Abstract
Purpose
The health-related quality of life (HRQoL) of patients completing multidrug-resistant tuberculosis (MDR-TB) treatment in Namibia and whether the occurrence of adverse events influenced patients’ rating of their HRQoL was evaluated.
Patients and methods
A cross-sectional analytic survey of patients completing or who recently completed MDR-TB treatment was conducted. The patients rated their HRQoL using the simplified Short Form-™ (SF-8) questionnaire consisting of eight Likert-type questions. Three supplemental questions on the adverse events that the patients may have experienced during their MDR-TB treatment were also included. Scoring of HRQoL ratings was norm-based (mean =50, standard deviation =10) ranging from 20 (worst health) to 80 (best health), rather than the conventional 0–100 scores. We evaluated the internal consistency of the scale items using the Cronbach’s alpha, performed descriptive analyses, and analyzed the association between the patients’ HRQoL scores and adverse events.
Results
Overall, 36 patients (20 males, 56%) aged 17–54 years (median =40 years) responded to the questionnaire. The median (range) HRQoL score for the physical component summary was 58.6 (35.3–60.5), while the median score for the mental component summary was 59.3 (26.6–61.9), indicating not-so-high self-rating of health. There was good internal consistency of the scale scores, with a Cronbach’s alpha value of >0.80. In all, 32 (89%) of the 36 patients experienced at least one adverse drug event of any severity during their treatment (median events =3, range 1–6), of which none was life-threatening. The occurrence of adverse events was not related to HRQoL scores. For patients reporting zero to two events, the median (range) HRQoL score was 56.8 (44.4–56.8), while for those reporting three or more events, the median score was 55.2 (38.6–56.8); P=0.34 for difference between these scores.
Conclusion
Patients completing treatment for MDR-TB in Namibia tended to score moderately low on their HRQoL, using the generic SF-8 questionnaire. The occurrence of adverse events did not lead to lower HRQoL scores upon treatment completion.
Keywords: drug safety, patient-reported health outcomes, SF-8™ questionnaire, second-line tuberculosis drugs, Namibia
Video abstract
Introduction
Multidrug-resistant tuberculosis (MDR-TB) has become a major public health problem, especially in developing countries, where the MDR-TB burden is the highest.1 Unlike the treatment of drug-sensitive Mycobacterium tuberculosis, the treatment of MDR-TB takes a long time, is complex and is frequently associated with the occurrence of various adverse drug reactions.2–8 Some of these adverse drug reactions, such as ototoxicity, nephrotoxicity, and hepatotoxicity, could severely diminish a person’s health-related quality of life (HRQoL).9–12 Besides, the success rates of global MDR-TB treatment have been generally poor, at ~48%, due to several factors that included the patients’ difficulties with adhering to prescribed MDR-TB treatment regimens.13,14 The occurrence of severe or serious treatment-related adverse events, along with other disease-related sequelae, may impair patients’ ability to perform activities of daily life during or after MDR-TB treatment.12 This calls for the routine assessment of the HRQoL of patients undergoing MDR-TB treatment.12
Over the past decade, there has been an increasing interest on the impact of tuberculosis (TB) treatment on patient’s HRQoL.12,15 However, most of the research published on this topic has primarily focused on drug-sensitive TB. For example, out of the 27 studies reviewed by Brown et al,12 only one study pertained to MDR-TB. Similarly, in the systematic review by Guo et al,15 only one study included patients diagnosed with drug-resistant TB. Notably, none of these studies analyzed the relationship between patients’ HRQoL and the occurrence of adverse events in the context of MDR-TB treatment.
Several instruments for measuring patients’ HRQoL have been used in previous studies.12,15 The instruments include the Short Form-36 (SF-36) questionnaire, the World Health Organization Quality of Life-BREF tool (WHOQOL-BREF), the EuroQol five dimensions questionnaire (EQ-5D), the EuroQol visual analogue scale (EQ-VAS), the Dhingra and Rajpal-12 questionnaire (DR-12), the Functional Assessment of Chronic Illness Therapy-TB questionnaire (FACIT-TB), the Liebowitz Social Anxiety Scale, and the Airway Questionnaire 20. All these instruments except the EQ-5D and the EQ-VAS are fairly long and require substantial effort by the respondent to complete.
