Abstract
Objective
Myelomeningocele (MMC) is one of the most common reason of neurogenic bladder dysfunction in children. Although neurogenic bladder dysfunction occurrence is related with bladder innervation, also there are some changes seen in the smooth muscle and neural cells of the bladder. Interstitial cells of Cajal (ICC) are the pacemaker cells found in organs with peristaltic activity. Although it has been shown that ICC are diminished in the rat urinary bladder with traumatic spinal cord injury, there is no data about ICC in fetal rat bladders with MMC. This study has been conducted to investigate the ICC in the bladders of fetal rats with retinoic acid induced MMC.
Materials and methods
Time dated pregnant Wistar albino rats were divided into 3 groups. In MMC group, dams were fed with gavage solution containing 60 mg/kg all-trans retinoic acid dissolved in olive oil on 10. embryologic day. Sham group animals were fed only olive oil. Control group dams were fed with standard rat chow. Fetuses were delivered by cesarean section and harvested on 22. embryologic day. MMC was identified by observing MMC sacs at the back of the fetuses. Distribution of ICCs were evaluated using immunohistochemical staining.
Results
ICCs were found in all groups, which have the same morphological features that had been described earlier in the gastrointestinal tract and the bladder. The density of the ICC in the MMC group was found to be significantly decreased when compared with the control and the sham groups (p<0.05).
Conclusion
The density of the ICC in the urinary bladder decreased in the neurogenic bladder developed in MMC.
Keywords: Bladder contractility, bladder dysfunction, interstitial cells of Cajal, myelomeningocele, neurogenic bladder
Introduction
A common variant of non-lethal neural tube defects is Myelomeningocele (MMC) resulting in the development of neurogenic bladder (NB) and bowel dysmotility.[1,2] Besides, the bladder innervation defects, several changes in the smooth muscle and neural cells have been described in histopathology reports of NB.[3,4] The interaction between the smooth muscle and neural cells is mediated through the interstitial cells of Cajal (ICC) which also controls bladder contractility.[5,6]
Interstitial cells of Cajal are the pacemaker cells found in human organs with peristaltic activity that were thought to be originating from the precursors of the smooth muscle cells.[7,8] Several studies have shown that ICCs are widely distributed in the urinary tract of animals and humans.[5,9–13] Although the gastrointestinal and bladder ICCs seem to share the same morphological and receptor expressions, they function differently.[14] Different neurological and functional conditions of the bladder have been associated with changes in the number of ICCs.[9] It has been shown that the number of ICCs decrease in the rat bladder after traumatic spinal cord injury.[12] Recently, the distribution of ICC in humans has been evaluated in children with neurogenic bladder.[13] However, there is no data on the presence and distribution of ICCs in fetal bladders with spinal cord injury due to MMC. Therefore, a study has been conducted to investigate the effect of neural injury on ICC in the bladders of fetal rats with retinoic acid- induced MMC.
Material and methods
The study protocol was approved by the Institutional Animal Care and Use Committee (13/2009). Time-dated pregnant Wistar albino rats (210–300 g) were used in this study. Before starting the experiment, time-dated primigravida Wistar albino rats were fed with standard rat chow and water, ad libitum. They were housed in separate cages with controlled temperature and 12 hour light/dark cycle for at least 1 week. There were 3 experimental groups;
Control group (n=5)
Comprised of fetuses of dams which did not undergo any treatment and manipulation.
Sham group (n=7)
Comprised of fetuses of dams fed with 2 mL olive oil through a feeding tube on the 10. embryologic day (E).
MMC Group (n=7)
Comprised of fetuses of dams fed with 60 mg/kg all-trans retinoic acid (Vesanoid, Roche, Basel, Switzerland) dissolved in 2 mL olive oil through a feeding tube on E 10 as described by Danzer et al.[15,16]
On the E 22, all dams were anesthetized with ether and the surviving fetuses were harvested by cesarean section, then the dams were sacrificed by cervical dislocation. Fetuses that had a MMC sac at their dorsum were included in the MMC group (Figure 1). Experiments were continued until 7 surviving rat fetuses were obtained in the MMC group. Fetal urinary bladders were harvested under ×10 magnification and the ICCs were evaluated in the anterior wall of corpus.
Figure 1.
