Abstract
Laparoscopic surgery is routinely used to treat many urological conditions and it is the gold standard treatment option for many surgeries such as radical nephrectomy. Due to the difficulty of learning, laparoscopic training should start outside the operating room. Although it is a very different model of laparoscopic training; the aim of this review is to show the value of human cadaveric model for laparoscopic training and present our experience in this area. Fresh frozen cadaveric model in laparoscopic training, dry lab, cadaveric model, animal models and computer based simulators are the most commonly used models for laparoscopic training. Cadaveric models mimic the live setting better than animal models. Also, it is the best way in demonstrating important anatomic landmarks like prostate, bladder, and pelvic lymph nodes templates. However, cadaveric training is expensive, and should be used by multiple disciplines for higher efficiency. The laparosopic cadaveric training starts with didactic lectures with introduction of pelvic surgical anatomy. It is followed by hands-on dissection. A typical pelvic dissection part can be completed in 6 hours. Surgical robot and some laparoscopy platforms are equipped with 3-D vision. In recent years, we have use the stereoscopic laparoscopy system for training purposes to show exact anatomic landmarks. Cadavers are removed from their containers 3 to 5 days prior to training session to allow enough time for thawing. Intracorporeal suturing is an important part of laparoscopic training. We believe that suturing must be practiced in the dry lab, which is significantly cheaper than cadaveric models. Cadaveric training model should focus on the anatomic dissection instead. In conclusion, fresh-frozen cadaveric sample is one of the best 3D simulation models for laparoscopic training purposes. Major aim of cadaveric training is not only mimicking the surgical technique but also teaching true anatomy. Lack of availability and higher financial cost are the two setbacks for the use of cadavers. Surgeon should learn basic laparoscopic skills with box trainers prior to cadaveric skill training.
Keywords: Cadaver, laparoscopic surgery, training
Introduction
Laparoscopic surgery has revolutionized modern urological surgery and it is routinely used to treat many urological conditions.[1] Since the first case of laparoscopic nephrectomy[2], laparoscopy has evolved significantly and it is now the gold standard treatment option replacing many open surgeries such as radical nephrectomy.[3] Despite availability of laparoscopy, the learning curve can be mastered gradually.[4] Most urology residents in Europe consider their laparoscopic skills to be inadequate.[1] This begs the question as to what can be done additionally in laparoscopic training. Laparoscopic training needs to be intensified and should start outside the operating room (OR).[5] Laparoscopic simulators[6], animal models[7] and cadaveric models[6,8] are the most commonly used training methods for basic training. In the practice of urologic surgery, laparoscopic surgery mostly can be used in upper retroperitoneal and pelvic regions. The aim of this review is both to show the value and details of human fresh frozen cadaveric model which is a well-known 3D model for laparoscopic training in kidney, prostate and bladder surgery, and present our experience in this area.
