Abstract
Peyronie’s disease (PD) is an inflammatory condition of penile tunica albuginea which commonly ends with penile curvature and difficulty in vaginal penetration. Unfortunately, the pathophysiology of PD has not been completely understood. In this paper, we will review what is known about the pathophysiology of PD and the nonsurgical medical treatment options that have been trialed as a result. In the last 5 years, commonly used oral medications left their places to intralesional therapies. Clostridium collagenase, which is the only Food and Drug Administration (FDA) approved treatment for PD, is now the most prescribed intralesional therapy in the last years. Clostridium collagenase is advised for patients whose penile curvature is > 30° and < 90°. Because of its side effects, patients should be counseled before intralesional Clostridium collagenase treatment. Until finding best treatment solution for PD, more investigations in regards to the basic science of PD need to be carried out in order to elucidate the exact mechanisms of the fibrosis.
Keywords: Humans, male, penile induration, phosphodiesterase inhibitors
Introduction
As a connective tissue disorder of penile tunica albuginea (TA) Peyronie’s disease (PD) commonly causes penile curvature and difficulty in vaginal penetration. In PD, in addition to fibroblastic proliferation, and alterations in the elastin framework, there is a fibrous plaque that contains excessive amounts of collagen.[1] The PD plaque is inelastic and therefore results in penile deformity including curvature, indentation, hinge effect, and penile shortening. PD is relatively common and affects up to 20.3% of adult men to some degree.[2]
In the early phase of PD, plaque formation, inflammation and edema irritate nerve endings, thereby producing pain. As the inflammatory reaction matures and settles, the trapped nerve fibers may become ischemic and necrotic. In the chronic phase of PD, the process of plaque formation, and fibrosis gains momentum and impairs the erectile tissue often resulting in ED.[3] In the chronic phase of PD, although surgical correction of curvature is the gold-standard treatment, its effectiveness is restricted by significant morbidity including worsening erectile dysfunction. The devastating sexual and psychological impact of PD on patients necessitates implementation of improved treatment and prevention strategies. Satisfactory therapeutic interventions need to be elucidated to maximize patient satisfaction and mental well-being. Current therapies frequently do not meet expectations. Therefore, we need a complete understanding of the precise cellular and pathophysiologic processes of PD and gather knowledge about interventions at molecular level in order to develop new strategies.[2,4]
Pathophysiology of PD
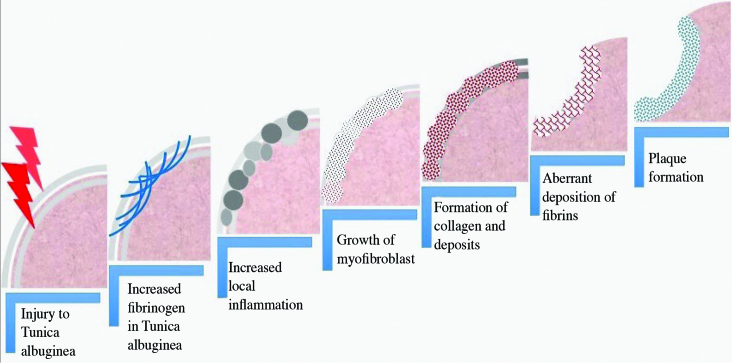
Peyronie’s disease is a wound-healing disorder, similar to keloids, hypertrophic scars, or Dupuytren’s contractures, all of which may present with overlapping findings (Figure 1).[5] The dense plaques identified in PD are a result of an imbalance between fibrosis and fibrinolysis. Fibrosis is an outcome of tissue injuries, chronic inflammation, autoimmune reactions, allergic responses, chemical insults and radiation.[6] Normal tissue healing restores the baseline levels and organization of extracellular matrix (ECM), whereas fibrosis involves the overgrowth, hardening, and scarring of tissues which are attributed to excess deposition of ECM components. Collagen is the most abundant protein in ECM and provides the structural and tensile strength in most human tissues.[7] Accumulation of ECM occurs via increasing expression and deposition of connective tissue proteins and preventing the catabolism of ECM proteins.[8] Excess production of ECM and the failure to degrade it are the hallmarks of fibrosis.
Figure 1.
Schematic representation of the pathophysiology of the fibrotic process leading to Peyronie’s disease (Drawing by O.C.).
Nonsurgical treatment options
Once the diagnosis is made, the patient should be counseled on both surgical and nonsurgical interventions (Figure 2). In this article, we aimed to review latest recommendations and highlight recent advances in the nonsurgical management of PD.
