Abstract
Background
Operative site drainage (OSD) after elective hepatectomy remains widely employed despite data suggesting limited benefit. Multi-institutional quality-driven databases and analytic techniques offer a unique source from which the utility of OSD can be assessed.
Study Design
Elective hepatectomies from the 2014 American College of Surgeons (ACS) NSQIP Targeted Hepatectomy Database were propensity score matched on OSD utilization using preoperative and intraoperative variables. The influence of OSD on the diagnosis of postoperative bile leaks, rates of subsequent intervention, and other outcomes within 30 days were assess using paired testing.
Results
OSD was used in 42.2% of 2583 eligible hepatectomies. 1868 cases were matched, with 7.2% experiencing a post-hepatectomy bile leak. The incidence of bile leak initially requiring intervention was no different between the OSD and no OSD groups (n=32 vs n=24, p=.278). OSD was associated with a greater number of drainage procedures to manage post-hepatectomy bile leak (n=27 in the OSD group, n=13 in the no OSD group, p=.034, RR 2.1 [95% CI 1.1 - 4.0]). The OSD group had a greater mean length of stay (+0.8 days, p=.004) and more 30-day readmissions (p<.001, RR 1.6 [95% CI 1.2 - 2.1]). On multivariate analysis, post-hepatectomy bile leak and receipt of additional drainage procedures were stronger predictors of increased length of stay and readmissions than OSD.
Conclusions
In a propensity score matched cohort, OSD did not improve the rate of diagnosis of major bile leaks and was associated with increased interventions, greater length of stay, and greater 30-day readmissions. These data suggest that routine OSD after elective hepatectomy may not be helpful in capturing clinically relevant bile leaks and has additional consequences.
Graphical Abstract
Precis
Operative site drainage is associated with a greater number of drainage procedures and contributes to greater length of stay and increased 30-day unplanned readmissions. It is not associated with the diagnosis of post-hepatectomy bile leaks requiring intervention.
INTRODUCTION
Increasing rates of liver disease and advancements in procedural techniques have resulted in greater numbers of liver resection and increasing complexity of patients undergoing such treatments (1-3). Improving outcomes for complex patients demands quality care through evidence-based practice. One particular component of surgical care for which there exists significant data yet high variability in practice is the use of operative site drainage (OSD) after hepatectomy.
OSD refers to leaving an externalized closed-suction drain (Jackson-Pratt®, BLAKE®, or similar) at or near the operative site prior to completion of the surgical case. The benefits of OSD after hepatectomy are believed to be the potential for early diagnosis of hemorrhage or bile leaks and the prevention and possible management of postoperative fluid collections (4). Post-hepatectomy bile leak has an estimated of incidence between 3 and 12% (5-7) and can greatly impact additional postoperative outcomes. However, randomized trials and observational studies (8-21), including several published since 2014 (8, 20-22), have suggested that routine OSD offers no benefit in managing bile leak. Recent prospective data has reinforced the message by suggesting that OSD increases the ability to diagnose bile leaks but, instead of managing these leaks, actually increases the need for additional drainage procedures (22). Admittedly, many of the studies have had some important limitations, including selection bias, being underpowered, and protocol non-adherence. To some extent, these limitations have diminished the strength of the recommendations not to drain by authors (22). As a result, practice patterns have been slow to change as evidenced by the percentage of patients receiving OSD. Recent analysis of practice patterns suggest that one-third to as many as all patients receive OSD, depending on protocols, extent of resection, and surgical approach (21, 23, 24).
The mismatch between the evidence and rates of OSD utilization suggests that the utility of OSD after hepatectomy remains a significant and relevant clinical question warranting further appropriately designed investigations. The choice of OSD is multifactorial, following a complex interaction of factors including the patient's surgical and comorbid diseases, the extent and difficulty of resection, and surgeon training, technique, skill, and preference. The availability of large multi-institutional quality-driven datasets presents an opportunity to review outcomes associated with surgical practices such as OSD. The addition of procedure-targeted variables to the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) represents more granular data with which to undertake such investigations. Drawing on existing data, we hypothesized that operative site drainage offers minimal or no benefit in preventing or treating post-hepatectomy bile leaks, leads to over-diagnosis bias and additional procedures, and may be associated with increased infection rates. When analyzing groups within large datasets, one must consider imbalances due to treatment selection bias or confounding by indication (25). Analytic techniques like propensity score matching can help reduce the effects of this and other biases (26). As such, we developed a propensity score matched analysis from the ACS-NSQIP targeted hepatectomy file to assess the implications of the use of OSD on post-hepatectomy bile leak and other outcomes after elective hepatectomy.
METHODS
Data Source and Eligibility Criteria
Patients were identified from the ACS-NSQIP Procedure Targeted Database for Hepatectomy, 2014. This data file contains 42 hepatectomy-specific variables and 3,064 patient entries. Similar to the general ACS-NSQIP Participant User File (PUF), the procedure targeted database records preoperative comorbidities, intraoperative details, and tracks outcomes up to postoperative day 30 for a unique set of hepatectomy-specific variables. The general PUF dataset was merged with the hepatectomy variables by case ID.
