Summary
Objectives
We previously reported inferior outcomes for locally-advanced head and neck squamous cell carcinoma (LAHNSCC) patients treated with concurrent cetuximab vs. high-dose cisplatin with intensity-modulated radiation therapy (IMRT). Prior to FDA approval of cetuximab for LAHNSCC, non-cisplatin eligible patients at our institution received 5-fluorouracil (5FU)/carboplatin. We sought to compare concurrent cetuximab vs. 5FU/carboplatin vs. high-dose cisplatin with IMRT for LAHNSCC.
Materials and methods
Retrospective review was performed for LAHNSCC patients treated at Memorial Sloan-Kettering Cancer Center from 11/02 to 04/08 with concurrent cetuximab (n = 49), 5FU/carboplatin (n = 52), or cisplatin (n = 259) and IMRT. Overall survival (OS), locoregional failure (LRF), distant metastasis-free survival, and late toxicity were analyzed using univariate and multivariate analyses. OS analysis was confirmed by propensity score adjustment.
Results
Treatment groups were similar with regard to primary tumor site, overall stage, and alcohol and tobacco history. Cetuximab and 5FU/carboplatin patients were older, with lower performance status, more comorbidities, higher T classification, and worse renal function. On multivariate analysis, compared with cisplatin and 5FU/carboplatin, cetuximab was associated with inferior 4-year OS (86.9% vs. 70.2% vs. 40.9%; P < .0001) and 4-year LRF (6.3% vs. 9.7% vs. 40.2%; P < .0001). Late toxicity was highest with 5FU/carboplatin (25.0%) vs. cisplatin (8.0%) vs. cetuximab (7.7%).
Conclusions
Although 5FU/carboplatin patients were sicker and experienced greater toxicity than cisplatin patients, no significant difference was found in all endpoints. In contrast, despite similar pretreatment characteristics, outcomes for cetuximab vs. 5FU/carboplatin were significantly worse. We feel that caution should be used with routine use of cetuximab in the management of LAHNSCC.
Introduction
Treatment with chemoradiation is an accepted standard for locally advanced head and neck squamous cell carcinoma (LAHNSCC). The addition of concurrent chemotherapy to radiotherapy (RT) for LAHNSCC results in an absolute survival benefit of 6.5% at 5-years, with greater benefit with platinum-based chemotherapy [1]. Among the concurrent platinum agents, single-agent cisplatin is superior to single-agent carboplatin and equivalent to carbo-platin with 5-fluorouracil (5FU) in retrospective analyses [2,3].
Although chemoradiation with high-dose cisplatin improves survival versus RT alone, it is associated with higher toxicity [4]. Therefore less toxic agents that will achieve equivalent or superior outcomes have been sought. Bonner et al. reported that cetuximab/RT resulted in improved locoregional control and survival with little increase in toxicity compared with RT alone [5]. This led to the adoption of cetuximab as one alternative to cisplatin concurrent with RT for LAHNSCC. Although cisplatin is still the most commonly used agent (51%), cetuximab is being used in approximately 20% of patients [6].
Importantly, the study by Bonner was conducted when RT alone was still an accepted standard for LAHNSCC. Only recently has cetuximab/RT been compared to concurrent platinum/RT in prospective randomized trials, although results are not yet reported. Our initial retrospective report showed that concurrent cisplatin/RT versus cetuximab/RT was associated with superior locoregional control, failure-free survival, and overall survival [7]. Nonetheless, unmeasured confounders limited this study. Prior to FDA approval of cetuximab for LAHNSCC, non-cisplatin candidates were routinely treated with alternative platinum-based regimens, namely, 5FU/carboplatin [8] at our center. We hypothesized that characteristics of these patient groups would be similar and hence we sought to compare the outcomes of concurrent IMRT with high-dose cisplatin, 5FU/carboplatin, or cetuximab.
Methods and materials
Study design
In this Institutional Review Board-approved (WA0654-10) study, we retrospectively identified patients with a diagnosis of LAHNSCC of the oropharynx, hypopharynx, or larynx treated with curative intent with IMRT and concurrent cisplatin, 5FU/carboplatin, or cetuximab, from 11/02 to 4/08. Reasons for exclusion were surgery to the primary site, prior RT for a non-basal cell carcinoma of the head and neck, induction or adjuvant chemotherapy, weekly cisplatin, or prior active malignancy. Three hundred sixty patients were eligible for analysis. Comorbid conditions were scored using the Charlson criteria [9]. Alcohol use was defined as none/mild (≤7 drinks/week) versus moderate/heavy (>7 drinks/week). Smoking history was recorded as >10 or ≤10 pack-years. Creatinine clearance was calculated using the Cockcroft-Gault formula. Pretreatment Karnofsky performance status (KPS) was recorded for all except two patients.
Treatment
Radiotherapy techniques have been previously described [7,10]. Patients were treated with IMRT with a median dose of 70 Gy. Treatment with cisplatin and cetuximab was delivered as previously reported [7]. Patients treated with 5FU/carboplatin received a planned three cycles (carboplatin 70 mg/m2 and 5FU 600 mg/m2 daily continuous infusion, both for 4 days) every 3 weeks, with recycling time and dosing adjusted based on toxicity concerns. Patients in whom chemotherapy was switched after one or two cycles (n = 30; 24 cisplatin, 6 5FU/carboplatin, 0 cetuximab) were analyzed according to the initial prescribed drug regimen.