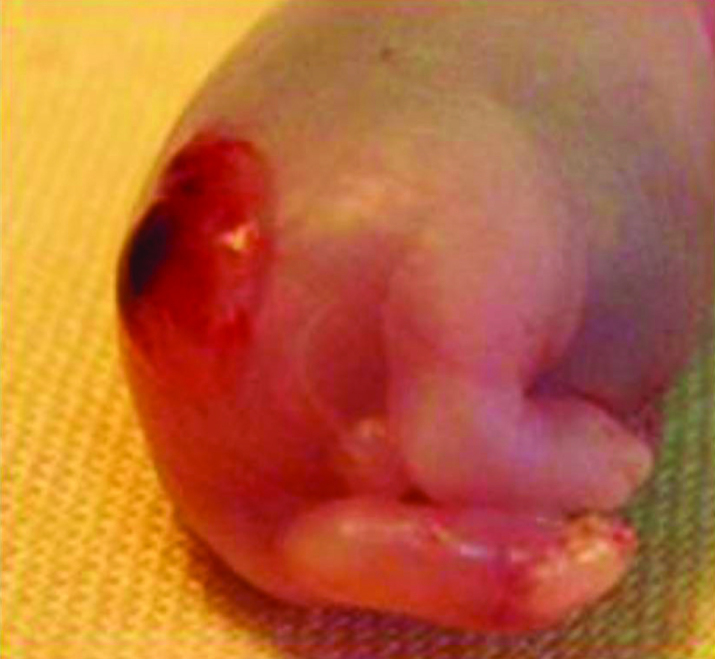
Macroscopic appearance of rat fetus with myelomeningocele
Histolopathological analysis
Fetal bladders were excised en bloc and fixed with 4% paraformaldehyde for 12 hours. Bladders were embedded in paraffin, 4 μm serial sections were obtained and mounted on positively charged slides. Slides were deparaffinized in xylene and used for the immunohistochemical study.
Immunoperoxidase staining was done to determine the c-kit (CD117) protein expressing cells. After deparaffinization, to expose the antigens, the tissue sections were boiled with 10 mM ethylenediamine tetraacetic acid (EDTA) buffer at pH 6.0 for 10–20 min followed by cooling down to room temperature for 20 min. Mouse Monoclonal Antibody c-kit Oncoprotein (CD117) (Novacastra, Newcastle, UK) primary antibody in a 1:50 dilution and an anti-rabbit immuno-conjugate secondary antibody were incubated with horseradish peroxide (HRP) - streptavidin solution (LSAB2 System-HRP, Code K0675; Dako Cytomation, Glostrup, Denmark). Slides were developed with 3-3′-diaminobenzidine (DAB; D4293; Sigma Aldrich), counterstained with Mayer’s hematoxylin, and examined under light microscope (Olympus BX-51). Brown coloration was accepted as positive staining.
Sections were also stained with 1% toluidin blue (CAS-92-31-9, Chem Cruz, Santa Cruz Biotechnology Inc., Texas, USA) to identify Cajal cells in c-kit positive mast cells. Negative control samples were identically stained with study samples without primary antibodies.
Under light microscope at 40 xmagnification, ICCs were counted on 10 different microscopic fields of view which were selected randomly by the pathologist and expressed as count per unit area (100 μm2). The pathologist was blinded with respect to experimental groups when preparing and evaluating the specimens. Morphological differences that had been described earlier in the gastrointestinal tract and the urinary bladder were used to distinguish the ICC from the mast cells.[5,17] The mean number of ICCs in 10 different microscopic fields of view were calculated for each fetus and scored to show the density of the ICC as: no count was scored as 0, 1–2 counts as 1, 3–4 counts as 2 and more than 4 counts as 3 points.
After ANOVA test, Tukey’s test was used for statistical comparisons among groups. Statistical level of significance was set at p<0.05.
Results
In the present study, MMC was induced using retinoic acid in 70% of the cases.
With immunohistochemical staining, two groups of cells with c-kit positive staining were determined in all groups as ICCs and mast cells. ICCs were found within the muscle fibers and suburothelium of the bladder. ICCs had fusiform cell bodies, large oval nuclei and two dendritic processes. Mast cells had large central round nuclei and they were localized at the mucosa and submucosal area (Figure 2). ICCs were found in all the groups (Figures 3 and 4).
Figure 2.

