Abstract
Background
Childhood cancer survivors have an increased risk of developing cardiovascular disease following treatment, yet few interventions have been evaluated to reduce this risk. Purple grape juice (pGJ), a rich source of flavonoids with antioxidant properties, has been shown in adults to reduce oxidative stress and improve endothelial function. We examined the effects of supplementing meals with pGJ on microvascular endothelial function and markers of oxidative stress and inflammation in 24 cancer survivors (ages 10–21 years).
Procedure
In a randomized controlled crossover trial consisting of two, 4 week intervention periods, each preceded by a 4 week washout period, subjects received in random order 6 ounces twice daily of pGJ and clear apple juice (cAJ; similar in calories but lower in flavonoids). Measurements were obtained before and after each supplementation period; change was evaluated using mixed effects ANOVA.
Results
pGJ did not improve endothelial function, measured using digital reactive hyperemia, compared with cAJ (mean change: pGJ 0.06, cAJ 0.22; difference of mean change [95% CI]: −0.16 [−0.42 – 0.11], P = 0.25). No significant changes in plasma concentrations of oxidized-LDL, myeloperoxidase, or high sensitivity C-reactive protein were observed.
Conclusion
After 4 weeks of daily consumption of flavonoid-rich pGJ, no measurable change in vascular function was observed in these childhood cancer survivors. Pediatr Blood Cancer 2014;61:2290–2296.
Keywords: cardiovascular disease, childhood cancer survivors, endothelial function, grape juice, intervention
INTRODUCTION
Survivors of childhood cancer are at an increased risk for premature atherosclerosis and manifest cardiovascular disease (CVD) early in adult life [1]. Endothelial dysfunction plays a key role in the initiation and progression of atherosclerosis and is an independent predictor of CVD events such as myocardial infarction and stroke [2,3]. Compared with age- and sex-matched controls, childhood cancer survivors have impaired vascular structure (increased carotid intima-media thickness and arterial stiffness) and function (endothelium-dependent vasodilation) [4–10]. Survivors are also at increased risk of cardiovascular risk factors such as obesity, hypertension, diabetes, and dyslipidemia [11–13], which contribute to increased vascular oxidative stress and impairment. Recent studies in adolescent survivors suggest that vascular damage begins soon after completion of therapy [7,8,10,14]. Treatment protocols that include anthracyclines, platinum agents, or radiation therapy (cranial, neck, or chest) are most associated with (cardio) vascular toxicity [4,5,9,10, 15–17]; however, treatment with surgery alone has been associated with having multiple adverse metabolic conditions [15]. Despite the increased risk of premature atherosclerosis, few interventions have been conducted in childhood cancer survivors to alter the rate of progression of disease and prevent or delay serious cardiovascular events in adulthood.
Dietary strategies have been evaluated as potential means to prevent, attenuate, or reverse the CVD process. There is considerable interest in the cardio-protective properties of red wine [18], which is abundant in flavonoids, a class of polyphenols known for their strong antioxidant activities [19]. Purple grape juice (pGJ), made from the skin, seeds, and flesh of purple grapes, is a rich source of flavonoids, similar in flavonoid type and quantity, and antioxidant activity to a light red wine (e.g., Valpolicella, Beaujolais) [20]. Clinical studies in adults suggest that daily consumption of pGJ contributes to increased antioxidant capacity, reduced LDL oxidation, and improved vasodilation [21–24]. Studies of the health effects of pGJ have focused on older individuals at high risk for or with existing CVD. Few studies have evaluated the effects of antioxidant vitamin therapies in pediatric populations [25–27], but none have been conducted in cancer survivors.
We hypothesized that pGJ may reduce some of the negative vascular effects resulting from cancer therapy. We conducted a randomized controlled crossover trial to evaluate the feasibility and preliminary efficacy of an intervention to assess the effects of supplementing meals with pGJ on the vascular health in childhood cancer survivors. Our primary endpoint was microvascular endothelial function, a key measure of vascular health and considered a barometer for cardiovascular disease risk [28]. Secondary endpoints included biomarkers of vascular and systemic oxidative stress and inflammation.
SUBJECTS AND METHODS
Study Design
The intervention utilized a randomized, controlled crossover design, which provided a more efficient way to examine an outcome (endothelial function) with likely greater between- versus within-subject variability. Each subject consumed both pGJ and the control beverage, clear apple juice (cAJ), which was similar in calories, but without the flavonoid content of pGJ. Eligible subjects were asked to refrain from consuming any type of juice, juice drink (less than 100% fruit juice), wine, or grapes for the duration of the study. The remaining diet was unchanged to determine whether an effect could be observed in a free-living population, that is, whether daily pGJ, either as an addition to the diet or a replacement for another type of juice, could improve endothelial function regardless of diet or vitamin use. After a 4 week run-in period, whereby subjects were asked to refrain from consuming the restricted items, subjects were randomized to the juice sequence in equal allocation. The participants were instructed to drink six ounces of juice twice daily (with morning and evening meals) for a duration of 4 weeks. A 4 week washout period separated the two supplementation periods.
Clinic visits to assess outcomes were scheduled prior to and after each supplementation period and were conducted at the University of Minnesota Clinical and Translational Science Institute (CTSI). Participants were asked to fast and refrain from exercise for at least 12 hours prior to each visit. The study protocol was approved by the Institutional Review Board at the University of Minnesota. All participants provided written informed consent or assent (clinicalTrials.gov NCT01043939).