Toxicity
Acute/chronic toxicity was graded by chart review according to the Common Toxicity Criteria for Adverse Events (CTCAE), v3. Duration of percutaneous endoscopic gastrostomy (PEG) dependence was measured from the time RT was completed. Late toxicity was defined as the presence of PEG or tracheostomy 12 months from RT start.
Statistical methods
Time to locoregional failure (LRF), distant metastasis (DM), or death was calculated from the first day of RT for all patients. OS was censored at the date of last information. The Kaplan–Meier method was used to calculate OS. Univariate analysis was performed using a Cox proportional hazards model. Variables included sex, age, race, KPS, primary site of disease, T and N classification, Charlson Index, alcohol and tobacco history, creatinine clearance, and drug. Variables with P < .05 on univariate analysis were entered into the multivariate model. Results were then confirmed using propensity score adjustment in the Cox model.
LRF was defined as persistent or recurrent disease in the head/neck. LRF-free survival (LRFS) was calculated from the first day of RT to the date of LRF, date of last follow-up, or date of death from any cause without LRF. DM-free survival (DMFS) was defined as the time from the first day of RT to the diagnostic date of DM, date of last follow-up, or date of death from any cause. For the determination of LRFS and DMFS, univariate and multivariate competing-risks analysis was performed with death without LRFS or DMFS regarded as a competing risk.
Univariate and multivariate analyses using logistic regression were used to analyze variables contributing to late toxicity, including age, T and N classification, and drug. The Chi-squared test and Fisher's exact test were used to compare clinical characteristics of patients receiving cetuximab vs. cisplatin vs. 5FU/carboplatin. P values and 95% confidence intervals were two-sided. A P value less than .05 was considered statistically significant. SAS 9.2 (SAS Institute) and R 2.9.2 were used for statistical analysis.
Results
Study population
Characteristics of all patients are shown in Table 1. Patient selection for cetuximab or 5FU/carboplatin, respectively, was based on the following factors: audiogram/poor hearing (30.6% and 53.8%), renal insufficiency (4.1% and 9.6%), cardiac history (2.0% and 1.9%), performance status (16.3% and 3.8%), patient preference (16.3% and 5.8%), neuropathy (4.1% and 9.6%), and a combination of factors (24.5% and 15.4%). Treatment groups were balanced with regard to all factors except age, KPS, T-stage, creatinine clearance, and comorbidities. Patients treated with cetuximab and 5FU/carboplatin were older (P < .0001), had lower performance status (P = .02), worse renal function (P < .0001), higher percentage of T4 tumors (P = .04), and more comorbidities (P < .0001) compared with cisplatin. The number of patients with Charlson score ≥2 was highest for 5FU/carboplatin (36.5% vs. 14.3% vs. 8.9%, P < .0001). With the exception of KPS, which was balanced between the groups, these findings were upheld in the oropharynx subset. As shown in Table 2, compared with 5FU/carboplatin, cetuximab patients had fewer comorbidities (P = .04), and were less likely to switch treatment drug (P = .03). Although cetuximab patients were more likely to be over age 71 (P = .03), the median age of cetuximab and 5FU/carboplatin patients was similar (66 vs. 64 years, respectively).
Table 1.
Characteristics of all patients.
| Characteristic | Drug | P value | ||
|---|---|---|---|---|
|
| ||||
| Cisplatin (n = 259) | 5FU/carboplatin (n = 52) | Cetuximab (n = 49) | ||
| n (%) | n (%) | n (%) | ||
| Age | <.0001 | |||
| <71 | 247 (95.4) | 41 (78.8) | 29 (59.2) | |
| ≥71 | 12 (4.6) | 11 (21.2) | 20 (40.8) | |
| Race | 0.33 | |||
| Caucasian | 234 (90.4) | 45 (86.5) | 41 (83.7) | |
| Non-Caucasian | 25 (9.7) | 7 (13.5) | 8 (16.3) | |
| Sex | 0.22 | |||
| Male | 225 (86.9) | 43 (82.7) | 38 (77.6) | |
| Female | 34 (13.1) | 9 (17.3) | 11 (22.4) | |
| Primary site | 0.22 | |||
