Abstract
Purpose
To compare the effect of topical prednisolone acetate 1% (PA) used after routine cataract surgery to the effect of difluprednate 0.05% (DFBA) used for the same indication on intraocular pressure (IOP).
Methods
An electronic query was created to gather information from all cataract surgeries between January 2010 and January 2015 within the electronic health record database at Barnet Dulaney Perkins, a multicenter, multiphysician private practice in Phoenix, Arizona. Information collected included age, sex, diabetes status, glaucoma history, medication regimen (use of PA or DFBA), and IOP before surgery, 5–10 days postoperatively (TP1) and 3–6 weeks postoperatively (TP2). Postoperative IOP measurements were compared to baseline IOP measurement in each patient.
Results
Regardless of steroid used, all patients in this study experienced an increase in IOP within TP1 and returned to baseline IOP (±2.0 mmHg) by TP2. Patients who received DFBA showed a statistically significant increase in IOP at TP1 compared to those on PA (P<0.001) with the mean IOP an average 0.60 mmHg higher (95% CI =0.3, 0.9). The odds ratio of a clinically significantly increased IOP at TP1 (defined as overall IOP ≥21 mmHg and an increase of ≥10 mmHg) in DFBA-treated patients was 1.84 (95% CI =1.4, 2.6). In patients treated with PA, 3% reached a significantly increased IOP, compared to 4.4% of patients in the DFBA group (P<0.05). Risk factors for increased IOP were identified, and include advanced age (>75) (P<0.005) and a history of glaucoma (P<0.001).
Conclusion
In postoperative cataract patients, use of DFBA increased the risk of a clinically significant IOP increase.
Keywords: intraocular pressure, cataract surgery, steroid responder, glaucoma
Introduction
Topical corticosteroids are commonly used after cataract surgery to decrease inflammation and improve visual outcomes. Inflammation is a natural result of tissue injury and can be damaging to the ocular environment. Steroids inhibit the production of leukotrienes and prostaglandins post-surgery, thereby reducing potentially hazardous ocular inflammation.1 Although steroids help in decreasing inflammation by speeding up recovery and improving visual outcomes, they are known to have significant side effects, whether topically or systemically administered.2,3
One of the common side effects of topical steroids include increased intraocular pressure (IOP).3–5 Interestingly, use of systemic steroids may also result in increased IOP in a set of patients.6 Patients with a tendency to experience corticosteroid-induced significantly increased IOP have been referred to as “steroid responders” and may experience worsening of side effects. While the mechanism of steroid response is not yet elucidated, it has been estimated that 5% of the population may be classified as a steroid responder and thus may develop a significantly increased IOP.7 Various factors have been identified which increase the risk of corticosteroid-induced increase in IOP, including a history of glaucoma,8,9 advanced age,10,11 diabetes,9–13 and high myopia, among others.5 Chronically elevated IOP may lead to vision loss by optic nerve damage.14–16
Prednisolone acetate 1% (PA) has been commonly used off-label in the treatment of postoperative inflammation after cataract surgery. Difluprednate ophthalmic emulsion 0.05% (DFBA) (Durezol, Alcon Laboratories, Fort Worth, TX, USA) is a recent medication used in the treatment of postoperative inflammation. It was FDA approved in July 2008 for the treatment of inflammation and pain after ocular surgery.17 DFBA differs from PA in its chemical structure. Due to the altered structure of DFBA, the synthetic steroid has 56 times increased receptor-binding affinity to glucocorticoid receptors when compared to PA,18 increased anti-inflammatory capacity, and increased tissue penetration.19,20 In ophthalmologic clinical practice, DFBA has been shown to be effective in reducing postoperative inflammation and decreasing corneal edema and retinal thickness.2,21–24 However, reports have shown an increase in IOP when used after ophthalmologic surgery.23–29
While studies have compared DFBA versus PA for vitreoretinal surgery28,29 and anterior uveitis,21,30 few have investigated IOP increase with the use of these drugs in the context of cataract surgery.2,24 Here, the results of a large (n=4,184) retrospective study evaluating the potential for corticosteroid-induced ocular hypertension with the use of either PA (n=2,151) or DFBA (n=1,337) in post-cataract surgery patients are reported. IOP measurements were collected at various time points, including preoperative, 5–10 days postoperatively (TP1), and 3–6 weeks postoperatively (TP2), allowing for examination of time-dependent changes in IOP after cataract surgery.