Study Population
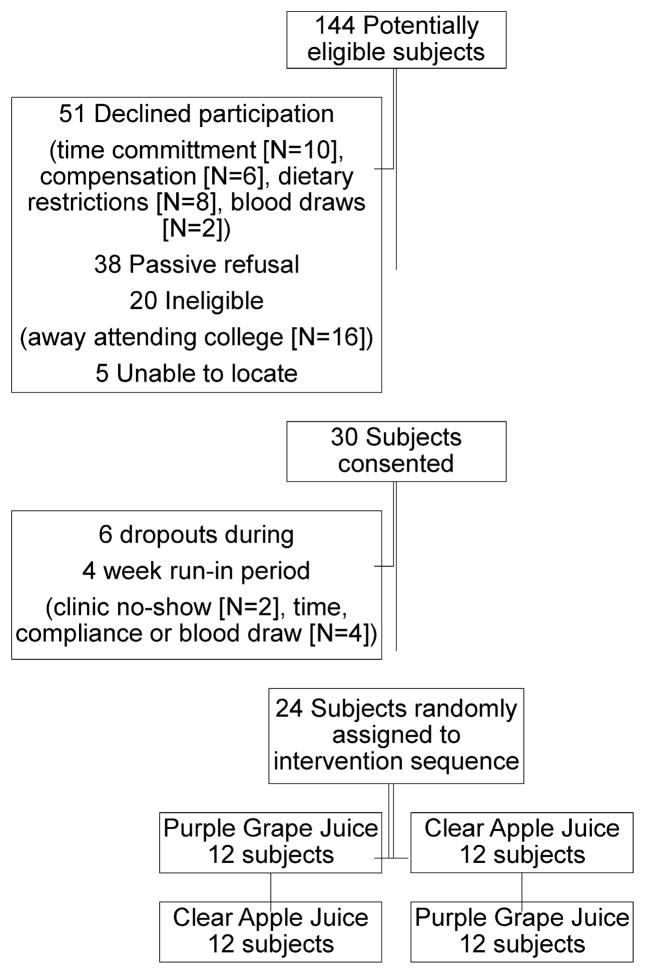
The targeted accrual for this pilot study was 24 childhood cancer survivors. From October 2009–April 2010, potentially eligible survivors who had recently completed an NIH funded study on metabolic syndrome or received healthcare at the Long-Term Follow-Up Clinic (University of Minnesota), and who provided consent for future contact, were invited to participate in the pilot intervention. Eligibility criteria included 10–22 years of age, off therapy for ≥3 years, and resided within a 50 mi radius of the CTSI. Exclusion criteria included pregnant or planning to become pregnant, start of oral contraceptives ≤3 months of study enrollment, current smoker, antibiotic use ≤2 weeks of study enrollment, and diabetes. One hundred nineteen eligible survivors were identified and contacted, 30 individuals commenced the four week run-in period, and 24 subjects completed the four month study (Fig. 1). Compared with study participants, non-participants were older (16.8 vs. 15.5 years, P = 0.05), female (50.0% vs. 29.2%, P = 0.07), and non-White (15.0% vs. 0%, P = 0.04). Time since cancer diagnosis and the proportion diagnosed with a hematologic malignancy were similar between groups; however, survivors of central nervous system tumors, Wilms tumor, or soft tissue sarcomas were less likely to participate.
Fig. 1.
CONSORT flowchart for recruitment and retention of childhood cancer survivors in a randomized controlled crossover trial to evaluate the effects of purple grape juice on vascular health.
Supplementation
The study beverages were Old Orchard 100% Grape Juice (pGJ) and Mott’s 100% Apple Juice (cAJ), which contained identical vitamin C supplementation (a common preservative for bottled juice). The control beverage, cAJ, was chosen because the flavonoid type and quantity is substantially different compared with pGJ. The majority of flavonoids are found in apple peels, which are retained in the pomace rather than extracted into the juice during processing of clear apple juice [29]. In comparative studies, pGJ and cAJ ranked the highest and lowest, respectively, among fruit juices in polyphenol content, and antioxidant potency and functionality (e.g., ability to inhibit LDL oxidation) [20,30]. The dose (six ounces twice daily) and duration (four weeks) were chosen to be consistent with prior research in adults on pGJ and cardiovascular health [22,24], and was within the limit recommended by the American Academy of Pediatrics of 8–12 oz/day for children 7–18 years old [31]. To evaluate compliance, subjects were asked to return their calendar of daily juice intake along with any non-empty juice containers at the following clinic visit.
Endothelial Function Assessment
Peripheral microvascular endothelial function, assessed during the morning after a 12 hour fast in a quiet, temperature controlled room, was measured by digital reactive hyperemia peripheral arterial tonometry (RH-PAT) (EndoPAT 2000, Itamar Medical, Caesarea, Israel) as previously described [32,33]. After patients rested quietly for 15 minutes in the supine position, probes were placed on the index fingers of both hands to measure baseline and reactive hyperemic pulse amplitude. These probes inflate to apply a uniform pressure on the fingers, and sensors inside the probes detect small pulse volume changes throughout the cardiac cycle. Following the collection of 5 minutes of baseline data, a blood pressure cuff was placed on the upper forearm (just below the elbow) and inflated to a suprasystolic level for 5 minutes. Following cuff release, the change in pulse amplitude during reactive hyperemia was measured for 5 minutes. The ratio of the hyperemic and the baseline pulse amplitude (corrected for the same ratio on the control finger) were calculated and expressed as the reactive hyperemia index (RHI) score. Lower RHI scores reflect worse endothelial function.