We searched for short versions of the generic HRQoL questionnaires and identified the SF-8™ (SF-8) questionnaire, developed by QualityMetric. The SF-8 questionnaire is the shortest of the short form family of HRQoL questionnaires and has one question for each of the eight concepts (health dimensions) that are measured by the longer version of SF-36 questionnaire.16 This questionnaire has been tested for reliability and validated in two large settings in Uganda17 and in Japan (among teachers after enforcement of a smoke-free school policy).18
To date, no published study has evaluated the association between the reporting of adverse events and patients’ HRQoL scores at the end of MDR-TB treatment. Our study objective, therefore, was to investigate the impact of adverse events on perceived HRQoL in patients at the end of MDR-TB treatment in Namibia using the SF-8 questionnaire.
Patients and methods
Study design and patient selection
This was a cross-sectional analytic survey conducted among a consecutive sample of patients treated for MDR-TB in Namibia. The patients were considered eligible for the study if they were treated for MDR-TB using second-line drugs, were at the age of 16 years or older, were in their final month of treatment or had completed their treatment within the past 3 months, were reachable, and were willing to participate in the study. Those who defaulted or did not complete treatment were not considered for the study. Participants were recruited as outpatients using the MDR-TB register maintained at the main clinic where they received their treatment. For persons who had finished their treatment within the past 3 months, the nurse at the clinic called their phone numbers, inviting them to participate in the survey. Since there were few eligible patients, each patient was approached to participate in the study. The target sample size was 138 patients. This was determined based on the following assumptions: anticipated minimum score differences of 0.5 to consider change when assessing differences between a group mean and a fixed norm, an alpha score of 0.05, two-tailed t-test, and a statistical power of 80%.19
Setting and MDR-TB treatment
The study was conducted within the public sector health service of Namibia. Once diagnosed with MDR-TB infection, the patients were admitted to the MDR-TB treatment ward nearest to them where they were initiated on the intensive phase of treatment that included a course of kanamycin injections and at least four other oral second-line anti-TB drugs for at least 6 months until the patient converted to sputum smear and culture negative. The oral anti-TB drugs used were cycloserine, ethionamide, levofloxacin, pyrazinamide, and sometimes ethambutol. This drug regimen was in accordance with Namibia’s clinical guidelines for the treatment of MDR-TB that were current at the time of the study.20 After the intensive phase, the patients were discharged on oral anti-TB drugs and referred to the outpatient clinic closest to them for their continuation phase of treatment. This treatment phase often lasted for at least 12 months depending on how long it took the patient to be cured of the infection.20 The continuation phase treatment was administered daily on an outpatient basis at a hospital, health center, or clinic nearest to the patient and was supervised by a nurse. A medical doctor periodically reviewed the patients for their progress on treatment. The patients were prompted to report to the medical doctor or nurse any adverse events or concerns they had regarding their medication, throughout the course of their treatment. As part of routine care, doctors and nurses read to the patients a list of 18 adverse events commonly encountered with second-line anti-TB drugs, to trigger the patients’ recollection. The adverse events reported by the patients were documented in the patients’ medical records, as previously described elsewhere.21,22 If a patient failed to appear at the outpatient clinic for their medication appointment, the patient was immediately traced by a community health care worker so that treatment is not interrupted.
Questionnaire
The SF-8 questionnaire is a simple tool consisting of eight questions about a person’s self-assessment of his or her HRQoL at a particular point in time.16 Four questions of the SF-8 questionnaire address the physical component of health, while the other four questions address the mental health component. The physical health dimensions are physical functioning (PF), role physical (RP), general health (GH), and bodily pain (BP). The mental health dimensions are vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). Each question has five or six Likert-type responses. We also included three supplemental questions on the occurrence of adverse events during MDR-TB treatment and on the age and sex of the study participants (Figure S1).
Data collection
The nurses at the MDR-TB treatment clinics were informed about the study and were trained to use the SF-8 questionnaire. Consenting patients were invited to respond to the survey that was administered by the trained nurses. The patients were asked to rate their health for each of the eight items of the SF-8 questionnaire. The patients also reported the adverse events that they recalled having experienced during their treatment. This was further supplemented by the information on adverse events that was recorded on the patients’ treatment card. The survey was consecutively conducted for each consenting participant from January 1, 2015 to April 30, 2015.