| Oropharynx | 206 (79.5) | 38 (73.1) | 34 (69.4) | |
| Hypopharynx/larynx | 53 (20.5) | 14 (26.9) | 15 (30.6) | |
| T classification | 0.04 | |||
| T1 | 58 (22.4) | 9 (17.3) | 9 (18.4) | |
| T2 | 104 (40.2) | 12 (23.1) | 17 (34.7) | |
| T3 | 66 (25.5) | 17 (32.7) | 12 (24.5) | |
| T4 | 31 (12.0) | 14 (26.9) | 11 (22.4) | |
| N classification | 0.74 | |||
| N0 | 33 (12.7) | 3 (5.8) | 9 (12.2) | |
| N1 | 64 (24.7) | 14 (26.9) | 11 (22.5) | |
| N2 | 158 (61.0) | 34 (65.4) | 30 (61.2) | |
| N3 | 4 (1.5) | 1 (1.9) | 2 (4.1) | |
| Overall stage | 0.65 | |||
| II | 7 (2.7) | 0 (0) | 0 (0) | |
| III | 79 (30.5) | 13 (25.0) | 16 (32.7) | |
| IV | 173 (66.8) | 39 (75.0) | 33 (67.3) | |
| Alcohol history | 0.21 | |||
| Low/mild | 97 (37.5) | 17 (32.7) | 24 (49.0) | |
| Moderate/heavy | 162 (62.5) | 35 (67.3) | 25 (51.0) | |
| >10 pack-yr tobacco | 0.32 | |||
| No | 124 (47.9) | 19 (36.5) | 22 (44.9) | |
| Yes | 135 (52.1) | 33 (63.5) | 27 (55.1) | |
| KPS | ||||
| NA | 1 (0.4) | 1 (1.9) | 0 (0) | 0.02 |
| >80 | 201 (77.6) | 37 (71.2) | 29 (59.2) | |
| ≤80 | 57 (22.0) | 14 (26.9) | 20 (40.8) | |
| Cr clearancea | <.0001 | |||
| ≥60 | 245 (94.6) | 42 (80.8) | 35 (71.4) | |
| <60 | 14 (5.4) | 10 (19.2) | 14 (28.6) | |
| Charlson index | <.0001 | |||
| 0 | 187 (72.2) | 24 (46.2) | 29 (59.2) | |
| 1 | 49 (18.9) | 9 (17.3) | 13 (26.5) | |
| ≥2 | 23 (8.9) | 19 (36.5) | 7 (14.3) | |
| Pre-RT trach | ||||
| No | 250 (96.5) | 47 (90.4) | 45 (91.8) | |
| Yes | 9 (3.5) | 5 (9.6) | 4 (8.2) | |
| Neck dissection | .36 | |||
| No | 247 (95.4) | 51 (98.1) | 45 (91.8) | |
| Yes | 12 (4.6) | 1 (1.9) | 4 (8.2) | |
| Switched chemotherapy | .04 | |||
| No | 235 (90.7) | 46 (88.5) | 49 (100.0) | |
| Yes | 24 (9.3) | 6 (11.5) | 0 (0) | |
| Radiation length (days), median (range) | 46 (35–76) | 47 (42–58) | 46 (6–70) | .06 |
Abbreviations: RT = radiation therapy; KPS = Karnofsky performance status; Trach = tracheostomy; Cr = creatinine.
Calculated using Cockcroft-Gault formula.
Table 2.
Characteristics of 5FU/carboplatin versus cetuximab patients.
| Characteristic | Drug | P value | |
|---|---|---|---|
|
| |||
| 5FU/carboplatin (n = 52) | Cetuximab (n = 49) | ||
| n (%) | n (%) | ||
| Age | .03 | ||
| <71 | 41 (78.8) | 29 (59.2) | |
| ≥71 | 11 (21.2) | 20 (40.8) | |
| Race | .69 | ||
| Caucasian | 45 (86.5) | 41 (83.7) | |
| Non-Caucasian | 7 (13.5) | 8 (16.3) | |
| Sex | .52 | ||
| Male | 43 (82.7) | 38 (77.6) | |
| Female | 9 (17.3) | 11 (22.4) | |
| Primary site | .68 | ||
| Oropharynx | 38 (73.1) | 34 (69.4) | |
| Hypopharynx/larynx | 14 (26.9) | 15 (30.6) | |
| T classification | .57 | ||
| T1 | 9 (17.3) | 9 (18.4) | |
| T2 | 12 (23.1) | 17 (34.7) | |
| T3 | 17 (32.7) | 12 (24.5) | |
| T4 | 14 (26.9) | 11 (22.4) | |
| N classification | .61 | ||
| N0 | 3 (5.8) | 9 (12.2) | |
| N1 | 14 (26.9) | 11 (22.5) | |
| N2 | 34 (65.4) | 30 (61.2) | |
| N3 | 1 (1.9) | 2 (4.1) | |
| Overall stage | .40 | ||
| II | 0 (0) | 0 (0) | |
| III | 13 (25.0) | 16 (32.7) | |
| IV | 39 (75.0) | 33 (67.3) | |
| Alcohol history | .10 | ||
| Low/mild | 17 (32.7) | 24 (49.0) | |
| Moderate/heavy | 35 (67.3) | 25 (51.0) | |
| >10 pack-yr tobacco | .39 | ||
| No | 19 (36.5) | 22 (44.9) | |
| Yes | 33 (63.5) | 27 (55.1) | |
| KPS | .16 | ||
| NA | 1 (1.9) | 0 (0) | |
| >80 | 37 (71.2) | 29 (59.2) | |
| ≤80 | 14 (26.9) | 20 (40.8) | |
| Cr clearancea | .27 | ||
| ≥60 | 42 (80.8) | 35 (71.4) | |
| <60 | 10 (19.2) | 14 (28.6) | |
| Charlson index | .04 | ||
| 0 | 24 (46.2) | 29 (59.2) | |
| 1 | 9 (17.3) | 13 (26.5) | |
| ≥2 | 19 (36.5) | 7 (14.3) | |
| Pre-RT trach | 1.00 | ||
| No | 47 (90.4) | 45 (91.8) | |
| Yes | 5 (9.6) | 4 (8.2) | |
| Neck dissection | .20 | ||
| No | 51 (98.1) | 45 (91.8) | |
| Yes | 1 (1.9) | 4 (8.2) | |
| Switched chemotherapy | |||
| No | 46 (88.5) | 49 (100.0) | .03 |
| Yes | 6 (11.5) | 0 (0) | |
| Radiation length (days), median (range) | 47 (42–58) | 46 (6–70) | .34 |
Abbreviations: RT = radiation therapy; KPS = Karnofsky performance status; Trach = tracheostomy; Cr = creatinine.
Calculated using Cockcroft-Gault formula.