Biochemical Analyses
Assays for fresh plasma samples were conducted at Fairview Diagnostic Laboratories. Cholesterol, triglycerides, and glucose were determined by colorimetric reflectance spectrophotometry. Insulin was determined by chemiluminescent immunoassay. Plasma samples for biomarkers were stored at −70°C until analyses at study completion. Biomarkers were selected to determine if the hypothesized improvement in endothelial function was due to reductions in oxidative stress or inflammation: oxidized LDL (oxLDL): a marker of vascular and systemic oxidative stress reduced by daily consumption of pGJ in previous studies [23,24]; myeloperoxidase (MPO): secreted during the vascular inflammatory response to oxidative stress [34]; and high-sensitivity C-reactive protein (hs-CRP): a marker of inflammation thought to play a role in the early stages of atherosclerosis, including endothelial dysfunction [35]. oxLDL (Mercodia, Winston-Salem, NC), MPO (R&D Systems, Minneapolis, MN), and hs-CRP (ALPCO, Salem, NH) were analyzed in the University of Minnesota Cytokine Reference Laboratory using enzyme-linked immunosorbent assay.
Other Measurements
Study participants or their guardian were asked about factors that may affect vascular function, including over-the-counter (e.g., NSAIDS and cold medicine) and prescription medications, vitamin supplements, caffeine consumption, and phase of menstrual cycle. Measured height and weight were used to calculate body mass index (BMI; kg/m2) and age- and sex-specific BMI percentiles. Seated blood pressure and heart rate were obtained after 5 minutes of quiet-rest. Metabolic equivalent hours per week of physical activity during the past year were calculated using data from the Modifiable Activity Questionnaire [36,37]. A dietary assessment of flavonoid rich foods and beverages was obtained during each clinic visit to evaluate potential seasonal trends in flavonoid intake.
Statistical Analysis
Baseline continuous variables are presented as medians and interquartile range (IQR). P-values are from the chi-square test (categorical variables) or the Wilcoxon rank-sum test (continuous variables). Change from baseline was compared between pGJ and cAJ for endothelial function and each biomarker using a mixed effects analysis of covariance (ANCOVA) model, which included period, group, juice, and baseline as fixed effects. Subject within group was modeled as a random effect, to test and adjust for potential carry-over effects. Normally distributed outcome variables (RHI scores and oxLDL) are reported as mean changes from baseline (SD) and group differences of least square mean changes from baseline (LSMeans; 95% CI). MPO and hs-CRP were log transformed prior to analyses; change from baseline is reported as median (IQR) and the difference of LSMeans was back transformed (exponentiated) to obtain the ratio of means in the two juice groups. The 95% CIs were also back transformed. For all outcomes, the adjusted differences and P-values reported are for the comparison of the effects of pGJ to cAJ. No significant nor clinically meaningful changes in clinical characteristics or BMI percentile were expected as a result of the juice intervention; however, the before and after juice comparison was made to verify that no adverse effects were associated with the dose and duration of juice consumption (changes were evaluated using the Wilcoxon signed rank test).
Limited data were available to inform a power/sample size estimate at the time this pilot study was designed; thus one of the goals was to obtain preliminary data to be used in the power calculation for a future, larger trial. A 2 × 2 crossover design with 24 participants provided 80% power using a two-sided significance level of 0.05 to detect a mean difference of 0.3 in the RHI score between the juice groups, assuming the standard deviation of the difference was 0.25.
RESULTS
Baseline demographic, lifestyle, and clinical characteristics are presented in Table I. The majority of participants (21 of 24) received one or more potentially vascular toxic therapies as part of their treatment protocol (anthracyclines, platinum agents, and radiation). The juice sequences (pGJ vs. cAJ first) were balanced with regard to baseline characteristics, except more survivors of hematopoietic malignancies (P = 0.01) and higher BMIs (P = 0.05) were randomized to start the intervention with cAJ.
TABLE I.
Baseline Characteristics of Study Participants
| N (%) or Median (interquartile range) | |
|---|---|
| Demographic and lifestyle characteristics | |
| Males (N, %) | 17 (71%) |
| Non-Hispanic white (N, %) | 22 (92%) |
| ≥6 times/2 weeks hard exercise (N, %)a | 15 (65%) |
| ≥4 hours/day TV/computer/video (N, %)b | 11 (46%) |
| Age (years) | 16.4 (13.7–17.2) |
| Fruit/vegetable servings/day | 2.0 (0.8–4.0) |
| MET hours/week (past year)c | 42.3 (20.2–78.6) |
| Cancer characteristics | |
| Cancer diagnosis (N, %) | |
| Hematopoietic malignancy | 12 (50%) |
| ALL | 9 |
| AML | 1 |
| Hodgkin disease | 1 |
| Non-Hodgkin lymphoma | 1 |
| Solid tumor | 12 (50%) |
| CNS tumors | 3 |
| Bone tumors | 2 |
| Retinoblastoma | 2 |
| Germ cell tumor | 2 |
| Neuroblastoma | 1 |
| Hepatoblastoma | 1 |
| Soft tissue sarcoma | 1 |
| Cancer treatment (N, %) | |
| Chemotherapy and radiation | 5 (21%) |
| Chemotherapy | 14 (58%) |
| Radiation | 2 (8%) |
| Surgery only | 3 (13%) |
| Radiation (N, %) | |
| None | 17 (71) |
| Any | 7 (29) |
| Brain | 3 (13) |
| Other head | 2 (8) |
| Spine | 2 (8) |
| Chest/Mediastinum | 1 (4) |
| Total body irradiation | 1 (4) |
| Chemotherapy (N, %) | |
| None | 5 (21) |
| Any | 19 (79) |
| Protocol included: | |
| Anthracyclines (doxorubicin, daunorubicin, idarubicin)d | 15 (63) |
| Antimetabolites (cytosine arabinoside, methotrexate) | 11 (46) |
| Platinum agents (cisplatinum, carboplatinum)d | 5 (21) |
| Alkylating agents (cyclophosphamide, ifosfamide) | 16 (67) |
| Anti-tumor antibiotics (bleomycin) | 2 (8) |
| Topoisomerase inhibitors (etoposide) | 5 (21) |
| Age at cancer diagnosis (years) | 3.6 (1.5–6.1) |
| Years since cancer treatment | 8.5 (6.4–13.0) |
| Clinical characteristics | |
| Weight (kg) | 60.7 (50.4–72.1) |
| Height (cm) | 166.4 (158.8–171.5) |
| BMI percentilese | 70.5 (47.1–82.3) |
| Systolic blood pressure (mmHg) | 111 (103–118) |
| Diastolic blood pressure (mmHg) | 59 (57–63) |
| Fasting glucose (mg/dL) | 78.0 (75.0–83.0) |
| Fasting insulin (mU/L) | 5.0 (3.0–8.0) |
| LDL-C (mg/dL) | 93.0 (78.0–111.0) |
| HDL-C (mg/dL) | 50.0 (42.0–56.0) |
| Triglycerides (mg/dL) | 72.0 (56.0–86.0) |
| Total cholesterol (mg/dL) | 159.0 (142.0–177.0) |
BMI, body mass index; MET, metabolic equivalent; LDL-C, low-density lipoprotein cholesterol; and HDL-C, high-density lipoprotein cholesterol.
