Abstract
Until recently, the literature on learning-related synaptic plasticity in invertebrates has been dominated by models assuming plasticity is mediated by presynaptic changes, whereas the vertebrate literature has been dominated by models assuming it is mediated by postsynaptic changes. Here I will argue that this situation does not reflect a biological reality and that, in fact, invertebrate and vertebrate nervous systems share a common set of mechanisms of synaptic plasticity.
Introduction
Some years ago a Nobel Prize Winner (NPW) visited my university to deliver a major lecture. The NPW’s host arranged for him to meet with interested faculty during his visit, and I managed to snare one of the coveted meetings. During our rendezvous I summarized for the NPW results of my laboratory’s recent research. The NPW listened to my recitation, and when I was finished asked me point blank why I continued to work on Aplysia. “After all,” he said, “the problem of learning and memory in Aplysia has been basically solved.” Somewhat taken aback, I protested that our understanding of learning in simple organisms, including Aplysia, remained quite incomplete. The NPW replied dismissively, “Well, if you want to spend your career merely tying up loose ends, that’s your business.” Our meeting ended shortly afterward. As he departed the NPW recommended that I switch my area of research, and work instead on memory in either mice or rats.
I suspect that the NPW’s remarks reflect a widespread attitude among neuroscientists who study mammalian learning and memory. I further suspect that many, perhaps most, mammalian neuroscientists believe that basic synaptic mechanisms of learning and memory in vertebrates, such as those underlying long-term potentiation (LTP) and long-term depression (LTD), differ significantly from those in invertebrates, such as those underlying long-term facilitation (LTF) in Aplysia. I will argue here that this idea is incorrect. Recent data from work on invertebrates suggest that the cellular and molecular processes of learning and memory have been conserved to a remarkable degree over hundreds of millions of years of evolution. This fact has been obscured, however, by the biases of some investigators and, to some extent, by bad luck. With the dust having settled on formerly fierce controversies, now is an opportune moment to reevaluate the extent to which invertebrate and vertebrate synaptic plasticity share basic synaptic mechanisms.
Invertebrate Synaptic Plasticity 1970–1995: The Presynaptic Dogma
Beginning around 1970, a systematic investigation of the synaptic mechanisms that underlie two simple forms of nonassociative learning in Aplysia, habituation and sensitization (or dishabituation), was initiated. This investigation was spearheaded by the laboratory of Eric Kandel, although other laboratories also made significant contributions. By 1975, it had been determined that habituation and sensitization were accompanied by, respectively, depression and facilitation of the monosynaptic connection between the central sensory and motor neurons that mediate the gill- and siphon-withdrawal reflex (the sensorimotor synapse) [1]. Both types of synaptic plasticity were originally attributed to presynaptic changes: specifically, depression of the sensorimotor synapse was ascribed to a decrease in transmitter released from the sensory neuron [2], whereas facilitation was ascribed to increased presynaptic release [3]. Depression and facilitation of the sensorimotor synapse were also shown to differ with respect to whether or not heterosynaptic input is required. Presynaptic (or homosynaptic) depression is intrinsic to the sensorimotor synapse [1]; by contrast, facilitation of the sensorimotor synapse during sensitization depends on heterosynaptic modulatory input [1]. The monoamine serotonin (5-HT) plays a major role in sensitization-related facilitation, although other endogenous transmitters can also facilitate the sensorimotor synapse and may play roles in sensitization as well [4].
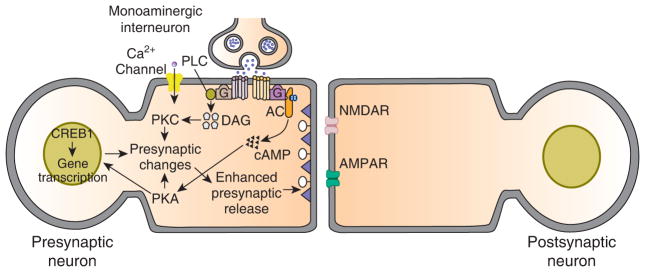
The discovery of 5-HT’s role in sensitization set the stage for a powerful cellular and molecular analysis of synaptic facilitation in Aplysia. This analysis revealed that facilitation involves a coordinated set of changes within the sensory neuron. The binding of 5-HT to its G protein-coupled receptor within the sensory cell membrane causes the synthesis of cyclic adenosine monophosphate (cAMP) and subsequent activation of protein kinase A (PKA). Activation of PKA, in turn, produces closure of two types of potassium channel within the cell membrane, the S-channel and the voltage-dependent potassium channel, as well as enhanced mobilization of presynaptic vesicles. These changes lead to increased presynaptic release of transmitter, as well as an increase in the intrinsic excitability of the sensory neurons [4] (Figure 1). More recent studies have shown that presynaptic facilitation also involves activation of protein kinase C [4], as well as an all-or-none switching on of release sites [5].