Figure 2.
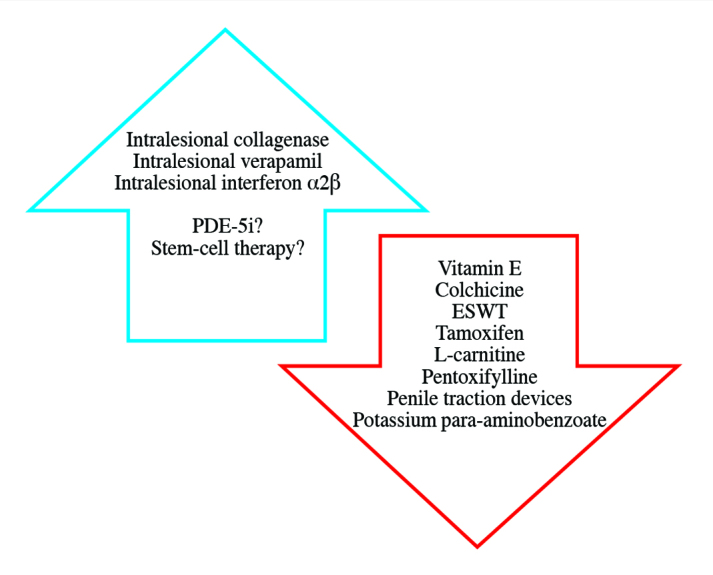
Recommendations for nonsurgical treatment modalities in patients with Peyronie’s disease
In 2015, the American Urological Association (AUA) developed its first-ever clinical guidelines on the diagnosis and management of PD.[2] To date, pharmacologic treatment of PD consists of oral, topical and injection therapies. Despite a wide variety of options, treatment outcomes have been disappointing. Multiple well-designed, prospective studies have evaluated oral agents however their clinical significance is currently limited by conflicting outcomes, single-center data, and small sample sizes which limit statistical power of studies. Advances in our understanding of molecular mechanisms of PD pathogenesis have revealed promising molecular targets for antifibrotic treatments. Based on the best available evidence, there are currently no recommended oral agents for the routine treatment of PD.
Oral therapies
Vitamin E
Vitamin E has been extensively investigated for its potential use in the treatment of PD. This fat-soluble vitamin has antioxidant effects that are thought to limit the oxidative stress of reactive oxygen species (ROS). There is an overexpression of ROS during the acute and proliferative phases of plaque formation in PD.[9] This target makes vitamin E an ideal option for medical intervention. However, Gelbard et al.[10] examined effect of vitamin E on PD, and demonstrated that vitamin E does not have an impact on the natural history of the disease. According to guidelines and earlier research, vitamin E is not currently recommended for the treatment of PD.[2]
Colchicine
The mechanism of action of colchicine involves the binding of tubulin to inhibit microtubule polymerization. Through its inhibition of neutrophil microtubules, colchicine has been shown to prevent fibrosis and collagen deposition.[11] Depolymerization of tubulin results in the inhibition of cell mitosis, leukocyte adhesion, and transcellular collagen transport. Colchicine also had a role in the activation of collagenases and therefore in promoting fibrinolysis and plaque breakdown.[12] Initial studies using colchicine as a treatment for PD were promising, but they were nonrandomized studies with small sampling size. These studies did show improvement in the correction of curvature, but did not reveal any significant clinical benefit.[13,14] Due to the undesirable gastrointestinal and hematologic side effects of colchicine, conduction of further studies exploring this medication as a treatment for PD has been abandoned.