Overall survival
Median OS was not reached. With a median follow-up time of 53.1 months (range, 5.3–93.0), 273 (75.8%) patients were alive. There were 44 (17.7%), 17 (32.7%), and 26 (53.1%) deaths in the cisplatin, 5FU/carboplatin, and cetuximab groups, respectively. The 4-year OS was 86.9% (95% confidence interval [CI], 82.0–90.6%) for cisplatin, 70.2% (95% CI, 55.5–80.9%) for 5FU/carboplatin, and 40.9% (95% CI, 26.0–55.2%) for cetuximab (Fig. 1A). On univariate analysis, treatment with 5FU/carboplatin or cetuximab, age ≥ 71, non-Caucasian race, female sex, KPS ≤ 80, hypopharynx or larynx primary, advanced T classification (T4 vs. T1–3), higher comorbidity index, creatinine clearance <60, and >10 pack-year tobacco history were associated with significantly shorter OS (Table 3). After adjusting for all other variables with P < .05 on univariate analysis in a multivariate Cox model, cetuximab remained associated with shorter OS compared with cisplatin (HR 3.61, P < .0001). There was no significant difference in OS between patients treated with 5FU/carboplatin and cisplatin (HR 1.34, P = .37). Further multivariate analysis including the same variables but treating 5FU/carboplatin as a reference revealed that patients treated with cetuximab also experienced worse OS compared with 5FU/carboplatin (HR 2.69, 95% CI 1.32–5.45, P = .006). Because no significant difference in OS existed between platinum-based regimens, they were combined for propensity score analysis, which yielded a HR of 2.93 (P = .0002) for treatment with cetuximab.
Figure 1.
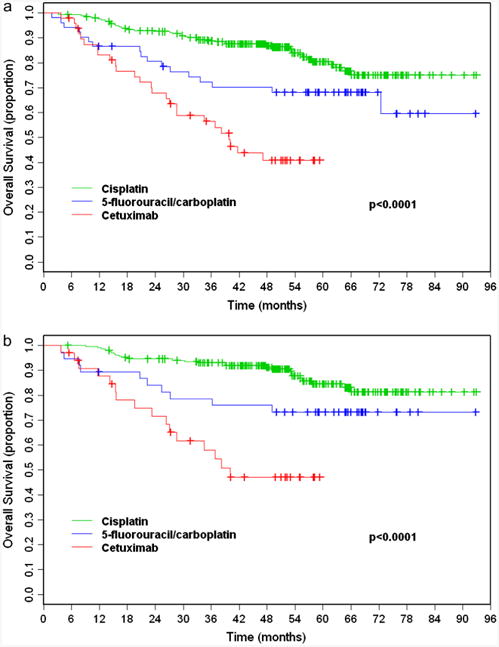
Kaplan–Meier overall survival from start of radiation therapy for all patients (a) and oropharynx patients (b) receiving concurrent high-dose cisplatin (green), 5-fluorouracil/carboplatin (blue), or cetuximab (red).
Table 3.
Univariate and multivariate analysis of prognostic factors for overall survival using the Cox proportional hazards regression model and locoregional failure-free survival using the competing-risks method (all patients).
| Variable | Overall survival | Locoregional failure-free survival | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
|
|
|
|
|
|||||
| Hazard ratio | P value | Hazard ratio | P value | Hazard ratio | P value | Hazard ratio | P value | |
| Drug | ||||||||
| 5FU/carboplatin vs. cisplatin | 1.92 | .02 | 1.34 | .37 | 1.61 | .35 | 1.51 | .44 |
| Cetuximab vs. cisplatin | 4.89 | <.0001 | 3.61 | <.0001 | 8.20 | <.0001 | 8.31 | <.0001 |
| Sex (female vs. male) | 1.98 | .009 | 1.24 | .44 | 1.26 | .58 | ||
| Age (≥71 vs. <71) | 2.81 | <.0001 | .85 | .63 | 2.39 | .02 | .76 | .50 |
| Race (non-Caucasian vs. Caucasian) | 2.36 | .002 | 1.45 | .26 | 2.73 | .008 | 1.83 | .18 |
| KPS (≤80 vs. >80) | 2.83 | <.0001 | 1.74 | .03 | 2.35 | .008 | 1.17 | .71 |
| Primary site (HPX/LX vs. OPX) | 2.97 | <.0001 | 1.82 | .02 | 3.38 | .0001 | 2.79 | .003 |
| T classification (T4 vs. T1–T3) | 3.27 | <.0001 | 3.29 | <.0001 | 1.92 | .07 | ||
| N classification (N2–N3 vs. N0–N1) | 1.05 | .84 | 0.56 | .07 | ||||
| Charlson index | ||||||||
| (1 vs. 0) | 1.58 | .09 | 1.14 | .64 | 1.67 | .18 | 1.21 | .67 |
| (≥2 vs. 0) | 2.41 | .001 | 1.27 | .46 | 2.26 | .04 | 1.45 | .37 |
| Alcohol history (moderate/heavy vs. low/mild) | 1.17 | .49 | 0.90 | .75 | ||||
| Tobacco history >10 pack-year (yes vs. no) | 1.71 | .02 | 1.18 | .49 | 1.84 | .07 | ||
| Creatinine clearance (<60 vs. ≥60) | 2.67 | .0003 | 1.46 | .27 | 2.43 | .03 | .68 | .47 |
Abbreviations: HPX = hypopharynx; KPS = Karnofsky performance status; LX = larynx; OPX = oropharynx.
