Abstract
Previous studies have shown CD34 family member Podocalyxin is required for epithelial lumen formation in vitro. We demonstrate that Endoglycan, a CD34 family member with homology to Podocalyxin, is produced prior to lumen formation in developing nephrons. Endoglycan localizes to Rab11-containing vesicles in nephron progenitors, and then relocalizes to the apical surface as progenitors epithelialize. Once an apical/luminal surface is formed, Endoglycan (and the actin-binding protein Ezrin) localize to large, intraluminal structures that may be vesicles/exosomes. We generated mice lacking Endoglycan and found mutants had timely initiation of lumen formation and continuous lumens, similar to controls. Mice with conditional deletion of both Endoglycan and Podocalyxin in developing nephrons also had normal tubular lumens. Despite this, Endoglycan/Podocalyxin is required for apical recruitment of the adaptor protein NHERF1, but not Ezrin, in podocyte precursors, a subset of the epithelia. In summary, while CD34 family members appear dispensable for lumen formation, our data identify Endoglycan as a novel pre-luminal marker and suggest lumen formation occurs via vesicular trafficking of apical cargo that includes Endoglycan.
Keywords: kidney development, lumen, tubulogenesis, Endoglycan, Podocalyxin, CD34, polarity, nephron, exosomes
Introduction
Epithelial tubules are a component of many organs, including the kidney [1, 2]. A central feature of tubulogenesis is the segregation of spatially and functionally distinct plasma membrane surfaces, called apical-basal polarization, within each cell. The polarization of individual cells is coordinated with surrounding cells, forming a continuous apical surface at the site of the tubule lumen.
Renal epithelial tubules are characterized by a single, central lumen at the apical surface of cells. Despite this commonality, renal tubules derive from two different embryonic sources, the ureteric bud epithelium and the metanephric mesenchyme, which form tubules by different cellular mechanisms [3]. It is the reciprocal interactions between these two tissues that allow for the generation of nephron tubules. Signals from the metanephric mesenchyme induce the branching of the ureteric bud epithelia, which buds from a pre-existing tubule. Conversely, the ureteric bud epithelia signals to the metanephric mesenchyme, inducing a subset of the mesenchyme containing nephron progenitor cells (the cap mesenchyme, CM) to undergo compaction and then a mesenchymal-to-epithelial transition (MET), forming a sphere of polarized epithelia with a central lumen (Fig 1A). This polarized sphere, called a renal vesicle (RV), subsequently elongates to form a primordial tubule called the s-shaped body (SB). Each developing s-shaped body will fuse with the tip of an adjacent ureteric bud tubule, thus forming a continuous lumen. The s-shaped body subsequently undergoes extensive morphogenesis to form the epithelial tubules of nephrons, while the UB gives rise to the collecting duct system.
Fig 1. Endoglycan and Podocalyxin localization during lumen formation.
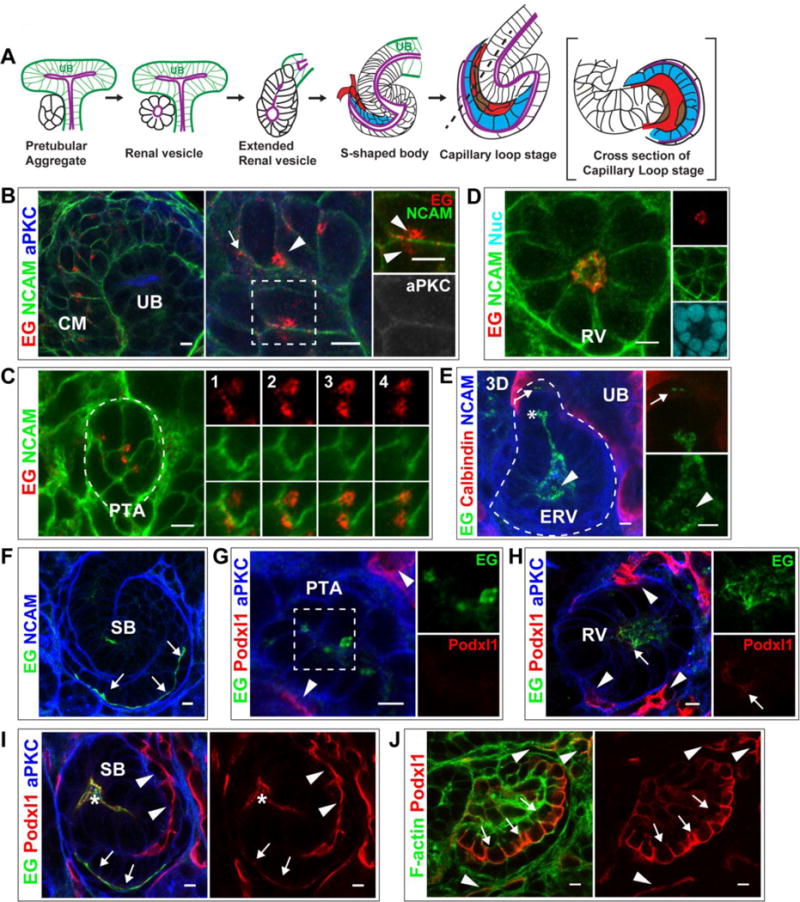
(A) General schematic of lumen formation in developing nephrons. The cap mesenchyme (not shown) compacts to form a pretubular aggregate (PA). The PA becomes a polarized, epithelial sphere called the renal vesicle (RV) containing a central lumen (purple). The RV becomes an extended structure, then forms the s-shaped body (SB), whose lumen is continuous with the ureteric bud (UB) lumen (purple). As the SB elongates, endothelial (red) and mesangial (brown) precursors invade the SB cleft and the SB “tail” forms a primordial glomerulus known as the capillary loop stage. Podocyte precursors are indicated (blue).