Potassium para-aminobenzoate
Potassium para-aminobenzoate (Potaba, NJ, USA) has both anti-inflammatory and antifibrotic effects and it is used to treat fibrotic conditions such as Dupuytren’s contracture. Potassium para-aminobenzoate stabilizes tissue serotonin–monoamine oxidase activity and has a direct inhibitory effect on fibroblast glycosaminoglycan secretion.[15] There is currently no evidence supporting the clinically beneficial role for potassium para-aminobenzoate in PD and therefore it is not recommended for use in these patients.[2]
Tamoxifen
Tamoxifen citrate is a selective estrogen receptor used in the treatment of breast cancer. This medication has been explored as a therapeutic option for PD due to its inhibitory effects both on the release of transforming growth factor (TGF) from fibroblasts and TGF-receptors.[16] Studies with small patient populations have been unable to confirm a benefit of tamoxifen in reducing the size of PD plaque or correcting clinical curvature compared to placebo.[17] Adverse reactions of oral tamoxifen include hot flashes, ED, rashes, and gastrointestinal upset. As a treatment for PD, tamoxifen is not recommended in the AUA guidelines.[2]
Pentoxifylline
Pentoxifylline (PTX) is a nonspecific phosphodiesterase (PDE) inhibitor with anti-inflammatory and antifibrogenic properties. PTX down-regulates TGF-β and TNF and increases fibrinolytic activity. PTX is not recommended in the guidelines for the treatment of PD.[2]
Carnitine
Carnitine facilitates the entry of long-chain fatty acids into muscle mitochondria and allows them to be used as an energy substrate. Carnitine is an inhibitor of acetyl coenzyme-A, which helps repair damaged DNA and prevents the formation of free radicals during cell stress.[18] Carnitine is currently not recommended in the guidelines for the treatment of PD.[2]
Phosphodiesterase-5 inhibitors
Activation of the nitric oxide pathway by phosphodiesterase type 5 inhibitors (PDE5is) has a role in improving erectile function, in suppressing collagen synthesis and initiating apoptosis of myofibroblasts.[19] Long-term administration of PDE5is in PD rat models has demonstrated an increase in collagen and decrease in fibroblasts in TA cells.[20,21] PDE5is have therefore been investigated for their implications concerning plaque formation and remodeling in human PD. Ozturk et al.[22] compared 39 PD patients with ED by randomizing them to either daily sildenafil or vitamin E treatment groups for 3 months. They showed a similar reduction in plaque size in both groups, but the PDE5i group had a statistically significant improvement in erectile function and pain reduction scores. Chung et al.[23] have provided evidence of scar remodeling in patients with isolated septal scars without evidence of penile deformity in patients taking daily doses of tadalafil for 6 months. In the latter study, patients had not visual impairment, and therefore the authors concluded that PDE5i plays a role in the acute phase rather than during degradation process of an ossified plaque. Larger- scale, placebo-controlled trials for the use of PDE5is need to be performed to help support their potential use as a treatment option for PD.
Non-steroidal anti-inflammatory medications
The clinician may offer oral nonsteroidal anti-inflammatory medications to the patient suffering from active PD who is in need of pain management.[2]
Intralesional therapies
Intralesional injection therapy has been used for years to treat PD. Compared to oral medications these choices (Figure 2) help ensure adequate drug concentrations reach the ECM affected by PD at the level of TA. Adequate drug penetration may significantly slow, prevent, or reverse PD plaque formation. Higher concentrations injected immediately into cells should hopefully negate the need for prolonged treatment as seen with some oral medications. Unfortunately, results have been limited and many medications are riddled with local side effects including pain, bruising, and local inflammation.
Intralesional interferon
Initial reports on the impact of interferon (IFN) as an intralesional treatment modality for PD were encouraging. In 1991, Duncan et al.[24] demonstrated that in vitro IFN promotes fibrinolysis by decreasing fibroblast proliferation, decreasing ECM collagen, and increasing collagenase within PD plaques. In 2006, Hellstrom et al.[25] published their data on a placebo-controlled, multicenter trial of 117 PD patients who had bi-weekly injections of 5×106 units of IFN-2α for a total of 12 weeks. Results showed an average improvement of penile deviation of 13° compared to only 4° change with placebo. Pain resolution was observed in 67% of the treatment group versus 28% in the placebo group. Wegner et al.[26] demonstrated low rates of improvement and a high incidence of side effects, including myalgia and fever. Limited improvements in curvature observed in these studies are unlikely to have a significant clinical benefit and therefore intralesional IFN is currently not recommended as a treatment for PD.
On the other hand, recent studies have suggested that IFN- α2β leads to an improvement in penile hemodynamics, supporting improved erectile function.[27] One of the randomized-controlled trials (RCTs) evaluated the efficacy of intralesional IFN- α2β compared to placebo.[25] In this trial, the IFN group exhibited significant improvements in penile curvature, plaque size, and pain. The mean curvature reduction was 13.5% in this study. Overall, the drug was very well tolerated, with the most common side effect being flu-like symptoms that lasted for less than 36 hours. Based on the exclusion criteria of that study, intralesional IFN α2β can be utilized in men with curvature of at least 30° without calcified plaques.[2] A recent retrospective study similarly showed that IFN- α2β had resulted in significant reduction (mean, 9°) in penile curvature. They further showed that this decrease in curvature was independent of both disease duration and the location of the curvature (ventral versus dorsal/lateral).[28] This finding is particularly important because this is one of the few studies which examined ventral plaques, with important implications for the generalizability of this treatment modality to patients with ventral PD. In conclusion, IFN- α2β is a reasonable alternative as an intralesional treatment for PD, with modest efficacy and an overall excellent safety profile. Further studies are needed to better compare its efficacy to other treatments and to assess its functional significance for patients with PD.