Fresh frozen cadaveric model in laparoscopic training in surgery
Dry lab, cadaveric model, animal models and computer based simulators are the most commonly used models for laparoscopic training. Dry lab allows basic training in a low- cost environment. However, it doesn’t mimic the live setting as well as the other aforementioned models. In urology, animal models are suboptimal due to the absence of prostate in many animal models. In addition, unlike human kidney, porcine kidney is not surrounded by fat tissue and minimal dissection allows the adequate exposure for nephrectomy. Therefore, cadaveric models mimic the live setting better than animal models. They provide 3D visual aspect of real human anatomy compared to the other training modalities. However, cadaveric training is expensive, and should be jointly used by multiple disciplines for higher efficiency.[9]
Different training models have been compared by general surgeons. Leblanc et al.[10] compared the laparoscopic skills during a laparoscopic colectomy training session. They divided the participants in two groups, where one group was trained by augmented reality simulators and the other group was trained with cadavers. Group trained with cadavers showed superior performance in hand-assisted sigmoid colectomy. Sharma et al.[11] reported similar findings favoring the cadaveric surgical training over virtual reality simulators, in which they compared the skill sets of three groups based on their surgical experiences. In a different study, Sharma et al.[6] assessed the baseline laparoscopic performance of two groups and then trained one group with cadaveric models. The practice group showed significant enhancement in laparoscopic skills compared to the control group. Cadaveric setting is used for robot- assisted surgery, laparoscopic, urologic surgery and minimally invasive stone surgery as well.[8,12,13] Vlaovic et al.[13] showed that a week long intensive laparoscopic and robotic urologic surgery training improves test scores significantly. McDougall et al.[12] reported that a five days of robotic training course which includes cadaveric training encourages the experienced laparoscopic urologists to incorporate robotic surgery into their practice. The efficiency of cadaver-based training has been demonstrated in obstetrics and gynecology as well.[14]
In addition to developing surgical skills, laparoscopic dissection- based teaching has been shown to allow better identification of anatomy by medical students and surgical residents.[4] The three dimensional environment of laparoscopic teaching effectively contributes to the teaching of abdominopelvic anatomy.[15] Also, cadaveric models have been used to assess the feasibility of new procedures such as perineal robot- assisted laparoscopic prostatectomy[16] and laparoscopic kidney transplantation[17] or evaluation of newer surgical instruments.[18]
Laparoscopic cadaveric radical nephrectomy model
Laparoscopic radical nephrectomy is recommended for patients with T2 tumours and localized renal masses not treatable by nephron-sparing surgery.[3] Torso of the fresh frozen cadaver is used for all abdominal training modules, including laparoscopic nephrectomy. Cadavers are removed from their containers 3 to 5 days prior to training session to allow enough time for thawing. The degree of thawing is important to provide best tissue for tactile perception and appropriate dissection. The torso is positioned in a modified (45 to 60 degrees) lateral decubitus position for transperitoneal nephrectomy. Standard flank positioning is used for retroperitoneal training. Three trocars are used for both retroperitoneal and transperitoneal approach. For right nephrectomy, an additional 4th trocar can be used if required for liver retraction. Standard torso positioning and duties of each person during training session (primary surgeon, camera assistant, instructor and observer) are shown in Figure 1. The other important issue is that ethical information concerning training on fresh-frozen cadaveric tissue should be fully, and accurately explained in detail to trainees before starting the surgery. The training session should be evaluated with psychometric analysis to obtain objective feedback and determine the quality of educational level.
Figure 1.
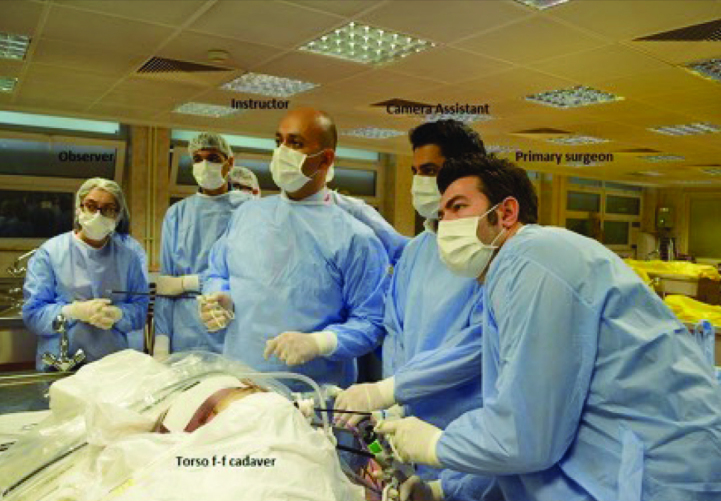
Positioning and setup for transperitoneal laparoscopic nephrectomy
The transperitoneal approach is preferred, especially for beginners, due to the larger workspace. Cadaveric Research on Laparoscopic Training (CRLT) study group identified the steps of transperitoneal cadaveric approach for training purpose on renal surgery. The steps and the duration periods for each section are; abdominal access and trocar insertion with intra-abdominal insufflations, insertion of abdominal trocars (30 min), identification of ureter (15 min), and renal artery and vein (15 min), performing pyeloplasty (45 min), and partial nephrectomy (excision of the tumor/kidney tissue-30 min, internal renoraphy-30 min, external renoraphy-30 min), and nephrectomy including application of endoclips on renal artery and vein (application of an endoclip on renal artery or renal vein-15 min, and nephrectomy-15 min). The procedure starts with the medialization of the colon. After the kidney is visualized, the ureter is identified and dissected to the posterior direction. We believe that the gonadal vein and the ureter must be dissected as distally as possible for training purposes. The gonadal vein must be dissected to the superior to find the renal vein. It is sometimes difficult to distinguish veins and arteries of a cadaveric model. The lack of oozing makes the dissection of the renal hilum easier than the live surgery. We prefer clipping the renal veins and the renal arteries separately, rather than using staplers. The rational for this is improving fine surgical skills similar to the dissection of distal ureter and the gonadal vein.