Methods
Study design
This study was approved by Quorum Institutional Review Board and was conducted in accordance with the Declaration of Helsinki. The Quorum Institutional Review Board did not require written informed consent be obtained from the participants, as this was a retrospective study, and all data was anonymous. The study was completed at Barnet Dulaney Perkins Eye Center (BDPEC), a multi-physician, multi-center eye center in Phoenix, Arizona. An electronic query was created to collect the following information from the charts of cataract patients operated on at BDPEC from January 2010 to January 2015: age at time of service, sex, diabetes status, glaucoma history, preoperative IOP, and postoperative IOP at TP1 and TP2. For data collection, a custom query was designed to identify patients who had undergone uncomplicated cataract surgery and who fit the inclusion criteria: having been treated with either DFBA or PA and having had no other ocular surgeries in the study period. The patients were identified based on the CPT code 66984 and retrieved from a database which had been initially created with NextGen (NextGen Healthcare, Horsham, PA, USA). Exclusion criteria included any patient with missing data values for IOP, if it could not be ascertained with certainty which corticosteroid drops were used after cataract surgery, and any patient who required further surgical procedures on the same eye within the study time period, in addition to exclusion of patients with diabetic retinopathy. The postoperative eye drop regimen was standardized across all surgeons in the group practice and consisted of a corticosteroid (PA or DFBA), a fourth-generation fluoroquinolone, and a nonsteroidal anti-inflammatory agent (Nepafenac ophthalmic).
The prescribed dosing for PA (n=2,151) was 1 drop four times a day starting the day of surgery and continuing for 2 weeks after surgery. After this time the patients decreased the drops to one drop twice a day for 2 weeks. The prescribed dosing for DFBA (n=1,337) was DFBA twice a day for 2 weeks, then once a day for 2 weeks. All physicians in the practice adhered to the same prescription regimens during these different time frames.
IOP was measured using Goldmann applanation tonometry on different instruments with standardized calibrations. No patient had both eyes included in the study, nor did any eye undergo a secondary intraocular surgery during the study period.
Statistical analysis
Statistical analyses were conducted using STATA version 14 (College Station, TX, USA). Demographic and clinical characteristics of the DFBA and PA groups were evaluated using descriptive statistics including means, standard deviations for continuous variables, and frequencies and proportions for categorical variables. The independent t-test was implemented to determine differences in means between the treatment and control groups. The Fisher’s exact test was employed to test differences in proportions. Mean IOP (95% CI) was estimated for the DFBA and PA groups at three time points (baseline, TP1, and TP2). Mean differences in IOP change from baseline between treatment groups at each time point were estimated using analysis of covariance adjusting for baseline IOP levels. Furthermore, paired t-tests were used to assess the differences in the mean change of IOP from baseline in each treatment group. The same procedure mentioned above was implemented after stratifying by age (>75 and ≤75 years) glaucoma status and diabetes status, respectively. Multiple comparisons were adjusted for by the Bonferroni method considering a P-value <0.05 divided by 3 (number of comparisons made) or <0.0167 as significant. Finally, proportions of patients were ascertained for the aforementioned categories if the patients’ IOP measured ≥21 mmHg and exhibited increases of IOP of ≥10 mmHg at TP1. These values were chosen based on previously published studies and seemed both most rigorous in the definition of steroid response as well as clinically significant.2,28,30 Fisher’s exact test was assessed to evaluate any relationship between the proportions of clinical significant increases of IOP and potential risk factors (age, glaucoma, and diabetes). The generalizing estimating equation using the logit construct was used to estimate odds ratios and 95% confidence intervals to ascertain the likelihood of obtaining an IOP of ≥21 mmHg with a ≥10 mmHg increase at TP1 between treatment groups after adjusting for time.
Results
A total of 3,488 eyes of 3,488 patients at BDPEC who underwent cataract surgery between the period of January 2010 and January 2015 and fit all of the inclusion criteria were identified. Of these, a total of 2,151 patients received PA and 1,337 patients received DFBA. Patient demographics are shown in Table 1 and there was no significant difference in patient populations between the two groups stratified by age, sex, history of glaucoma, diabetes, or baseline IOP.
Table 1.
Baseline demographic and clinical characteristics
| Variables | Overall population, N=3,488 | Difluprednate, n=1,337 | Prednisolone, n=2,151 | P-valuea |
|---|---|---|---|---|
| Age in years, mean (SD) | 70.6 (8.6) | 70.6 (8.5) | 70.6 (8.7) | 0.85 |
| Male gender, n (%) | 1,384 (51.0) | 310 (53.1) | 1,074 (50.5) | 0.26 |
| Glaucoma history, n (%) | 548 (15.7) | 195 (14.6) | 353 (16.4) | 0.15 |
| Diabetes history, n (%) | 641 (18.4) | 253 (18.9) | 388 (18.0) | 0.51 |
| Intraocular pressure in mmHg, mean (SD) | 14.7 (3.2) | 14.6 (3.2) | 14.7 (3.2) | 0.46 |
Note:
P-value calculated using independent t-test for continuous variables and chi squared for continuous variables.