Data analysis
The patients’ responses were entered into the SF-8 QualityMetric Health Outcomes™ Scoring Software 4.5, which was supplied by the proprietor of the SF-8 questionnaire – QualityMetric, Optuminsight Life Sciences.16 The software automatically computes individual patient scores based on their self-ratings of each item on the questionnaire, using a norm-based scoring method.23 In a norm-based scoring approach, each scale is scored to have a standardized mean and standard deviation (SD), relative to the general population scores.24 For the SF-8 questionnaire, the scale item values are normed by the scoring tool so that 50 is equal to the mean of the norm sample and 10 is equal to the SD of the norm sample.23 The norm sample has been selected by the developers of the questionnaire based on the US general population.25 Scores above or below 50 were considered above or below the general average, respectively. The physical component summary (PCS) and the mental component summary (MCS) scores were also computed by the tool. Higher PCS and MCS scores indicate better health. A two-sided Wilcoxon signed-rank test, with an alpha of 0.05, was used to determine whether the difference in the median PCS and MCS scores was statistically significant. Furthermore, the HRQoL scores for each of the eight items were then exported to SPSS version 12.0.1 and R for further analysis and for calculating the Cronbach’s alpha for the physical component and the mental component scale items, respectively. The Cronbach’s alpha is a measure of the internal consistency (reliability) of a psychometric scale. Generally, a Cronbach’s alpha of ≥0.70 is considered to indicate satisfactory reliability of a scale.26 In addition, we used 2×2 contingency tables to perform the Fisher’s exact test (rather than the chi-square test) due to the small cell values and to compute P-values of the association between the proportion of patients who experienced three or more adverse events and those who rated their HRQoL scores <50 points. The level of statistical significance was 0.05.
Ethical statement
Participation in this study was voluntary. Nurses at the participating MDR-TB treatment sites explained the study aim and objectives to eligible patients and sought their written informed consent. Only consenting patients were invited to respond to the questionnaire. All participants provided written informed consent. In the event that a patient declined to participate in the study, the patient’s decision did not compromise the care that the patient received from the clinic. Furthermore, the patients could stop responding to the questionnaire at any time, without reprisals. The data were analyzed anonymously, and the results were reported in an aggregate manner, for patient confidentiality. The study was approved by the institutional review board of Utrecht University (reference: UP1307) and the research and ethics committee of the Namibian Ministry of Health and Social Services (reference: 17/3/3, dated on December 19, 2013).
Results
A total of 36 patients responded to the SF-8 questionnaire as well as the supplementary questions. Of these respondents, 20 (56%) were males, and the median age of the patients was 40 years, ranging from 17 to 54 years.
The HRQoL scores of individual patients for each of the eight SF-8 dimensions ranged between 25 and 65 points (Figure 1). There was considerable interpersonal variation in the patients’ scores. Patient scores were highest for BP, GH, and VT. However, the PF, RP, and the RE dimensions tended to be rated poorly by the patients, with each of these dimensions achieving mean ratings of 52.4, 52.1, and 51.0, respectively. For the entire group, the median (range) HRQoL score for the PCS was 58.6 (35.3–60.5), while it was 59.3 (26.6–61.9) for the MCS. The difference in the median PCS and MCS scores was small (0.68), but was statistically significant (P=0.005).
Figure 1.
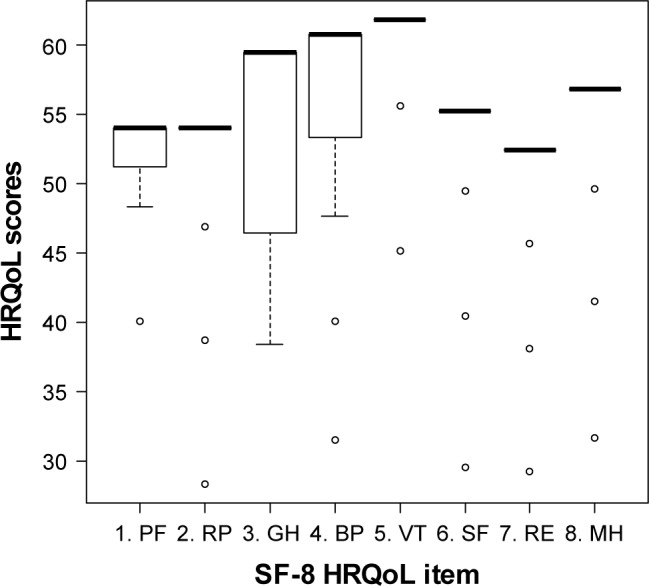
Boxplots comparing the variation in HRQoL scores for each SF-8 item.