(B–F) Localization of Endoglycan (EG) in P0 kidney in CM (B), PA (C), and RV (D) stages. EG localizes largely to punctate structures in CM and PA, then relocalizes to the nascent nephron lumen. NCAM marks the plasma membrane in CM and PA and becomes restricted to the lateral surfaces in RVs. aPKC marks apical, luminal surfaces (B). In extended RVs (E), EG localizes to the lumen, including the leading portion (asterisks), and is in contiguous cells prior to lumen formation (arrow). EG also localizes to intraluminal structures in the widened lumen (arrowhead). Calbindin marks the adjacent UB. (F) EG is apical (and intraluminal) in the SB stage, including the apical surface of podocyte precursors (arrows).
(G–J) Localization of Podocalyxin (Podxl1) in P0 kidney shows it is absent from CM (not shown) and PA (G), but is present at low levels in RV (H, arrow). Podxl1 also localizes to endothelia (H, arrowheads), where levels are higher. (I) Podxl1 levels increase at the SB stage, where it is apical/luminal (asterisks indicate mid-SB and arrows indicate proximal SB where podocyte precursors reside). (J) Further increase in Podxl1 is observed in early glomeruli at the apical surface of immature podocytes (arrows) and in endothelia (arrowheads). Results are representative of 3 experiments. Scale bars: 5 μm.
The lumen of the UB arises as an extension of a pre-existing lumen, as it generally does when a tubule forms as a bud of a pre-existing tubule. In contrast, the lumen of the nascent nephron arises de novo during MET and polarization [4]. How the nephron lumen arises is not clear, and this is an outstanding question in the field. Much of our knowledge of the cellular and molecular mechanisms of nephron lumen formation has arisen from 3D culture of a well-established renal epithelial cell line, Madin Darby Canine Kidney (MDCK) cells. Indeed use of this culture model has allowed for dissection of many aspects of epithelial polarity and lumen formation [5].
One protein that has a central role in MDCK epithelial polarity is Podocalyxin. Podocalyxin is a member of the CD34 family of sialomucins, which is comprised of CD34, Podocalyxin and Endoglycan (Podocalyxin-2). CD34 family members have been shown to be markers of tissue-specific stem cells and play roles in several biological functions, including tubulogenesis, adhesion, cell morphology, and homing of hematopoietic stem cells [6]. Numerous in vitro studies have shown a central role for Podocalyxin in epithelial polarity. Podocalyxin interacts directly with the adaptor proteins NHERF1/2 and Ezrin, forming a ternary complex at the apical surface that is tethered to the actin cytoskeleton through Ezrin [7–10]. Polarized distribution of Podocalyxin/NHERF to the free surface of single MDCK cells attached to a culture dish is an early event in epithelial polarization, and Podocalyxin allows segregation of apical and basolateral domains in an epithelial monolayer [11]. In addition, Podocalyxin (and Ezrin) expression drives expansion of apical surfaces by increasing microvilli number and length [12–14]. Finally, Podocalyxin appears to function as an anti-adhesive, causing cell-cell repulsion via its negatively charged sialic acids [15].
Depletion of Podocalyxin from 3D MDCK cultures leads to formation of multiple lumens with cytosolic redistribution of NHERF1 [10]. Podocalyxin-depleted cysts mislocalize several apically located proteins, such as Crb3, to sub-apical Rab11-positive vesicles. Interestingly, the phenotype is not one of absent or collapsed lumens, as might be expected from Podocalyxin’s numerous functions. Consistent with these findings, individual depletion of Ezrin or NHERF1, -2, or -3 in vitro also leads to multiple lumens [10]. Collectively, these results suggest a critical role for Podocalyxin/NHERF/Ezrin complexes in generating apical-basal polarity and a continuous apical surface.
Despite its essential role in in vitro renal epithelia, Podocalyxin expression in the kidney is reported to be limited to hematopoietic progenitors, vascular endothelia, and podocytes [16, 17]. It localizes to the apical surface of developing and mature podocytes [18, 19], where it plays a critical role in the formation of foot processes and slit diaphragms [20]. Mice lacking Podocalyxin die in the neonatal period with anuric renal failure [20]. Podocalyxin function is also important in human podocytes: exome sequencing has identified a mutation in Podxl1 as a likely candidate for autosomal dominant focal segmental glomerulosclerosis [21].
The lack of significant Podocalyxin in vivo during renal epithelial tubulogenesis and lumen formation led us to examine if other CD34 family members are expressed in developing and mature renal epithelia. Herein we find that Endoglycan localizes to intracellular vesicles within progenitors of nephron epithelia and at the apical/luminal surface coincident with lumen initiation. Although we find that Endoglycan is not required for lumen formation per se, our results support a model in which intracellular vesicles containing apical components traffic to a forming apical surface to generate a tubular lumen.
Materials And Methods
Animals
We generated and tested mice from three validated clones of Podxl2tm1a(EUCOMM)Wtsi ES cells through use of the UTSW Transgenic core facility. Mice derived from clone EPD0647_7_C06 were used for this paper. The Podxl2tm1a allele is a targeted trap allele that functions as a gene-trap knockout. Mice carrying the Podxl2tm1a/tm1a allele were screened by PCR for correct targeting, and lack of Endoglycan (EG or Podocalyxin-like 2) was verified by immunofluorescence. We also generated mice with a conditional EG allele by mating Podxl2tm1a mice to mice carrying ubiquitous expression of FlpE recombinase (β-actin-FlpE mice) [22]. In this conditional allele of EG, exons 3 and 4 are flanked by loxP sites. Exons 3 and 4 are comprised of 869 base pairs that encode for amino acids 48 to 337 of Endoglycan. The excision of these two exons causes a frameshift mutation in subsequent exons, resulting in a stop codon after 114 base pairs.
Conditional Podxl2flox/flox (hereafter referred to as EGflox/flox) mice were mated with mice carrying conditional alleles of Podocalyxin (Podxl1flox/flox) [23] to generate EGflox/flox; Podxl1flox/flox mice. The doubly conditional mice were crossed to Six2-cre and Pax3-cre transgenic mice [24, 25] to obtain EGflox/flox; Podxl1flox/flox; Six2-eGFP-cre and EGflox/flox; Podxl1flox/flox; Pax3-cre mutant embryos. Recombination of the conditional allele of EG by Pax3-cre was verified by PCR of exons 3–7 from cDNA of E13.5 kidney lysates of EGflox/flox; Podxl1flox/flox; Pax3-cre and EGflox/flox; Podxl1flox/flox mice. See Supplemental Materials and Methods for details. Mice were maintained on mixed genetic backgrounds and genotyped by PCR. Procedures were performed according to UTSW IACUC-approved guidelines.