The majority of renal masses are diagnosed as organ-confined disease.[19] Therefore partial nephrectomy makes up a significant percentage of kidney surgeries for small renal masses. Intracorporeal suturing is an important part of laparoscopic training. We believe that suturing must be practiced in the dry lab, which is significantly cheaper than cadaveric models. By this way, surgeon will have adequate suturing skill during cadaveric surgery. Suturing can be practiced by incising the ureter, in addition to performing partial nephrectomy.
Anatomic laparoscopic prostatectomy model
Pelvic anatomy is one of the most complicated areas for urologists. Better understanding of pelvic anatomy results in achievement of better oncological and functional outcomes from operations. However, there is no satisfactory model in facilitating the teaching and learning pelvic anatomy. Pelvic trainers and computer-based simulators can teach basic laparoscopic skills. Cadaveric model is the best way in demonstrating important anatomic landmarks like prostate, bladder, and pelvic lymph nodes. The cadaver model is applicable for both open and laparoscopic surgical approaches. In the era of minimal invasive surgery, while laparoscopic and robot-assisted radical prostatectomies are commonly performed, the unique perspective of operating surgeon can only be clearly addressed in laparoscopic cadaver model. In this section, preparation, usage of cadaver model for laparoscopic pelvic surgery, and relevant expectations will be discussed. The training methodology of laparoscopic cadaveric training model of The Chinese University of Hong Kong will be introduced.
Due to the limited source of cadavers, we will use the torso only. The head and limbs will be removed and reserved for dissection workshops of ear nose throat and orthopedic surgeons. Therefore, this approach provides more cost-effectivity and efficient usage of cadaveric samples. The fresh-frozen cadaver model allows creation of pneumoperitoneum after adequate thawing, which is a critical part of laparoscopic training model. The body will be placed in Trendelenburg/head down position. Five ports will be inserted as shown in Figure 2. The camera port should be placed at umbilical level with working ports below in order to provide unlimited access to prostate apex.
Figure 2.
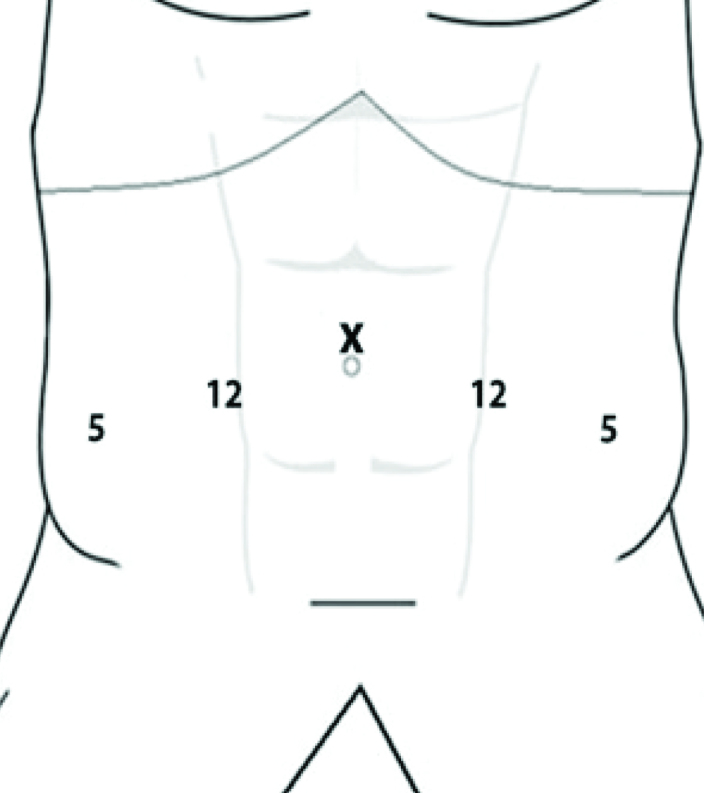
Port locations for laparoscopic pelvic dissection
The steps in laparoscopic cadaver dissection will be the same as laparoscopic or robotic radical prostatectomy. Transperitoneal approach is preferable due to exposure of larger working space and better appreciation of the anatomical relationship with different pelvic structures. Urethral catheterization may be difficult in some cases due to stiffness of tissues. It can be attempted in the later part of the dissection course after the body is sufficiently thawed and softened. After mobilization of bladder, the detrusor apron and pubovesical ligaments can be visualized. Special attention in cadaver training method is made on posterior dissection of the prostate, nerve-sparing technique and apical/urethral dissection. These steps are probably the most crucial part of radical prostatectomy to achieve trifecta outcomes.