Intralesional verapamil
Verapamil, a common calcium channel blocker (CCB), has shown promising evidence for the treatment of PD when used intralesionally. In vitro studies have revealed that verapamil inhibits local ECM production by reducing fibroblast proliferation, increasing local collagenase activity, and altering the cytokine environment of fibroblasts.[29,30] CCBs modify the release of cytokines, IL-6, IL-8, and plaque growth factor (PGF) and therefore reduce fibrinogenesis and the formation of a stable plaque. Verapamil was firstly used as an intralesional treatment for PD by Levine et al. in 1994.[31] In a nonrandomized study they used intralesional verapamil bi-weekly in 14 men for 6 months and reported about efficacy and toxicity of dose escalation. Intralesional verapamil in these men had no significant side effects and provided a significant improvement in plaque-associated narrowing, curvature, plaque volume, and firmness. Rehman et al.[32] published the first randomized, single-blind trial concerning intralesional verapamil used for the treatment of PD, and revealed improvement in erection quality and softening of plaque. Subsequently randomized placebo-controlled studies comparing intralesional verapamil to intralesional saline injections were performed. A total of 80 patients with PD were treated, but the verapamil group had failed to demonstrate any significant improvements in penile deformity, pain, plaque softening or sexual function.[33] Overall, inconsistent results regarding the use of intralesional verapamil have been reported in these studies. Discrepancies in patient selection, plaque size, plaque calcification, injection technique, and drug concentration have been noted. Probably, intralesional verapamil has a role as a non-surgical intervention for PD, but further randomized studies need to be undertaken to discover which patients will achieve the maximum benefit from this treatment modality.
Intralesional collagenase
The newest treatment of PD is intralesional injection of collagenase of Clostridium histolyticum (CCh). CCh is the only FDA approved treatment for PD. Collagenases are enzymes which catalyze the breakdown of collagen. This natural enzyme degrades type I and III collagen, which are the most abundant types found in the plaques formed in PD. CCh has also been found to directly increase apoptosis of fibroblasts to prevent tissue fibrosis. Clinicians may administer intralesional CCh in combination with modeling performed by the clinician and the patient for the reduction of penile curvature in patients with stable PD (patients with penile z >30° and <90°, and intact erectile function with or without the use of medications).[2] Direct injectable forms of CCh are marketed under Xiaflex, and Xiapex (Auxilium Pharmaceuticals in the USA and by Sobi in Europe, respectively) as a novel local therapy for PD. The impact of collagenase as a potential intralesional agent for PD treatment was first examined by Gelbard et al.[34] in the 1980s. Intralesional injection of CCh in 31 men showed clinical improvement in plaque size and resolution of penile pain within 4 weeks of treatment without significant adverse reactions.[34] Later, Gelbard’s group published a prospective double-blind placebo-controlled trial of intralesional CCh in 49 patients suffering from PD.[35] Results confirmed their previous findings and demonstrated significant reduction in penile curvature and plaque size, especially in patients with a penile curvature of less than 60° and plaques less than 4 cm in size.[35] More recently, the outcomes of Investigation for Maximal Peyronie’s Reduction Efficacy and Safety Studies (IMPRESS) I and II were published in the United States and Australia respectively to support the use of CCh for the treatment of PD. These large multi-institutional RCTs enrolled patients in a 6-week cycle of two intralesional CCh or saline injections followed by manual remodeling of the plaque. Enrolled patients were chronic phase PD patients with primarily mean dorsal curvatures of 50 degrees. Patients were evaluated with respect to penile length, plaque size, pain, erectile function scores, and PD Questionnaire (PDQ) values. Significant results at one-year follow-up were as follows: a mean improvement of 17° in penile curvature, and an improvement of erectile function and PDQ scores. CCh is associated with minor local adverse events including penile ecchymosis, swelling, and pain. Since serious adverse events did occur in six patients including three cases of corporeal ruptures requiring surgical repair and three penile hematomas, it has been recommended that patients should avoid intercourse for at least 2 weeks after intralesional CCh injection.[36,37] Since the IMPRESS trials, the safety and efficacy of CCh has been supported by a phase-3 open-label trial and it is currently approved by FDA for the intralesional use for the treatment of PD.