Histology and immunofluorescence
For paraffin sections, embryonic day 14.5 (E14.5) and post-natal day 0 (P0) kidneys were fixed in 4% paraformaldehyde (PFA) in PBS overnight at 4°C, embedded in paraffin and sectioned at 5 μm. E10.5 pancreases were collected and embedded in paraffin as previously described [26, 27]. For nonparaffin sections, E14.5 and P0 kidneys were fixed for 2h in 4% PFA/PBS, and E10.5 pancreases were fixed in 4% PFA/PBS overnight 4°C. Sections were permeabilized with 0.3% Triton X-100/PBS (PBST) and blocked with 10% donkey sera/PBST. Sections were incubated with primary antibodies overnight (4°C), and then with fluorophore-conjugated secondary antibodies and mounted with Prolong Gold (Invitrogen).
Antibodies
Primary antibodies were diluted 1:100 unless stated otherwise: Endoglycan (R&D, AF3534, 1:200), Podocalyxin (R&D, MAB1556), Calbindin (Swant, CB-38a, 1:1000), NCAM (DSHB, 5B8; GeneTex H28-123), Rab11 (Invitrogen, 71-5300), Ezrin (Abcam, ab41672), CD34 (eBioscience, RAM34), Par-6b (SCBT, sc-67392, 1:400), Par-3 (Millipore, 07-330), aPKC (SCBT, C-20, 1:300), NHERF1 (Abcam, ab3452), Phalloidin 647 (Invitrogen, 42008A, 1:200). Secondary antibodies were purchased from Jackson ImmunoResearch, PA, USA.
Imaging and statistical analysis
Confocal imaging was performed on a Zeiss LSM510 META laser scanning confocal microscope. Immunofluorescence and light microscopy were performed with a Nikon TE300 inverted fluorescence microscope (AxioCam HRc camera and AxioVision 4.5 software). Images were minimally processed and resampled to 300 dpi using Adobe Photoshop. The 3-D reconstruction of a z-stack was performed with the LSM510 3-D module and ImageJ.
Transmission electron microscopy
Dissected embryonic kidneys at E14.5 were fixed in 2.5% glutaraldehyde in 0.1 M Na+cacodolate. Kidneys were post-fixed in 1% buffered OsO4, en bloc stained in 2% uranyl acetate, dehydrated and embedded in EMbed-812 resin. Sections were cut on a Leica EM UC6 ultramicrotome and stained with 2% uranyl acetate and lead citrate. Images were acquired on a FEI Tecnai G2 Spirit.
Results
Endoglycan localizes to intracellular vesicles in nephron progenitor cells
A schematic of the early steps of nephron tubulogenesis is depicted as Fig 1A. The cap mesenchyme containing nephron progenitor cells compact to form a pretubular aggregate, which subsequently undergoes a MET to form a polarized epithelial sphere called the renal vesicle. The renal vesicle undergoes morphogenesis to form an intermediate structure called the extended renal vesicle, and then forms an s-shaped tubule called the s-shaped body. At the s-shaped body stage, the lumen is continuous with the lumen of the ureteric bud [4]. As the s-shaped body continues to elongate, endothelial and mesangial precursors invade the proximal “tail” of the s-shaped body. When viewed in cross-section, this appears to form a loop, and is aptly named the capillary loop stage.
The Genitourinary Developmental Molecular Anatomy Project (GUDMAP) database showed Endoglycan is expressed in the developing kidney, particularly in the metanephric mesenchyme and renal vesicles [28]. In the cap mesenchyme, which contains the nephron epithelial progenitors, we found Endoglycan (EG) localized to numerous intracellular puncta that appeared vesicular in nature (Fig 1B). We also noted the presence of EG in the renal stroma (not shown). The distribution of EG-containing puncta in the cap mesenchyme was not polarized with respect to the CM-UB interface. When progenitors compacted to form a pretubular aggregate (Fig 1C), the puncta appeared to cluster and form higher-order structures. During lumen formation at the renal vesicle stage, these puncta relocalized to the apical cell surface (Fig 1D). When a renal vesicle elongates, its lumen extends toward the UB and forms small discrete lumens in the connecting region that eventually become continuous at the s-shaped body stage [4]. EG was present at the leading edge of an extending lumen (Fig 1E, asterisks) and at the discrete de novo lumens (not shown), indicating it is an early luminal component in developing nephrons. EG was also observed in intracellular puncta of adjacent cells that had not yet polarized (Fig 1E, arrow). Interestingly, in widened portions of the maturing lumen, such as the extended renal vesicle and mid-s-shaped body tubule, EG often appeared to partially fill the lumen with large, circular structures (Fig 1E, arrowhead) suggestive of extracellular vesicles. In s-shaped body tubules, EG was localized to the apical/luminal surface, including the proximal portion containing podocyte precursors (Fig 1F, arrows). A low level of EG was also present in the capillary loop stage, but EG was not present in maturing tubules or glomeruli of neonatal kidneys or in adult kidneys (not shown).
To determine if EG expression in developing epithelial tubules was specific to the kidney, we also examined its expression in the developing pancreas (Fig S1). The pancreas bud emerges from the endoderm ~E8.75 in mice, and undergoes rapid stratification. By E10.5, the stratified epithelium remodels to form rosettes that then open to form central microlumens. These microlumens then coalesce as the epithelium remodels into a single-layered epithelial tubular network, beginning around E12.5 [26]. Using immunofluorescence, we found that EG colocalizes with many, but not all, aPKC+ microlumens in the pancreas at E10.5. As microlumens form asynchronously in the pancreas and EG expression decreases as micolumens mature, it may explain why EG is not observed in all microlumens.