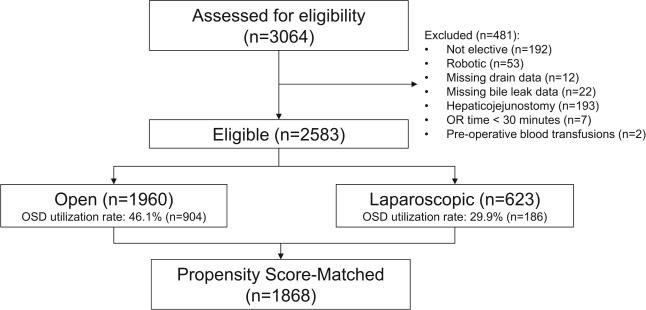
The study population was then limited to patients who underwent elective inpatient (length of stay ≥1 day) hepatectomy. Hepatectomy as coded by ACS-NSQIP includes cases with a primary Current Procedural Terminology (CPT) code of 47120 (partial lobectomy), 47122 (trisegmentectomy), 47125 (total left lobectomy), and 47130 (total right lobectomy). Patients were excluded if data was missing for OSD or post-hepatectomy bile leak, if the procedure was performed robotically or hybrid, and if the patient required >4 units of blood transfused preoperatively. Cases with hepaticojejunostomy reconstruction were excluded due to anticipated differences in rates of OSD utilization. Cases less than 30 minutes were eliminated to reduce the possible inclusion of liver biopsies. Laparoscopic procedures were analyzed on an intention-to-treat basis. The inclusion schema is reviewed in Figure 1.
Figure 1.
Inclusion schema. OR, operating room; OSD, operative site drainage.
The primary outcome, post-hepatectomy bile leak, is defined by ASC-NSQIP using a documented clinical diagnosis from the attending surgeon or International Study Group of Liver Surgery (ISGLS) criteria (27): drain bilirubin at least three times serum on or after postoperative day 3 or need for radiologic or operative intervention for biliary collection. Coding of this diagnosis includes a subsequent initial treatment decision, which was used to distinguish between bile leaks requiring intervention (“major” bile leaks) and those initially treated without additional intervention (“minor” bile leaks). Secondary outcomes included surgical site infections, length of stay, and unplanned readmissions within thirty days after surgery.
Statistical analysis
Descriptive statistics, chi-square test for proportions, and student's t-test for means were used to characterize differences among patient groups. Statistical analysis was performed using SAS software version 9.4 (SAS Institute, Cary, NC) with two-sided testing and significance set at p<.05. For propensity scoring, preoperative and intraoperative variables were used, as these would be available to a surgeon at the time of OSD decision (Table 1). Variables were included if they met a crude univariate chi-square or t-test p<.50 and had <15% of cases missing data. Missing data was most common for preoperative laboratory values (range 2.1% - 14.9%). There were no statistical differences (p>.05) for all laboratory variables entered into the model on Wilcoxon-Mann-Whitney test between the group missing any one of these values (n=645) and the population missing no values. Laboratory values were therefore determined to be missing at random and were imputed to the group median due to non-normal distributions. Five other variables had <3% missing data and were also imputed to the group median. Categorical variables were assessed for non-linear effects by graphing independent subset chi-square-derived odds ratios and additional non-linear adjustment variables were then added to the logistic regression using the formula suggested for the line-of-best-fit from Excel 2013 (Microsoft Corp., Redmond, WA). Propensity score matching was performed 1:1 using a caliper of 0.2 times the standard deviation of the logit of the propensity score (28). Heterogeneity of the propensity score match was assessed between the OSD and no OSD groups using absolute standardized differences, with a threshold of <10% considered appropriate balance(29). Propensity scores were graphed using Stata v14.1 (StataCorp, College Station, TX). Statistical analysis of the primary and secondary endpoints, post-hepatectomy bile leak and additional drainage procedures, was done using paired relative risk and McNemar's tests (26). Length of stay was assessed using linear regression and readmissions were assessed using chi-square test and multivariate logistic regression. Outcomes analysis in the subset of patients with major bile leak was performed in a non-paired fashion using Fisher's exact test and Wilcoxon-Mann-Whitney testing. The project was approved as IRB-exempt.
Table 1.
Variables Included in the Propensity Score Logistic Regression
| Categorical |
| Preoperative |
| Sex (M/F*) |
| ASA Class (1*-4) |
| Diabetes (Y/N*) |
| Current smoker within 1 y (Y/N*) |
| Chronic steroid use (Y/N*) |
| Receipt of neoadjuvant therapy for hepatic disease within 90 d of operation (Y/N*) |
| Intraoperative |
| Operative approach: laparoscopic* or open |
| Extent of resection: CPT 47120*, 47122, 47125, or 47130 |
| Pringle maneuver (Y/N*) |
| Concurrent intraoperative ablation (Y/N*) |
| Intraoperative or postoperative (< 72 h) transfusion (Y/N*) |
| Continuous |
| Preoperative |
| Age |
| BMI |
| Albumin |
| Creatinine |
| INR |
| Platelet count |
| Serum glutamic oxaloacetic transaminase |
| Alkaline phosphatase |
| Intraoperative |
| Operating room time |
| Concurrent partial resections |
Group variable: operative site drainage
Reference group.
INR, International Normalized Ratio
RESULTS
2583 hepatectomies met inclusion criteria (Figure 1). 1960 (75.9%) cases were performed open and 623 (24.1%) cases were performed laparoscopically. 30-day mortality was 1.2% (n=23). OSD was used in 1090 cases (42.2%), including in 46.1% (n=904) of open procedures and 29.9% (n=186) of laparoscopic procedures (p<.001). Preoperative and intraoperative factors associated with the use of OSD included ASA class greater than 1, greater extent of resection, longer procedure time, and increasing preoperative alkaline phosphatase and platelet count. Receipt of any neoadjuvant therapy within the 90 days prior to surgery was associated with decreased use of OSD (Table 2).
Table 2.