[20] Careful incision of Denonvillier’s fascia is practiced in order to prevent rectal injury. The anatomic relationship of seminal vesicles, and artery to vas deference, and the perirectal fat plane should be easily identified. Increased knowledge on the anatomy of the prostate, and neurovascular bundle results in good functional outcomes in continence and potency after radical prostatectomy. However, due to variable condition of the cadaver, the intra-, inter- and extra-fascial planes may be difficult to identify. Trainers should give clear instructions and guidance on this step. During the apical dissection of the prostate, the close proximity between urethra and rectum is emphasized. Good functional length of urethral stump should be preserved after apical dissection. Urethral anastomosis may be performed with intracorporeal suturing technique. However, laparoscopic suturing skill can be practiced in dry lab or animal models. Cadaveric training model should focus on the anatomic dissection instead. We should stress the importance of fascia-sparing radical prostatectomy (prostate in-out phenomen) as anatomic identification of nerve-sparing technique during the cadaveric training session, so as to show anatomic continuation of the pelvic, and prostatic fascial layers at the anterior, lateral and posterior aspects of the prostate.[21]
Anatomic laparoscopic cystectomy and bilateral extended pelvic lymph node dissection
For radical cystectomy, an anatomic dissection approach that follows the course of natural avascular plane is used. There are a few important anatomic landmarks, which should be identified for successful and bloodless operation. Ureter, medial umbilical ligament, and superior vesical artery should be firstly identified. The ureter can be found above iliac vessel. The dissection is continued along the ureter. On the lateral side of the ureter, medial umbilical ligament will be encountered. After transecting the medial umbilical ligament, superior vesical artery can be identified medial to the ureter. The bloodless plane can be developed posteriorly down to prostate apex and laterally down to endopelvic fascia. Vascular pedicle will be found at the posterior-lateral part of the bladder. The rest of the procedure is similar as radical prostatectomy.
There is evidence that bilateral pelvic lymph node dissection has both diagnostic and therapeutic values. Extended lymph node dissection up to ureter crossing over common iliac arteries or aortic bifurcation becomes the current “standard”. The number of lymph nodes which can be removed is related to the extent of the dissection template. Meticulous dissection is needed for complete removal of lymph node(s). Accidental injury to common and internal iliac veins can result in torrential bleeding. In cadaver- training model, the anatomy of iliac vein will be emphasized. In the cadaveric model, trainees can have a chance to fully expose the the entire course of iliac arteries and veins (Figure 3). The surgeons’ perspectives on open and minimal invasive cystectomy are entirely different. As compared with open approach, laparoscopic surgeons view the iliac vessels in higher and steeper angles (Figure 4). The lymph node packet behind the common iliac bifurcation, or fossa of Marcille, is largely obscured. This area needs to be approached from the lateral side of external iliac artery (Figure 5). Surgeons can get familiar with this specific area by using cadaveric training model.