Figure 1.
General presynaptic model for learning-related synaptic enhancement in invertebrates circa 1995.
This model is a hybrid and is based on results from cellular and molecular work on Aplysia and the leech, and genetic work on Drosophila. The main basis for plasticity is heterosynaptic modulatory input from monoaminergic interneurons. In Aplysia, as well as in the leech [48], at least some of the modulatory interneurons contain 5-HT, whereas in Drosophila the transmitter used by the modulatory interneurons is dopamine or octopamine [49]. According to the model, the monoamine binds to G protein-coupled receptors on the presynaptic neuron, and stimulates the activity of several kinases: prominent among these is PKA; presynaptic PKC has also been implicated in synaptic facilitation in Aplysia, and has been shown to play a role in associative learning in Drosophila. In Aplysia, PKA and PKC phosphorylate substrate proteins that lead to closure of K+ channels, as well as enhance mobilization of presynaptic vesicles. Pprolonged activation of PKA results in its translocation to the nucleus of the presynaptic cell, where it activates the transcription factor CREB1, and thereby triggers long-term (≥ 24 hour) changes. Activation of CREB1 involves its release from repression by the inhibitory isoform CREB2 in Aplysia and Drosophila; once activated, CREB1 stimulates gene transcription. Some of the induce genes themselves encode transcription factors, such as C/EBP. The products of the gene transcription mediate a variety of long-term cellular changes, including persistent closure of ion channels and growth of new presynaptic varicosities.
The basic model illustrated represents a cellular mechanism for sensitization, a nonassociative form of memory. The model originally proposed to explain classical conditioning in invertebrates is a modification of the presynaptic model for sensitization. According to the original presynaptic model, delivery of the conditioned stimulus (siphon touch in Aplysia) causes an influx of Ca2+ into the neuron through voltage-dependent Ca2+ channels. The combined Ca2+/calmodulin activates the adenylyl cyclase, which can be stimulated by both Ca2+/calmodulin and G-protein activation via the monoamine. Release of the monoamine is produced by the unconditioned stimulus (tail shock in Aplysia). Paired delivery of the conditioned and unconditioned stimuli results in dual stimulation of the cyclase by Ca2+/calmodulin and the monoamine. This dual stimulation of the cyclase yields greater synthesis of cAMP and, hence, greater activation of PKA, than either Ca2+/calmodulin or the monoamine alone. This dual stimulation of the presynaptic adenylyl cyclase, according to the model, is the basis for associative synaptic enhancement.
In the case of Aplysia the presynaptic neuron is a central mechanosensory neuron, whereas the postsynaptic neuron is a central motor neurons. In the leech the presynaptic neuron is also a central mechanosensory neuron, and the postsynaptic neuron is the S-cell interneuron [24]. In Drosophila the presynaptic neuron is thought to be a mushroom body Kenyon cell [49].
Both habituation and sensitization exhibit long-term (≥ 24 hour) forms, and these long-term forms of learning, like the short-term forms, were initially associated with presynaptic changes. Ultrastructural studies of sensory neurons in animals subjected to long-term habituation training found that there was a decrease in the number of release sites (presynaptic varicosities) on the axons of sensory neurons, as well as a decrease in the size of the axonal arbors. By contrast, in animals that underwent long-term sensitization training, sensory neurons exhibited an increase in number of presynaptic varicosities and an enlarged axonal arborization [6].
Although the molecular basis of long-term habituation in Aplysia remains obscure, significant progress has been made toward a molecular understanding of long-term sensitization. Long-term behavioral sensitization involves long-term (≥ 24 hour) facilitation (LTF) of sensorimotor synapses in the abdominal ganglion [7]. In 1986, Montarolo et al. [8] demonstrated that LTF could be induced in synapses in dissociated cell culture by repeated, spaced treatment with 5-HT. They also showed that this form of in vitro long-term synaptic plasticity depends on protein synthesis and gene transcription. Subsequently, Dash et al. [9] reported that LTF depends on presynaptic activity of the transcription factor cyclic AMP response element binding protein 1 (CREB1). This advance was followed by the discovery that LTF requires relief of the repression of CREB1 by the inhibitory isoform CREB2 [10]. Activation of CREB1 results in the activation of several immediate-early genes, among which is one encoding a ubiquitin hydrolase that regulates proteolysis required for LTF. The ubiquitin hydrolase facilitates the proteosomal degradation of the regulatory subunit of PKA, producing persistent activity of this enzyme within sensory neurons [7]. Another immediate-early gene implicated in LTF encodes CCAAT enhancer binding protein (C/EBP). C/EBP is itself a transcription factor, and its activity within sensory neurons has been implicated in the activation of several late response genes that contribute to the growth of new synaptic connections [7].