Abbreviation: SD, standard deviation.
IOP measurements within 1 month before surgery for baseline, TP1, and TP2 postoperative in all patients were collected. As a general trend, mean IOP increase over baseline was within ±2 mmHg by TP1 and returned to baseline at TP2 for all patients. This trend was seen consistently across all groups regardless of steroid use (Table 2; Figure 1A). For patients taking PA, the mean IOP at baseline was 14.7 mmHg, which increased to 15.6 mmHg by TP1 (SD =4.3; P<0.001) and was reduced to 14.2 mmHg by TP2 (SD =3.2; P<0.001). For patients taking DFBA, the mean IOP at baseline was 14.6 mmHg, which increased to 16.2 mmHg at TP1 (SD =4.2; P<0.001) and decreased to 14.6 mmHg by TP2 (SD =3.5; P>0.05) (Table 2; Figure 1A).
Table 2.
IOP (in mmHg) changes in prednisolone versus difluprednate
| Overall population | Categories
|
|||||
|---|---|---|---|---|---|---|
| Baselineb | TP1b | Δ from baselinea | TP2b | Δ from baselinea | ≥21 mmHg overall and ≥10 mmHg increase at TP1c | |
| Prednisolone (n=2,151) | 14.7 (3.2) | 15.6 (4.3)** | 0.89 (4.6)** | 14.2 (3.2)** | −0.49 (3.8)** | 65 (3.02%) |
| Difluprednate (n=1,337) | 14.6 (3.2) | 16.2 (4.2)** | 1.6 (4.7)** | 14.6 (3.5) | 0.04 (4.2) | 59 (4.41%) |
| Δ mean IOP (95% CI) | −0.08 (−0.30, 0.13) | 0.60 (0.30, 0.90) | 0.21 (0.12, 0.31) | 0.46 (0.23, 0.68) | 0.16 (0.09, 0.23) | N/A |
| P-value | 0.46 | <0.001 | <0.001 | <0.001 | <0.001 | 0.04 |
Notes: Data shown as mean (SD), mean change (SD), n (%), Δ mean (95% CI), or P-value.
P-values calculated using the analysis of covariance adjusted for baseline IOP.
P-values calculated using the Independent t-test.
P-values calculated using Fisher’s exact test at TP1.
P-value <0.001 comparing mean IOP change at each time point from baseline after multiple comparison adjustments.
Abbreviations: IOP, intraocular pressure; SD, standard deviation; TP1, 5–10 days postoperatively; TP2, 3–6 weeks postoperatively.
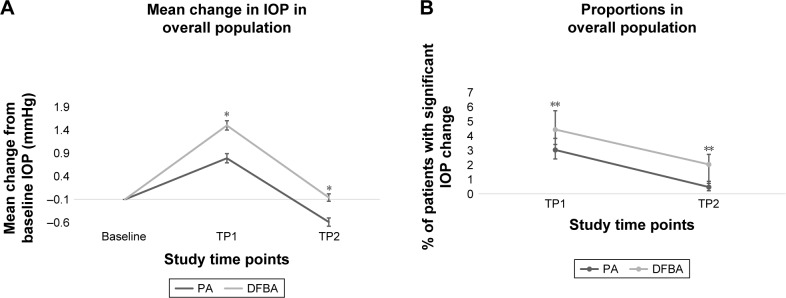
Figure 1.
General trends in IOP (mmHg) changes across study time points (TP1; TP2).
Notes: (A) Mean change (SE) in IOP from baseline among PA versus DFBA treatment arms. (B) Proportion (95% CI) of patients with IOP change ≥10 mmHg and overall IOP ≥21 mmHg at TP1 and TP2, respectively. *Analysis of covariance adjusted for baseline IOP to report P-values of <0.05 for the difference in mean change in IOP from baseline at each study time point. **Fisher’s exact test ≥ to report P-values of <0.05 for the difference in % of patients with IOP change ≥10 mmHg and overall IOP ≥21 mmHg in IOP from baseline at each study time point.
Abbreviations: CI, confidence interval; DFBA, difluprednate; IOP, intraocular pressure; PA, prednisolone acetate; SE, standard error; TP1, 5–10 days postoperatively; TP2, 3–6 weeks postoperatively.
Among the total population, patients taking DFBA experienced a statistically higher increase in mean IOP compared to PA patients at TP1 (Table 2; Figure 1A; P<0.001) with the mean increase in IOP being 0.60 mmHg higher (95% CI =−0.30, 0.90). The odds ratio of significantly increased IOP (defined as ≥21 mmHg that was also an increase of ≥10 mmHg from baseline) at TP1 in DFBA-treated patients was 1.84 (95% CI =1.4, 2.6, P<0.001; data not shown). In patients treated with PA, 3% reached a significantly increased IOP, compared to 4.4% of patients in the DFBA group (P<0.05; Table 2; Figure 1B).