Figure 3 a, b.
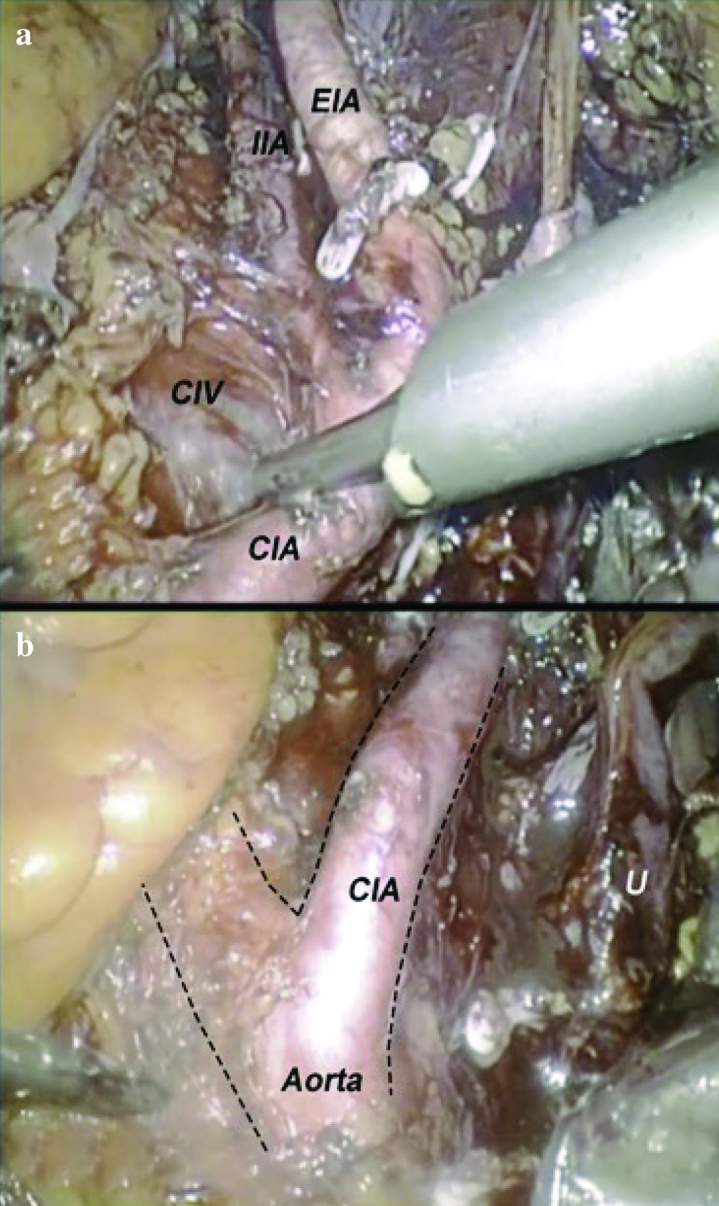
(a) Pelvic lymph node dissection. (i) EIA: external iliac artery, (ii) IIA: internal iliac artery, (iii) CIA: common iliac artery and (iv) CIV: common iliac vein. (b) aortic bifurcations and ureter (U) can be clearly identified
Figure 4 a, b.