Preoperative and Intraoperative Variables Associated with the Use of Operative Site Drainage
| Variable | p Value | OR | 95% CI |
|---|---|---|---|
| ASA Classification | |||
| 1 | - | - | -- |
| 2 | <0.001 | 5.2 | 2.6-10.2 |
| 3 | <0.001 | 4.3 | 2.2-8.5 |
| 4 | <0.001 | 4.1 | 1.9-8.6 |
| Neoadjuvant therapy within 90 d prior to surgery | 0.010 | 0.8 | 0.7-0.9 |
| Preoperative alkaline phosphatase ≥ 100 U/L | <0.001 | 1.4 | 1.2-1.7 |
| Preoperative platelet count ≥ 450,000/μL | 0.019 | 2.0 | 1.1-3.7 |
| Operative approach | |||
| Laparoscopic | - | - | -- |
| Open | <0.001 | 1.7 | 1.4-2.1 |
| CPT | |||
| 47120 Partial lobectomy | - | - | -- |
| 47122 Trisegmentectomy | 0.012 | 1.5 | 1.1-2.1 |
| 47125 Left lobectomy | <0.001 | 1.7 | 1.3-2.3 |
| 47130 Right lobectomy | 0.109* | 1.2 | 1.0-1.5 |
| Transfusion requirement† | <0.001 | 2.0 | 1.6-2.5 |
| Operative time ≥ 300 min | 0.004 | 1.3 | 1.1-1.6 |
C statistic for model = 0.663.
ASA, American Society of Anesthesiologists Physical Status Classification; CPT, Current Procedural Terminology.
Not significant but included for comparison (n=508).
Includes transfusions intraoperatively and up to 72 hours postoperatively.
The OSD and no OSD groups differed with respect to a number of comorbid and intraoperative variables (Table 3). Post-hepatectomy bile leak occurred in 167 patients (6.5%) and was more frequent after open procedures (n=146, 7.5%) compared to laparoscopic (n=21, 3.4%; p<.001). The incidence of bile leak was significantly different between the OSD (n=134, 12.3%) and no OSD groups (n=33, 2.21%; p<.001). This significance held for both open hepatectomy (n=119, 13.2%, with OSD vs n=27, 2.6%, without OSD; p<.001) and laparoscopic hepatectomy (n=15, 8.1% with OSD vs. n=6, 1.4% without OSD; p<.001). 95 cases (3.7%) experienced a minor bile leak (initially managed with observation with OSD in place) and 68 cases (2.6%) experienced a major leak, requiring intervention with either percutaneous drainage (n=53) or reoperation (n=15). Of these 68 patients, OSD had been in place in 36 (52.9%).
Table 3.
Descriptive and Comparative Distribution of Preoperative and Intraoperative Variables, Eligible Patient Population
| Total | OSD | No OSD | Standardized differences, % | |
|---|---|---|---|---|
| Comorbidities | ||||
| n (%) | 2,583 | 1,090 (42.2) | 1,493 (57.8) | |
| Age, y ±SD | 58.5 ± 13.5 | 59.2 ± 13.1 | 58.0 ± 13.8 | 8.8 |
| Male, n (%) | 1,234 (47.8) | 555 (50.9) | 794 (53.2) | 4.5 |
| ASA Class, n (%) | ||||
| 1 | 70 (2.7) | 11 (1.0) | 59 (4.0) | 19.0* |
| 2 | 690 (26.7) | 304 (27.9) | 386 (25.9) | 4.6 |
| 3 | 1,663 (64.4) | 706 (64.8) | 959 (64.2) | 1.1 |
| 4 | 158 (6.1) | 69 (6.3) | 89 (6.0) | 1.5 |
| BMI, kg/m2 ±SD | 28.4 ± 6.3 | 28.2 ± 6.4 | 6.0 | |
| Hypertension, n (%) | 1,101 (42.6) | 497 (45.6) | 604 (40.5) | 10.4* |
| COPD, n (%) | 87 (3.4) | 45 (4.1) | 42 (2.8) | 7.2 |
| Diabetes, n (%) | 401 (15.5) | 178 (16.3) | 223 (14.9) | 3.8 |
| Current smoker, n (%) | 370 (14.3) | 162 (14.9) | 208 (13.9) | 2.7 |
| Neoadjuvant therapy within 90 d prior to surgery, n (%) | 838 (32.8) | 352 (32.3) | 486 (32.6) | 0.6 |
| Systemic steroid use, n (%) | 101 (3.9) | 44 (4.0) | 57 (3.8) | 1.1 |
| Preoperative lab values | ||||
| Bilirubin, mg/dL ±SD | 0.59 ± 0.38 | 0.60 ± 0.41 | 0.58 ± 0.36 | 6.6 |
| INR ±SD | 1.03 ± 0.16 | 1.04 ± 0.16 | 1.02 ± 0.15 | 9.2 |
| Alkaline phosphatase, U/L ±SD | 104 ± 70 | 115 ± 91 | 96 ± 47 | 25.4* |
| SGOT, U/L ±SD | 32.7 ± 31.7 | 34.4 ± 27.2 | 31.5 ± 34.6 | 9.0 |
| Creatinine, mg/dL ±SD | 0.89 ± 0.42 | 0.89 ± 0.32 | 0.89 ± 0.49 | 2.2 |
| Platelets per μL ±SD | 231 ± 87 | 237 ± 96 | 227 ± 79 | 11.7* |
| Albumin, g/dL ±SD | 4.0 ± 0.4 | 3.98 ± 0.45 | 4.02 ± 0.41 | 8.0 |
| Intraoperative variables | ||||
| CPT, n (%) | ||||
| 47120 | 1,640 (63.5) | 620 (56.9) | 1,020 (68.3) | 23.8 |
| 47122 | 205 (7.9) | 113 (10.4) | 92 (6.2) | 15.3* |
| 47125 | 230 (8.9) | 122 (11.2) | 108 (7.2) | 13.7* |
| 47130 | 508 (19.7) | 235 (21.6) | 273 (18.3) | 8.2 |
| Laparoscopic approach, n (%) | 623 (24.1) | 186 (17.1) | 437 (29.3) | 29.2* |
| Procedure length, min ±SD | 241 ± 110 | 259 ± 112 | 228 ± 107 | 28.5* |
| Concurrent partial resection, n (%) | 1,421 (55.0) | 634 (58.2) | 787 (52.7) | 4.4 |
| Concurrent ablation, n (%) | 381 (14.8) | 167 (15.3) | 214 (14.3) | 2.8 |
| Pringle maneuver used, n (%) | 677 (26.2) | 325 (29.8) | 352 (23.6) | 14.1* |
| Transfusion, n (%) | 393 (15.2) | 239 (21.9) | 154 (10.3) | 32.0* |
Absolute standardized differences for variables with imbalance (≥10%) between groups.