Corporal rupture (penile fracture) was reported as an adverse reaction in 5 of 1044 (0.5%) Xiaflex-treated patients in clinical studies.[38] In Xiaflex-treated 9 patients (0.9%), a combination of penile ecchymosis or hematoma, sudden penile detumescence, and/or a penile “popping” sound or sensation was reported, and in these cases, a diagnosis of corporal rupture could not be excluded. Severe penile hematoma was also reported as an adverse reaction in 39 of 1044 (3.7%) Xiaflex-treated patients.[38] Because of these side effects, clinicians should inform patients with PD prior to beginning treatment with intralesional CCh regarding potential occurrence of adverse events, including penile ecchymosis, swelling, pain, and corporal rupture.
Non-pharmacologic therapies
Penile traction devices
Penile traction devices (PTDs) have been studied as a treatment for straightening the penile curvature inflicted on men with PD. Specifically, researchers have been considering PTDs as a treatment during the acute phase of PD. Some studies have shown up to a 25-degree reduction in curvature, an improvement in sexual function, and a significantly lower risk of surgical indication.[39] It is likely that the penile traction devices will play a more important role in the future as part of combination therapy for early-stage PD.
Extracorporeal shock wave therapy
In recent years many investigators have examined the effects of extracorporeal shock wave therapy (ESWT) for its potential effects on PD. Although molecular mechanism of action of ESWT has not been clearly defined, shock waves are used to disrupt the dense scar tissue.[40] No improvement in penile curvature deformity or plaque size has been shown in placebo-controlled trials using ESWT. Compared to baseline, ESWT patients had not demonstrated significant changes in mean plaque size or mean penile curvature deformity.[15,40] Even with modern modifications, the most recent studies have not observed any significant improvement with this treatment option.[25] ESWT is not currently used as a treatment option for PD, however alterations to the technique and further studies may show some benefit of ESWT in the treatment of chronic plaques. As recommended, clinicians should not use ESWT for the reduction of penile curvature or plaque size.[2]
Stem cell treatment
Innovative treatment strategies are looking at alternative ways to treat PD. In the age of individualized medicine, the time has come to treat PD according to each patient’s specific needs, disease subtype, and likelihood of progression. Lin and Lue have been using stem cells to treat PD.[41] Similarly, Castiglione’s group has been using stem cells to assess improvement of both PD and ED in rat models. Despite lack of any long-term results, they showed that they could induce PD and ED with TGF-β injections. Still when they used human adipose tissue-derived stem cells, they noted a reduction in fibrosis and improvement in erectile function.[42] Therefore, early treatment with stem- cell therapy seems to have a positive effect on diseased TA cells.[43] This is very promising for treatment of PD in men. This would be a minimally invasive approach that could treat both PD and ED simultaneously.
Conclusion
Currently nonsurgical treatment options for PD are suboptimal and can leave patients with physical and psychological morbidity. Many treatments discussed above should have provided more benefit given their proposed mechanism of action. To determine a more effective treatment option, one must first fully understand fully the cellular pathophysiologic basis of this disease. Preventing plaque formation would be a much more effective approach than attempting to reduce the size of an already calcified scar. Further research should investigate human cells at a molecular level and novel fibrotic pathway inhibitors. When this is accomplished, the early recognition of PD patients with their disease-specific issues will allow physicians to select optimal treatment approach for their patients.
Footnotes
Peer-review: This manuscript was prepared upon request of the Editorial Board and its scientific evaluation was carried out by the Editorial Board.