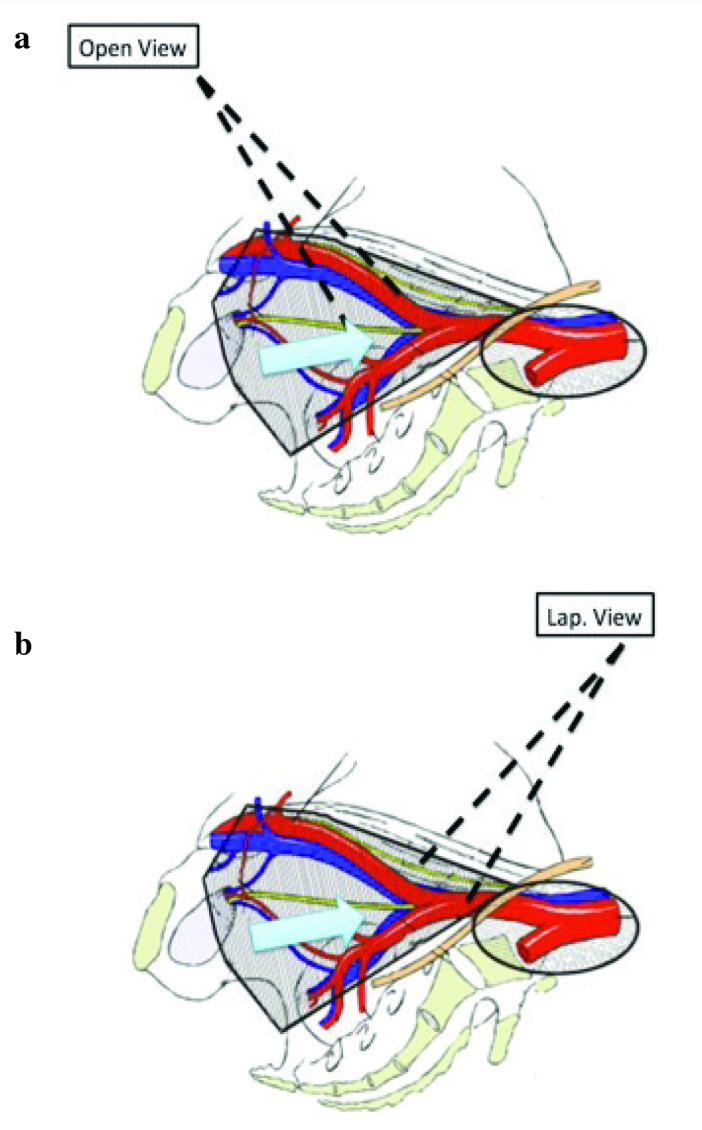
Angle of Marcille view from (a) anterior in open approach, and (b) umbilicus level in laparoscopic or robotic approach
Figure 5.
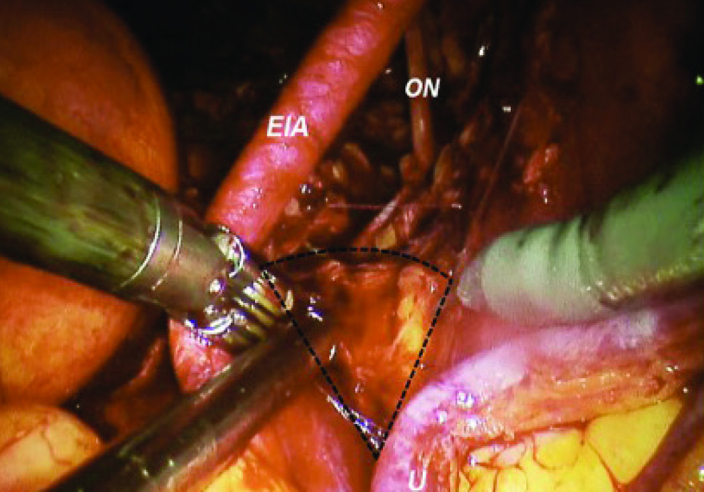
Lymph nodes at the angle of Marcille can be approached from lateral to the external and common iliac arteries (Photos at robot-assisted radical cystectomy)
The laparosopic cadaveric training starts with didactic lectures on the introduction of pelvic surgical anatomy. It is followed by hands-on dissection. A typical pelvic dissection can be completed in 6 hours. Surgical robot and some laparoscopy platforms are equipped with 3-D vision. In recent years, we have used the stereoscopic laparoscopy system for training purposes to show anatomic landmarks precisely (Figure 6). It provides sense of depth, which is very important for dissection and suturing in confined surgical field like pelvis. It also simulates the same working environment and allows the trainees to adopt and familiarize with the technology.