Number of days during the past 14 days of hard exercise for at least 20 minutes (question available only from the Modifiable Activity Questionnaire for Adolescents, N = 23).
Average number of hours a day spent watching television or videos, or playing computer games, after school or work.
Metabolic equivalent (MET) hours/week during the past year were calculated using the activity codes and MET intensities from the Compendium of Physical Activities.
Cumulative dose mean ± SD (range): anthracyclines: 189 ± 99 mg/m2 (75–375) and platinum agents: 575 ± 307 mg/m2 (250–1075).
Age- and sex-specific BMI percentiles for study participants <20 were determined according to the 2000 CDC Growth Charts for the United States (N = 23). The BMI for the one adult was within normal range (<25.0 kg/m2). Overweight/obese: BMI ± 85th age- and sex-specific percentile for children; ≥25 kg/m2 for adults.
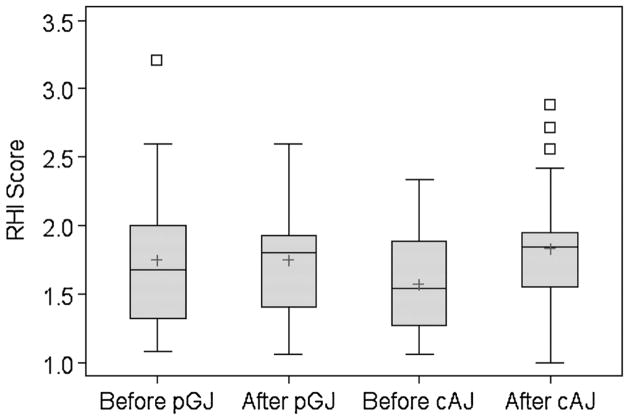
Pre- and post-supplementation RHI scores by juice type are presented in Figure 2. Pre-supplementation mean scores were lower for cAJ (1.57 ± 0.36 vs. 1.75 ± 0.52 for pGJ), but inter-individual variability was greater for pGJ (range 1.08–3.21 vs. 1.06–2.34 for cAJ). Compared with baseline, there was a negligible change in RHI scores after consumption of pGJ (LSMeans ± SE: 0.06 ± 0.089) and an increase in scores after consumption of cAJ (LSMeans ± SE: 0.22 ± 0.093). Thus the differential effect of juice consumption on RHI favored cAJ but was not statistically significant (Table II: difference of LSMeans [95% CI]: −0.16 [−0.42 – 0.11], P = 0.25). Adjustment for cancer type, baseline BMI, length of supplementation period, age at diagnosis, or length of time since treatment did not appreciably alter the effect estimates. There was no evidence of either a significant period effect (P = 0.91) or carry-over effect (P = 0.89).
Fig. 2.
RHI scores before and after four weeks of twice daily purple grape juice (pGJ) and clear apple juice (cAJ) supplementation in childhood cancer survivors. Lower scores represent worse endothelial function.
TABLE II.
Change in Endothelial Function and Biomarkers of Inflammation and Oxidative Stress
| Purple grape juice
|
Clear apple juice
|
Adjusted difference (95% CI) | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | Before | After | Before | After | |||
| Endothelial functiona | |||||||
| RH-PAT Index Score | 24 | 1.75 (0.52) | 1.75 (0.39) | 1.57 (0.36) | 1.83 (0.47) | −0.16 (−0.42–0.11) | 0.25 |
| Biomarkersb | |||||||
| Oxidized LDL (U/L) | 24 | 61.72 (15.11) | 66.73 (17.20) | 66.19 (13.43) | 66.57 (13.76) | 3.09 (−2.73–8.91) | 0.29 |
| MPO (ng/ml) | 24 | 117.3 (98.6,138) | 107.0 (91.7,131) | 116.2 (92.6,142) | 116.1 (99.1,144) | 0.92 (0.82–1.03) | 0.15 |
| hs-CRP (mg/L) | 24 | 0.19 (0.09,0.41) | 0.33 (0.15,0.73) | 0.24 (0.07,0.55) | 0.24 (0.11,0.85) | 1.34 (0.69–2.62) | 0.37 |
Data are reported as means (SD) and difference of least square means (95% CI) for normally distributed variables. Median (IQR) is reported for MPO and hs-CRP, which were log transformed prior to analyses; the difference of log-transformed least square means (95% CI) were back transformed to obtain the ratio of means (95% CI). The adjusted differences and P-values are from a mixed effects ANCOVA model with baseline included as a covariate, subject as a random effect, and juice, period, and group as fixed effects. RH-PAT, reactive hyperemia peripheral arterial tonometry; MPO, myeloperoxidase; and hs-CRP, high sensitivity C-reactive protein.