Author Contributions: Concept – Ö.C.; Design – R.A.T., M.A.I.; Supervision – Ö.C.; Resources – R.A.T., M.A.I.; Materials – R.A.T.; Data Collection and/or Processing – R.A.T., M.A.I.; Analysis and/or Interpretation – R.A.T., Ö.C.; Literature Search – R.A.T., M.A.I.; Writing Manuscript – R.A.T., Ö.C.; Critical Review – R.A.T., Ö.C.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Mulhall JP, Creech SD, Boorjian SA, Ghaly S, Kim ED, Moty A, et al. Subjective and objective analysis of the prevalence of Peyronie’s disease in a population of men presenting for prostate cancer screening. J Urol. 2004;171:2350–3. doi: 10.1097/01.ju.0000127744.18878.f1. https://doi.org/10.1097/01.ju.0000127744.18878.f1. [DOI] [PubMed] [Google Scholar]
- 2.Nehra A, Alterowitz R, Culkin DJ, Faraday MM, Hakim LS, Heidelbaugh JJ, et al. Peyronie’s Disease: AUA Guideline. J Urol. 2015;194:745–53. doi: 10.1016/j.juro.2015.05.098. https://doi.org/10.1016/j.juro.2015.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lue TF. Peyronie’s disease: an anatomically-based hypothesis and beyond. Int J Impot Res. 2002;14:411–3. doi: 10.1038/sj.ijir.3900876. https://doi.org/10.1038/sj.ijir.3900876. [DOI] [PubMed] [Google Scholar]
- 4.Hatzimouratidis K, Eardley I, Giuliano F, Hatzichristou D, Moncada I, Salonia A, et al. EAU guidelines on penile curvature. Eur Urol. 2012;62:543–52. doi: 10.1016/j.eururo.2012.05.040. https://doi.org/10.1016/j.eururo.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 5.Hellstrom WJ, Bivalacqua TJ. Peyronie’s disease: etiology, medical, and surgical therapy. J Androl. 2000;21:347–54. [PubMed] [Google Scholar]
- 6.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. https://doi.org/10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davila HH, Ferrini MG, Rajfer J, Gonzalez-Cadavid NF. Fibrin as an inducer of fibrosis in the tunica albuginea of the rat: a new animal model of Peyronie’s disease. BJU Int. 2003;91:830–8. doi: 10.1046/j.1464-410x.2003.04224.x. https://doi.org/10.1046/j.1464-410X.2003.04224.x. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Cadavid NF, Rajfer J. Mechanisms of Disease: new insights into the cellular and molecular pathology of Peyronie’s disease. Nat Clin Pract Urol. 2005;2:291–7. doi: 10.1038/ncpuro0201. https://doi.org/10.1038/ncpuro0201. [DOI] [PubMed] [Google Scholar]
- 9.Sikka SC, Hellstrom WJ. Role of oxidative stress and antioxidants in Peyronie’s disease. Int J Impot Res. 2002;14:353–60. doi: 10.1038/sj.ijir.3900880. https://doi.org/10.1038/sj.ijir.3900880. [DOI] [PubMed] [Google Scholar]
- 10.Gelbard MK, Dorey F, James K. The natural history of Peyronie’s disease. J Urol. 1990;144:1376–9. doi: 10.1016/s0022-5347(17)39746-x. [DOI] [PubMed] [Google Scholar]
- 11.Furst DE, Munster T. Nonsteroidal anti-inflammatory drugs, disease-modifying anti-rheumatic drugs, non-opioid analgesics & drugs used in gout. In: Bertram G, editor. Basic and Clinical Pharmacology. Katzung Lange; New York: 2001. [Google Scholar]
- 12.Ehrlich HP, Bornstein P. Microtubules in transcellular movement of procollagen. Nat New Biol. 1972;238:257–60. doi: 10.1038/newbio238257a0. https://doi.org/10.1038/newbio238257a0. [DOI] [PubMed] [Google Scholar]
- 13.Akkus E, Carrier S, Rehman J, Breza J, Kadioglu A, Lue TF. Is colchicine effective in Peyronie’s disease? A pilot study. Urology. 1994;44:291–5. doi: 10.1016/s0090-4295(94)80155-x. https://doi.org/10.1016/S0090-4295(94)80155-X. [DOI] [PubMed] [Google Scholar]
- 14.Kadioglu A, Tefekli A, Koksal T, Usta M, Erol H. Treatment of Peyronie’s disease with oral colchicine: long-term results and predictive parameters of successful outcome. Int J Impot Res. 2000;12:169–75. doi: 10.1038/sj.ijir.3900519. https://doi.org/10.1038/sj.ijir.3900519. [DOI] [PubMed] [Google Scholar]