Figure 6.
Stereoscopic laparoscope facilitates the three dimensional perception of pelvic anatomy
Sorensen et al.[22] showed that 3D laparoscopic vision improves speed and reduces the number of performance mistakes when compared to 2D laparoscopic vision. In this manner, the efficacy and visual perception can be increased in fresh-frozen cadaveric model for laparoscopic training purpose by using 3D laparoscopic system.
In conclusion, fresh-frozen cadaveric sample is one of the best 3D simulation models for laparoscopic training purposes. Surgical anatomy training during the laparoscopic surgery can be indicated as an advantage of this model. It has been well established that cadaveric models are suited for laparoscopic training better than other models. Major aim of cadaveric training is not only mimicking the surgical technique but also teaching the true life anatomy. Lack of availability and higher financial cost are the two setbacks for the use of cadavers. Surgeons should learn basic laparoscopic skills with box trainers prior to cadaveric skill training.
Acknowledgements
The authors thank to Erdem Canda, Özer Ural Çakıcı ve İlkan Tatar, for their academic support at training sessions.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.H.; Design - E.H., E.C.; Supervision - E.H., E.C.; Resources - E.H., M.E.; Materials - E.H.; Data Collection and/or Processing - M.E.; Analysis and/or Interpretation - E.H., E.C.; Literature Search - E.H., M.E.; Writing Manuscript - E.H., E.C.; Critical Review - E.C.; Other - E.H., M.E., E.C.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Furriel FT, Laguna MP, Figueiredo AJ, Nunes PT, Rassweiler JJ. Training of European urology residents in laparoscopy: results of a pan-European survey. BJU Int. 2013;112:1223–8. doi: 10.1111/bju.12410. https://doi.org/10.1111/bju.12410. [DOI] [PubMed] [Google Scholar]
- 2.Albala DM, Kavoussi LR, Clayman RV. Laparoscopic nephrectomy. Semin Urol. 1992;10:146–51. [PubMed] [Google Scholar]
- 3.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–24. doi: 10.1016/j.eururo.2015.01.005. https://doi.org/10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 4.ten Brinke B, Klitsie PJ, Timman R, Busschbach JJ, Lange JF, Kleinrensink GJ. Anatomy education and classroom versus laparoscopic dissection-based training: a randomized study at one medical school. Acad Med. 2014;89:806–10. doi: 10.1097/ACM.0000000000000223. https://doi.org/10.1097/ACM.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 5.Martin JA, Regehr G, Reznick R, MacRae H, Murnaghan J, Hutchison C, et al. Objective structured assessment of technical skill (OSATS) for surgical residents. Br J Surg. 1997;84:273–8. doi: 10.1046/j.1365-2168.1997.02502.x. https://doi.org/10.1046/j.1365-2168.1997.02502.x. [DOI] [PubMed] [Google Scholar]
- 6.Sharma M, Macafee D, Horgan AF. Basic laparoscopic skills training using fresh frozen cadaver: a randomized controlled trial. Am J Surg. 2013;206:23–31. doi: 10.1016/j.amjsurg.2012.10.037. https://doi.org/10.1016/j.amjsurg.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 7.La Torre M, Caruso C. The animal model in advanced laparoscopy resident training. Surg Laparosc Endosc Percutan Tech. 2013;23:271–5. doi: 10.1097/SLE.0b013e31828b895b. https://doi.org/10.1097/SLE.0b013e31828b895b. [DOI] [PubMed] [Google Scholar]
- 8.Prasad Rai B, Tang B, Eisma R, Soames RW, Wen H, Nabi G. A qualitative assessment of human cadavers embalmed by Thiel’s method used in laparoscopic training for renal resection. Anat Sci Educ. 2012;5:182–6. doi: 10.1002/ase.1267. https://doi.org/10.1002/ase.1267. [DOI] [PubMed] [Google Scholar]