Age
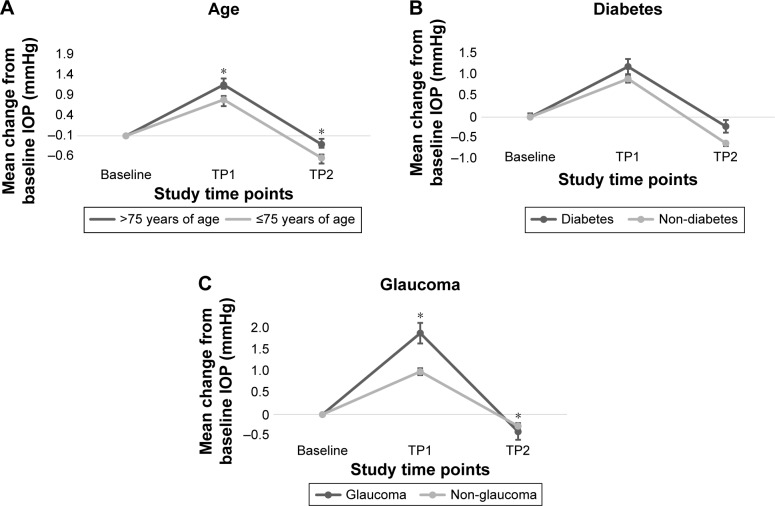
Baseline preoperative IOP in patients with more advanced age (>75 versus ≤75) was not statistically significant (P=0.47; Table 3), patients >75 (n=1,001) versus those ≤75 (n=2,487) did experience a higher increase over baseline IOP at both TP1 (P=0.003) and TP2 (P=0.001), regardless of steroid treatment group (Table 3; Figure 2A). At TP1, the difference was 0.48 mmHg between the two groups (P=0.003). At TP2, the difference in mean IOP between those ≤75 and those >75 was −0.41 mmHg (P=0.001).
Table 3.
IOP (mmHg) changes in patients stratified by risk factors: age, diabetes, and glaucoma
| Categories | Baselineb | TP1b | Δ from baselinea | TP2b | Δ from baselinea | ≥21 mmHg, overall and ≥10 mmHg, increase at TP1c |
|---|---|---|---|---|---|---|
| >75 years old (n=1,001) | 14.7 (3.2) | 15.9 (4.5)** | 1.2 (4.7)** | 14.5 (3.4)* | −0.20 (4.0)* | 30 (3.0) |
| ≤75 years old (n=2,487) | 14.6 (3.2) | 15.4 (4.0)** | 0.85 (4.5)** | 14.1 (2.9)** | −0.52 (3.6)** | 94 (3.8) |
| Δ mean IOP (95% CI) | −0.09 (−0.32, 0.15) | 0.48 (−0.80, −0.16) | −0.45 (−0.75, −0.14) | −0.41 (−0.65, −0.16) | −0.38 (−0.62, −0.15) | N/A |
| P-value | 0.47 | 0.003 | 0.004 | 0.001 | 0.001 | 0.25 |
| >75 years of age | ||||||
| Prednisolone (n=617) | 14.7 (3.1) | 15.4 (4.1)** | 0.68 (4.5)** | 14.1 (3.0)** | −0.57 (3.5)** | 19 (3.1) |
| Difluprednate (n=384) | 14.5 (3.3) | 15.6 (3.8)** | 1.1 (4.4)** | 14.0 (2.8)* | −0.44 (3.7)* | 11 (2.9) |
| Δ mean IOP | −0.19 (−0.60, 0.21) | 0.22 (−0.29, 0.73) | 0.09 (−0.08, 0.26) | −0.05 (−0.42, 0.32) | 0.001 (−0.12, 0.12) | N/A |
| P-value | 0.36 | 0.40 | 0.28 | 0.78 | 0.991 | 0.84 |
| Diabetes (n=641) | 15.0 (3.2) | 15.9 (4.3)** | 1.2 (4.6)** | 14.4 (3.1)** | −0.22 (3.9)** | 100 (3.5) |
| Non-diabetes (n=2,847) | 14.6 (3.2) | 15.8 (4.4)** | 0.91 (4.7)** | 14.4 (3.4)* | −0.62 (3.9)* | 24 (3.7) |
| Δ mean IOP (95% CI) | 0.47 (0.19, 0.74) | 0.20 (−0.16, 0.58) | 0.03 (−0.33, 0.39) | 0.07 (−0.21, 0.35) | −0.06 (−0.33, 0.21) | N/A |
| P-value | <0.001 | 0.28 | 0.87 | 0.60 | 0.67 | 0.78 |
| Diabetes patients | ||||||
| Prednisolone (n=388) | 15.1 (3.3) | 15.9 (4.6)* | 0.76 (4.9)* | 14.4 (3.2)** | −0.74 (4.03)** | 15 (3.9) |
| Difluprednate (n=356) | 14.9 (3.1) | 16.1 (3.9)** | 1.1 (4.3)** | 14.6 (3.1) | −0.42 (3.9) | 9 (3.6) |
| Δ mean IOP | −0.13 (−0.64, 0.39) | 0.25 (−0.43, 0.93) | 0.10 (−0.12, 0.32) | 0.20 (0.29, 0.70) | 0.08 (−0.09, 0.23) | N/A |
| P-value | 0.63 | 0.47 | 0.87 | 0.43 | 0.36 | 0.84 |
| Glaucoma (n=548) | 15.6 (3.6) | 17.5 (5.4)** | 1.9 (5.6)** | 15.2 (3.8)* | −0.40 (4.4)* | 41 (7.5) |
| Non-glaucoma (n=2,940) | 14.5 (3.1) | 15.5 (4.1)** | 1.0 (4.4)** | 14.2 (3.2)** | −0.27 (3.8)** | 83 (2.8) |
| Δ mean IOP (95% CI) | 1.1 (0.81, 1.4) | 2.0 (1.6, 2.4) | 1.6 (1.2, 2.0) | 0.97 (0.67, 1.3) | 0.67 (0.38, 0.96) | N/A |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Glaucoma patients | ||||||