In 1981, the gill- and siphon-withdrawal reflex of Aplysia was demonstrated to exhibit classical conditioning, an associative form of learning [11,12]. Subsequent cellular studies showed that associative enhancement of the sensorimotor synapse could be induced using physiological stimuli that mimicked the effects of the conditioned stimulus (CS, siphon touch) and unconditioned stimulus (US, tail shock) [13,14]. It was also shown that paired stimulation with the CS and US produced a presynaptic associative change, enhanced broadening of the sensory neuron’s action potential [13]. Biochemical analyses suggested that the mechanism underlying this conditioning-related associative presynaptic change involves an adenylyl cyclase that is dually regulated by CS-induced elevated intracellular Ca2+ and US-induced release of 5-HT. The consequence of paired CS–US stimulation was suggested to be an associative increase in the synthesis of presynaptic cAMP. This mechanism for classical conditioning is referred to as activity-dependent (enhancement of) presynaptic facilitation. The possibility that a Hebbian mechanism — that is, a mechanism based on a correlation between presynaptic and postsynapic activity — might contribute to classical conditioning in Aplysia was recognized; but an explicit test of this possibility failed to find evidence for a postsynaptic contribution to associative plasticity of the sensorimotor synapse [15].
Independent support for the presynaptic model of associative learning in Aplysia came from studies of olfactory conditioning in Drosophila. These studies made use of forward genetics to identify mutant flies that were defective learners. Two of the first mutants identified in the learning screens, dunce and rutabaga, had defects in the cAMP signaling pathway. The dunce mutation affects the gene for cAMP-dependent phosphodiesterase II, whereas the rutabaga mutation affects a gene for adenylyl cyclase [16]. In addition, experiments on transgenic flies showed that CREB-dependent signaling was critical for long-term olfactory memory in flies [17]. Therefore, by 1995 there was a general consensus that several forms of learning in invertebrates, particularly classical conditioning, depend on monoaminergic modulatory activity and cAMP-dependent presynaptic signaling pathways.
Despite the significant empirical support for the presynaptic model of invertebrate learning, there were hints that postsynaptic neurons were not simply passive followers of the learning-related presynaptic neuronal changes. For example, Bailey and Chen [18] observed that long-term sensitization training resulted in the growth of new spine-like processes on the identified gill motor neuron L7. Also, Glanzman et al. [19] reported that the long-term structural changes in sensory neurons that accompanied LTF depended on unidentified postsynaptic signals. But the implications of these findings were largely ignored for over a decade.
Vertebrate Synaptic Plasticity: Emphasis on Postsynaptic Mechanisms
The two leading candidates for neuronal mechanisms of learning and memory in mammals are LTP and LTD [20]. Discovered in 1973 by Tim Bliss and Terje Lömo in the dendate gyrus of the hippocampus, LTP has since been identified at several other classes of excitatory glutamatergic synapses in the mammalian brain, both in the hippocampus and other brain regions, including the amygdala, cerebellum and cerebral cortex (see [20]). The mechanisms that underlie the induction of LTP are heterogeneous; but one prominent form of LTP, expressed at synapses in the dentate gyrus and CA1 regions of the hippocampus, as well at synapses in other parts of the central nervous system, including the cortex, is induced by activation of N-methly-D-aspartate (NMDA)-type glutamate receptors [20].
NMDA receptor-dependent LTP in the hippocampus has been the object of a contentious, decades-long effort to determine the proximate cause of the increase in amplitude of synaptic potentials or currents at potentiated synapses (commonly referred to as the mechanism of LTP ‘expression’). A major analytic technique employed in this effort has been the statistical method of quantal analysis. This physiological technique was originally developed by Bernard Katz and his colleagues in the 1950s to examine synaptic transmission and plasticity at the vertebrate neuromuscular junction (see [21]). Quantal analysis involves a set of assumptions about the nature of synaptic transmission that, while appropriate for the neuromuscular junction, are not always so for central synapses [21]. Initial quantal analytic studies of hippocampal LTP indicated that a presynaptic mechanism — enhanced presynaptic release — accounted for LTP expression. This conclusion was based on the experimental finding that the probability that a presynaptic action potential would produce a synaptic response increased after the induction of LTP. According to the original quantal analytic model, such a result would be presumed to be due to an increase in the probability of presynaptic release; however, later experiments established that the probability of successful synaptic transmission could also increase following LTP induction through the insertion of additional α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors into the postsynaptic membrane at synaptic sites that initially lacked functional AMPA receptors (so-called ‘silent synapses’) [20].