Endoglycan expression precedes Podocalyxin expression during nephron development
Because EG was expressed intracellularly in the cap mesenchyme and pretubular aggregates, we investigated if Podocalyxin was also expressed prior to lumen formation, as previously observed in MDCK cells [29]. Podocalyxin was not evident at these stages (not shown and Fig 1G). In renal vesicles, faint immunostaining of Podocalyxin was evident at the luminal surface (Fig 1H). Low levels of Podocalyxin were also present at the apical surface of s-shaped bodies, especially at the apical surface of podocyte precursors (Fig 1I, arrows). Podocalyxin is also produced by endothelia [30], and this is indicated in the images (arrowheads in Fig 1I–J) to serve as a reference for image intensity. Note that Podocalyxin was more abundant in the capillary endothelium compared with the s-shaped body lumen. At a later developmental stage, the capillary loop stage of glomerulogenesis, increased levels of Podocalyxin were observed at the apical surface of podocytes (Fig 1J, arrows, and [31]), exceeding levels in the endothelia (Fig 1J, arrowheads). Lastly, we found that CD34 was not present in developing renal epithelia, although it was abundantly produced by the endothelia (not shown). From these studies we concluded that EG is the only CD34 family member to be expressed in nephron progenitors prior to lumen formation, suggesting it may participate in nephron lumen formation akin to the role of Podocalyxin in MDCK cells. We also found that EG is produced by the epithelia only during early tubulogenesis, suggesting a role in lumen formation but not maintenance. Finally, we noted a temporal relationship between EG and Podocalyxin levels. EG levels were high at the onset of lumen formation, declining thereafter, while Podocalyxin levels increased as EG declined. In the maturing nephron, Podocalyxin levels were highest in podocytes.
Endoglycan is present in Rab11-containing endosomes prior to lumen formation
Electron microscopy studies conducted by Saxen and Wartiovaara in the 1960s showed subapical vesicles in pretubular aggregates [32]. Because EG was observed in small puncta prior to lumen formation, we examined whether EG colocalized with Rab11, a known marker of recycling endosomes [33]. Prior to lumen formation, EG colocalized highly with Rab11 vesicles (Fig 2A). The amount of colocalization decreased after lumen initiation in the renal vesicle stage, where EG was mostly apical (Fig 2B). These results suggest that EG is transported to the apical surface via vesicular transport, and specifically via Rab11-containing endosomes. Together the data suggest a model in which intracellular vesicles containing apical components move to a forming apical surface and coalesce to form a lumen.
Fig 2. Endoglycan is present in Rab11-vesicles prior to lumen formation.
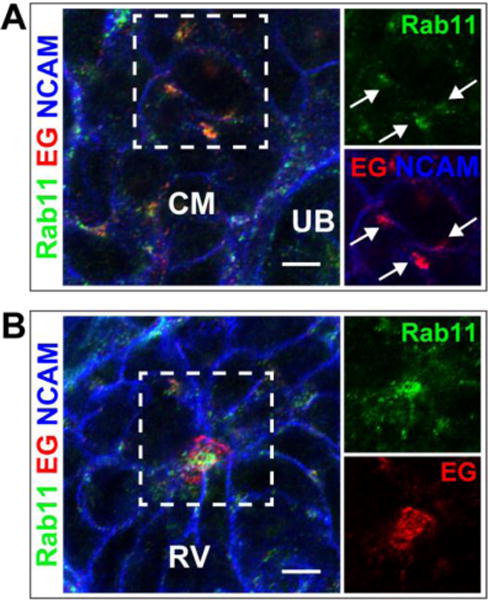
Immunolocalization of Endoglycan (EG) and Rab11a is shown in the cap mesenchyme (CM) (A) and early RV stage (B). Arrows in A indicate colocalization of EG and Rab11a. NCAM delineates the plasma membrane in the CM. Results are representative of 2 experiments. Scale bars: 5 μm.
Lumens of developing nephrons contain extracellular vesicles
The large EG-containing circular structures observed in the widened lumens of the extended renal vesicle and s-shaped body suggested the presence of extracellular vesicles (Fig 1D). The size of these structures, ~1 μm, was much larger than the intracellular EG-, Rab11-containing endosomes. This suggested that EG is not only a component of recycling endosomes, but may also be a component of extracellular vesicles that are being shed into the lumen. We examined other apical markers for a similar pattern of immunolocalization, and found that Ezrin, a known interactant of Podocalyxin, also localized to intraluminal structures (Fig 3A, B). Ezrin was not observed prior to lumen formation (not shown). Surprisingly, EG and Ezrin only partially colocalized within the lumen. We observed the vesicular structures adjacent to the apical surface in some images (Fig 3B), suggesting they might be budding from the plasma membrane. Electron microscopy revealed similarly-sized structures that also are consistent with a budding process from the plasma membrane (Fig 3C) although further experiments are needed to test this hypothesis. To determine if other known components of exosomes also localized to the lumens, we examined nephron lumens for CD63, a tetraspanin present in a subset of exosomes. However, we did not observe CD63 in nephron epithelia (not shown).
Fig 3. Endoglycan and Ezrin are present in intraluminal vesicles.
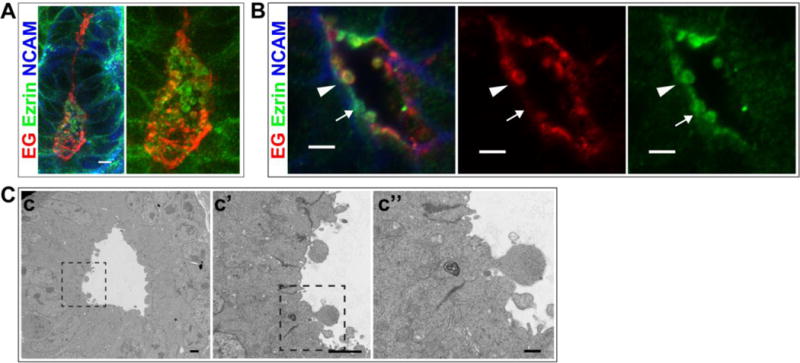
(A,B) Immunolocalization of EG and Ezrin in the intraluminal space of P0 kidney. NCAM marks the lateral plasma membrane in developing nephrons. B shows a high magnification image of a lumen (arrowhead indicates an extracellular vesicle with EG and Ezrin; arrow indicates an extracellular vesicle with Ezrin alone). (C) Transmission EM images showing intraluminal protrusions that appear to be budding from the plasma membrane. Results are representative of 2 experiments. Scale bars: 5 μm A,B; 2 μm c,c′ and 0.5 μm c″.