| Prednisolone (n=353) | 15.8 (3.6) | 17.4 (5.2)** | 1.6 (5.4)** | 15.2 (3.8)* | −0.54 (4.1)* | 23 (5.6) |
| Difluprednate (n=195) | 15.3 (3.7) | 17.6 (5.7)** | 2.4 (5.9)** | 15.1 (3.9) | −0.15 (4.3) | 18 (9.2) |
| Δ mean IOP | −0.52 (−1.1, 0.12) | 0.23 (−0.71, 1.2) | 0.15 (−0.15, 0.46) | −0.13 (−0.80, 0.54) | 0.02 (−0.18, 0.23) | N/A |
| P-value | 0.11 | 0.62 | 0.33 | 0.70 | 0.82 | 0.25 |
Notes: Data shown as mean (SD), mean change (SD), n (%), Δ mean (95% CI), or P-value.
P-values calculated using the analysis of covariance adjusted for baseline IOP.
P-values calculated using the Independent t-test.
P-values calculated using Fisher’s exact test.
Denotes P-value <0.05 comparing mean IOP change at each time point from baseline after multiple comparison adjustments.
Denotes P-value <0.001 comparing mean IOP change at each time point from baseline after multiple comparison adjustments.
Abbreviations: IOP, intraocular pressure; TP1, 5–10 days postoperatively; TP2, 3–6 weeks postoperatively.
Figure 2.
Mean change (SE) in IOP (mmHg) from baseline in the total patient population.
Notes: Data stratified by (A) age categories, (B) diabetes status, and (C) glaucoma status. *Analysis of covariance adjusting for baseline IOP to report P-values of <0.05 for the difference in mean change in IOP from baseline at each study time point.
Abbreviations: IOP, intraocular pressure; SE, standard error; TP1, 5–10 days postoperatively; TP2, 3–6 weeks postoperatively.
When comparing whether patients >75 years of age were more likely to experience an increase in IOP on PA versus DFBA, no difference was found between these groups at any time point.
Diabetes
Diabetic patients in this study did present with a higher IOP at baseline (P<0.001; Table 3) prior to surgery compared to non-diabetics. However, there was no significant difference in IOP increase over baseline in diabetics (n=614) versus non-diabetics (n=2,847) at any TP postoperatively (Table 3; Figure 2B).
There was an equal distribution of diabetic patients between the PA (n=388) and DFBA (n=356) groups. When comparing whether diabetic patients were more likely to have increased IOP on PA versus DFBA, no significant difference was found between the groups at any TP (Table 3).
Glaucoma history
Compared to those without glaucoma, patients who reported a history of glaucoma (n=548) presented with a higher IOP at baseline prior to surgery (P<0.001; Table 3). There was a statistically significant increase in mean IOP in glaucoma patients than non-glaucoma patients at both TP1 and TP2 (P<0.001; Table 3; Figure 2C).
Of the patients who reported glaucoma history, there were 353 in the PA group and 195 in the DFBA group. There were no statistically significant differences between these groups at any time point (Table 3).