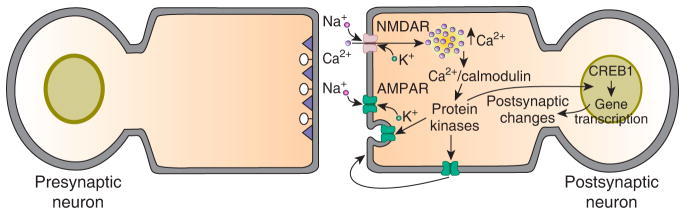
This plasticity mechanism provided a postsynaptic explanation for electrophysiological phenomena associated with LTP, particularly a decrease in the number of synaptic failures and an increase the coefficient of variation (CV) of the synaptic response. (CV is a statistical parameter that, according to the original mathematical model used for quantal analysis, provides a measure of changes in presynaptic release independent of postsynaptic changes.) It is now widely accepted that NMDA receptor-dependent LTP involves insertion of AMPA receptors into postsynaptic membranes, either by exocytosis or lateral diffusion from extrasynaptic sites [22] (Figure 2), although in some cases this may be accompanied by enhanced presynaptic release as well (see below).
Figure 2.
Standard model for NMDA receptor-dependent LTP in the mammalian hippocampus and cortex.
There are two phases to this form of LTP, an early phase that lasts for one to two hours and a late phase that can last for eight hours or more in slice preparations. In the model activation of postsynaptic NMDA receptors, either by high-frequency stimulation or paired pre- and postsynaptic (or Hebbian) stimulation, produces an influx of Ca2+ into postsynaptic dendrites via the open NMDA receptor channels. This postsynaptic influx of Ca2+ activates several kinases that are involved in the induction and expression of LTP. Among these kinases are Ca2+/calmodulin-dependent protein kinase II (CaMKII), PKA, mitogen-activated protein kinases (MAPK) and protein kinase M zeta (PKMζ) [20]. Activation of these kinases causes insertion of new AMPA receptors into the postsynaptic membrane, which is believed to be the major expression mechanism for this form of synaptic plasticity. The mode of AMPA receptor insertion is controversial; the main competing schemes are lateral diffusion of extrasynaptic receptors into postsynaptic sites [22] and exocytotic insertion of the receptors into the postsynaptic membrane [20]. Prolonged activation of the postsynaptic kinases, such as occurs with the multiple, spaced trains of high-frequency stimulation that are used to induce late-LTP, cause gene transcription and protein synthesis, both of which are required for late-LTP. There is evidence for a role for CREB1-dependent transcription in late-LTP. The gene products are believed to be involved in structural remodeling of the postsynaptic neuron, particularly the growth and stabilization of new dendritic spines.
LTD, like LTP, can be induced through several different signaling pathways. Perhaps the most well studied form can be induced at excitatory glutamatergic synapses by low frequency (typically 1–3 Hz) stimulation and depends, somewhat paradoxically, on NMDA receptor activity [20]. The low frequency stimulation causes modest depolarization of postsynaptic dendrites, and a consequent influx of Ca2+ into the dendrites through open NMDA receptors. The modest rise in intracellular Ca2+ activates protein phosphatases and this, in turn leads to endocytosis of postsynaptic AMPA receptors. Thus, within the last 15 years, a coherent and generally accepted model for synaptic plasticity in the mammalian brain has emerged. According to this model, both strengthening and weakening of excitatory, glutamatergic synapses are accomplished, at least in part, by modulation of AMPA receptor trafficking [22].
Postsynaptic Mechanisms of Plasticity in Invertebrates: Return of the Repressed
In 1994, evidence was published that, contrary to an earlier conclusion [15], the sensorimotor synapse of Aplysia possesses the capacity for Hebbian, NMDA receptor-dependent LTP [23]. The idea that invertebrate nervous systems could express LTP mechanistically akin to that observed in the mammalian brain was originally greeted with skepticism. It is now clear, however, that LTP and NMDA receptor-dependent plasticity are by no means unique to vertebrate nervous systems. NMDA receptor-dependent LTP has been reported in the leech [24]; and both the vertical lobe of the octopus [25] and the brain of the honeybee [26] express Hebbian — albeit NMDA receptor-independent — LTP. Furthermore, recent data support a role for NMDA receptor activity in associative learning in Aplysia [27,28], Drosophila [29] and Caenorhabditis elegans [30], and for Hebbian LTP in associative learning in the octopus [31]. (But it has not yet been shown that NMDA receptor-dependent LTP at glutamatergic synapses mediates learning in flies and worms.) Behavioral dishabituation and sensitization in Aplysia [32,33], as well as long-term habituation in C. elegans [34], which were originally ascribed entirely to presynaptic phenomena, have also now been demonstrated to involve modulation of postsynaptic AMPA receptor trafficking.