Unable to obtain RHI scores after the vascular test for two subjects before the apple juice supplementation; a weighted estimate was calculated using complete and incomplete measurements.
The blood draw was unsuccessful for four subjects at one of the four clinic visits; the weighted estimate includes available data from all 24 participants as above.
In post-hoc analyses, exclusion of potential outlying observations (N = 4 RHI scores = 1.5 times >75th% or <25th%) resulted in substantial attenuation of both the difference in RHI scores between the juice types (LSMean difference [95% CI]: −0.01 [−0.25 – 0.22], P = 0.92) and the change during the cAJ supplementation (LSMeans [95% CI]: 0.13 [−0.04 – 0.29], P = 0.14). There was no significant difference between juice effects when the analysis was restricted to subjects who received anthracyclines during cancer treatment (N = 15; 93% doxorubicin [Adriamycin]). Small sample size precluded additional subgroup analyses by treatment type or dose.
No significant differences were detected between the two juice types for biomarkers of inflammation or oxidative stress (Table II), nor did results change after adjustment. There was no evidence for either a period effect or a carryover effect for MPO or hs-CRP; however, there was a trend towards carryover effect observed for the analysis of oxidized LDL (P = 0.12).
Unexpectedly, HDL cholesterol significantly decreased during the cAJ supplementation (median change [IQR]: −5.0 mg/dL [IQR: −10.0–0.0]; P = 0.001), which was significantly different from change during pGJ supplementation (P =0.04; Table III). No significant effects on BMI percentile, blood pressure, other lipids, glucose, or insulin were observed for either juice, nor were any adverse side effects reported.
TABLE III.
Clinical Characteristics of Study Participants at Baseline and After Four Weeks Supplementation With Purple Grape Juice or Clear Apple Juice
| Purple grape juice
|
Clear apple juice
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na | Before | After | Difference | P | Na | Before | After | Difference | P | |
| BMI percentile | 23 | 71.6 (47.1–80.0) | 69.7 (41.9–82.6) | −0.27 (−2.9–2.9) | 0.75 | 23 | 70.5 (49.0–82.3) | 70.9 (44.5–82.8) | 0.93 (−1.8–4.4) | 0.10 |
| Heart rate (bpm) | 24 | 65.3 (58.5–72.2) | 68.3 (63.3–74.2) | 3.00 (−3.3–7.3) | 0.11 | 24 | 70.0 (62.8–78.3) | 67.8 (61.2–73.3) | −1.17 (−7.8–4.2) | 0.44 |
| SBP (mmHg) | 24 | 108.8 (102–116) | 108.8 (104–114) | 0.25 (−5.0–3.5) | 0.86 | 24 | 111.0 (103–120) | 110.8 (104–116) | −0.50 (−8.8–7.5) | 0.59 |
| DBP (mmHg) | 24 | 57.8 (54.8–61.0) | 55.8 (53.3–60.8) | −2.50 (−4.8–1.5) | 0.21 | 24 | 57.8 (54.5–61.3) | 56.0 (50.8–60.0) | −2.75 (−6.0–2.5) | 0.09 |
| Fast. glucose (mg/dL) | 21 | 82.0 (78.0–83.0) | 81.0 (76.0–86.0) | 1.00 (−4.0–4.0) | 0.70 | 23 | 77.0 (73.0–83.0) | 80.0 (77.0–86.0) | 3.00 (−4.0–6.0) | 0.19 |
| Fast. insulin (mU/L) | 21 | 5.0 (3.0–6.0) | 4.0 (3.0–8.0) | 0.00 (−1.0–2.0) | 0.79 | 23 | 4.0 (2.0–10.0) | 5.0 (2.0–12.0) | 0.00 (−2.0–2.0) | 0.61 |
| LDL chol. (mg/dL) | 21 | 92.0 (79.0–111) | 99.0 (86.0–109) | 4.00 (−3.0–9.0) | 0.20 | 23 | 97.0 (78.0–106) | 95.0 (73.0–117) | −2.00 (−8.0–3.0) | 0.46 |
| HDL chol.b (mg/dL) | 21 | 50.0 (42.0–55.0) | 50.0 (43.0–52.0) | −1.00 (−4.0–4.0) | 0.91 | 23 | 52.0 (44.0–56.0) | 46.0 (41.0–50.0) | −5.00 (−10.0–0.0) | 0.001 |
| Triglycerides (mg/dL) | 21 | 73.0 (50.0–80.0) | 67.0 (53.0–87.0) | −4.00 (−11.0–12.0) | 0.79 | 23 | 62.0 (57.0–86.0) | 72.0 (48.0–110) | 2.00 (−14.0–24.0) | 0.62 |
| Total chol.c (mg/dL) | 21 | 153.0 (142–176) | 159.0 (144–171) | 5.00 (−7.0–18.0) | 0.30 | 23 | 161.0 (148–181) | 157.0 (135–183) | −6.00 (−14.0–3.0) | 0.12 |
Data are presented as medians and 25th –75th percentile (IQR) given the non-Gaussian distribution of the majority of variables. P-values for change (difference) within juice are from the Wilcoxon signed rank test. For comparisons between juices (presented below), P-values for significant tests are from the Wilcoxon signed rank test adjusted for period effects. SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL chol., low-density lipoprotein cholesterol; HDL chol., high-density lipoprotein.