Discussion
Despite the known risks,5 the prescribed use of steroids after cataract surgery is a standard among ophthalmologists, because the benefits of steroid use outweigh the risks in most cases. The choice of corticosteroid is dependent on the physician. For various applications, difluprednate 0.05% has been shown to be more effective in reducing inflammation when compared to PA 1%.2,21–24 However, among all intraocular corticosteroids, studies have reported an increase in IOP with DFBA.23–30 Jeng et al conducted a retrospective chart review of 100 patients treated with either PA or DFBA after vitreoretinal surgery where patients received PA or DFBA four times a day for a minimum of 1 month. Similar to this present study, the authors found that compared to PA-treated patients, DFBA-treated patients had a significantly higher percentage of patients with an IOP of ≥21 mmHg and an increase from baseline of >10 mmHg.28 The percentages were higher in the study conducted by Jeng et al possibly related to the higher steroid dosage used (four times a day for 1 month). A multicenter randomized study conducted by Sheppard et al compared PA to DFBA usage in anterior uveitis (n=110) and found that DFBA-treated patients had a significantly increased IOP at 72 h but not at any other time points throughout the study.30 Patients in this study received PA eight times daily for 2 weeks or DFBA 4 times daily for 2 weeks which is a higher dosage regimen than in the current study. The mean IOP in this study remained within normal limits for both PA- and DFBA-treated patients. However, the study end point was examining the corticosteroid effect on acute endogenous anterior uveitis, for which they concluded DFBA was noninferior to PA.30 Donnenfeld et al conducted a multicenter randomized controlled trial of PA versus DFBA in cataract surgery using 104 eyes. Patients began administering one drop QID for 1 week, followed by one drop BID for a second week after surgery.2 As with this present study, all patients in their study had a mean IOP within the normal range throughout the trial and the highest increase in IOP was seen at postoperative day 1. However, their study found no difference in IOP increase between DFBA- and PA-treated patients.2
A more recent study of post-cataract surgery pediatric patients (0–3 years old) compared DFBA and PA and found similar safety and efficacy profiles between the two corticosteroids.31 Patients received a regimen of either DFBA or PA QID for 14 days, followed by a 14 day taper. IOP was measured on days 8, 15, 29 (end of treatment), 1 week post-treatment, and 3 months post-treatment. Results showed that mean IOP values were 2–3 mmHg higher in DFBA-treated patients on days 8, 15, and 29. However, mean IOP changes were similar after drug cessation and remained similar at 3 months. These results, while in pediatric patients, mirror the results presented here with more significant differences arising acutely and resolution by 3–6 weeks.
Here, the electronic health records of 3,488 patients who underwent cataract surgery at BDPEC and received either PA or DFBA were queried. The data from this study confirmed earlier reports showing an increase in IOP when DFBA is compared to PA.25,28,30 Importantly, in this study, the mean IOP remained ≤21 mmHg for both PA and DFBA patients. Strict criteria for the definition of a significant increase in IOP (defined by an IOP ≥21 mmHg overall and an increase from baseline of ≥10 mmHg at specified time point) were used. This was based on previously published studies and seemed the most rigorous in the definition of steroid response and the most clinically significant.2,28,30
Advanced age was confirmed to be a risk factor for higher elevations in IOP, as well as a history of glaucoma. Diabetes was not found to be a risk factor in this study. Compared to the aforementioned studies which previously examined PA versus DFBA, our study included a larger sample size and thus more statistical power. Trends were similar for patients using either corticosteroid; the mean IOP remained <21 mmHg for both groups. However, there was a statistically higher percentage (although still low at 4% on DFBA vs 3% on PA at TP1 and 2% on DFBA versus 0.46% on PA at TP2) of patients experiencing an IOP increase (as defined by an IOP ≥21 mmHg overall and an increase from baseline of ≥10 mmHg at specified time point). It also appears that the IOP increase in response to PA may decrease more rapidly than the response to DFBA because the difference between the two groups increased at TP2, although these differences may not pose true clinical significance. A prospective study evaluating IOP changes at various time points postoperative would further elucidate this. Interestingly, the dosage regimen used in the present study is half of the recommended dosing (QID for 2 weeks followed by BID for 2 weeks). Therefore, it is expected that higher IOPs would be seen using the recommended dosing.