- 9.Blaschko SD, Brooks HM, Dhuy SM, Charest-Shell C, Clayman RV, McDougall EM. Coordinated multiple cadaver use for minimally invasive surgical training. JSLS. 2007;11:403–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Leblanc F, Senagore AJ, Ellis CN, Champagne BJ, Augestad KM, Neary PC, et al. Hand-assisted laparoscopic sigmoid colectomy skills acquisition: augmented reality simulator versus human cadaver training models. J Surg Educ. 2010;67:200–4. doi: 10.1016/j.jsurg.2010.06.004. https://doi.org/10.1016/j.jsurg.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M, Horgan A. Comparison of fresh-frozen cadaver and high-fidelity virtual reality simulator as methods of laparoscopic training. World J Surg. 2012;36:1732–7. doi: 10.1007/s00268-012-1564-6. https://doi.org/10.1007/s00268-012-1564-6. [DOI] [PubMed] [Google Scholar]
- 12.McDougall EM, Corica FA, Chou DS, Abdelshehid CS, Uribe CA, Stoliar G, et al. Short-term impact of a robot-assisted laparoscopic prostatectomy ‘mini-residency’ experience on postgraduate urologists’ practice patterns. Int J Med Robot. 2006;2:70–4. doi: 10.1002/rcs.71. https://doi.org/10.1002/rcs.71. [DOI] [PubMed] [Google Scholar]
- 13.Vlaovic PD, Sargent ER, Boker JR, Corica FA, Chou DS, Abdelshehid CS, et al. Immediate impact of an intensive one-week laparoscopy training program on laparoscopic skills among postgraduate urologists. JSLS. 2008;12:1–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Levine RL, Kives S, Cathey G, Blinchevsky A, Acland R, Thompson C, et al. The use of lightly embalmed (fresh tissue) cadavers for resident laparoscopic training. J Minim Invasive Gynecol. 2006;13:451–6. doi: 10.1016/j.jmig.2006.06.011. https://doi.org/10.1016/j.jmig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Saberski ER, Orenstein SB, Matheson D, Novitsky YW. Real-time cadaveric laparoscopy and laparoscopic video demonstrations in gross anatomy: an observation of impact on learning and career choice. Am Surg. 2015;81:96–100. [PubMed] [Google Scholar]
- 16.Laydner H, Akca O, Autorino R, Eyraud R, Zargar H, Brandao LF, et al. Perineal robot-assisted laparoscopic radical prostatectomy: feasibility study in the cadaver model. J Endourol. 2014;28:1479–86. doi: 10.1089/end.2014.0244. https://doi.org/10.1089/end.2014.0244. [DOI] [PubMed] [Google Scholar]
- 17.He B, Mou L, Delriviere L, Hamdorf J. A human cadaver model for laparoscopic kidney transplant. Exp Clin Transplant. 2014;12:21–4. doi: 10.6002/ect.2013.0173. https://doi.org/10.6002/ect.2013.0173. [DOI] [PubMed] [Google Scholar]
- 18.Kaouk JH, Autorino R, Laydner H, Hillyer S, Yakoubi R, Isac W, et al. Robotic single-site kidney surgery: evaluation of second-generation instruments in a cadaver model. Urology. 2012;79:975–9. doi: 10.1016/j.urology.2012.02.004. https://doi.org/10.1016/j.urology.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–62. doi: 10.1016/j.juro.2008.09.017. https://doi.org/10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66(Suppl 5):83–94. doi: 10.1016/j.urology.2005.06.116. https://doi.org/10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 21.Huri E. Novel anatomical identification of nerve-sparing radical prostatectomy: fascial-sparing radical prostatectomy. Prostate Int. 2014;2:1–7. doi: 10.12954/PI.13038. https://doi.org/10.12954/PI.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorensen SM, Savran MM, Konge L, Bjerrum F. Three-dimensional versus two-dimensional vision in laparoscopy: a systematic review. Surg Endos. 2016;30:11–23. doi: 10.1007/s00464-015-4189-7. https://doi.org/10.1007/s00464-015-4189-7. [DOI] [PubMed] [Google Scholar]