OSD, operative site drainage; ASA Class, American Society of Anesthesiologists Physical Status Classification System; INR, international normalized ratio; SGOT, serum glutamic oxaloacetic transaminase.
After applying exclusion criteria, including removing 34 patients missing data for OSD usage or post-hepatectomy bile leak, 1868 patients were propensity score matched between the OSD and no-OSD groups. Distributions of propensity scores before and after matching can be seen in eFigure 1. 1490 cases (79.8%) were performed open and 378 (20.2%) were performed laparoscopically. 30-day mortality was 1.3% (n=25). All comorbid and intraoperative variables met statistical balance between the OSD and no OSD groups after propensity score matching (Table 4).
Table 4.
Descriptive and Comparative Distribution of Preoperative and Intraoperative Variables, Propensity Score Matched Patient Population
| Total | OSD | No OSD | Standardized differences, % | |
|---|---|---|---|---|
| Comorbidities | ||||
| n (%) | 1,868 | 934 (50.0) | 934 (50.0) | |
| Age, y ±SD | 59.3 ± 13.1 | 59.3 ± 13.1 | 59.2 ± 13.2 | 0.6 |
| Male, n (%) | 920 (49.3) | 450 (48.2) | 470 (50.3) | 4.3 |
| ASA Class, n (%) | ||||
| 1 | 20 (1.1) | 11 (1.2) | 9 (1.0) | 2.1 |
| 2 | 509 (27.2) | 252 (27.0) | 257 (27.5) | 1.2 |
| 3 | 1,224 (65.5) | 610 (65.3) | 614 (65.7) | 0.9 |
| 4 | 115 (6.2) | 61 (6.5) | 54 (5.8) | 3.1 |
| BMI, kg/m2, ±SD | 28.6 ± 6.3 | 28.6 ± 6.3 | 28.5 ± 6.4 | 2.6 |
| Hypertension, n (%) | 847 (45.3) | 425 (45.5) | 422 (45.2) | 0.6 |
| COPD, n (%) | 66 (3.5) | 32 (3.4) | 34 (3.6) | 1.2 |
| Diabetes, n (%) | 317 (17.0) | 158 (16.9) | 159 (17.0) | 0.3 |
| Current smoker, n (%) | 272 (14.6) | 134 (14.3) | 138 (14.8) | 1.2 |
| Neoadjuvant therapy within 90 d prior to surgery, n (%) | 620 (33.2) | 322(34.5) | 316 (33.8) | 1.4 |
| Systemic steroid use, n (%) | 69 (3.7) | 33 (3.5) | 36 (3.9) | 1.7 |
| Preoperative lab values | ||||
| Bilirubin, mg/dL ±SD | 0.58 ± 0.38 | 0.60 ± 0.38 | 0.58 ± 0.39 | 3.4 |
| INR ±SD | 1.03 ± 0.15 | 1.03 ± 0.15 | 1.03 ± 0.15 | 1.5 |
| Alkaline phosphatase, U/L ±SD | 102 ± 53 | 102 ± 53 | 101 ± 53 | 1.5 |
| SGOT, U/L ±SD | 33 ± 26 | 33 ± 25 | 33 ± 27 | 1.4 |
| Creatinine, mg/dL ±SD | 0.89 ± 0.32 | 0.89 ± 0.32 | 0.89 ± 0.31 | 0.5 |
| Platelets per μL ±SD | 229 ± 85 | 229 ± 85 | 230 ± 84 | 1.0 |
| Albumin, g/dL ±SD | 4.0 ± 0.4 | 4.0 ± 0.4 | 4.0 ± 0.4 | 1.3 |
| Intraoperative variables | ||||
| CPT, n (%) | ||||
| 47120 | 1,127 (60.3) | 568 (60.8) | 559 (59.9) | 2.0 |
| 47122 | 168 (9.0) | 80 (8.6) | 88 (9.4) | 3.0 |
| 47125 | 178 (9.5) | 84 (9.0) | 94 (10.1) | 3.6 |
| 47130 | 395 (21.1) | 202 (21.6) | 193 (20.7) | 2.4 |
| Laparoscopic approach, n (%) | 378 (20.2) | 184 (19.7) | 194 (20.8) | 2.7 |
| Procedure length, min ±SD | 250 ± 111 | 251 ± 111 | 249 ± 110 | 1.0 |
| Concurrent partial resection, n (%) | 1,042 (55.8) | 542 (58.0) | 527 (56.4) | 1.5 |
| Concurrent ablation, n (%) | 296 (15.8) | 149 (16.0) | 149 (16.0) | 0.0 |
| Pringle maneuver used, n (%) | 527 (28.2) | 265 (28.4) | 262 (28.1) | 0.7 |
| Transfusion, n (%) | 301 (16.1) | 154 (16.5) | 147 (15.7) | 2.0 |
OSD, operative site drainage; ASA Class, American Society of Anesthesiologists Physical Status Classification System; INR, international normalized ratio; SGOT, serum glutamic oxaloacetic transaminase.