Histopathology of a fetal rat bladder with an arrow indicating a mast cell (C-kit, ×40)
Figure 3.
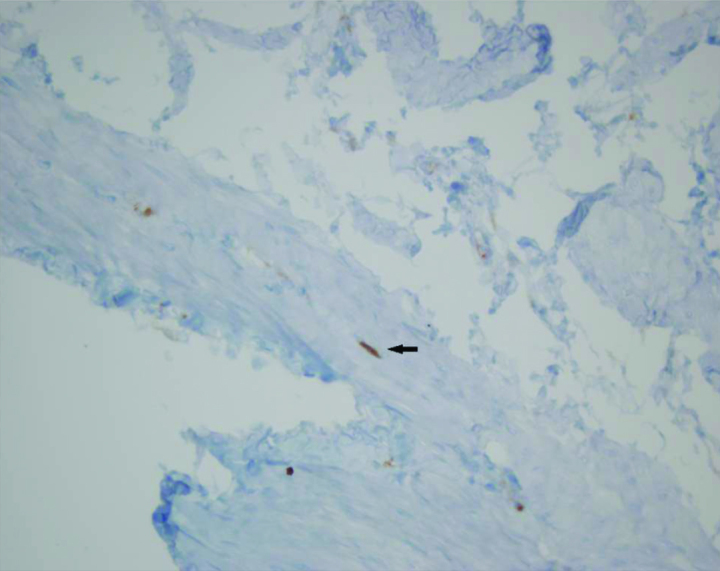
Histopathology of the fetal rat bladder of the control group (arrow indicates interstitial cells of Cajal located in the muscular layer with fusiform cell body, thin cytoplasm, wide ovoid nucleus and two dentritic processes) (C-kit, ×40)
Figure 4.
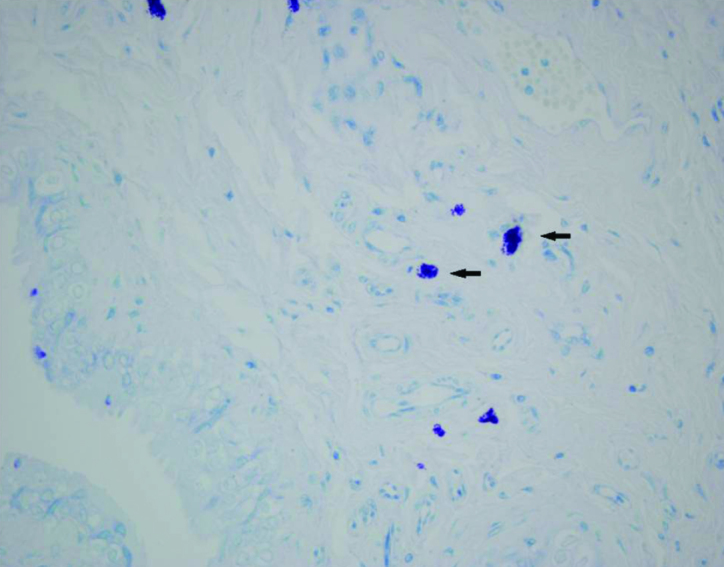
Histopathology of the fetal rat bladder of the myelomeningocele group (arrow indicates mast cells) (Toluidin blue, ×40)
The mean ICC score was 0.86±0.75 in the MMC, 2.14±0.89 in the sham and 2.00±0.71 in the control group, respectively. The ICC score of the MMC group was found to be significantly less when compared with the control and sham groups (p<0.05) (Figure 5).
Figure 5.
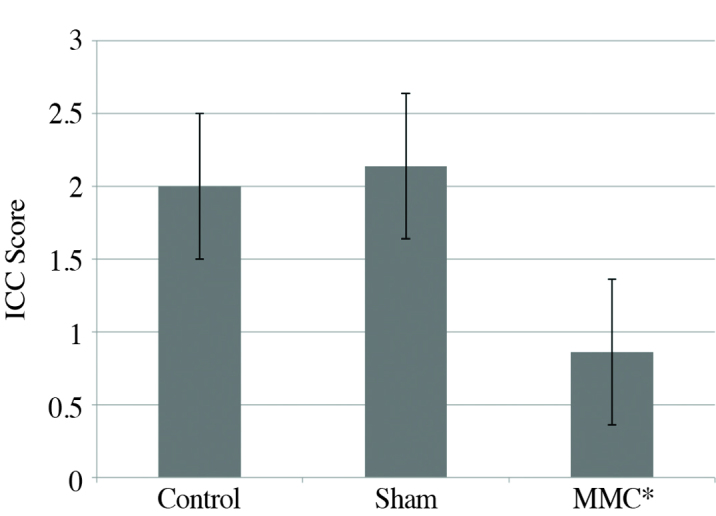
Scores of interstitial cells of Cajal in all groups (*myelomeningocele)
*p<0.05. When compared with the control and sham groups.