Unsuccessful blood draw at a clinic visit before or after purple grape juice or clear apple juice from three subjects and one subject, respectively.
The change in HDL cholesterol was significantly different between purple grape juice and apple juice (N = 20, P = 0.04).
The change in total cholesterol was significantly different between both juices (N = 20, P = 0.03).
DISCUSSION
Our pilot study was the first intervention to evaluate the effects of supplementing meals with flavonoid-rich pGJ on the vascular function of adolescent and young adult survivors of childhood cancer. After four weeks of consuming six ounces of juice twice daily, pGJ did not improve endothelial function compared with cAJ, nor were significant improvements in biomarkers of oxidative stress or inflammation observed.
In post-hoc analyses, we evaluated the effects of each juice separately. Interestingly, despite the lower flavonoid content of cAJ, we unexpectedly observed a significant improvement in RHI scores after the cAJ supplementation. This improvement in endothelial function was not accompanied by a decrease in glucose, insulin, LDL cholesterol, or biomarker levels. Nor was it correlated with any demographic, cancer, or treatment factors. We did not assess endothelium-independent vasodilation, thus we cannot rule out the role of vascular smooth muscle cells on the improvement in vasodilation. Reduced vascular inflammation could explain improvement in vascular function; however, there was no change in the only inflammatory biomarker (hsCRP) measured. Only a few intervention studies have demonstrated beneficial effects of flavonoids on inflammatory markers in humans. Nevertheless, cAJ ranks low in antioxidant potency and function due to removal of the flavonoid-rich apple peels during cAJ processing [20,30].
Conversely, there was virtually no change in RHI scores after the pGJ supplementation. Interestingly, this was accompanied by a significant increase in oxidized LDL, a marker of vascular oxidative stress, previously shown to be reduced by daily consumption of grape juice in adults [23,24]. This increase was not correlated with other biomarkers, or demographic or cancer/treatment related factors. It is unlikely that there were carry-over effects after a four week washout period, as flavonoids are rapidly metabolized and their metabolites rapidly eliminated from plasma, suggesting that flavonoid-rich foods should be eaten on a daily basis to maintain high concentrations in the blood [38]. There is no evidence that pGJ was consumed in smaller quantities than cAJ; overall, adherence to the protocol was good, as only two participants reported missing more than a few juice doses. However, a more accurate and reliable measure of adherence (e.g., computerized container) may have been more revealing. Given the role of oxidative stress in endothelial dysfunction, we would not expect to see an improvement in vasodilation during a period of elevated oxidative stress.
The change in HDL during the cAJ supplementation was unexpected. The change was not correlated with any demographic factors, other clinical variables, or the time of year (as diet and exercise are seasonally influenced). Change in lipid levels is common midway through pubertal development; however, it is an unlikely explanation in a trial of short duration.
We selected RH-PAT technology over flow mediated dilation (FMD) as it is less operator dependent, more mobile, and therefore easier to implement in larger studies. This is important for studies of rare diseases (like childhood cancer) that require multiple test sites, and thus a measurement technique with minimal intra- and inter-observer variability. RH-PAT measures correlate well with coronary endothelial function [39], CAD [32,40], CVD risk factors [32,41], and predict cardiovascular adverse events [42] in adults. Significant differences and improvements in peripheral endothelial function using RH-PAT have been demonstrated in studies of adolescents at high risk for CVD (e.g., type I diabetes, obesity and insulin resistance, and cancer survivors) [6,17,43–45] and in clinical or dietary interventions, including flavonoid studies [46–48].
While both FMD and RH-PAT induce a hyperemic response to evaluate changes in peripheral arterial function, there are important differences. FMD measures change in diameter of a single large conduit artery and requires highly trained sonographers and readers for image acquisition and analysis. RH-PAT measures pulse volume changes in small conduit vessels as well as resistance vessels, corrects for systemic vascular changes (e.g., transient environmental effects) by taking measurements on the un-occluded arm, and measurement and interpretation are determined by computer software. Unfortunately, few studies compared FMD and RH-PAT measures in the same subject, and the results are conflicting [32,49,50]. It has been suggested that FMD and RH-PAT may reflect different aspects of vascular function, potentially due to size and location of the arterial vessels being measured [49].
The duration of the intervention may have been too short to observe an effect in young, relatively healthy cancer survivors. Dose and duration were selected based on studies that demonstrated improved endothelial function in adults: 4–8 ml/kg/d for 2–4 weeks. Our participants consumed 355 ml/d or (mean ± SD) 6.3 ± 1.8 ml/kg/d for 32 ± 5.7 days. However, grape products, including pGJ, have been shown to demonstrate acute effects (<3 hours since consumption) on endothelial function in healthy adults, including counteracting the well-known deleterious effects of a high fat meal [21]. Alternatively, the low cardiovascular risk profile of our study population may have limited our ability to detect an effect, as presumably there is less room for improvement in individuals with limited vascular impairment. While the ideal time to intervene is before clinically evident disease, this may require different interventions in terms of dose, duration, or other parameters.
CONCLUSIONS
After four weeks of daily consumption of flavonoid-rich pGJ, no measurable change in vascular function was observed in this group of young, relatively healthy, survivors. A focused study among survivors with a greater degree of vascular impairment may be more revealing.