Post-hepatectomy bile leak occurred in 135 patients (7.2%) and was more frequent after open procedures (n=116, 7.8%) compared to laparoscopic (n=19, 5.0%; p=.064). OSD was associated with increased diagnosis of post-hepatectomy bile leak (p<.001, RR 4.4 [95% Confidence Interval (CI) I 2.9 – 6.7]). Minor bile leaks accounted for this increase (overall incidence 4.2%; n=78 in OSD group, n=1 in no OSD group; p<.001) as there was no significant difference in the incidence of major bile leaks between the OSD (overall incidence 3.0%, n=32 in OSD group, n=24 in no OSD group, p=.278). The one case of minor bile leak diagnosed without OSD occurred via spontaneous wound drainage. 15 cases of minor bile leak initially managed with OSD subsequently required percutaneous intervention (19.5%). The OSD group underwent more postoperative interventions for aspiration or drainage of bile (n=27 in OSD group, n=13 in the no OSD group; p=.034, RR 2.1 [95% CI 1.1 – 4.0]) and aspiration or drainage of abscess (n=28 in OSD group, n=14 in the no OSD group; p=.044, RR 2.0 [95% CI 1.1 – 3.8]). Twelve patients with bile leak were initially managed with reoperation: six in both the OSD group and the no OSD group.
OSD was independently associated with a higher incidence of organ space SSI (n=64, 6.9% with OSD, vs n=42, 4.5% with no OSD; p=.028, RR 1.5 [95% CI 1.04 – 2.2]). However, post-hepatectomy bile leak was also independently associated with organ space SSI (p<.001, OR 13.7, 95% CI 8.8 – 21.3). Controlling for this confounding effect, OSD was no longer associated with organ space SSI (p=.704). An interaction variable between OSD and post-hepatectomy bile leak was also not associated with organ space SSI (p=.226). The rate of other infectious complications was no different between the OSD and no OSD groups (Table 5). In the subset of patients with major bile leak, there were no statistical differences in the assessed outcomes between the OSD and no OSD groups (data not shown). OSD was utilized more frequently in resections for primary hepatobiliary malignancies but less frequently for secondary malignancies.
Table 5.
Descriptive and Comparative Distribution of Postoperative Variables, Propensity Score Matched Patient Population
| Total | OSD | No OSD | Standardized difference, % | |
|---|---|---|---|---|
| Overall, n (%) | 1,868 | 934 (50.0) | 934 (50.0) | |
| Length of stay, d ±SD | 6.5 ± 6.4 | 7.0 ± 6.3 | 6.1 ± 6.5 | 14.2* |
| Pathology, n (%) | ||||
| Primary hepatobiliary cancer | 489 (26.2) | 286 (30.6) | 203 (21.7) | 20.3* |
| Metastatic tumor | 980 (52.5) | 449 (48.1) | 531 (56.9) | 17.6* |
| Benign lesion | 333 (17.8) | 180 (19.3) | 153 (16.4) | 7.6 |
| Other/unknown | 66 (3.5) | 19 (2.0) | 47 (5.0) | 16.3* |
| Wound complications, n (%) | ||||
| Superficial | 61 (3.3) | 35 (3.7) | 26 (2.8) | 5.4 |
| Deep incisional | 12 (0.6) | 8 (0.9) | 4 (0.4) | 5.4 |
| Organ space | 106 (5.7) | 64 (6.9) | 42 (4.5) | 10.2* |
| Disruption, dehiscence | 14 (0.7) | 6 (0.6) | 8 (0.9) | 2.5 |
| Other infectious complications, n (%) | ||||
| Sepsis, septic shock | 93 (5.0) | 55 (5.9) | 38 (4.1) | 8.4 |
| Pneumonia | 66 (3.5) | 27 (2.9) | 39 (4.2) | 7.0 |
| Urinary tract infection | 48 (2.6) | 26 (2.8) | 22 (2.4) | 2.7 |
| Other outcomes, n (%) | ||||
| Post-hepatectomy liver failure | 78 (4.2) | 38 (4.1) | 40 (4.3) | 1.1 |
| Myocardial infarction | 13 (0.7) | 6 (0.6) | 7 (0.7) | 1.3 |
| Stroke | 7 (0.4) | 3 (0.3) | 4 (0.4) | 1.8 |
| DVT and/or PE | 54 (2.9) | 31 (3.3) | 23 (2.5) | 5.1 |
| Any reoperation | 49 (2.6) | 21 (2.2) | 28 (3.0) | 8.4 |
| Mortality | 25 (1.3) | 8 (0.9) | 17 (1.8) | 8.4 |
| Unplanned readmission | 181 (9.7) | 111 (11.9) | 70 (7.5) | 14.9* |
Absolute standardized differences for variables with imbalance (≥10%) between groups.
OSD, operative site drainage; DVT, deep venous thrombosis; PE, pulmonary embolism.