Limitations of this study include its retrospective nature lending itself to selection bias and the inclusion of data from multiple surgeons. Despite the use of the same protocol, there may have been individual differences which were not recorded. No data were available on the use of additional medications to decrease IOP postoperatively, including additional prescribed ocular medications for glaucoma, which may have been relevant to assess. However, the authors believe that given the large sample size, these effects, if any, should equally come to bear on both groups. The authors were unable to obtain HbA1c levels for diabetic patients in order to examine diabetic status. In addition, patients with a history of glaucoma were asked to self-report upon obtaining their ophthalmological history, and thus this account may lack rigorous classification, which may have led to a misrepresentation of patients with true glaucoma history. Furthermore, it has been shown that post-surgical use of DFBA may significantly reduce corneal thickness as compared to PA,2,32 which may affect estimations of IOP via Goldmann applanation tonometry.33,34 In this study, it was not possible to measure central corneal thickness in order to control for these potential differences. However, central corneal thickness changes have been shown to be most significant within 24 h of corticosteroid use and return to baseline within 30 days.2 Given that IOP was not measured at the 24 h time point, corneal thickness changes may not significantly alter the results.
Conclusion
All patients should be notified of the potential for increased IOP with the use of ocular corticosteroids and the importance for physician follow-up, as the data from this study have confirmed corticosteroid-induced increased IOP after cataract surgery, regardless of steroid choice. Patients on DFBA should be monitored closely because they are more likely to experience an IOP increase than those on PA.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rugstad HE. Antiinflammatory and immunoregulatory effects of glucocorticoids: mode of action. Scand J Rheumatol Suppl. 1988;76:257–264. doi: 10.3109/03009748809102977. [DOI] [PubMed] [Google Scholar]
- 2.Donnenfeld ED, Holland EJ, Solomon KD, et al. A multicenter randomized controlled fellow eye trial of pulse-dosed difluprednate 0.05% versus prednisolone acetate 1% in cataract surgery. Am J Ophthalmol. 2011;152(4):609.e1–617.e1. doi: 10.1016/j.ajo.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11(6):478–483. doi: 10.1097/00055735-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Armaly MF, Becker B. Intraocular pressure response to topical corticosteroids. Federation Proceedings. 1965;24(6):1274–1278. [PubMed] [Google Scholar]
- 5.Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye (Lond) 2006;20(4):407–416. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 6.Sapir-Pichhadze R, Blumenthal EZ. Steroid induced glaucoma. Harefuah. 2003;142(2):137–140. 157. [PubMed] [Google Scholar]
- 7.Razeghinejad MR, Katz LJ. Steroid-induced iatrogenic glaucoma. Ophthalmic Res. 2012;47(2):66–80. doi: 10.1159/000328630. [DOI] [PubMed] [Google Scholar]
- 8.Becker B, Mills DW. Corticosteroids and intraocular pressure. Archives of Ophthalmology. 1963;70(4):500–507. doi: 10.1001/archopht.1963.00960050502012. [DOI] [PubMed] [Google Scholar]
- 9.Dielemans I, de Jong PT, Stolk R, Vingerling JR, Grobbee DE, Hofman A. Primary open-angle glaucoma, intraocular pressure, and diabetes mellitus in the general elderly population: the Rotterdam Study. Ophthalmology. 1996;103(8):1271–1275. doi: 10.1016/s0161-6420(96)30511-3. [DOI] [PubMed] [Google Scholar]
- 10.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics: I. The effect of dexamethasone in the normal eye. Arch Ophthalmol. 1963;70(4):482–491. doi: 10.1001/archopht.1963.00960050484010. [DOI] [PubMed] [Google Scholar]
- 11.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics: II. The effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol. 1963;70(4):492–499. doi: 10.1001/archopht.1963.00960050494011. [DOI] [PubMed] [Google Scholar]
- 12.Becker B. Diabetes mellitus and primary open-angle glaucoma: The XXVII Edward Jackson memorial lecture. Am J Ophthalmol. 1971;71(1):1–16. doi: 10.1016/0002-9394(71)91088-9. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell P, Smith W, Chey T, Healey PR. Open-angle glaucoma and diabetes: the blue mountains eye study, Australia. Ophthalmology. 1997;104(4):712–718. doi: 10.1016/s0161-6420(97)30247-4. [DOI] [PubMed] [Google Scholar]
- 14.Quigley H, Sanchez R, Dunkelberger G, L’Hernault N, Baginski T. Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987;28(6):913–920. [PubMed] [Google Scholar]