Discussion
Loss of voluntary control of micturition, detrusor/sphincter dyssynergia and bladder overactivity are the symptoms of NB dysfunction coexisting with MMC.[1,2] In addition to morphological problems of the urinary bladder, the other main problems in the MMC are the impairment of the contractility response of the smooth muscle cells and lack of the relaxation response against contractile forces.[16] It has been shown that programmed trans-differentiation from skeletal muscle cell to smooth muscle cell in the fetal detrusor occurs.[18] However, this differentiation was arrested in the fetuses with MMC. Intact neural support is necessary both for normal smooth muscle development and for survival of the ICC in the fetal bladder.[18]
The presence of ICCs in the urinary tract has been first described at guinea pig bladders by McCloskey and Gurney[19] in 2002 and at human urinary tract by Solari et al.[20] in 2003. Various studies have showed that ICCs are widely distributed in the urinary tract including renal pelvis, ureter, bladder and urethra.[20,21] Shafik et al.[22] displayed that ICC are abundant in the human bladder dome and appears to constitute the primary vesical pacemaker that initiates the slow waves spreading to the rest of the bladder wall. They also suggested that a deficiency or absence of these cells may be involved in the bladder motility disorders.
A close relation between the neurons and ICC has been shown in the detrusor wall.[12] Disruption in neural integrity has been resulted in concomitant degeneration of ICC. They suggested that existence and survival of ICC mainly rely on neural release of trophic factors.[12] A recent study by Johnston et al.[6] showed that the human bladder contains a network of ICCs in the lamina propria, which is connected to cholinergic nerve plexus. Perivascular ICCs are localized close to the vascular smooth muscle cells, whereas the detrusor ICCs are observed in smooth muscle bundles and associated with nerves, controlling the bladder contractility.[6]
Piaseczna-Piotrowska et al.[13] had examined the distribution of ICCs in different parts of the overactive neurogenic bladder wall of the children with MMC. They had found that the ICC are widely located in the trigone than the anterior wall of the corpus of the urinary bladder. Additionally there is no significant difference in the distribution of ICC between the normal and overactive neurogenic bladders. [13] In the present study, our aim was to show the effect of neural injury occurred due to MMC on the distribution of ICC in the detrusor of fetal rats. Contrary to the literature, we found that the density of ICC decreased significantly in the MMC group than the control and sham groups. We may hypothesize that neural trophic factors in fetal tissue may be also responsible from fetal development and/or survival of ICC.
Different neurological or functional conditions of urinary bladder have been associated with the increase or decrease in the number of ICCs. Relaxed, dilated, and unobstructed bladders are related with decreased number of ICCs. Conversely overactive and obstructed bladders are related to increased number of ICCs.[9,23,24] This phenomenon was explained as follows. The ICCs increased in number as a result of increased peristaltic activity so as to overcome the bladder outlet obstruction.[25] We found that fetal cord injury due to MMC decreases the number of ICCs in detrusor. It is difficult to predict which clinical form of neuropathic bladder will occur postnatally due to decrease in the number of ICCs in the fetal bladder. Our results may indicate relaxed hyper-compliant, and unobstructed bladder. However, changing clinical form to obstructed non-compliant bladder postnatally may associate with increase in the number of ICCs as a result of increased peristaltic activity to overcome the bladder outlet obstruction. Since the fetus has a tiny and very fragile bladder, we could not evaluate the volume and intravesical pressures of the fetal bladders. It was impossible to perform urodynamic studies on fetal rat bladders.