Notes: Physical component: PF, RP, GH, and BP. Mental component: VT, SF, RE, and MH.
Abbreviations: HRQoL, health-related quality of life; SF-8, Short Form-8™ questionnaire; PF, physical functioning; RP, role physical; GH, general health; BP, bodily pain; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health.
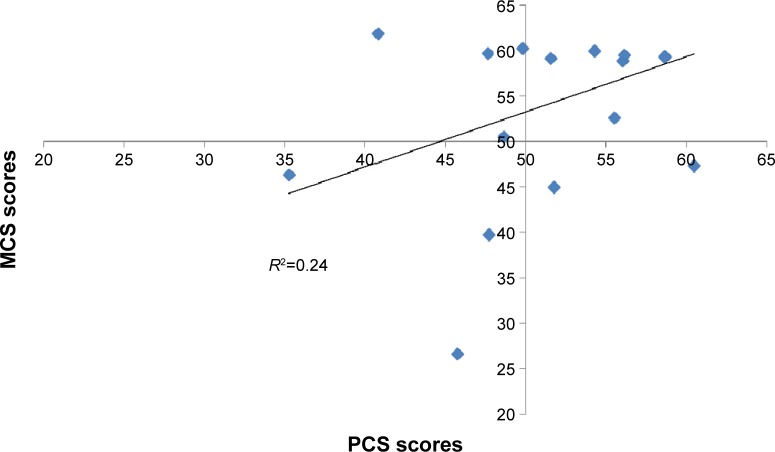
There was good internal consistency of the scale scores, with a Cronbach’s alpha of 0.83 and 0.94 for the PCS and MCS, respectively. Furthermore, as shown in Figure 2, there was essentially no correlation between the PCS and MCS scores (R2=0.24). However, the MCS scores were more variable than the PCS scores. The variance for the MCS was 51.7, while it was 33.8 for the PCS.
Figure 2.
Correlation of the physical and mental component scores.
Abbreviations: MCS, mental component summary; PCS, physical component summary.
A total of 32 (89%) of the 36 patients in this study reported experiencing at least one adverse drug event of any severity during their treatment (median events =3, range 1–6). The frequency of the reported adverse events is shown in Table 1. None of the adverse events were life-threatening. Except for hearing loss, the other adverse events were not permanent and they subsided when the treatment ended. In addition to these adverse events, the patients complained of painful injections during the intensive phase of treatment, taking too many tablets, having to undergo lengthy daily treatment schedules and some tablets tasting awful. Some of the patients lamented that the entire treatment experience was very stressful for them. Despite these medication-related challenges, all the patients completed their treatment and were cured of the MDR-TB infection after 20–24 months of taking anti-TB medicines on a daily basis.
Table 1.
Frequency of patients’ self-reported adverse events
| Adverse event | Patients reporting (N=36), n (%) |
|---|---|
| Hearing loss | 9 (25) |
| Vomiting | 8 (22) |
| Joint pain | 8 (22) |
| Nausea | 7 (19) |
| Tinnitus | 6 (17) |
| Dizziness | 6 (17) |
| Abdominal pain | 6 (17) |
| Headache | 5 (14) |
| Fatigue | 5 (14) |
| Vision problems | 4 (11) |
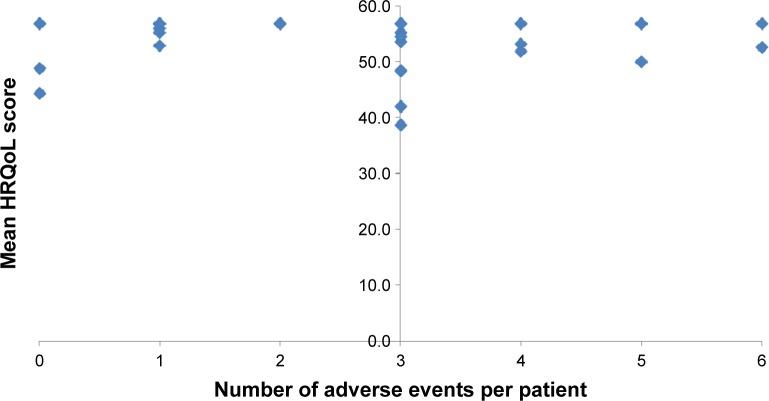
Only four of the 32 patients (12.5%) who experienced at least one adverse event rated their HRQoL <50 points. The HRQoL scores for these four patients were 38.6, 42.0, 48.4, and 49.9. Figure 3 shows the association between the total number of adverse events reported per patient and their overall HRQoL score. No association was found between the occurrence of adverse events and patients’ ratings of their HRQoL (P=0.34) at the end of MDR-TB treatment. Neither did the occurrence of ototoxicity (P=0.45), gastrointestinal adverse events (P=0.70), joint pain, or neuropathy (P=0.30) significantly influence the patients’ HRQoL scores (Table 2).