- 15.Quigley HA, Dunkelberger GR, Green WR. Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology. 1988;95(3):357–363. doi: 10.1016/s0161-6420(88)33176-3. [DOI] [PubMed] [Google Scholar]
- 16.Quigley HA, Katz J, Derick RJ, Gilbert D, Sommer A. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992;99(1):19–28. doi: 10.1016/s0161-6420(92)32018-4. [DOI] [PubMed] [Google Scholar]
- 17.Patel M, Singhal D. Difluprednate in Ophthalmic Practice. Natl J Physiol Pharm Pharmacol. 2014;4(1):92–94. [Google Scholar]
- 18.Tajika T, Waki M, Tsuzuki M, Kida T, Sakaki H. Pharmacokinetic features of difluprednate ophthalmic emulsion in rabbits as determined by glucocorticoid receptor-binding bioassay. J Ocul Pharmacol Ther. 2011;27(1):29–34. doi: 10.1089/jop.2010.0106. [DOI] [PubMed] [Google Scholar]
- 19.Bodor N, Harget AJ, Phillips EW. Structure-activity relationships in the antiinflammatory steroids: a pattern-recognition approach. J Med Chem. 1983;26(3):318–328. doi: 10.1021/jm00357a003. [DOI] [PubMed] [Google Scholar]
- 20.Chak G, Kiely AE, Challa P. Topical Corticosteroid and NSAID Therapies for Ocular Inflammation. Cataract Refract Surg Today. 2014;14(11):15–21. [Google Scholar]
- 21.Foster CS, DaVanzo R, Flynn TE, McLeod K, Vogel R, Crockett RS. Durezol (difluprednate ophthalmic emulsion 0.05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. J Ocul Pharmacol Ther. 2010;26(5):475–483. doi: 10.1089/jop.2010.0059. [DOI] [PubMed] [Google Scholar]
- 22.Jamal KN, Callanan DG. The role of difluprednate ophthalmic emulsion in clinical practice. Clin Ophthalmol. 2009;3:381–390. doi: 10.2147/opth.s4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korenfeld MS, Silverstein SM, Cooke DL, Vogel R, Crockett RS, (Durezol) Study Group Difluprednate ophthalmic emulsion 0.05% for postoperative inflammation and pain. J Cataract Refract Surg. 2009;35(1):26–34. doi: 10.1016/j.jcrs.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Smith S, Lorenz D, Peace J, McLeod K, Crockett R, Vogel R. Difluprednate ophthalmic emulsion 0.05% (Durezol®) administered two times daily for managing ocular inflammation and pain following cataract surgery. Clin Ophthalmol. 2010;4:983–991. doi: 10.2147/opth.s10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meehan K, Vollmer L, Sowka J. Intraocular pressure elevation from topical difluprednate use. Optometry. 2010;81(12):658–662. doi: 10.1016/j.optm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Birnbaum AD, Jiang Y, Tessler HH, Goldstein DA. Elevation of intraocular pressure in patients with uveitis treated with topical difluprednate. Arch Ophthalmol. 2011;129(5):667–668. doi: 10.1001/archophthalmol.2011.82. [DOI] [PubMed] [Google Scholar]
- 27.Cable M. Intraocular pressure spikes using difluprednate 0.05% for postoperative cataract inflammation. Invest Ophthalmol. 2010;51(13):1981. [Google Scholar]
- 28.Jeng KW, Fine HF, Wheatley HM, Roth D, Connors DB, Prenner JL. Incidence of steroid-induced ocular hypertension after vitreoretinal surgery with difluprednate versus prednisolone acetate. Retina. 2014;34(10):1990–1996. doi: 10.1097/IAE.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 29.Prenner JL, Roth DB, Fine HF, Wheatley HM, Connors D. Incidence of steroid induced ocular hypertension following vitreoretinal surgery with difluprednate versus prednisolone acetate. Invest Ophthalmol. 2012;53(14):5383. doi: 10.1097/IAE.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 30.Sheppard JD, Toyos MM, Kempen JH, Kaur P, Foster CS. Difluprednate 0.05% versus prednisolone acetate 1% for endogenous anterior uveitis: a phase III, multicenter, randomized study. Invest Ophthalmol Vis Sci. 2014;55(5):2993–3002. doi: 10.1167/iovs.13-12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson ME, O’Halloran H, VanderVeen D, et al. Difluprednate versus prednisolone acetate for inflammation following cataract surgery in pediatric patients: a randomized safety and efficacy study. Eye (Lond) 2016;30(9):1187–1194. doi: 10.1038/eye.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donnenfeld ED. Difluprednate for the prevention of ocular inflammation postsurgery: an update. Clin Ophthalmol. 2011;5:811–816. doi: 10.2147/OPTH.S6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton KE, Pye DC, Hali A, Lin C, Kam P, Ngyuen T. The effect of contact lens induced corneal edema on Goldmann applanation tonometry measurements. J Glaucoma. 2007;16(1):153–158. doi: 10.1097/01.ijg.0000212277.95971.be. [DOI] [PubMed] [Google Scholar]
- 34.Lau W, Pye D. Changes in corneal biomechanics and applanation tonometry with induced corneal swelling. Invest Ophthalmol Vis Sci. 2011;52(6):3207–3214. doi: 10.1167/iovs.10-6754. [DOI] [PubMed] [Google Scholar]