Yet another shift in mechanistic outlook pertains to the role of postsynaptic protein synthesis in LTF. Until recently, it has been claimed that LTF depends only on presynaptic protein synthesis [35,36]. But new data indicate that, to the contrary, postsynaptic protein synthesis is critical for LTF [37]. The reasons for the initial failure to detect a role for postsynaptic protein synthesis in LTF are unclear. One possibility is that the large size of the target motor neuron (the giant gill neuron L7) used in the earlier studies hampered access of the cell membrane-impermeant inhibitor, which was injected into the motor neuron’s cell body, to distal postsynaptic sites, although other explanations are also possible.
Thus, invertebrate synaptic plasticity is characterized by postsynaptic mechanisms of induction and expression, contrary to long-held views. Why has recognition of this fact been so tardy, especially in the case of Aplysia? One might have anticipated a much earlier appreciation for the critical contribution of postsynaptic processes to learning in this organism, given that Aplysia has been the invertebrate in which synaptic plasticity has been studied the longest and most intensively, and to such seminal effect. Possibly, the early success of presynaptic explanations of learning reduced receptivity to the potential importance of postsynaptic ones. This might help to explain why the discovery of silent synapses and their significance for interpretations of mammalian quantal analytic experiments has not triggered, even at this late date, a reexamination of the results from quantal analyses of synaptic plasticity in Aplysia [2,3] that provided some of the main support for the presynaptic models of learning in this organism. Regardless of the reason, the predominately presynaptic focus of Aplysia research has been unfortunate for the field of invertebrate learning as a whole, because it has encouraged the idea that invertebrates and vertebrates use different mechanisms of learning; this, in turn, has retarded the integration of mammalian and invertebrate research.
Toward a General Model of Synaptic Plasticity
While there is a strong empirical basis for the AMPA receptor trafficking model of synaptic plasticity in mammals, there is also evidence that LTP and LTD may involve presynaptic mechanisms of expression in addition to postsynaptic mechanisms [38]. (It is generally agreed that NMDA receptor-independent LTP of mossy fiber-to-CA3 hippocampal synapses involves primarily presynaptic expression [39,40], but the situation for the CA1 and dentate synapses remains controversial.) Recent evidence in favor of presynaptic expression mechanisms for NMDA receptor-dependent LTP and LTD relies on modern ultrastructural and optical techniques; these techniques are not subject to the interpretive ambiguities that plagued the earlier quantal analytic studies. Despite this fact, there is still not general agreement that the expression of LTP and LTD in CA1 and the dentate gyrus involve, at least in part, presynaptic changes. A possible resolution of this controversy is that expression of NMDA receptor-dependent LTP and LTD involves coordinated presynaptic and postsynaptic changes. If this idea is correct, then because induction of these forms of synaptic plasticity is postsynaptic, there must be one or more retrograde signals that mediate the presynaptic expression changes. A plethora of retrograde signaling molecules have been proposed for LTP and LTD [41]; although some are controversial, empirical support for others is firmer (see below).
Interestingly, retrograde signaling has also emerged as a prominent feature of persistent synaptic plasticity in Aplysia. Antonov et al. [27] found that induction of associative enhancement of the sensorimotor synapse during classical conditioning is accompanied by an increase in the excitability of the presynaptic sensory neuron. This associative increase in presynaptic excitability depends not only on presynaptic PKA activity, but also on postsynaptic Ca2+ because the increase is blocked by injecting the rapid Ca2+ chelator BAPTA into the motor neuron prior to conditioning. Furthermore, two recent studies have shown that LTF involves Ca2+-dependent retrograde signaling. Cai et al. [37] reported that LTF of sensorimotor synapses in cell culture due to spaced 5-HT treatment was blocked by prior injection of BAPTA into the motor neuron. This study also showed that the increased expression of a specific presynaptic neuropeptide, sensorin, which is required for LTF [42], depends on elevated postsynaptic Ca2+. In a related and elegant study, Wang et al. [43] have found that local translation of the reporter for presynaptic sensorin also depends on postsynaptic Ca2+. The identity of the retrograde signal activated by postsynaptic Ca2+ has not been identified, but one of its presynaptic targets may be PKA, because PKA mediates the increased expression of sensorin during LTF [42].