Endoglycan is not required for lumen formation in nephrons
To investigate if Endoglycan is an apical membrane protein required for nephron lumen formation, we generated mice (Podxl2tm1a(EUCOMM)Wtsi) globally lacking Endoglycan. Immunostaining of neonatal (P0) kidneys showed Endoglycan was absent (Fig 4A). Immunostaining with an apical marker such as aPKC revealed no delay of normal lumen formation in renal vesicles (Fig 4A). Three-dimensional reconstruction of confocal z-stacks of s-shaped bodies from mutant mice at P0 showed that nephron lumens were continuous and indistinguishable from controls (Fig 4B). Neonatal mice lacking EG had kidneys with normal histology, as assessed by H&E staining (Fig 4C). The mutant renal vesicles did not exhibit increased Podocalyxin (or CD34, not shown) at the luminal surface compared with controls (Fig 4D), so functional family member compensation did not account for normal-appearing lumens. We also examined mutant mice at 3 months of age (n = 3), and found the mice to be healthy with normal-appearing renal histology (not shown). The tubules had normal diameter and no interstitial fibrosis. As mentioned earlier, Endoglycan is not produced in adult renal tubules, but this result supports the absence of a developmental defect. Defects in lumen formation can lead to profound renal dysgenesis in post-natal kidneys, as we have shown previously [4]. Similar to our results in kidney, mice lacking EG appeared to have normal lumen formation and tubule structure in developing pancreases (not shown).
Fig 4. Endoglycan is not required for nephron lumen formation.
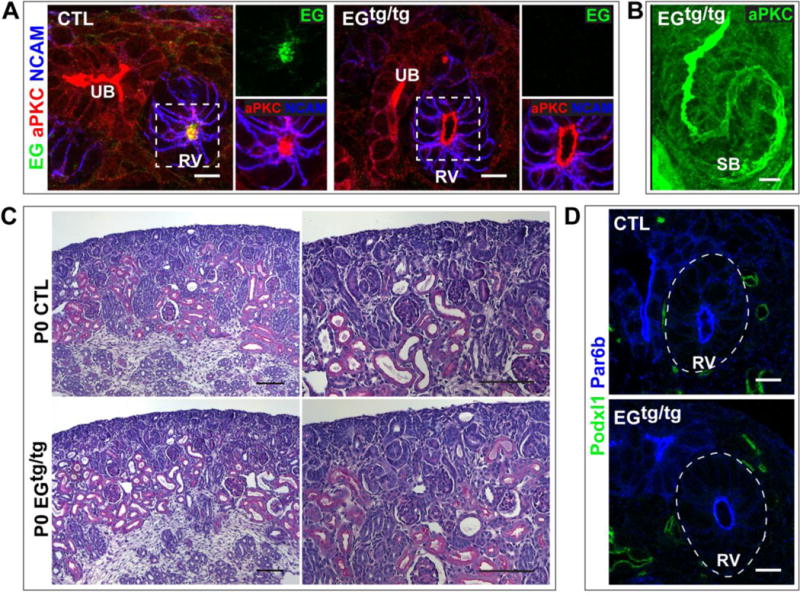
(A) Immunolocalization of EG and aPKC in P0 kidneys from control and mutant Podxl2tm1a/tm1a (EGtg/tg) mice shows EG is absent from the renal vesicle in the EGtg/tg kidney. aPKC, a marker of the luminal surface, shows the renal vesicle has a central lumen in control and EGtg/tg images. (B) A 3-D reconstruction of a confocal z-stack shows an s-shaped body with a continuous lumen from the EGtg/tg kidney. (C) Hematoxylin and eosin stain of P0 sections from a control and EGtg/tg kidney. (D) Immunolocalization of Podocalyxin (Podxl1) shows little/no Podocalyxin in control and EGtg/tg renal vesicles. Par6b marks the apical surface. Results are representative of 2 experiments. Scale bars: 5 μm A,B,D; 100 μm C.
Because we had observed faint immunostaining of Podocalyxin at the lumen of renal vesicles (Fig 1G), we questioned if there might be functional redundancy between EG and Podocalyxin, and decided to remove both EG and Podocalyxin from developing nephrons. To do this, we generated mice with conditional deletion of both EG and Podocalyxin from nephron progenitors using Six2-cre to generate EGflox/flox; Podxl1flox/flox; Six2-cre mice [24]. The mutant mice died in the neonatal period, similar to mice null for Podocalyxin [20]. As podocalxyin is already known to have a critical role in podocyte function [20], we limited our analysis to early stages of nephrogenesis. These mice had no obvious defects in timely lumen formation or luminal continuity (not shown). We next generated mice with conditional deletion of EG and Podocalyxin using the Pax3-cre, a Cre line with more widespread recombination, but importantly, with earlier recombination within nephron progenitors [19]. These mutant mice had no delay in timely lumen formation (Fig 5A), despite confirmation that both EG (Fig 5A) and Podocalyxin (Fig 5B) were absent from mutant nephrons by immunofluorescence. We confirmed that EG was absent from the cap mesenchyme (not shown). There was also no compensatory expression of CD34 in the developing nephrons at the s-shaped body lumens, including the apical surface of developing podocytes (Fig 5C). Mutant lumens were not dilated or convoluted (n = 3 mutants). In addition to normal lumen initiation, the lumens of mutants were continuous at the s-shaped body stage, similar to controls (Fig 5D). Together, these results suggest that lumen formation in developing epithelial tubules, both in the kidney and pancreas, does not require CD34 family members.
Fig 5. Absence of Endoglycan and Podocalyxin from developing nephron tubules.