Figure 3.
Patients’ mean HRQoL scores by number of adverse events experienced by the patients.
Abbreviation: HRQoL, health-related quality of life.
Table 2.
Occurrence of adverse events and patients’ rating of their HRQoL
| Type of adverse event | Categories | Proportion of patients with HRQoL scores <50, n (%) |
P-value (Fisher’s exact test) |
|---|---|---|---|
| Any adverse event | 0–2 events | 3/17 (18) | 0.45 |
| ≥3 events | 6/19 (32) | ||
| Ototoxicity (hearing loss and tinnitus) | Absent | 6/20 (30) | 0.70 |
| Present | 3/16 (19) | ||
| Gastrointestinal eventsa | Absent | 6/22 (27) | 1.00 |
| Present | 3/14 (21) | ||
| Joint pain and neuropathy | Absent | 5/25 (20) | 0.41 |
| Present | 4/11 (36) |
Note:
Includes nausea, vomiting, abdominal pain, and diarrhea.
Abbreviation: HRQoL, health-related quality of life.
Discussion
In our study, patients’ HRQoL ratings were moderately low at the end of their MDR-TB treatment. The maximum overall HRQoL score of the patients was 61 points, while the lowest was 25 points, which is way below the ideal HRQoL rating of 80 norm-based points. PF, RP, and RE were the lowest-rated dimensions of health, barely achieving ratings >55 points. No association was found between these HRQoL scores and the occurrence of adverse events.
The majority of the surveyed patients experienced at least one adverse event during their treatment. These adverse events were hardly debilitating or life-threatening. The occurrence of the adverse events was unrelated to the patients’ HRQoL scores. This finding might appear surprising and counterintuitive, but it has to be interpreted carefully. First, we have previously reported that almost all the adverse events experienced by the patients during MDR-TB treatment occur within the first 8 months of treatment (also known as the intensive phase of treatment).3 Very few new adverse events, if any, occur in the continuation phase of treatment. However, some adverse events that originally developed in the intensive phase, such as the permanent adverse events, may persist into the continuation phase of treatment. The continuation phase typically lasts for at least 12 months depending on how long it takes for a patient to be bacteriologically cured. Except for the few persistent or permanent adverse events, most adverse events occurring within the first 8 months of treatment resolve by the time the patient progresses into the continuation phase of treatment. At the time of treatment completion, almost all the adverse events have fully resolved, thereby negligibly impacting on a patient’s assessment of his/her HQRoL at the end of treatment.
Second, one would ask whether the occurrence of persistent or permanent adverse events may influence a patient’s HRQoL rating at treatment completion. In the current study, hearing loss was the only permanent adverse event that was most frequently cited by the patients. Yet, the occurrence of hearing loss did not influence the patients’ rating of their HRQoL. A potential explanation is that the patients may have already adjusted to their hearing loss by the time they completed their MDR-TB treatment; hence, they did not consider the hearing loss to be a major handicap to worry about. Indeed, the patients may have resigned themselves into accepting these adverse events as part of their MDR-TB treatment knowing that the benefits far outweigh any adverse event, making the patients not to complain about the events. Alternatively, some of the patients may have experienced only mild forms of hearing loss, while others may have received hearing aids that corrected for the hearing deficit, perhaps further explaining why they rated their HRQoL similarly to those who did not experience hearing loss.
Third, we could have surveyed a biased sample of well-motivated and tolerant patients who were determined to go through their entire MDR-TB treatment schedule despite the challenges posed by any adverse event(s) they may have encountered during the course of treatment. Such a group of treatment “survivors” might be the patients who did not suffer from the potentially severe or serious adverse events, which may have lowered their HRQoL rating. This is an inherent limitation of our study design because we did not compare the HQRoL of patients completing MDR-TB treatment with those who might have dropped off from their treatment at an earlier stage.