Given the increasing number of childhood cancer survivors and the identification of treatment-related late effects, it is of great import to design and implement interventions to prevent, delay, or mitigate these adverse effects. An important next step is to identify survivors most at risk before vascular disease is clinically evident. Future studies in childhood cancer survivors should consider designing interventions that target the major risk factors for CVD, such as obesity, insulin resistance or diabetes, or hypertension, which may directly or indirectly affect endothelial function. Interventions involving pleiotropic effects (e.g., physical activity) may provide a more successful approach to improve vascular function in young survivors, as has been recently demonstrated [8].
Acknowledgments
Grant sponsor: Children’s Cancer Research Fund (Minneapolis), a University of Minnesota Graduate School Doctoral Dissertation Fellowship, the University of Minnesota Masonic Cancer Center, NIH; Grant numbers: R25 CA047888; K05 CA157439; Grant sponsor: National Center for Research Resources; Grant number: UL1RR033183
The authors thank the cancer survivors and their parents for their time and participation in the study. This study was supported by the Children’s Cancer Research Fund (Minneapolis), the University of Minnesota Graduate School Doctoral Dissertation Fellowship, the University of Minnesota Masonic Cancer Center, NIH R25 CA047888, NIH K05 CA157439, and Award Number UL1RR033183 from the National Center for Research Resources.
Abbreviations
- BMI
body mass index
- HDL-C
high-density lipoprotein cholesterol
- hs-CRP
high sensitivity C-reactive protein
- IQR
interquartile range
- LDL-C
low-density lipoprotein cholesterol
- MPO
myeloperoxidase
- RH-PAT
reactive hyperemia peripheral arterial tonometry
Footnotes
Conflict of Interest: The authors do not have a financial relationship with any of the organizations that sponsored the research.
References
- 1.Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: A scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: Endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 2.Widlansky ME, Gokce N, Keaney JF, Jr, et al. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 3.Landmesser U, Hornig B, Drexler H. Endothelial function: A critical determinant in atherosclerosis? Circulation. 2004;109:II27–33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 4.Chow AY, Chin C, Dahl G, et al. Anthracyclines cause endothelial injury in pediatric cancer patients: A pilot study. J Clin Oncol. 2006;24:925–928. doi: 10.1200/JCO.2005.03.5956. [DOI] [PubMed] [Google Scholar]
- 5.Dengel DR, Ness KK, Glasser SP, et al. Endothelial function in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30:20–25. doi: 10.1097/MPH.0b013e318159a593. [DOI] [PubMed] [Google Scholar]
- 6.Ruble K, Davis CL, Han HR. Endothelial health in childhood acute lymphoid leukemia survivors: Pilot evaluation with peripheral artery tonometry. J Pediatr Hematol Oncol. 2014 Feb 26; doi: 10.1097/MPH.0000000000000122. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dengel DR, Kelly AS, Zhang L, et al. Signs of early sub-clinical atherosclerosis in childhood cancer survivors. Pediatr Blood Cancer. 2014;61:532–537. doi: 10.1002/pbc.24829. [DOI] [PubMed] [Google Scholar]
- 8.Jarvela LS, Niinikoski H, Heinonen OJ, et al. Endothelial function in long-term survivors of childhood acute lymphoblastic leukemia: Effects of a home-based exercise program. Pediatr Blood Cancer. 2013;60:1546–1551. doi: 10.1002/pbc.24565. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer CA, Postma A, Hooimeijer HL, et al. Endothelial damage in long-term survivors of childhood cancer. J Clin Oncol. 2013;31:3906–3913. doi: 10.1200/JCO.2012.46.6086. [DOI] [PubMed] [Google Scholar]
- 10.Jenei Z, Bardi E, Magyar MT, et al. Anthracycline causes impaired vascular endothelial function and aortic stiffness in long term survivors of childhood cancer. Pathol Oncol Res. 2013;19:375–383. doi: 10.1007/s12253-012-9589-6. [DOI] [PubMed] [Google Scholar]
- 11.Chow EJ, Pihoker C, Hunt K, et al. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 12.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 13.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: A report for the childhood cancer survivor study. Arch Intern Med. 2009;169:1381–1388. doi: 10.1001/archinternmed.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinberger J, Sinaiko AR, Kelly AS, et al. Cardiovascular risk and insulin resistance in childhood cancer survivors. J Pediatr. 2012;160:494–499. doi: 10.1016/j.jpeds.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker KS, Chow EJ, Goodman PJ, et al. Impact of treatment exposures on cardiovascular risk and insulin resistance in childhood cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22:1954–1963. doi: 10.1158/1055-9965.EPI-13-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaughn DJ, Palmer SC, Carver JR, et al. Cardiovascular risk in long-term survivors of testicular cancer. Cancer. 2008;112:1949–1953. doi: 10.1002/cncr.23389. [DOI] [PubMed] [Google Scholar]
- 17.Zelcer S, Chen B, Mangel J, et al. Impaired vascular function in asymptomatic young adult survivors of Hodgkin Lymphoma following mediastinal radiation. J Cancer Surviv. 2010;4:218–224. doi: 10.1007/s11764-010-0138-6. [DOI] [PubMed] [Google Scholar]
- 18.Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutr Res. 2008;28:729–737. doi: 10.1016/j.nutres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Erdman JW, Jr, Balentine D, Arab L, et al. Flavonoids and heart health: Proceedings of the ILSI North America Flavonoids Workshop, May 31–June 1, 2005, Washington, DC. J Nutr. 2007;137:718S–737S. doi: 10.1093/jn/137.3.718S. [DOI] [PubMed] [Google Scholar]