OSD was independently associated with a significant increase in the mean length of stay by 0.8 days (p=.004, standard error 0.3) and a greater number of unplanned 30-day readmissions (p=.001, RR 1.6 [95% CI 1.2 – 2.1]) (Table 6 and 7). However, post-hepatectomy bile leak and receipt of additional drainage procedures were also independent predictors of increased length of stay and readmissions. Patients experiencing post-hepatectomy bile leak had an estimated increase in length of stay by 6.0 days compared to the entire cohort, and those who underwent an additional procedure for bile leak had an estimated increase of 5.0 days. On multivariate analysis of the effects of OSD, bile leak, and drainage procedures on length of stay, only post-hepatectomy bile leak was predictive (Table 6). For unplanned 30-day readmission, each of these three variables are significant predictors of unplanned 30-day readmissions on univariate analysis, and bile leak and additional procedures are significant on multivariate analysis (Table 7).
Table 6.
Univariate and Multivariate Analysis of Operative Site Drainage, Post-Hepatectomy Bile Leak, and Additional Drainage Procedures on Index Admission Length of Stay
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Length of stay | p Value | Estimated effect size, d (standard error) | p Value | Estimated effect size, d (standard error) |
| Operative site drainage | 0.004 | + 0.8 (0.3) | 0.328 | + 0.3 (0.3) |
| Post-hepatectomy bile leak | <0.001 | + 6.0 (0.5) | <0.001 | + 5.4 (0.6) |
| Additional procedure for bile leak | <0.001 | + 5.0 (0.7) | 0.053 | + 1.5 (0.8) |
| Intercept 6.0 d | ||||
Table 7.
Univariate and Multivariate Analysis of Operative Site Drainage, Post-Hepatectomy Bile Leak, and Additional Drainage Procedures on 30-Day Unplanned Readmissions
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| 30-d Unplanned readmission | p-value | Incidence, exposed vs unexposed* | Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI |
| Operative site drainage | 0.001 | 12.3% (115/934) vs 7.7% (72/934) | 1.7 | 1.2 - 2.3 | 0.092 | 1.3 | 1.0 - 1.9 |
| Post-hepatectomy bile leak | <0.001 | 39.3% (53/135) vs 7.7% (134/1733) | 7.7 | 5.2 - 11.4 | <0.001 | 2.6 | 1.6 - 4.6 |
| Additional procedure for bile leak | <0.001 | 69.4% (50/72) vs 7.6% (137/1796) | 27.5 | 16.2 - 46.8 | <0.001 | 15.3 | 8.4 - 28.0 |
Incidence for univariate analysis of 30-day readmission is presented as incidence, % (n), of readmission in the exposed group (operative site drainage, bile leak, or additional procedure) vs non-exposed group.
CI, confidence interval.
DISCUSSION
Using a multi-institutional procedure-specific database, we identified variables associated with usage of OSD after elective hepatectomy and demonstrated that OSD did aid in the diagnosis of bile leaks. However, OSD was not associated with the incidence of bile leaks requiring intervention and patients who received OSD required additional drainage procedures, had increased length of stay, and experienced a greater number of unplanned readmissions after elective hepatectomy.
There is mounting evidence that routine OSD lacks benefit after hepatectomy and other major abdominal operations, including elective pancreas (30) and colorectal surgery (31). Despite literature suggesting routine OSD in hepatectomy lacks sufficient evidence and support, OSD continues to be frequently employed – 42% of cases in this dataset of elective resections – and therefore studying its effectiveness is an appropriate and clinically relevant question. Several randomized controlled trials comparing OSD to no OSD have been published(13-17), which, when summarized in a meta-analysis, demonstrated no significant differences in any outcome variable between the groups(18); the authors ultimately concluded that there is no evidence to support routine OSD after uncomplicated liver resections. Common criticisms of the available literature include the lack of a generalizable dataset and disagreement on the definition of post-hepatectomy bile leak, which has only recently been standardized (27) and validated (22). Another criticism is the potential bias by treatment selection for OSD. Retrospective (8-11) and prospective studies (12, 22) have adequately described the clinical characteristics associated with usage of OSD and the differences in outcomes between drainage and non-drainage groups but the conclusions may be invalid if imbalances between the OSD and no OSD groups are not properly adjusted for in the analyses.
This work utilized a multi-institutional quality-driven database with procedure-specific outcomes combined with an analytic technique allowing for control of treatment-selection bias accounted for by the available variables. Propensity score matching uses several variables to create a single score modeling the likelihood of exposure, here OSD. For each patient in the OSD group, a complimentary patient is paired who did not get an OSD but has a nearly-identical overall score or likelihood of having received an OSD. Using this technique, we adjust for a degree of treatment selection bias by eliminating patients without a suitable complimentary pair and, in effect, create a virtual or statistical allocation of patients within groups that mimics a randomized controlled trial. In doing so with the ACS-NSQIP database, we hope to have presented a cohort from which clinicians can draw less biased conclusions using a database representing the outcomes of multiple institutions and surgeons that may be more generalizable than single-institution reports.
This strategy does exclude patients who do not have a complimentary match and may therefore introduce some selection bias, although these excluded patients tend to exist at both ends of the propensity score spectrum, while the matched population represents the majority of the eligible population (eFigure 1). Although the incidence of our primary outcome, post-hepatectomy bile leak, is low (7.1%), this is in line with reported incidence rates of 3 – 12% (5-7) and the propensity score matching process did not exclude a significant number of these events, as the incidence in the matched population was actually greater than that of the total eligible ACS-NSQIP cohort. The low incidence rate must be considered when evaluating the conclusions here, and greater power for subsequent testing can be expected as this database continues to build.