- 15.Hauck EW, Diemer T, Schmelz HU, Weidner W. A critical analysis of nonsurgical treatment of Peyronie’s disease. Eur Urol. 2006;49:987–97. doi: 10.1016/j.eururo.2006.02.059. https://doi.org/10.1016/j.eururo.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 16.Safarinejad MR, Asgari MA, Hosseini SY, Dadkhah F. A double-blind placebo-controlled study of the efficacy and safety of pentoxifylline in early chronic Peyronie’s disease. BJU Int. 2010;106:240–8. doi: 10.1111/j.1464-410X.2009.09041.x. https://doi.org/10.1111/j.1464-410X.2009.09041.x. [DOI] [PubMed] [Google Scholar]
- 17.Teloken C, Rhoden EL, Grazziotin TM, Ros CT, Sogari PR, Souto CA. Tamoxifen versus placebo in the treatment of Peyronie’s disease. J Urol. 1999;162:2003–5. doi: 10.1016/S0022-5347(05)68087-1. https://doi.org/10.1016/S0022-5347(05)68087-1. [DOI] [PubMed] [Google Scholar]
- 18.Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983;63:1420–80. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- 19.Vernet D, Ferrini MG, Valente EG, Magee TR, Bou-Gharios G, Rajfer J, et al. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie’s fibrotic plaque and in its rat model. Nitric Oxide. 2002;7:262–76. doi: 10.1016/s1089-8603(02)00124-6. https://doi.org/10.1016/S1089-8603(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 20.Valente EG, Vernet D, Ferrini MG, Qian A, Rajfer J, Gonzalez-Cadavid NF. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie’s fibrotic plaque and related fibroblast cultures. Nitric Oxide. 2003;9:229–44. doi: 10.1016/j.niox.2003.12.002. https://doi.org/10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Ferrini MG, Kovanecz I, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Effects of long-term vardenafil treatment on the development of fibrotic plaques in a rat model of Peyronie’s disease. BJU Int. 2006;97:625–33. doi: 10.1111/j.1464-410X.2006.05955.x. https://doi.org/10.1111/j.1464-410X.2006.05955.x. [DOI] [PubMed] [Google Scholar]
- 22.Ozturk U, Yesil S, Goktug HN, Gucuk A, Tuygun C, Sener NC, et al. Effects of sildenafil treatment on patients with Peyronie’s disease and erectile dysfunction. Ir J Med Sci. 2014;183:449–53. doi: 10.1007/s11845-013-1036-5. https://doi.org/10.1007/s11845-013-1036-5. [DOI] [PubMed] [Google Scholar]
- 23.Chung E, Deyoung L, Brock GB. The role of PDE5 inhibitors in penile septal scar remodeling: assessment of clinical and radiological outcomes. J Sex Med. 2011;8:1472–7. doi: 10.1111/j.1743-6109.2011.02217.x. https://doi.org/10.1111/j.1743-6109.2011.02217.x. [DOI] [PubMed] [Google Scholar]
- 24.Duncan MR, Berman B, Nseyo UO. Regulation of the proliferation and biosynthetic activities of cultured human Peyronie’s disease fibroblasts by interferons-alpha, -beta and -gamma. Scand J Urol Nephrol. 1991;25:89–94. doi: 10.3109/00365599109024539. https://doi.org/10.3109/00365599109024539. [DOI] [PubMed] [Google Scholar]
- 25.Hellstrom WJ, Kendirci M, Matern R, Cockerham Y, Myers L, Sikka SC, et al. Single-blind, multicenter, placebo controlled, parallel study to assess the safety and efficacy of intralesional interferon alpha-2B for minimally invasive treatment for Peyronie’s disease. J Urol. 2006;176:394–8. doi: 10.1016/S0022-5347(06)00517-9. https://doi.org/10.1016/S0022-5347(06)00517-9. [DOI] [PubMed] [Google Scholar]
- 26.Wegner HE, Andresen R, Knispel HH, Miller K. Local interferon-alpha 2b is not an effective treatment in early-stage Peyronie’s disease. Eur Urol. 1997;32:190–3. [PubMed] [Google Scholar]
- 27.Kendirci M, Usta MF, Matern RV, Nowfar S, Sikka SC, Hellstrom WJ. The impact of intralesional interferon alpha-2b injection therapy on penile hemodynamics in men with Peyronie’s disease. J Sex Med. 2005;2:709–15. doi: 10.1111/j.1743-6109.2005.00110.x. https://doi.org/10.1111/j.1743-6109.2005.00110.x. [DOI] [PubMed] [Google Scholar]
- 28.Stewart CA, Yafi FA, Knoedler M, Mandava SH, McCaslin IR, Sangkum P, et al. Intralesional Injection of Interferon-alpha2b Improves Penile Curvature in Men with Peyronie’s Disease Independent of Plaque Location. J Urol. 2015;194:1704–7. doi: 10.1016/j.juro.2015.06.096. https://doi.org/10.1016/j.juro.2015.06.096. [DOI] [PubMed] [Google Scholar]