The small sample size in our study was a major limitation. We were able to survey only 36 of the targeted 138 (26%) respondents. This low sample coverage underpowered the ability of the study to detect the predefined differences if they would exist. However, the current data show that there is no association between the occurrence of adverse events and the patients’ HRQoL at the end of MDR-TB treatment.
Last, as postulated by Stewart and Nápoles-Springer27 and by Lee et al,28 the patients’ perception of their HRQoL may vary according to the patients’ socioeconomic background and cultural context. It is, therefore, possible that a patient raised up in a developing country context, such as Namibia, may rate his/her health in the presence of aminoglycoside-induced hearing loss differently from a patient raised up and living in a developed country setting who experiences a similar condition. Such differences in people-perceived HRQoL may depend on the individual’s tolerance and acceptance to live with some health conditions, as well as the support availed to the patient through the social structures or the health system in which he or she lives. It would be advisable to confirm this postulation in a larger, multi-country comparative study.
The high Cronbach’s alpha values (>0.8) and the lack of correlation between the physical component and mental component scores show good psychometric properties of the SF-8 questionnaire. This compares favorably with the good Cronbach’s alpha values of 0.82 for the physical dimension and 0.87 for the mental dimension as reported by Severo et al,29 who used the Portuguese version of the SF-36 questionnaire. However, it is important to note that some scholars have cautioned against the use of Cronbach’s alpha in assessing the reliability of tools for measuring HRQoL.30,31 Our findings indicate that the SF-8 questionnaire is a simple, reliable tool that could be used for the routine measurement and clinical monitoring of changes in the HRQoL of patients treated for MDR-TB, especially at an aggregate or group level.
The findings of the current study have important programmatic and clinical implications for the treatment of MDR-TB, particularly in Namibia. Although there was no correlation between the occurrence of treatment-related adverse events and the patients’ HRQoL scoring, we encourage TB program managers and clinicians to pay closer attention to changes in HRQoL in the patients undergoing MDR-TB treatment. While we could not demonstrate it, there is a possibility that the HRQoL of the patients in our study may have transiently diminished at the earlier stages of their MDR-TB treatment (in the intensive phase), due to the occurrence of adverse events. Our argument is informed by the various studies of patients treated for drug-susceptible TB, which have shown that the patients’ HRQoL ratings change at various stages in the course of their TB treatment.12,15,32,33
Among the patients who successfully completed their prescribed MDR-TB treatment, it appears that disease factors, rather than treatment-related adverse events, may have a bigger role in influencing the HRQoL of the patients.34 Better clinical management of the potentially serious or severe adverse events experienced by the patients will ensure that the adverse events do not significantly contribute to the decrement of patients’ HRQoL. We recommend that a larger longitudinal study be conducted to determine the relative role that MDR-TB-disease and its treatment may play in influencing the patients’ HRQoL ratings.
Since the SF-8 questionnaire is a reliable, simple, and easy-to-apply tool, we recommend TB program managers and clinicians to routinely use it to monitor changes in HRQoL in the patients. Such routine patient HRQoL measurements could be aggregated at a programmatic level to monitor the groupwise impact of MDR-TB treatment on the patients’ HRQoL as a quality of care indicator for the MDR-TB treatment program.
A major strength of the current study is that the questionnaire used was short and easy to administer. However, being cross-sectional, the study only collected data at one point in time (at the end of MDR-TB treatment). Consequently, it was not possible for us to compare the patients’ baseline HRQoL scores with their subsequent scores at various points during the treatment and at the end of the treatment. Moreover, there could have been biases caused by the patients’ recall and selective self-reporting of adverse events and also by TB clinic nurses administering the questionnaire to the patients because they were the same nurses who provided care to the patients. However, we addressed this challenge by extracting supplemental data on adverse events from the patients’ MDR-TB treatment records.
Conclusion
Patients who completed their MDR-TB treatment in Namibia tended to score moderately low on their HRQoL using the generic SF-8 questionnaire. No association was found between the patients’ HRQoL scores upon treatment completion and the occurrence of adverse events. This finding needs to be confirmed in a larger study that measures HRQoL at baseline, at multiple time points during the MDR-TB treatment phases and at the completion of treatment so that the changes in HRQoL may be ascertained.