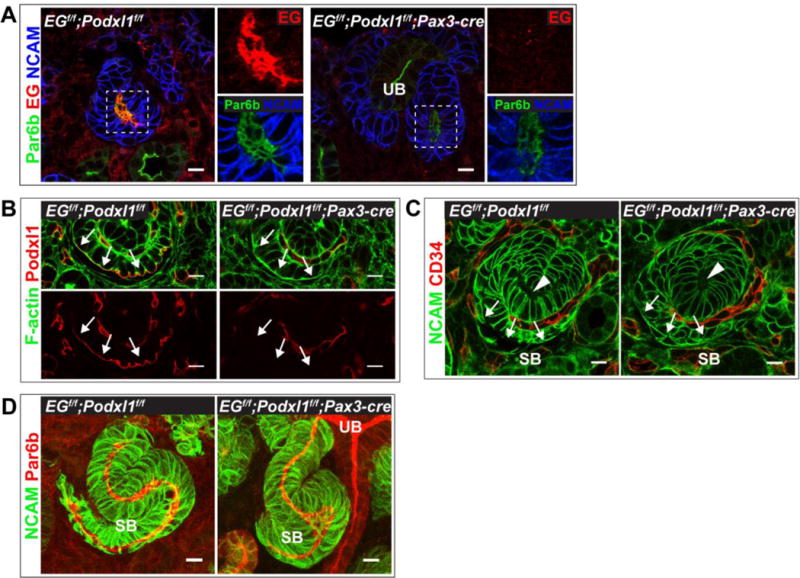
(A,B,C) Immunolocalization of Endoglycan (A), Podocalyxin (B), and CD34 (C) in control (EGflox/flox; Podxl1flox/flox) and mutant (EGflox/flox; Podxl1flox/flox; Pax3-cre) E14.5 kidneys. Panel A shows mutants have a central lumen in renal vesicles, as delineated by Par6b, but lack Endoglycan. (B) Podocalyxin is present in developing podocytes (arrows) of controls but not mutants. Other Podocalyxin+ structures are endothelial cells. (C) CD34 is absent from s-shaped body lumens (arrowhead), including developing podocytes (arrows), in controls and mutants. CD34+ structures are endothelial cells. (D) Continuous lumens, delineated by Par6b, are present in control and mutant s-shaped bodies. The mutant image (right) shows a lumen that is continuous with the UB lumen. Results are representative of 3 experiments. Scale bars: 10 μm A–D.
CD34 family members recruit NHERF1, but not Ezrin, to the forming apical surface of podocytes
Podocalyxin forms a ternary complex with NHERF1/2 and Ezrin, the latter tethering it to the actin cytoskeleton [7–10]. Ezrin was detected as early as the renal vesicle stage (not shown and Fig 3A). NHERF1, in contrast, was not present in appreciable amounts until the s-shaped body stage, where it was prominent in podocyte precursors at the apical surface (Fig 6A), similar to Podocalyxin localization. Mice lacking both EG and Podocalyxin (EGflox/flox; Podxl1flox/flox; Pax3-cre) showed a mislocalization of apically localized NHERF1 in developing podocytes (Fig 6A). Ezrin still localized to the apical surface of podocytes lacking both EG and Podocalyxin (Fig 6B), suggesting its localization is not dependent on these proteins. We also observed that NHERF1 localized to the apical surface of maturing tubules (Fig 6A, arrowheads) and this was not changed in the absence of Podocalyxin and EG in nephron tubules (not shown), suggesting that NHERF1 requires Podocalyxin/EG for recruitment to the apical surface specifically in podocytes. Mice lacking NHERF1, in contrast to mice lacking Podocalyxin, have not been reported to have developmental defects in podocyte structure and function [34], so the functional consequences of this are unknown.
Fig 6. CD34 family members recruit NHERF1, but not Ezrin, to the apical surface of podocytes.
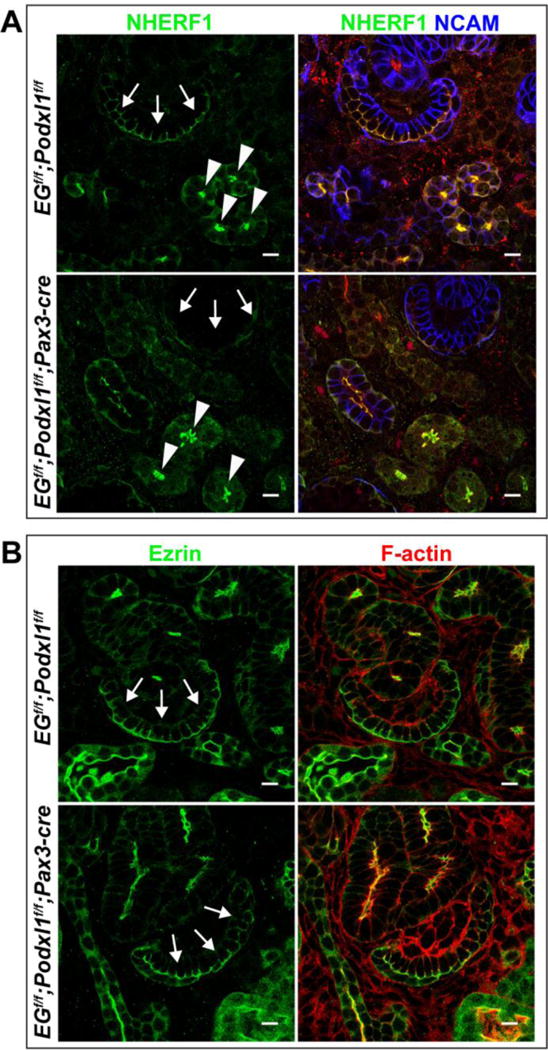
Immunolocalization of NHERF1 (A) and Ezrin (B) in control (EGflox/flox; Podxl1flox/flox) and mutant (EGflox/flox; Podxl1flox/flox; Pax3-cre) P0 kidneys. (A) NHERF1 is present at the apical surface of developing podocytes (arrows) of controls but not mutants. Arrowheads show the apical surface of maturing tubules. NCAM delineates the lateral plasma membrane of developing nephron tubules. (B) Ezrin localizes to the apical surface of developing podocytes (arrows) of controls and mutants. Results are representative of 2 experiments. Scale bars: 10 μm.