In the oropharynx subset, cetuximab (HR 5.90; P < .0001) but not 5FU/carboplatin (HR 2.03; P = .06) was also associated with significantly shorter OS (Table 4). These findings were maintained on multivariate analysis after adjusting for independent risk factors. KPS, T and N classification, and creatinine clearance remained significant on multivariate analysis. On further analysis including all variables with P < .05, patients in the OPX subset treated with cetuximab vs. 5FU/carboplatin experienced worse OS (HR 4.67, 95% CI 1.87–11.64, P = .001). Propensity score analysis for OPX patients yielded a HR of 3.58 (P = .0003) for treatment with cetuximab vs. platinum-based regimens.
Table 4.
Univariate and multivariate analysis of prognostic factors for overall survival in the oropharynx subset using the Cox proportional hazards regression model.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| Hazard ratio | P value | Hazard ratio | P value | |
| Drug | ||||
| 5FU/carboplatin vs. cisplatin | 2.03 | .06 | 1.17 | .70 |
| Cetuximab vs. cisplatin | 5.90 | <.0001 | 5.48 | <.0001 |
| Sex (female vs. male) | 1.85 | .11 | ||
| Age (≥71 vs.<71) | 2.73 | .006 | .37 | .06 |
| Race (non-Caucasian vs. Caucasian) | 2.46 | .02 | 1.65 | .26 |
| KPS (≤80 vs. >80) | 2.98 | .0002 | 2.40 | .01 |
| T classification (T4 vs. T1–T3) | 3.40 | <.0001 | 2.36 | .01 |
| N classification (N2–N3 vs. N0–N1) | 1.99 | .05 | 2.18 | .045 |
| Charlson index | ||||
| (1 vs. 0) | 1.46 | .28 | 1.21 | .61 |
| (≥2 vs. 0) | 2.45 | .02 | 1.91 | .12 |
| Alcohol history (moderate/heavy vs. low/mild) | 1.10 | .75 | ||
| Tobacco history >10 pack-year (yes vs. no) | 1.28 | .38 | ||
| Creatinine clearance (<60 vs. ≥60) | 3.01 | .003 | 3.48 | .006 |
Abbreviations: KPS = Karnofsky performance status; 5FU = 5-fluorouracil.
Locoregional failure
LRF occurred in 16/249 (6.2%), 5/52 (9.6%), and 19/49 (38.8%) cisplatin, 5FU/carboplatin, and cetuximab patients, respectively. Salvage treatment for LRF consisted of surgery (n = 18) with total laryngectomy in 9 patients, chemotherapy (n = 9), chemotherapy with re-irradiation (n = 2), no further therapy (n = 6), re-irradiation alone (n = 1), and unknown (n = 4). Neck dissection within 6 months of RT completion was considered part of the up-front management and was not counted as a regional failure event. Patients generally underwent neck dissection if there was clinical or radiographic suspicion of persistent disease. Among those undergoing neck dissection, 13 cisplatin patients, zero 5FU/carboplatin patients, and 1 cetuximab patient had pathological confirmation of persistent disease.
Median LRFS was not reached. The 4-year LRF rate was 6.3% (95% CI, 3.3–9.4%) for cisplatin, 9.7% (95% CI, 1.5–17.9%) for 5FU/carboplatin, and 40.2% (95% CI, 25.9–54.4%) for cetuximab (Figure 2A). Primary site of disease and drug were the factors most associated with LRFS (Table 3). Univariate competing-risks analysis showed that treatment with cetuximab versus cisplatin was associated with significantly shorter LRFS for all patients (HR 8.20, P < .0001) and oropharynx patients (HR 3.38; P = .0001). Additional analysis showed cetuximab patients also experienced worse LRFS than 5FU/carboplatin patients (multivariate HR 5.50, 95% CI, 1.88–16.10; P = .002). In contrast, there was no difference in LRFS between 5FU/carboplatin and cisplatin for all patients (HR 1.61; P = .35) or oropharynx patients (HR 1.22; P = .80). Multivariate competing-risks analysis confirmed shorter LRFS with cetuximab compared with cisplatin (HR 8.31; P < .0001). Within the oropharynx subset, treatment with cetuximab was the only factor that significantly correlated with shorter LRFS (HR 9.39; P < .0001).
Figure 2.
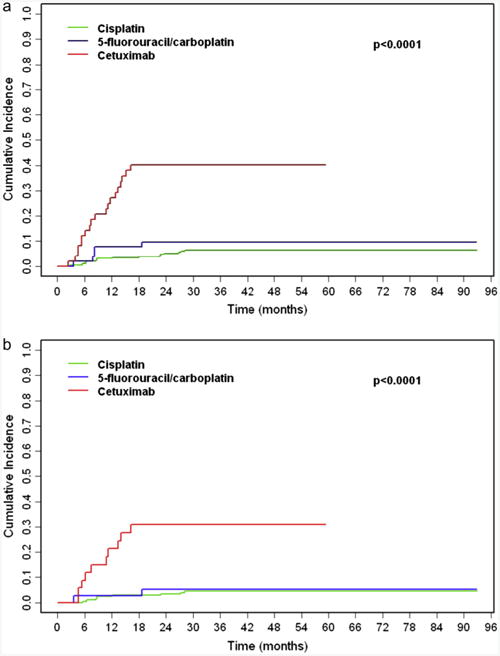
Cumulative incidence of locoregional failure for all patients (a) and oropharynx patients (b) receiving concurrent high-dose cisplatin (green), 5-fluorouracil/carboplatin (blue), or cetuximab (red).