Supplementary material
The three supplemental questions on the occurrence of adverse events during MDR-TB treatment.
Abbreviation: MDR-TB, multidrug-resistant tuberculosis.
Acknowledgments
We thank Elsie Muundjua, Genius Magweta, Isabel Haingura, Saima Nakangombe, and Lydia Haindongo for their assistance in administering the questionnaires for this study. Our special thanks go to the patients who participated in the study.
Footnotes
Author contributions
EL Sagwa conceived and designed the study, collected and analyzed the data, and drafted and finalized the manuscript. N Ruswa, F Mavhunga, T Rennie, and HGM Leufkens reviewed the study protocol and manuscript. AK Mantel-Teeuwisse guided the writing of the protocol, supported data analysis, and critically reviewed all drafts of the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization (WHO) Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 2.Sturdy A, Goodman A, José RJ, et al. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J Antimicrob Chemother. 2011;66(8):1815–1820. doi: 10.1093/jac/dkr221. [DOI] [PubMed] [Google Scholar]
- 3.Sagwa E, Mantel-Teeuwisse AK, Ruswa N, et al. The burden of adverse events during treatment of drug-resistant tuberculosis in Namibia. South Med Rev. 2012;5(1):6–13. [PMC free article] [PubMed] [Google Scholar]
- 4.Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2004;8(11):1382–1384. [PubMed] [Google Scholar]
- 5.Shin SS, Pasechnikov AD, Gelmanova IY, et al. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis. 2007;11(12):1314–1320. [PubMed] [Google Scholar]
- 6.Furin JJ, Mitnick CD, Shin SS, et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001;5(7):648–655. [PubMed] [Google Scholar]
- 7.Bloss E, Kuksa L, Holtz TH, et al. Adverse events related to multidrug-resistant tuberculosis treatment, Latvia, 2000–2004. Int J Tuberc Lung Dis. 2010;14(3):275–281. [PubMed] [Google Scholar]
- 8.Van der Walt M, Lancaster J, Odendaal R, Davis JG, Shean K, Farley J. Serious treatment related adverse drug reactions amongst anti-retroviral naïve MDR-TB patients. PLoS One. 2013;8(4):e58817. doi: 10.1371/journal.pone.0058817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel PG, Ramanuj V, Bala DV. Assessment Quality of Life (QoL) of TB patients registered in Tuberculosis units of Ahmedabad Municipal Corporation area by using WHO Short Form-36. Scholars J Appl Med Sci. 2014;2(6F):3303–3306. [Google Scholar]
- 10.Muniyandi M, Rajeswari R, Balasubramanian R, et al. Evaluation of post-treatment health-related quality of life (HRQoL) among tuberculosis patients. Int J Tuberc Lung Dis. 2007;11(8):887–892. [PubMed] [Google Scholar]
- 11.Sharma R, Yadav R, Sharma M, Saini V, Koushal V. Quality of life of multi drug resistant tuberculosis patients: a study of north India. Acta Med Iran. 2014;52(6):448–453. [PubMed] [Google Scholar]
- 12.Brown J, Capocci S, Smith C, Morris S, Abubakar I, Lipman M. Health status and quality of life in tuberculosis. Int J Infect Dis. 2015;32:68–75. doi: 10.1016/j.ijid.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO) Global Tuberculosis Report, 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 14.World Health Organization (WHO) MDR-TB 2014 Update. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 15.Guo N, Marra F, Marra CA. Measuring health-related quality of life in tuberculosis : a systematic review. Health Qual Life Outcomes. 2009;10:1–10. doi: 10.1186/1477-7525-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.OptumInsight Life Sciences Inc [webpage on the Internet] SF-8 Health Survey. [Accessed September 5, 2015]. Available from: https://www.optum.com/optum-outcomes/what-we-do/health-surveys/sf-8-health-survey.html.