- 20.Mullen W, Marks SC, Crozier A. Evaluation of phenolic compounds in commercial fruit juices and fruit drinks. J Agric Food Chem. 2007;55:3148–3157. doi: 10.1021/jf062970x. [DOI] [PubMed] [Google Scholar]
- 21.Chaves AA, Joshi MS, Coyle CM, et al. Vasoprotective endothelial effects of a standardized grape product in humans. Vascul Pharmacol. 2009;50:20–26. doi: 10.1016/j.vph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Chou EJ, Keevil JG, Aeschlimann S, et al. Effect of ingestion of purple grape juice on endothelial function in patients with coronary heart disease. Am J Cardiol. 2001;88:553–555. doi: 10.1016/s0002-9149(01)01738-6. [DOI] [PubMed] [Google Scholar]
- 23.O’Byrne DJ, Devaraj S, Grundy SM, et al. Comparison of the antioxidant effects of Concord grape juice flavonoids alpha-tocopherol on markers of oxidative stress in healthy adults. Am J Clin Nutr. 2002;76:1367–1374. doi: 10.1093/ajcn/76.6.1367. [DOI] [PubMed] [Google Scholar]
- 24.Stein JH, Keevil JG, Wiebe DA, et al. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999;100:1050–1055. doi: 10.1161/01.cir.100.10.1050. [DOI] [PubMed] [Google Scholar]
- 25.Deng YB, Li TL, Xiang HJ, et al. Impaired endothelial function in the brachial artery after Kawasaki disease and the effects of intravenous administration of vitamin C. Pediatr Infect Dis J. 2003;22:34–39. doi: 10.1097/00006454-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Engler MM, Engler MB, Malloy MJ, et al. Antioxidant vitamins C and E improve endothelial function in children with hyperlipidemia: Endothelial Assessment of Risk from Lipids in Youth (EARLY) trial. Circulation. 2003;108:1059–1063. doi: 10.1161/01.CIR.0000086345.09861.A0. [DOI] [PubMed] [Google Scholar]
- 27.Mietus-Snyder M, Malloy MJ. Endothelial dysfunction occurs in children with two genetic hyperlipidemias: Improvement with antioxidant vitamin therapy. J Pediatr. 1998;133:35–40. doi: 10.1016/s0022-3476(98)70174-x. [DOI] [PubMed] [Google Scholar]
- 28.Vita JA, Keaney JF., Jr Endothelial function: A barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 29.Van Der Sluis AA, Dekker M, Skrede G, et al. Activity and concentration of polyphenolic antioxidants in apple juice. 1. Effect of existing production methods. J Agric Food Chem. 2002;50:7211–7219. doi: 10.1021/jf020115h. [DOI] [PubMed] [Google Scholar]
- 30.Seeram NP, Aviram M, Zhang Y, et al. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem. 2008;56:1415–1422. doi: 10.1021/jf073035s. [DOI] [PubMed] [Google Scholar]
- 31.American Academy of Pediatrics. The use and misuse of fruit juice in pediatrics. Pediatrics. 2001;107:1210–1213. doi: 10.1542/peds.107.5.1210. [DOI] [PubMed] [Google Scholar]
- 32.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 33.Metzig AM, Schwarzenberg SJ, Fox CK, et al. Postprandial endothelial function, inflammation, and oxidative stress in obese children and adolescents. Obesity (Silver Spring) 2011;19:1279–1283. doi: 10.1038/oby.2010.318. [DOI] [PubMed] [Google Scholar]
- 34.Le Brocq M, Leslie SJ, Milliken P, et al. Endothelial dysfunction: From molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1631–1674. doi: 10.1089/ars.2007.2013. [DOI] [PubMed] [Google Scholar]
- 35.Jarvisalo MJ, Harmoinen A, Hakanen M, et al. Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arterioscler Thromb Vasc Biol. 2002;22:1323–1328. doi: 10.1161/01.atv.0000024222.06463.21. [DOI] [PubMed] [Google Scholar]
- 36.Aaron DJ, Kriska AM, Dearwater SR, et al. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. American journal of epidemiology. 1995;142:191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 37.Pereira MA, FitzerGerald SJ, Gregg EW, et al. A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc. 1997;29:S1–205. [PubMed] [Google Scholar]
- 38.Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 39.Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 40.Kuvin JT, Mammen A, Mooney P, et al. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12:13–16. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- 41.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham heart study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 43.Haller MJ, Stein J, Shuster J, et al. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatr Diabetes. 2007;8:193–198. doi: 10.1111/j.1399-5448.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 44.Mahmud FH, Van Uum S, Kanji N, et al. Impaired endothelial function in adolescents with type 1 diabetes mellitus. J Pediatr. 2008;152:557–562. doi: 10.1016/j.jpeds.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 45.Mahmud FH, Hill DJ, Cuerden MS, et al. Impaired vascular function in obese adolescents with insulin resistance. J Pediatr. 2009;155:678–682. doi: 10.1016/j.jpeds.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 46.Bonetti PO, Barsness GW, Keelan PC, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 47.Fisher ND, Hughes M, Gerhard-Herman M, et al. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Schroeter H, Heiss C, Balzer J, et al. (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamburg NM, Palmisano J, Larson MG, et al. Relation of brachial and digital measures of vascular function in the community: The Framingham heart study. Hypertension. 2011;57:390–396. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhindsa M, Sommerlad SM, DeVan AE, et al. Interrelationships among noninvasive measures of postischemic macro- and microvascular reactivity. J Appl Physiol. 2008;105:427–432. doi: 10.1152/japplphysiol.90431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]