Google Scholar]
- 8.Svensson LG, Al Kindi AH, Vivacqua A, Pettersson GB, Gillinov AM, Mihaljevic T, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg. 2014;97:1539–47. doi: 10.1016/j.athoracsur.2013.11.036. discussion 48. [DOI] [PubMed] [Google Scholar]
- 9.Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Composite valve graft replacement of the proximal aorta: comparison of techniques in 348 patients. Ann Thorac Surg. 1992;54:427–37. doi: 10.1016/0003-4975(92)90432-4. discussion 38–9. [DOI] [PubMed] [Google Scholar]
- 10.Svensson LG. Sizing for modified David’s reimplantation procedure. Ann Thorac Surg. 2003;76:1751–3. doi: 10.1016/s0003-4975(03)00439-9. [DOI] [PubMed] [Google Scholar]
- 11.Nakahira A, Shibata T, Sasaki Y, Hirai H, Hattori K, Hosono M, et al. Outcome after the modified Bentall technique with a long interposed graft to the left coronary artery. Ann Thorac Surg. 2009;87:109–15. doi: 10.1016/j.athoracsur.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Svensson LG. Approach for insertion of aortic composite valve grafts. Ann Thorac Surg. 1992;54:376–8. doi: 10.1016/0003-4975(92)91409-3. [DOI] [PubMed] [Google Scholar]
- 13.Beach JM, Mihaljevic T, Rajeswaran J, Marwick T, Edwards ST, Nowicki ER, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 2014;147:362–9. doi: 10.1016/j.jtcvs.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Svensson LG, Blackstone EH, Feng J, de Oliveira D, Gillinov AM, Thamilarasan M, et al. Are Marfan syndrome and marfanoid patients distinguishable on long-term follow-up? Ann Thorac Surg. 2007;83:1067–74. doi: 10.1016/j.athoracsur.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 15.Svensson LG, Cooper M, Batizy LH, Nowicki ER. Simplified David reimplantation with reduction of anular size and creation of artificial sinuses. Ann Thorac Surg. 2010;89:1443–7. doi: 10.1016/j.athoracsur.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 16.Miller DC. Valve-sparing aortic root replacement: current state of the art and where are we headed? Ann Thorac Surg. 2007;83:S736–9. doi: 10.1016/j.athoracsur.2006.10.101. discussion S785–90. [DOI] [PubMed] [Google Scholar]
- 17.Kvitting JP, Kari FA, Fischbein MP, Liang DH, Beraud AS, Stephens EH, et al. David valve-sparing aortic root replacement: equivalent mid-term outcome for different valve types with or without connective tissue disorder. J Thorac Cardiovasc Surg. 2013;145:117–26. 27 e1–5. doi: 10.1016/j.jtcvs.2012.09.013. discussion 26–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lytle BW, Sabik JF, Blackstone EH, Svensson LG, Pettersson GB, Cosgrove DM., III Reoperative cryopreserved root and ascending aorta replacement for acute aortic prosthetic valve endocarditis. Ann Thorac Surg. 2002;74:S1754–7. doi: 10.1016/s0003-4975(02)04129-2. discussion S1792–9. [DOI] [PubMed] [Google Scholar]
- 19.Tuzcu EM, Kapadia SR, Svensson LG. Valve in valve: another milestone for transcatheter valve therapy. Circulation. 2012;126:2280–2. doi: 10.1161/CIRCULATIONAHA.112.133777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.