These findings in Aplysia strikingly echo those of a study of NMDA receptor-independent LTP at mossy fiber synapses in the hippocampus. Although expression of this form of LTP is presynaptic, its induction appears to require elevated postsynaptic Ca2+ ([44], but see [45]). Contractor et al. [40] reported that a rise in intracellular Ca2+ within the postsynaptic CA3 neuron activates a transsynaptic pathway involving EphB-receptor–ephrinB ligand interaction. Activation of this pathway stimulates presynaptic PKA, which, in turn, produces enhanced release of transmitter from the mossy fibers. A related form of plasticity has recently been demonstrated in the retinotectal system of Xenopus. Here, activation of ephrin B1 signaling in the terminals of retinal ganglion cells by tectal perfusion with EphB2–Fc fusion proteins produces both an early enhancement of presynaptic release and a delayed (> 30 minutes) postsynaptic increase in the AMPA receptor-to-NMDA receptor ratio similar to that observed following the induction of LTP at retinotectal synapses [46]. Thus, ephrin B reverse signaling also mediates increased presynaptic release at retinotectal synapses.
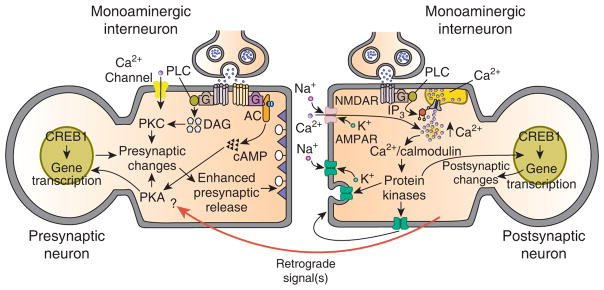
This general schema of postsynaptic induction and presynaptic, as well as in some cases, postsynaptic expression, applies equally to the Aplysia sensorimotor synapse. Indeed, the induction of every form of persistent (> 30 minutes) synaptic enhancement currently known in Aplysia can be disrupted by prior postsynaptic injection of BAPTA. It remains to be seen whether postsynaptic induction via elevated intracellular Ca2+ is ubiquitous for long-term plasticity at excitatory, glutamatergic synapses in the mammalian brain. At present, this issue is controversial [40,44,45]. However, a general model of persistent synaptic enhancement, one that incorporates postsynaptic induction and postsynaptic and/or presynaptic expression, and in which presynaptic expression is mediated by retrograde signaling [41], may prove generally applicable to excitatory, glutamatergic synapses in invertebrate and vertebrate nervous systems (Figure 3).
Figure 3.
General model for learning-related enhancement of excitatory glutamatergic synapses.
This model is based on recent data from studies of synaptic plasticity in invertebrates and vertebrates. Two prominent features of the model are postsynaptic modulatory input from monoaminergic interneurons and retrograde signaling. Evidence for a postsynaptic contribution of monoamines comes from recent studies in Aplysia [32,33] and mammals [50]. In Aplysia, prolonged stimulation with 5-HT causes modulation of AMPA receptor trafficking, which appears to involve exocytotic insertion of AMPA receptors into the cell membrane of the motor neuron. This process is mediated by G protein-stimulated release of Ca2+ from intracellular stores. In the mammalian hippocampus there is evidence that heterosynaptic modulatory input from the basolateral amygdala can convert early-LTP to late-LTP [50]. In this instance, the heterosynaptic input may be from norepinephrine- or acetylcholine-containing axons. In other instances, the heterosynaptic modulatory input may be from peptidergic axons as well. (Only monoaminergic interneurons are shown in the model for the purpose of simplicity.) As shown in the model, the conversion of early-LTP to late-LTP could be mediated by the summing of separate pools of intracellular Ca2+, one resulting from open NMDA receptor channels and the other from release of Ca2+ from intracellular stores, which is stimulated by the heterosynaptic monoaminergic input. The elevated intracellular Ca2+ is also responsible, either directly or indirectly (perhaps through protein synthesis), for triggering the activation of one or more retrograde signals; the retrograde signals, in turn, contribute critically to presynaptic changes and enhanced presynaptic release [41].
An important question is the identity of the presynaptic molecules that are the targets of the retrograde signals. In Aplysia there is evidence that one of these presynaptic targets may be PKA [37,42]. Another important question is to what extent the long-term presynaptic changes result from an interaction between the heterosynaptic input to the presynaptic neuron and the retrograde signal(s). An intriguing possibility is that retrograde signaling alone may be sufficient for long-term changes in the presynaptic neuron.
Much remains to be done to flesh out a comprehensive general model for synaptic plasticity. For example, there has been relatively little work done on identifying retrograde signaling pathways in synaptic plasticity in invertebrates. Furthermore, the potential contribution of monoaminergic modulation, which figures so prominently in invertebrate synaptic plasticity, has been given less attention in studies of synaptic plasticity in vertebrates. Despite the gaps in our knowledge, the convergence of the work on synaptic plasticity in invertebrates and vertebrates, as outlined here, is striking. The idea that the biological mechanisms of synaptic change have been highly conserved during evolution continues to face resistance. Nonetheless, it increasingly appears that invertebrate and vertebrate central nervous systems share, not only the same basic mechanisms for propagating action potentials and communicating across synapses, but those for altering the strength of synapses as well.