In the present study, we showed that ICCs are present in the bladders of both MMC and normal rat fetuses. When we compared the three groups of fetuses, we have found that the density of ICC in MMC fetuses was significantly less than the other groups. Nevertheless, either the quantitative or qualitative changes involving ICCs may cause detrusor dysfunction. We assume that the bladder dysfunction seen in MMC might be related to the decreased density of ICCs in the neurogenic bladder developed in MMC. For further studies should be conducted with larger-sized experimental animals, and the density of ICCs and the contractility of the detrusor should be evaluated in combination.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Animal Care and Use Committee of Dokuz Eylül University (13/2009).
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.T., G.H.; Design - G.H., F.M.A.; Supervision - G.H., O.A.; Resources - A.T., E.Ö., F.M.A.; Materials - A.T., E.Ö.; Data Collection and/or Processing - A.T., O.A., E.Ö., O.Z.K.; Analysis and/or Interpretation - A.T., O.Z.K., O.A., M.O.; Literature Search - O.Z.K., M.O., G.H.; Writing Manuscript - O.Z.K., F.M.A., M.O.; Critical Review - O.Z.K., M.O., F.M.A.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Yeung CK, Barker GM, Läckgren G. Pathophysiology of bladder dysfunction. In: Gearhart JP, Rink R, Mouriquand PDE, editors. Pediatric Urology. 2 Edition. Philadelphia: Saunders Elsevier; 2010. pp. 353–65. https://doi.org/10.1016/B978-1-4160-3204-5.00027-X. [Google Scholar]
- 2.Rien JM, Nijman ST. Pathophysiology of neurogenic bladder dysfunction. In: Ciro Esposito JMG, Gough D, Savanelli A, editors. Pediatric Neurogenic Bladder Dysfunction: Diagnosis, Treatment, Long-Term Follow-up. Berlin: Springer; 2006. pp. 33–8. [Google Scholar]
- 3.Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57–67. doi: 10.1016/s0090-4295(97)00591-8. https://doi.org/10.1016/S0090-4295(97)00591-8. [DOI] [PubMed] [Google Scholar]
- 4.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935–86. doi: 10.1152/physrev.00038.2003. https://doi.org/10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 5.Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–90. doi: 10.1097/01.ju.0000146272.80848.37. https://doi.org/10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- 6.Johnston L, Woolsey S, Cunningham RM, O’Kane H, Duggan B, Keane P, et al. Morphological expression of KIT positive interstitial cells of Cajal in human bladder. J Urol. 2010;184:370–7. doi: 10.1016/j.juro.2010.03.005. https://doi.org/10.1016/j.juro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders KM, Ordög T, Koh SD, Torihashi S, Ward SM. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. 1999;11:311–38. doi: 10.1046/j.1365-2982.1999.00164.x. https://doi.org/10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 8.Streutker CJ, Huizinga JD, Driman DK, Riddel RH. Interstitial cells of Cajal in health and disease. Part I: normal ICC structure and function with associated motility disorders. Histopathology. 2007;50:176–89. doi: 10.1111/j.1365-2559.2006.02493.x. https://doi.org/10.1111/j.1365-2559.2006.02493.x. [DOI] [PubMed] [Google Scholar]
- 9.McCloskey KD. Bladder interstitial cells: an updated review of current knowledge. Acta Physiol (Oxf) 2013;207:7–15. doi: 10.1111/apha.12009. https://doi.org/10.1111/apha.12009. [DOI] [PubMed] [Google Scholar]
- 10.Piotrowska AP, Rolle U, Chertin B, De Caluwe D, Bianchi A, Puri P. Alterations in smooth muscle contractile and cytoskeleton proteins and interstitial cells of Cajal in megacystis microcolon intestinal hypoperistalsis syndrome. J Pediatr Surg. 2003;38:749–55. doi: 10.1016/jpsu.2003.50159. https://doi.org/10.1016/jpsu.2003.50159. [DOI] [PubMed] [Google Scholar]