Discussion
Many of the cellular and molecular mechanisms that underlie lumen formation in the kidney are not well understood. In this study, we report the localization and function of a CD34 family member, Endoglycan, in nephron epithelial tubulogenesis. Examination of this protein has led to several novel observations that help elucidate general principles of nephron lumen formation. First, we demonstrate that Endoglycan localizes to Rab11+ intracellular vesicles prior to lumen formation, which relocalize to the luminal surface during lumen initiation. This finding suggests a model of lumen formation in which intracellular vesicles containing apical components traffic to and fuse with a forming apical surface. Our results are consistent with observations made in the 1960’s by Saxen and Wartiovaara, who reported the presence of subapical vesicles in pretubular aggregates. Furthermore, this model of lumen formation, dubbed “hollowing” has been previously described in MDCK cells [35], a known and well-studied renal epithelial cell line.
We also demonstrate that Endoglycan and Ezrin are present within the lumen. We frequently observed their localization in round, luminal structures of ~1 μm. Because the size of these structures is much larger than the Endoglycan-, Rab11-positive intracellular vesicles, we hypothesize that they represent a distinct cellular process. Electron microscopy images of the apical surface of the s-shaped body stage, which has a continuous lumen, show apical membrane blebs of ~1 μm diameter that appear to be budding from the apical plasma membrane. Together, this suggests that developing tubules may release Endoglycan-containing vesicles/exosomes into the lumen. This interpretation is bolstered by previous studies that have shown Podocalyxin is a component of exosomes in urine and cultured cells [36–38]. However, future studies with live imaging will determine if apical membrane blebs are indeed released into the lumen as extracellular vesicles/exosomes during normal tubulogenesis.
At this point, we can only speculate on the roles that intraluminal structures play in developing tubules. It was recently shown that exosomes increase HGF-mediated outward growth and cell migration in MDCK cells [39]. Perhaps the intraluminal structures observed are similarly required for intercellular communication to drive nephron tubulogenesis and/or extend the lumen to adjacent cells in vivo. They may also serve to regulate the size of the apical domain. Another possibility is that luminal structures provide a “filler” to expand the luminal space. Although this is not a known role of luminal structures or exosomes, the deposition (and removal) of a structured protein matrix in the lumen of developing Drosophila trachea affects both lumen diameter and tubule length [40, 41].
In developing nephrons, while Endoglycan localizes to intracellular vesicles and the nascent luminal surface of developing tubules, it is absent from more differentiated lumens, even during embryogenesis. This initially suggested it might be required to initiate lumen formation or expansion, as Podocalyxin does in MDCK cells, mouse aorta, and spheroids derived from human pluripotent stem cells [10, 20, 42]. However, neonatal and adult mice lacking Endoglycan were healthy and fertile without obvious renal abnormalities. This was not due to functional compensation by other known CD34 family members, Podocalyxin or CD34. Indeed, mice with nephron epithelia lacking both Endoglycan and Podocalyxin also had normal morphology in developing tubules, although as expected, these doubly deficient mice had defects in podocytes. One possibility is that, due to its paramount importance, multiple signaling pathways are functionally redundant to ensure the fidelity of apical-basal polarity. Alternatively, the CD34 family/NHERF/Ezrin axis, while not essential for lumen formation, may have other specific roles in renal epithelial function.
Doyonnas et al. [20] has previously demonstrated that mice lacking Podocalyxin die in the neonatal period with anuria and defective glomerular development [20]. While we did not examine the podocyte phenotype in depth in the current work, we found that EGflox/flox; Podxl1flox/flox; Pax3-cre mice, which lack both proteins from developing podocytes in addition to nephron tubular epithelia, had reduced NHERF1 at the apical surface of podocytes. This was not a disruption of apical-basal polarity per se, as Par6 and NCAM were apical and basal respectively. We did not observe a similar decrease in total Ezrin at the apical surface of podocytes (or other tubules). Interestingly, NHERF1 is present at the apical surface in proximal tubules [43], which lack Podocalyxin. This suggests Podocalyxin regulates NHERF1 localization in a cell type-specific manner.
In summary, we provide a morphological analysis of lumen formation in developing nephron tubules and identify Endoglycan as a novel pre-luminal marker in this process.
Supplementary Material
Immunolocalization of EG, aPKC, and E-cadherin (white) in a mouse E10.5 pancreas. aPKC marks the micolumens and E-cadherin marks the basolateral surfaces. Inset depicts colocalization of EG to an aPKC+ microlumen. Results are representative of 3 experiments. Scale bars: 10 μm.
Acknowledgments
We thank the EUCOMM consortium for ES cells and the UTSW transgenic core for generation of Podxl2tm1a(EUCOMM)Wtsi mice. We thank the UTSW EM core and Children’s Medical Center of Dallas for assistance with TEM. We thank Tom Carroll for discussion.