At present we have only partial cellular accounts of learning, even for simple forms in relatively simple organisms. Given this state of affairs, it seems unwise to focus the overwhelming majority of the field’s attention and resources on the study of learning and memory in a tiny number of mammalian species, especially if, as I have argued here, the cellular mechanisms of learning are conserved across species. Invertebrates continue to offer significant experimental advantages for students of learning and memory, including the relative simplicity of their nervous systems and the possession of identified neurons. Thus, Aplysia and other molluscs give one the ability to study synaptic plasticity in a single pair of identified neurons in dissociated cell culture; and due to the large size of these neurons, one has ready, simultaneous access to both pre- and postsynaptic compartments. Furthermore, Drosophila and C. elegans provide unparalleled opportunities for gaining fundamental genetic and molecular insights into learning and memory (for example [17]); and as genetic information for other invertebrates becomes available (for example [47]), reverse genetic analyses should prove feasible in a broad range of species. Finally, looking ahead to the day when understanding the operation of neural circuits in learning and memory, rather than plasticity at specific synapses, is the predominant concern of the field, more investigators may discover the reductionist charms of working with the considerably simpler neural circuits of invertebrates. Sometimes it’s better to ignore well-meaning advice, even if it comes from a Nobel Prize Winner.
Acknowledgments
I thank Diancai Cai for assistance with the figures, and Dean Buonomano, Diancai Cai and Frank Krasne for insightful comments on the manuscript.
References
- 1.Castellucci V, Pinsker H, Kupfermann I, Kandel ER. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- 2.Castellucci VF, Kandel ER. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci USA. 1974;71:5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellucci VF, Kandel ER. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976;194:1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- 4.Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royer S, Coulson RL, Klein M. Switching off and on of synaptic sites at Aplysia sensorimotor synapses. J Neurosci. 2000;20:626–638. doi: 10.1523/JNEUROSCI.20-02-00626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 7.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 8.Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- 9.Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- 10.Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 11.Lukowiak K, Sahley C. The in vitro classical conditioning of the gill withdrawal reflex of Aplysia californica. Science. 1981;212:1516–1518. doi: 10.1126/science.212.4502.1516. [DOI] [PubMed] [Google Scholar]
- 12.Carew TJ, Walters ET, Kandel ER. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981;1:1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins RD, Abrams TW, Carew TJ, Kandel ER. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983;219:400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- 14.Walters ET, Byrne JH. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983;219:405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- 15.Carew TJ, Hawkins RD, Abrams TW, Kandel ER. A test of Hebb’s postulate at identified synapses which mediate classical conditioning in Aplysia. J Neurosci. 1984;4:1217–1224. doi: 10.1523/JNEUROSCI.04-05-01217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- 17.Yin JCP, Wallach JS, Del Vecchio M, Wilder EL, Zhuo H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 18.Bailey CH, Chen M. Long-term sensitization in Aplysia increases the number of presynaptic contacts onto the identified gill motor neuron L7. Proc Natl Acad Sci USA. 1988;85:9356–9359. doi: 10.1073/pnas.85.23.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glanzman DL, Kandel ER, Schacher S. Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science. 1990;249:799–802. doi: 10.1126/science.2389145. [DOI] [PubMed] [Google Scholar]
- 20.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991;60:1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin XY, Glanzman DL. Hebbian induction of long-term potentiation of Aplysia sensorimotor synapses: partial requirement for activation of an NMDA-related receptor. Proc Biol Sci. 1994;255:215–221. doi: 10.1098/rspb.1994.0031. [DOI] [PubMed] [Google Scholar]
- 24.Burrell BD, Sahley CL. Multiple forms of long-term potentiation and long-term depression converge on a single interneuron in the leech CNS. J Neurosci. 2004;24:4011–4019. doi: 10.1523/JNEUROSCI.0178-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hochner B, Brown ER, Langella M, Shomrat T, Fiorito G. A learning and memory area in the octopus brain manifests a vertebrate-like long-term potentiation. J Neurophysiol. 2003;90:3547–3554. doi: 10.1152/jn.00645.2003. [DOI] [PubMed] [Google Scholar]