- 29.Mulhall JP, Anderson MS, Lubrano T, Shankey TV. Peyronie’s disease cell culture models: phenotypic, genotypic and functional analyses. Int J Impot Res. 2002;14:397–405. doi: 10.1038/sj.ijir.3900874. https://doi.org/10.1038/sj.ijir.3900874. [DOI] [PubMed] [Google Scholar]
- 30.Roth M, Eickelberg O, Kohler E, Erne P, Block LH. Ca2+ channel blockers modulate metabolism of collagens within the extracellular matrix. Proc Natl Acad Sci U S A. 1996;93:5478–82. doi: 10.1073/pnas.93.11.5478. https://doi.org/10.1073/pnas.93.11.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine LA, Merrick PF, Lee RC. Intralesional verapamil injection for the treatment of Peyronie’s disease. J Urol. 1994;151:1522–4. doi: 10.1016/s0022-5347(17)35291-6. [DOI] [PubMed] [Google Scholar]
- 32.Rehman J, Benet A, Melman A. Use of intralesional verapamil to dissolve Peyronie’s disease plaque: a long-term single-blind study. Urology. 1998;51:620–6. doi: 10.1016/s0090-4295(97)00700-0. https://doi.org/10.1016/S0090-4295(97)00700-0. [DOI] [PubMed] [Google Scholar]
- 33.Shirazi M, Haghpanah AR, Badiee M, Afrasiabi MA, Haghpanah S. Effect of intralesional verapamil for treatment of Peyronie’s disease: a randomized single-blind, placebo-controlled study. Int Urol Nephrol. 2009;41:467–71. doi: 10.1007/s11255-009-9522-4. https://doi.org/10.1007/s11255-009-9522-4. [DOI] [PubMed] [Google Scholar]
- 34.Gelbard MK, Lindner A, Kaufman JJ. The use of collagenase in the treatment of Peyronie’s disease. J Urol. 1985;134:280–3. doi: 10.1016/s0022-5347(17)47123-0. [DOI] [PubMed] [Google Scholar]
- 35.Gelbard MK, James K, Riach P, Dorey F. Collagenase versus placebo in the treatment of Peyronie’s disease: a double-blind study. J Urol. 1993;149:56–8. doi: 10.1016/s0022-5347(17)35998-0. [DOI] [PubMed] [Google Scholar]
- 36.Gelbard M, Hellstrom WJ, McMahon CG, Levine LA, Smith T, Tursi J, et al. Baseline characteristics from an ongoing phase 3 study of collagenase clostridium histolyticum in patients with Peyronie’s disease. J Sex Med. 2013;10:2822–31. doi: 10.1111/jsm.12312. https://doi.org/10.1111/jsm.12312. [DOI] [PubMed] [Google Scholar]
- 37.Lipshultz LI, Goldstein I, Seftel AD, Kaufman GJ, Smith TM, Tursi JP, et al. Clinical efficacy of collagenase Clostridium histolyticum in the treatment of Peyronie’s disease by subgroup: results from two large, double-blind, randomized, placebo-controlled, phase III studies. BJU Int. 2015;116:650–6. doi: 10.1111/bju.13096. https://doi.org/10.1111/bju.13096. [DOI] [PubMed] [Google Scholar]
- 38.Available from: http://www.endo.com/prescribing information.
- 39.Martinez-Salamanca JI, Egui A, Moncada I, Minaya J, Ballesteros CM, Del Portillo L, et al. Acute phase Peyronie’s disease management with traction device: a nonrandomized prospective controlled trial with ultrasound correlation. J Sex Med. 2014;11:506–15. doi: 10.1111/jsm.12400. https://doi.org/10.1111/jsm.12400. [DOI] [PubMed] [Google Scholar]
- 40.Palmieri A, Imbimbo C, Longo N, Fusco F, Verze P, Mangiapia F, et al. A first prospective, randomized, double-blind, placebo-controlled clinical trial evaluating extracorporeal shock wave therapy for the treatment of Peyronie’s disease. Eur Urol. 2009;56:363–9. doi: 10.1016/j.eururo.2009.05.012. https://doi.org/10.1016/j.eururo.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Lin CS, Lue TF. Adipose-derived stem cells for the treatment of Peyronie’s disease? Eur Urol. 2013;63:561–2. doi: 10.1016/j.eururo.2012.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castiglione F, Hedlund P, Van der Aa F, Bivalacqua TJ, Rigatti P, Van Poppel H, et al. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie’s disease. Eur Urol. 2013;63:551–60. doi: 10.1016/j.eururo.2012.09.034. https://doi.org/10.1016/j.eururo.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin CS, Xin ZC, Wang Z, Deng C, Huang YC, Lin G, et al. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev. 2012;21:343–51. doi: 10.1089/scd.2011.0303. https://doi.org/10.1089/scd.2011.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]