Funding
This work was supported by Basil O’Connor Starter Scholar Research Award No. 5-FY13-201 from the March of Dimes Foundation (DKM), Satellite Healthcare Coplon Grant (DKM), NIH R01DK099478, P30DK079328 (O’Brien Center grant), and FU2010-15237 from Ministerio de Economia y Competitividad of Spain (CG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schluter MA, Margolis B. Apical lumen formation in renal epithelia. J Am Soc Nephrol. 2009;20(7):1444–52. doi: 10.1681/ASN.2008090949. [DOI] [PubMed] [Google Scholar]
- 2.Marciano DK. A holey pursuit: lumen formation in the developing kidney. Pediatr Nephrol. 2016 doi: 10.1007/s00467-016-3326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxen L. Organogenesis of the Kidney. In: Barlow PW, Green PB, Wylie CC, editors. Developmental and Cell Biology Series. New York: Cambridge University Press; 1987. [Google Scholar]
- 4.Yang Z, et al. De novo lumen formation and elongation in the developing nephron: a central role for afadin in apical polarity. Development. 2013;140(8):1774–84. doi: 10.1242/dev.087957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryant DM, et al. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12(11):1035–45. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121(Pt 22):3683–92. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- 7.Orlando RA, et al. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol. 2001;12(8):1589–98. doi: 10.1681/ASN.V1281589. [DOI] [PubMed] [Google Scholar]
- 8.Schmieder S, et al. Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and Ezrin in MDCK cells. J Am Soc Nephrol. 2004;15(9):2289–98. doi: 10.1097/01.ASN.0000135968.49899.E8. [DOI] [PubMed] [Google Scholar]
- 9.Takeda T, et al. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001;108(2):289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant DM, et al. A molecular switch for the orientation of epithelial cell polarization. Dev Cell. 2014;31(2):171–87. doi: 10.1016/j.devcel.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meder D, et al. Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J Cell Biol. 2005;168(2):303–13. doi: 10.1083/jcb.200407072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen JS, et al. The CD34-related molecule podocalyxin is a potent inducer of microvillus formation. PLoS One. 2007;2(2):e237. doi: 10.1371/journal.pone.0000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonilha VL, Finnemann SC, Rodriguez-Boulan E. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J Cell Biol. 1999;147(7):1533–48. doi: 10.1083/jcb.147.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonilha VL, et al. Microvilli defects in retinas of ezrin knockout mice. Exp Eye Res. 2006;82(4):720–9. doi: 10.1016/j.exer.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Takeda T, et al. Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11(9):3219–32. doi: 10.1091/mbc.11.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin–the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984;98(4):1591–6. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen JS, McNagny KM. The role of podocalyxin in health and disease. J Am Soc Nephrol. 2009;20(8):1669–76. doi: 10.1681/ASN.2008070782. [DOI] [PubMed] [Google Scholar]
- 18.Hartleben B, et al. Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS One. 2012;7(5):e36705. doi: 10.1371/journal.pone.0036705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marciano DK, et al. p120 catenin is required for normal renal tubulogenesis and glomerulogenesis. Development. 2011;138(10):2099–109. doi: 10.1242/dev.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyonnas R, et al. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med. 2001;194(1):13–27. doi: 10.1084/jem.194.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barua M, et al. Exome sequencing and in vitro studies identified podocalyxin as a candidate gene for focal and segmental glomerulosclerosis. Kidney Int. 2014;85(1):124–33. doi: 10.1038/ki.2013.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez CI, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25(2):139–40. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 23.Pericacho M, et al. Diminished thrombogenic responses by deletion of the Podocalyxin Gene in mouse megakaryocytes. PLoS One. 2011;6(10):e26025. doi: 10.1371/journal.pone.0026025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi A, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3(2):169–81. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Chen F, Epstein JA. Neural crest expression of Cre recombinase directed by the proximal Pax3 promoter in transgenic mice. Genesis. 2000;26(2):162–4. doi: 10.1002/(sici)1526-968x(200002)26:2<162::aid-gene21>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 26.Villasenor A, et al. Epithelial dynamics of pancreatic branching morphogenesis. Development. 2010;137(24):4295–305. doi: 10.1242/dev.052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marty-Santos L, Cleaver O. Pdx1 regulates pancreas tubulogenesis and E-cadherin expression. Development. 2016;143(1):101–12. doi: 10.1242/dev.126755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harding SD, et al. The GUDMAP database–an online resource for genitourinary research. Development. 2011;138(13):2845–53. doi: 10.1242/dev.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrari A, et al. ROCK-mediated contractility, tight junctions and channels contribute to the conversion of a preapical patch into apical surface during isochoric lumen initiation. J Cell Sci. 2008;121(Pt 21):3649–63. doi: 10.1242/jcs.018648. [DOI] [PubMed] [Google Scholar]
- 30.Horvat R, et al. Endothelial cell membranes contain podocalyxin–the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol. 1986;102(2):484–91. doi: 10.1083/jcb.102.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber TB, et al. Loss of podocyte aPKClambda/iota causes polarity defects and nephrotic syndrome. J Am Soc Nephrol. 2009;20(4):798–806. doi: 10.1681/ASN.2008080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saxen L, Wartiovaara J. Cell contact and cell adhesion during tissue organization. Int J Cancer. 1966;1(3):271–90. doi: 10.1002/ijc.2910010307. [DOI] [PubMed] [Google Scholar]
- 33.Ullrich O, et al. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135(4):913–24. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morales FC, et al. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci U S A. 2004;101(51):17705–10. doi: 10.1073/pnas.0407974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol. 2011;21(3):R126–36. doi: 10.1016/j.cub.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheruvanky A, et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol Renal Physiol. 2007;292(5):F1657–61. doi: 10.1152/ajprenal.00434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogan MC, et al. Subfractionation, characterization, and in-depth proteomic analysis of glomerular membrane vesicles in human urine. Kidney Int. 2014;85(5):1225–37. doi: 10.1038/ki.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez D, et al. Release of podocalyxin into the extracellular space. Role of metalloproteinases. Biochim Biophys Acta. 2011;1813(8):1504–10. doi: 10.1016/j.bbamcr.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Kwon SH, Liu KD, Mostov KE. Intercellular transfer of GPRC5B via exosomes drives HGF-mediated outward growth. Curr Biol. 2014;24(2):199–204. doi: 10.1016/j.cub.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsarouhas V, et al. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev Cell. 2007;13(2):214–25. doi: 10.1016/j.devcel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Luschnig S, et al. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr Biol. 2006;16(2):186–94. doi: 10.1016/j.cub.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 42.Freedman BS, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715. doi: 10.1038/ncomms9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wade JB, et al. Localization and interaction of NHERF isoforms in the renal proximal tubule of the mouse. Am J Physiol Cell Physiol. 2003;285(6):C1494–503. doi: 10.1152/ajpcell.00092.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunolocalization of EG, aPKC, and E-cadherin (white) in a mouse E10.5 pancreas. aPKC marks the micolumens and E-cadherin marks the basolateral surfaces. Inset depicts colocalization of EG to an aPKC+ microlumen. Results are representative of 3 experiments. Scale bars: 10 μm.