Distant metastasis
Fifty-three patients developed distant metastasis with distribution as follows: cisplatin, 31/259; 5FU/carboplatin, 9/52; cetuximab, 13/49. Median DMFS was not reached. The 4-year rate of DM was 12.2% for cisplatin, 17.9% for 5FU/carboplatin, and 28.5% for cetuximab. On univariate analysis, advanced T classification (P < .0001) and treatment with cetuximab (P = .005) were associated with worse DMFS. These findings were upheld on multivariate analysis with T classification the greatest predictor of metastatic disease (HR 3.19; P < .0001), followed by cetuximab (HR 2.15; P = .03). These findings were consistent in the oropharynx subset.
Late toxicity
After excluding patients with less than 12 months of follow-up time, 333 patients remained for late toxicity analysis. Late toxicity was as follows: cisplatin, 8.0%; 5FU/carboplatin, 25.0%; cetuximab, 7.7%. Thirty-four patients were PEG dependent beyond one year from treatment start. Tracheostomy dependence occurred in 4 patients, of whom 3 were also PEG dependent. Two remain alive with tracheostomy; two underwent tracheostomy removal, 13 and 35 months from RT start, respectively. In the multivariate logistic regression, cisplatin and cetuximab were associated with less late toxicity than 5FU/carboplatin, respectively (OR = .40, P = .053; OR = .17, P = .02) after adjusting for all other variables (Table 5). Age over 70 (OR = 3.11, P = .04) and pre-treatment tracheostomy (OR = 4.55, P = .02) also correlated with a significantly higher rate of late toxicity. In a separate multivariate logistic regression, cetuximab did not significantly differ from cisplatin in late toxicity (OR = .43, P = .27) after adjusting for these additional factors.
Table 5.
Univariate and multivariate logistic regression analysis of predictors for late toxicity based on patients with follow-up time greater than 12 months.
| Variable | All Patients | Oropharynx Patients | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
|
|
|
|
|
|||||
| OR | P value | OR | P value | OR | P value | OR | P value | |
| Age (≥71 vs. <71) | 3.23 | .01 | 3.11 | .04 | 4.45 | .009 | 3.74 | .07 |
| KPS (≤80 vs. >80) | 1.24 | .60 | 1.05 | .93 | ||||
| T classification (T4 vs. T1–T3) | 2.72 | .02 | 1.97 | .16 | 2.34 | .12 | ||
| N classification (N2–N3 vs. N0–N1) | 1.18 | .66 | 1.50 | .44 | ||||
| Charlson index (≥2 vs. 0/1) | 2.48 | .04 | 1.45 | .47 | 2.94 | .08 | ||
| Tobacco history >10 pack-year (yes vs. no) | 1.33 | .44 | 1.28 | .58 | ||||
| Pre-RT trach (Yes vs. No) | 6.91 | .0006 | 4.55 | .02 | 18.95 | .002 | 20.67 | .02 |
| Drug | ||||||||
| Cisplatin vs. 5FU/carboplatin | 0.26 | .001 | 0.40 | .053 | 0.21 | .002 | 0.32 | .04 |
| Cetuximab vs. 5FU/carboplatin | 0.25 | .046 | 0.17 | .02 | 0.12 | .049 | 0.04 | .03 |
Abbreviations: 5FU = 5-fluorouracil; OR = odds ratio.
Discussion
Concurrent treatment with cetuximab and IMRT for LAHNSCC was associated with inferior OS, LRFS, and DMFS versus concurrent high-dose cisplatin or 5FU/carboplatin and IMRT in our series. Although 5FU/carboplatin and cetuximab patients had very similar pretreatment characteristics, cetuximab was associated with a 2.6-fold increased risk of death (P = .005). Furthermore, treatment with cetuximab but not 5FU/carboplatin resulted in significantly higher rates of LRF than cisplatin. These findings were consistent in the oropharynx subset. Aside from drug, other known negative prognostic factors such as lower KPS, primary disease of the hypopharynx or larynx, and advanced T classification were also confirmed in our study. These data support the use of concurrent platinum-based chemotherapy with IMRT for LAHNSCC in eligible patients.
We previously reported that concurrent high-dose cisplatin was superior to cetuximab for LAHNSCC without resulting in increased toxicity [7]. However, these results were viewed as controversial by some because cetuximab patients were older, with worse renal function, and included more T4 tumors. Since this publication, a group from Washington University conducted a similar comparison of LAHNSCC patients treated with concurrent cetuximab/RT (n = 33) versus cisplatin/RT (n = 30) at their institution [11]. At three years, they reported 83% disease-specific survival with cisplatin versus 31% with cetuximab. In their series, cisplatin patients had better performance status, younger age, and fewer comorbid conditions. Investigators from William Beaumont performed a matched-pair analysis and also found decreased cause-specific survival (P = .04) and overall survival (P = .001) with cetuximab (n = 31) compared with cisplatin (n = 62) [12]. Caudell et al. also compared concurrent cetuximab/RT versus platinum-based chemotherapy and RT at the University of Alabama and reported 3-year actuarial OS of 75.9% in the cetuximab/RT cohort versus 61.3% in the platinum-based/RT cohort [13]. However, the superior OS seen with cetuximab was not significant after controlling for T-stage, treatment on protocol, or on multivariate analysis. Treatment with cisplatin versus cetuximab was also compared in a retrospective series by Strom et al., finding equivalent actuarial 2 year survival (88% vs. 89%, respectively) despite significantly older age and lower percentage of ≥N2 nodal involvement among cetuximab patients [14]. In contrast to the present study, the study by Strom et al. also included patients with oral cavity tumors; however, both studies concluded that the two treatment regimens appeared to yield equivalent outcomes.