- 17.Roberts B, Browne J, Ocaka KF, Oyok T, Sondorp E. The reliability and validity of the SF-8 with a conflict-affected population in northern Uganda. Health Qual Life Outcomes. 2008;6:108. doi: 10.1186/1477-7525-6-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiyohara K, Itani Y, Kawamura T, Matsumoto Y, Takahashi Y. Changes in the SF-8 scores among healthy non-smoking school teachers after the enforcement of a smoke-free school policy: a comparison by passive smoke status. Health Qual Life Outcomes. 2010;8:44. doi: 10.1186/1477-7525-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prieto L, Alonso J, Antó JM. Estimating sample sizes for studies using the SF-36 health survey. J Epidemiol Community Health. 1996;50(4):473–474. doi: 10.1136/jech.50.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health and Social Services (MoHSS) National Guidelines for the Management of Tuberculosis. 3rd ed. Windhoek: MoHSS; 2012. [Google Scholar]
- 21.Sagwa E, Ruswa N, Musasa JP, Mantel-Teeuwisse AK. Adverse events during treatment of drug-resistant tuberculosis: a comparison between patients with or without human immunodeficiency virus co-infection. Drug Saf. 2013;36(11):1087–1096. doi: 10.1007/s40264-013-0091-1. [DOI] [PubMed] [Google Scholar]
- 22.Sagwa EL, Mantel-Teeuwisse AK, Ruswa NC. Occurrence and clinical management of moderate-to-severe adverse events during drug-resistant tuberculosis treatment: a retrospective cohort study. J Pharm policy Pract. 2014;7(1):14. doi: 10.1186/2052-3211-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ware JE. User’s Manual for the SF-36v2 Health Survey. 2nd ed. Lincoln, RI: QualityMetric, Inc; 2007. Advantages of norm-based scoring of SF health survey; pp. 81–84. [Google Scholar]
- 24.Supina AL, Feeny DH, Carroll LJ, Johnson JA. Misinterpretation with norm-based scoring of health status in adults with type 1 diabetes. Health Qual Life Outcomes. 2006;4(1):15. doi: 10.1186/1477-7525-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware JE. How to Score and Interpret Single-Item Health Status Measures: A Manual for Users of the SF-8 Health Survey. Lincoln, RI; Boston, MA: QualityMetric, Inc; 2001. [Google Scholar]
- 26.Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53–55. doi: 10.5116/ijme.4dfb.8dfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart AL, Nápoles-Springer A. Health-related quality-of-life assessments in diverse population groups in the United States. Med Care. 2000;38(9 suppl):II102–II124. [PubMed] [Google Scholar]
- 28.Lee JE, Fos PJ, Zuniga MA, Kastl PR, Sung JH. Health-related quality of life of cataract patients: cross-cultural comparisons of utility and psychometric measures. Ophthalmic Epidemiol. 2003;10(3):177–191. doi: 10.1076/opep.10.3.177.15084. [DOI] [PubMed] [Google Scholar]
- 29.Severo M, Santos AC, Lopes C, Barros H. Reliability and validity in measuring physical and mental health construct of the Portuguese version of MOS SF-36. Acta médica Port. 2006;19(4):281–287. [PubMed] [Google Scholar]
- 30.Streiner DL. Being inconsistent about consistency: when coefficient alpha does and doesn’t matter. J Pers Assess. 2003;80(3):217–222. doi: 10.1207/S15327752JPA8003_01. [DOI] [PubMed] [Google Scholar]
- 31.Konerding U. What does Cronbach’s alpha tell us about the EQ-5D? A methodological commentary to “Psychometric properties of the EuroQol Five-Dimensional Questionnaire (EQ-5D-3L) in caregivers of autistic children”. Qual Life Res. 2013;22(10):2939–2940. doi: 10.1007/s11136-013-0430-9. [DOI] [PubMed] [Google Scholar]
- 32.Atif M, Syed Sulaiman SA, Shafie AA, et al. Impact of tuberculosis treatment on health-related quality of life of pulmonary tuberculosis patients: a follow-up study. Health Qual Life Outcomes. 2014;12(1):1–11. doi: 10.1186/1477-7525-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louw J, Peltzer K, Naidoo P, Matseke G, Mchunu G, Tutshana B. Quality of life among tuberculosis (TB), TB retreatment and/or TB-HIV co-infected primary public health care patients in three districts in South Africa. Health Qual Life Outcomes. 2012;10(1):77. doi: 10.1186/1477-7525-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harries AD, Ade S, Burney P, Hoa NB, Schluger NW, Castro JL. Successfully treated but not fit for purpose: paying attention to chronic lung impairment after TB treatment. Int J Infect Dis. 2016;20(8):1010–1013. doi: 10.5588/ijtld.16.0277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The three supplemental questions on the occurrence of adverse events during MDR-TB treatment.
Abbreviation: MDR-TB, multidrug-resistant tuberculosis.