The primary limitation of this study is that the outcome of interest – post-hepatectomy bile leak – can be defined by the intervention itself, OSD. Our analysis supported our hypothesis, that OSD is associated with a greater number of diagnosed bile leaks. However, we also hypothesized that this diagnostic advantage may not be clinically significant and that OSD may, instead, lead to over-diagnosis bias, resulting in increased procedures for managing what could be clinically irrelevant asymptomatic bile leaks. Our analysis supports a component of this hypothesis by demonstrating that OSD utilization did not impact the rate of diagnosis of major bile leaks and was associated with the need for additional interventions for the management of these leaks. Furthermore, we adjusted for the interaction of OSD, post-hepatectomy bile leak, and additional interventions and identified that OSD contributes to, although is not the strongest predictor of, increased length of stay and readmissions. As a result, the association between OSD, length of stay, and readmissions may be due to OSD management. Unfortunately, the timing and duration of drainage relative to discharge dates and readmissions cannot be completely studied, as ACS-NSQIP records the date of removal of the last closed suction drain, which may or may not represent the initial surgical drain. We acknowledge that we cannot definitively say OSD has no benefit, particularly in that we could not test our hypothesis of whether OSD is an adequate management strategy of bile leaks, as predicting the progression of this outcome would not be possible without rigorous screening and the collection of additional variables not present in this dataset. Further investigation of the utility of OSD may require effectiveness and predictive decision modeling.
We also hypothesized that OSD may be associated with infectious complications and observed such an association with organ space SSI. However, this finding was confounded by the known effect of post-hepatectomy bile leak on this outcome (27). Such unintended negative consequences of drains after hepatectomy has been previously reported, including higher incidences of intraabdominal infection and drain-related complications such as subcutaneous abscess and drain-tract tumor recurrence with OSD (14). Higher rates of intraabdominal fluid collections have also been reported with OSD after pancreatectomy (32).
Modeling using a multi-institutional database provides ample opportunities for clinical analysis but leads to a number of limitations. The first is the inherent biases of the data and its collection. ACS-NSQIP is a robust, standardized multi-institutional database with intrinsic limitations including concerns of the hospital make-up, case volume, and surgeon volume at institutions participating in the ACS-NSQIP targeted hepatectomy database. The targeted ACS-NSQIP modules have been introduced to enhance the granularity of data collection and improve risk adjustment (33), and employ a highly reliable standardized data collection process (34). Another major limitation is the absence of variables that may be relevant to the outcomes of interest. Surgeon subjective and objective assessment of the risk of biliary leak and the potential benefit of OSD undoubtedly influences the decision to place a drain at time of surgery. This assessment is likely the result of assessing patient comorbidities and intraoperative events but also is a combination of training, preference, and experience. Such surgeon-specific variables cannot be accounted for in our dataset and may contribute to its modest C statistic. As such, the interpretation and application of the results of this study must be done using sound clinical judgement. Table 3 attempted to highlight associations of OSD utilization with specific variables, including increasing comorbidity (ASA Class) and extent of resection (CPT code), which seem reasonable. More curious are the significance thresholds of preoperative alkaline phosphatase > 100 IU/L and platelet count > 450,000/μL, as well as an inverse association between OSD and receipt of neoadjuvant therapy. We do not suggest that these variables or cut-offs should be used in the decision to use an OSD, but rather that these may shed light on the preoperative and intraoperative variables surgeons consciously or unknowingly consider when deciding whether or not to use an OSD. These may be a starting point for future risk stratification of the utility of OSD, but surgeon-specific factors and patient-specific assessments should take precedent.
CONCLUSION
Using the ACS-NSQIP procedure targeted dataset and a propensity score matched analysis, we described variables that were associated with OSD and found that OSD was not associated with the rate of diagnosis of major bile leaks. In our series, OSD was associated with increased interventions and contributed to greater length of stay and a greater number of unplanned 30-day readmissions. Propensity score matching from this large database has decreased bias to provide generalizable between-group comparisons of retrospective data to guide evidence-based best practice patterns in the use of OSD. These data suggest multiple limitations of OSD, although the conclusions here should be interpreted with knowledge of the limitations of this analytic approach.
Supplementary Material
ACKNOWLEDGMENT
The authors wish to thank Margie Olsen, PhD, MPH, for her guidance.