Prior to the approval of cetuximab, patients at our institution who were not candidates for cisplatin received 5FU/carboplatin. Consistent with this practice pattern, both the cetuximab and 5FU/carboplatin cohorts had lower performance status, greater percentage of age over 71, larger primary tumors, and higher Charlson comorbidity index versus cisplatin; all of these factors have previously been correlated with worse prognosis in oropharyngeal SCC [15,16]. Patients receiving cetuximab were older than 5FU/carboplatin, but had fewer comorbid conditions. Nevertheless, as compared to cisplatin, only cetuximab was associated with inferior OS, suggesting that the inferior survival in cetuximab patients in this study was unlikely to be related to escalated comorbidity in this patient group. The additional comparison with 5FU/carboplatin also showed cetuximab was associated with inferior OS after adjusting for all other variables in the model. Propensity score adjusted analysis reduced the patient-selection bias and further confirmed our previous findings.
Patients treated with cetuximab were five times more likely than 5FU/carboplatin and seven-times more likely than patients treated with cisplatin to experience LRF. Although LRC was the primary endpoint in the Bonner study of cetuximab with RT, the 5-year update included overall survival but long-term LRC outcomes were not reported. At a median follow-up of 24.4 months, Bonner initially reported 47% 3-year LRC in patients who received cetuximab/RT [5]. Four-year LRC for cetuximab patients on the present study was 59.8%, suggesting that it was not poor outcomes in our cetuximab patients that accounts for the inferiority to platinum-based treatment. The lower rates of LRF and DM in patients treated with concurrent cisplatin and 5FU/carboplatin in the present study likely translated to the observed OS benefit of these regimens compared with cetuximab.
Both tobacco use and human papillomavirus (HPV) have been identified as risk factors for development of SCC of the head and neck, particularly the oropharynx [17,18]. It has been suggested that the risk of SCC from tobacco use is independent of the association with HPV infection and that patients with HPV-negative tumors are more likely to be smokers [15,19]. Patients with HPV-positive tumors of the oropharynx and those with a history of less tobacco use have improved OS and PFS [15,17,20]. The percentage of oropharynx patients in this study with a less than 10 pack-year tobacco history, and thus those more likely to be HPV-positive, was 55.0% and did not vary significantly between treatment groups. Although we were unable to obtain the HPV status of the majority of patients, HPV and p16 status was determined for 31 cisplatin and 17 cetuximab patients, of which 86% and 74% were positive, respectively [21]. After accounting for HPV status in this small patient subset, cisplatin was still associated with superior outcomes. In keeping with these results, the Washington University study reported inferior disease-specific survival with cetuximab versus cisplatin despite the groups being matched with regard to HPV status [11]. Differences in HPV status therefore are unlikely to account for the improved survival in cisplatin and 5FU/carboplatin patients in the present study.
Despite cisplatin generally being considered a more toxic agent, 5FU/carboplatin was associated with higher rates of late toxicity. Patients have generally been selected to receive cetuximab due to concerns of toxicity associated with chemotherapy. There are conflicting reports of toxicity from cetuximab versus platinum-based chemotherapy and RT alone [7,22,23]. In our previous report, we found no difference in late Grade 3 and 4 toxicity or feeding tube dependence at 9 months between patients treated with cetuximab/RT and cisplatin/RT [7]. With regards to acute toxicity, cetuximab/RT has been reported to result in worse grade ≥3 radiation dermatitis and oral mucositis, and more weight loss and enteral feeding requirements than cisplatin/RT [22]. In the postoperative setting, concurrent RT and cisplatin versus carboplatin monotherapy yielded equivalent toxicity [2]. In a comparison of cisplatin/RT and 5FU/carboplatin/RT for oropharyngeal cancer, the only difference in toxicity observed was Grade 3 or 4 neutropenia, which was higher in cisplatin patients [3]. Our results suggest that while there was no difference between cisplatin/RT and cetuximab/RT, 5FU/carboplatin/RT may be a more toxic regimen. Though 5FU/carboplatin patients had the highest percentage of patients with Charlson comorbidity index ≥2, Charlson index was not found to independently correlate with late toxicity. Because of the similarities between cetuximab and 5FU/carboplatin patients, the increased toxicity with 5FU/carboplatin cannot be attributed to baseline patient heterogeneity.
The present study is limited by the retrospective nature, the lack of randomized patient populations, and is therefore subject to bias. Although all patients were treated with intensity-modulated radiation therapy, the cetuximab and 5FU/carboplatin patients were treated during different time periods, which may have also added to selection bias. Cetuximab and 5FU/carboplatin patients were similar, but baseline characteristics of all three patient groups differed. Furthermore, there were fewer patients in the 5FU/carboplatin and cetuximab patient groups which limited the statistical power to include a greater number of variables. We used propensity score adjustment to closely mimic randomized trials to reduce selection bias by equating treatment groups based on a set of known imbalanced factors. Nevertheless, a randomized controlled study is needed to verify our results. As previously described, HPV status has emerged as a significant prognosticator and was unknown in the majority of our patient cohort. Although we tried to use tobacco history as a potential marker of HPV status, we recognize this is not a true surrogate. Ongoing prospective studies have the advantage of randomization based on HPV status to yield important information regarding treatment for these patient groups that appear to have a differential treatment response.