- 11.Piaseczna Piotrowska A, Rolle U, Solari V, Puri P. Interstitial cells of Cajal in the human normal urinary bladder and in the bladder of patients with megacystis-microcolon intestinal hypoperistalsis syndrome. BJU Int. 2004;94:143–6. doi: 10.1111/j.1464-410X.2004.04914.x. https://doi.org/10.1111/j.1464-410X.2004.04914.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnston L, Cunningham RM, Young JS, Fry CH, McMurray G, Eccles R, et al. Altered distribution of interstitial cells and innervation in the rat urinary bladder following spinal cord injury. J Cell Mol Med. 2012;16:1533–43. doi: 10.1111/j.1582-4934.2011.01410.x. https://doi.org/10.1111/j.1582-4934.2011.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piaseczna-Piotrowska A, Dzieniecka M, Samolewicz E, Lesniak D, Kulig A. Distribution of interstitial cells of Cajal in the neurogenic urinary bladders of children with myelomeningocele. Adv Med Sci. 2013;58:388–93. doi: 10.2478/ams-2013-0002. https://doi.org/10.2478/ams-2013-0002. [DOI] [PubMed] [Google Scholar]
- 14.Kanai A, Fry C, Hanna-Mitchell A, Birder L, Zabbarova I, Bijos D, et al. Do we understand anymore about bladder interstitial cells?-ICI-RS 2013. Neurol Urodyn. 2014;33:573–6. doi: 10.1002/nau.22591. https://doi.org/10.1002/nau.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danzer E, Schwarz U, Wehrli S, Radu A, Adzick NS, Flake AW. Retinoic acid induced myelomeningocele in fetal rats: characterization by histopathological analysis and magnetic resonance imaging. Exp Neurol. 2005;194:467–75. doi: 10.1016/j.expneurol.2005.03.011. https://doi.org/10.1016/j.expneurol.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Danzer E, Kiddoo DA, Redden RA, Robinson L, Radu A, Zderic SA, et al. Structural and functional characterization of bladder smooth muscle in fetal rats with retinoic acid-induced myelomeningocele. Am J Physiol Renal Physiol. 2007;292:197–206. doi: 10.1152/ajprenal.00001.2006. https://doi.org/10.1152/ajprenal.00001.2006. [DOI] [PubMed] [Google Scholar]
- 17.Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43. doi: 10.1146/annurev.physiol.68.040504.094718. https://doi.org/10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro E, Seller MJ, Lepor H, Kalousek DK, Hutchins GM, Perlman EJ, et al. Altered smooth muscle development and innervation in the lower genitourinary and gastrointestinal tract of the male human fetus with myelomeningocele. J Urol. 1998;160:1047–53. doi: 10.1097/00005392-199809020-00023. https://doi.org/10.1097/00005392-199809020-00023. [DOI] [PubMed] [Google Scholar]
- 19.McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–6. https://doi.org/10.1016/S0022-5347(05)64752-0. [PubMed] [Google Scholar]
- 20.Solari V, Piotrowska AP, Puri P. Altered expression of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. J Urol. 2003;170:2420–2. doi: 10.1097/01.ju.0000097401.03293.f0. https://doi.org/10.1097/01.ju.0000097401.03293.f0. [DOI] [PubMed] [Google Scholar]
- 21.McCloskey KD. Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol. 2011;202:233–54. doi: 10.1007/978-3-642-16499-6_11. https://doi.org/10.1007/978-3-642-16499-6_11. [DOI] [PubMed] [Google Scholar]
- 22.Shafik A, El-Sibai O, Shafik AA, Shafik I. Identification of interstitial cells of Cajal in human urinary bladder: concept of vesical pacemaker. Urology. 2004;64:809–13. doi: 10.1016/j.urology.2004.05.031. https://doi.org/10.1016/j.urology.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 23.Deng T, Zhang Q, Wang Q, Zhong X, Li L. Changes in hyperpolarization-activated cyclic nucleotide-gated channel expression and activity in bladder interstitial cells of Cajal from rats with detrusor overactivity. Int Urogynecol J. 2015;26:1139–45. doi: 10.1007/s00192-015-2632-x. https://doi.org/10.1007/s00192-015-2632-x. [DOI] [PubMed] [Google Scholar]
- 24.Patra PB, Patra S. Research findings on overactive bladder. Curr Urol. 2014;8:1–21. doi: 10.1159/000365682. https://doi.org/10.1159/000365682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubota Y, Kojima Y, Shibata Y, Imura M, Sasaki S, Kohri K. Role of KIT-positive interstitial cells of Cajal in the urinary bladder and possible therapeutic target for overactive bladder. Adv Urol. 2011;2011:816342. doi: 10.1155/2011/816342. https://doi.org/10.1155/2011/816342. [DOI] [PMC free article] [PubMed] [Google Scholar]