- 26.Menzel R, Manz G. Neural plasticity of mushroom body-extrinsic neurons in the honeybee brain. J Exp Biol. 2005;208:4317–4332. doi: 10.1242/jeb.01908. [DOI] [PubMed] [Google Scholar]
- 27.Antonov I, Antonova I, Kandel ER, Hawkins RD. Activity-dependent presynaptic facilitation and Hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron. 2003;37:135–147. doi: 10.1016/s0896-6273(02)01129-7. [DOI] [PubMed] [Google Scholar]
- 28.Murphy GG, Glanzman DL. Mediation of classical conditioning in Aplysia californica by long-term potentiation of sensorimotor synapses. Science. 1997;278:467–471. doi: 10.1126/science.278.5337.467. [DOI] [PubMed] [Google Scholar]
- 29.Xia S, Miyashita T, Fu TF, Lin WY, Wu CL, Pyzocha L, Lin IR, Saitoe M, Tully T, Chiang AS. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr Biol. 2005;15:603–615. doi: 10.1016/j.cub.2005.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kano T, Brockie P, Sassa T, Fujimoto H, Kawahara Y, Iino Y, Mellem J, Madsen D, Hosono R, Maricq A. Memory in Caenorhabditis elegans is mediated by NMDA-type ionotropic glutamate receptors. Curr Biol. 2008;18:1010–1015. doi: 10.1016/j.cub.2008.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shomrat T, Zarrella I, Fiorito G, Hochner B. The octopus vertical lobe modulates short-term learning rate and uses LTP to acquire long-term memory. Curr Biol. 2008;18:337–342. doi: 10.1016/j.cub.2008.01.056. [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Roberts AC, Glanzman DL. Synaptic facilitation and behavioral dishabituation in Aplysia: dependence upon release of Ca2+ from postsynaptic intracellular stores, postsynaptic exocytosis and modulation of postsynaptic AMPA receptor efficacy. J Neurosci. 2005;25:5623–5637. doi: 10.1523/JNEUROSCI.5305-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li HL, Huang BS, Vishwasrao H, Sutedja N, Chen W, Jin I, Hawkins RD, Bailey CH, Kandel ER. Dscam mediates remodeling of glutamate receptors in Aplysia during de novo and learning-related synapse formation. Neuron. 2009;61:527–540. doi: 10.1016/j.neuron.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose JK, Kaun KR, Chen SH, Rankin CH. GLR-1, a non-NMDA glutamate receptor homolog, is critical for long-term memory in Caenorhabditis elegans. J Neurosci. 2003;23:9595–9599. doi: 10.1523/JNEUROSCI.23-29-09595.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trudeau LE, Castellucci VF. Postsynaptic modifications in long-term facilitation in Aplysia: upregulation of excitatory amino acid receptors. J Neurosci. 1995;15:1275–1284. doi: 10.1523/JNEUROSCI.15-02-01275.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin KC, Casadio A, Zhu H, EY, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- 37.Cai D, Chen S, Glanzman DL. Postsynaptic regulation of long-term facilitation in Aplysia. Curr Biol. 2008;18:920–925. doi: 10.1016/j.cub.2008.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krueger S, Fitzsimonds RM. Remodeling the plasticity debate: the presynaptic locus revisited. Physiology. 2006;21:346–351. doi: 10.1152/physiol.00013.2006. [DOI] [PubMed] [Google Scholar]
- 39.Mellor J, Nicoll RA, Schmitz D. Mediation of hippocampal mossy fiber long-term potentiation by presynaptic Ih channels. Science. 2002;295:143–147. doi: 10.1126/science.1064285. [DOI] [PubMed] [Google Scholar]
- 40.Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- 41.Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu JY, Wu F, Schacher S. Two signaling pathways regulate the expression and secretion of a neuropeptide required for long-term facilitation in Aplysia. J Neurosci. 2006;26:1026–1035. doi: 10.1523/JNEUROSCI.4258-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang DO, Kim SM, Zhao Y, Hwang H, Miura SK, Sossin WS, Martin KC. Synapse- and stimulus-specific local translation during long-term neuronal plasticity. Science. 2009;324:1536–1540. doi: 10.1126/science.1173205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mellor J, Nicoll RA. Hippocampal mossy fiber LTP is independent of postsynaptic calcium. Nat Neurosci. 2001;4:125–126. doi: 10.1038/83941. [DOI] [PubMed] [Google Scholar]
- 46.Lim BK, Matsuda N, Poo MM. Ephrin-B reverse signaling promotes structural and functional synaptic maturation in vivo. Nat Neurosci. 2008;11:160–169. doi: 10.1038/nn2033. [DOI] [PubMed] [Google Scholar]
- 47.Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L, et al. Neuronal transcriptome of Aplysia: neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burrell BD, Sahley CL, Muller KJ. Non-associative learning and serotonin induce similar bi-directional changes in excitability of a neuron critical for learning in the medicinal leech. J Neurosci. 2001;21:1401–1412. doi: 10.1523/JNEUROSCI.21-04-01401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 50.Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP. J Neurosci. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]