In summary, concurrent IMRT and platinum-based chemotherapy resulted in excellent outcomes, which were significantly superior to cetuximab. Preliminary results of RTOG 0522 demonstrated that the addition of cetuximab to cisplatin with concurrent RT did not improve PFS or OS [24]. Cetuximab and cisplatin with RT is being compared in the RTOG 1016 trial for p16-positive oropharyngeal cancer and a similar ongoing Phase II study is investigating the addition of cetuximab to 5FU/carboplatin with concurrent IMRT [25]. We encourage participation in these randomized trials and anticipate the final results to help select the optimal treatment for patients with LAHNSCC.
Footnotes
Presented in part at the American Society of Clinical Oncology Annual Meeting, 2012
Conflicts of interest statement: Eric J. Sherman has consulted for Bristol-Myers-Squibb.
Other conflicts of interest: None declared.
References
- 1.Pignon JP, Le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Rades D, Ulbricht T, Hakim SG, et al. Cisplatin superior to carboplatin in adjuvant radiochemotherapy for locally advanced cancers of the oropharynx and oral cavity. Strahlentherapie und Onkol Organ der Dtsch Röntgengesellschaft al. 2012;188:42–8. doi: 10.1007/s00066-011-0005-z. [DOI] [PubMed] [Google Scholar]
- 3.Barkati M, Fortin B, Soulières D, et al. Concurrent chemoradiation with carboplatin-5-fluorouracil versus cisplatin in locally advanced oropharyngeal cancers: is more always better? Int J Radiat Oncol Biol Phys. 2010;76:410–6. doi: 10.1016/j.ijrobp.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 4.Adelstein DJ. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–8. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 6.Wong SJ, Harari PM, Garden AS, et al. Longitudinal Oncology Registry of Head and Neck Carcinoma (LORHAN): analysis of chemoradiation treatment approaches in the United States. Cancer. 2011;117:1679–86. doi: 10.1002/cncr.25721. [DOI] [PubMed] [Google Scholar]
- 7.Koutcher L, Sherman E, Fury M, et al. Concurrent cisplatin and radiation versus cetuximab and radiation for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:915–22. doi: 10.1016/j.ijrobp.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–6. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82:291–8. doi: 10.1016/j.ijrobp.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 11.Ley J, Mehan P, Wildes TM, et al. Cisplatin versus cetuximab given concurrently with definitive radiation therapy for locally advanced head and neck squamous cell carcinoma. Oncology. 2013;85:290–6. doi: 10.1159/000355194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Baschnagel AM, Chen P, et al. A matched-pair comparison of intensity-modulated radiation therapy with cetuximab versus intensity-modulated radiation therapy with platinum-based chemotherapy for locally advanced head neck cancer. Int J Clin Oncol. 2014;19:240–6. doi: 10.1007/s10147-013-0540-y. [DOI] [PubMed] [Google Scholar]
- 13.Caudell JJ, Sawrie SM, Spencer SA, et al. Locoregionally advanced head and neck cancer treated with primary radiotherapy: a comparison of the addition of cetuximab or chemotherapy and the impact of protocol treatment. Int J Radiat Oncol Biol Phys. 2008;71:676–81. doi: 10.1016/j.ijrobp.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Strom TJ, Trotti AM, Kish J, et al. Comparison of every 3 week cisplatin or weekly cetuximab with concurrent radiation therapy for locally advanced head-and-neck cancer: definitive management of head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:484. [Google Scholar]
- 15.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh B, Bhaya M, Stern J, et al. Validation of the Charlson comorbidity index in patients with head and neck cancer: a multi-institutional study. Laryngoscope. 1997;107:1469–75. doi: 10.1097/00005537-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012;22:128–42. doi: 10.1016/j.semradonc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Rades D, Seibold ND, Gebhard MP, et al. Prognostic factors (including HPV status) for irradiation of locally advanced squamous cell carcinoma of the head and neck (SCCHN) Strahlentherapie und Onkol Organ der Dtsch Röntgengesellschaft al. 2011;187:626–32. doi: 10.1007/s00066-011-1139-8. [DOI] [PubMed] [Google Scholar]
- 19.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 20.Lau HY, Brar S, Klimowicz AC, et al. Prognostic significance of p16 in locally advanced squamous cell carcinoma of the head and neck treated with concurrent cisplatin and radiotherapy. Head Neck. 2011;33:251–6. doi: 10.1002/hed.21439. [DOI] [PubMed] [Google Scholar]
- 21.Riaz N, Sherman E, Koutcher L, et al. Concurrent chemoradiotherapy with cisplatin versus cetuximab for squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2014;00:1–5. doi: 10.1097/COC.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh L, Gillham C, Dunne M, et al. Toxicity of cetuximab versus cisplatin concurrent with radiotherapy in locally advanced head and neck squamous cell cancer (LAHNSCC) Radiother Oncol. 2011;98:38–41. doi: 10.1016/j.radonc.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–8. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 24.Ang KK, Zhang QE, Rosenthal DI, et al. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III–IV head and neck squamous cell carcinomas (HNC) J Clin Oncol. 2011;29 doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habl G, Jensen AD, Potthoff K, et al. Treatment of locally advanced carcinomas of head and neck with intensity-modulated radiation therapy (IMRT) in combination with cetuximab and chemotherapy: the REACH protocol. BMC Cancer. 2010;10:651. doi: 10.1186/1471-2407-10-651. [DOI] [PMC free article] [PubMed] [Google Scholar]