Support: Drs Brauer and Nywening were supported by a National Cancer Institute National Research Service Award to the Department of Surgery at the Washington University School of Medicine (T32 CA009621).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Disclaimer: The American College of Surgeons (ACS) NSQIP and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
REFERENCES
- 1.Minter RM, Alseidi A, Hong JC, et al. Training in hepatopancreatobiliary surgery: assessment of the hepatopancreatobiliary surgery workforce in North America. Ann Surg. 2015;262:1065–1070. doi: 10.1097/SLA.0000000000001096. [DOI] [PubMed] [Google Scholar]
- 2.Dimick JB, Wainess RM, Cowan JA, et al. National trends in the use and outcomes of hepatic resection. J Am Coll Surg. 2004;199:31–38. doi: 10.1016/j.jamcollsurg.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. e1. doi: 10.1016/j.cgh.2011.03.020. quiz e60. [DOI] [PubMed] [Google Scholar]
- 4.Messager M, Sabbagh C, Denost Q, et al. Is there still a need for prophylactic intra-abdominal drainage in elective major gastro-intestinal surgery? J Visc Surg. 2015;152:305–313. doi: 10.1016/j.jviscsurg.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita Y, Hamatsu T, Rikimaru T, et al. Bile leakage after hepatic resection. Ann Surg. 2001;233:45–50. doi: 10.1097/00000658-200101000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka S, Hirohashi K, Tanaka H, et al. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg. 2002;195:484–489. doi: 10.1016/s1072-7515(02)01288-7. [DOI] [PubMed] [Google Scholar]
- 7.Capussotti L, Ferrero A, Vigano L, et al. Bile leakage and liver resection: Where is the risk? Arch Surg. 2006;141:690–694. doi: 10.1001/archsurg.141.7.690. discussion 695. [DOI] [PubMed] [Google Scholar]
- 8.Squires MH, 3rd, Lad NL, Fisher SB, et al. Value of primary operative drain placement after major hepatectomy: a multi-institutional analysis of 1,041 patients. J Am Coll Surg. 2015;220:396–402. doi: 10.1016/j.jamcollsurg.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Guillaud A, Pery C, Campillo B, et al. Incidence and predictive factors of clinically relevant bile leakage in the modern era of liver resections. HPB (Oxford) 2013;15:224–229. doi: 10.1111/j.1477-2574.2012.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cauchy F, Fuks D, Nomi T, et al. Incidence, risk factors and consequences of bile leakage following laparoscopic major hepatectomy. Surg Endosc. 2015 Nov 17; doi: 10.1007/s00464-015-4666-z. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto K, Tamesa T, Yukio T, et al. Risk factors and managements of bile leakage after hepatectomy. World J Surg. 2015 Jul 10; doi: 10.1007/s00268-015-3156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donadon M, Costa G, Cimino M, et al. Diagnosis and management of bile leaks after hepatectomy: results of a prospective analysis of 475 hepatectomies. World J Surg. 2015 Jul 7; doi: 10.1007/s00268-015-3143-0. [DOI] [PubMed] [Google Scholar]
- 13.Belghiti J, Kabbej M, Sauvanet A, et al. Drainage after elective hepatic resection. A randomized trial. Ann Surg. 1993;218:748–753. doi: 10.1097/00000658-199312000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong Y, Brennan MF, Brown K, et al. Drainage is unnecessary after elective liver resection. Am J Surg. 1996;171:158–162. doi: 10.1016/s0002-9610(99)80092-0. [DOI] [PubMed] [Google Scholar]
- 15.Fuster J, Llovet JM, Garcia-Valdecasas JC, et al. Abdominal drainage after liver resection for hepatocellular carcinoma in cirrhotic patients: a randomized controlled study. Hepatogastroenterology. 2004;51:536–540. [PubMed] [Google Scholar]
- 16.Liu CL, Fan ST, Lo CM, et al. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg. 2004;239:194–201. doi: 10.1097/01.sla.0000109153.71725.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun HC, Qin LX, Lu L, et al. Randomized clinical trial of the effects of abdominal drainage after elective hepatectomy using the crushing clamp method. Br J Surg. 2006;93:422–426. doi: 10.1002/bjs.5260. [DOI] [PubMed] [Google Scholar]
- 18.Gurusamy KS, Samraj K, Davidson BR. Routine abdominal drainage for uncomplicated liver resection. Cochrane Database Syst Rev. 2007:CD006232. doi: 10.1002/14651858.CD006232.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Kim YI, Fujita S, Hwang VJ, Nagase Y. Comparison of abdominal drainage and no-drainage after elective hepatectomy: a randomized study. Hepatogastroenterology. 2014;61:707–711. [PubMed] [Google Scholar]
- 20.Amini N, Gani F, Margonis GA, et al. Tu1728 incidence and risk factors associated with bile leak and liver failure following hepatic resection among a cohort of 3,064 patients in the United States. Gastroenterology. 150:S1258–S1259. [Google Scholar]
- 21.Olthof PB, Coelen RJ, Wiggers JK, et al. External biliary drainage following major liver resection for perihilar cholangiocarcinoma: impact on development of liver failure and biliary leakage. HPB (Oxford) 2016;18:348–353. doi: 10.1016/j.hpb.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooke-Smith M, Figueras J, Ullah S, et al. Prospective evaluation of the International Study Group for Liver Surgery definition of bile leak after a liver resection and the role of routine operative drainage: an international multicentre study. HPB (Oxford) 2015;17:46–51. doi: 10.1111/hpb.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strasberg SMTV, Meng X, Hall BL, Pitt H. Laparoscopic right hepatectomy: comparative analysis of 2013 ACS-NSQIP Data.. The Society for Surgery of the Alimentary Tract: 56th Annual Meeting; Washington, DC.. [Google Scholar]
- 24.Rahbari NN, Elbers H, Koch M, et al. Bilirubin level in the drainage fluid is an early and independent predictor of clinically relevant bile leakage after hepatic resection. Surgery. 2012;152:821–831. doi: 10.1016/j.surg.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Salas M, Hofman A, Stricker BH. Confounding by indication: an example of variation in the use of epidemiologic terminology. Am J Epidemiol. 1999;149:981–983. doi: 10.1093/oxfordjournals.aje.a009758. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat Med. 2011;30:1292–1301. doi: 10.1002/sim.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–753. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 30.Witzigmann H, Diener MK, Kienkotter S, et al. No need for routine drainage after pancreatic head resection: the dual-center, randomized, controlled PANDRA trial (ISRCTN04937707). Ann Surg. 2016;264:528–537. doi: 10.1097/SLA.0000000000001859. [DOI] [PubMed] [Google Scholar]
- 31.Jesus EC, Karliczek A, Matos D, et al. Prophylactic anastomotic drainage for colorectal surgery. Cochrane Database Syst Rev. 2004;(4):CD002100. doi: 10.1002/14651858.CD002100.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conlon KC, Labow D, Leung D, et al. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001;234:487–493. doi: 10.1097/00000658-200110000-00008. discussion 493-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217:336–346. e1. doi: 10.1016/j.jamcollsurg.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 34.Shiloach M, Frencher SK, Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:6–16. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.