Abstract
Whereas the fear-avoidance model of chronic low back pain (CLBP) posits a generic avoidance of movement that is perceived as threatening, we have repeatedly shown that individuals with high fear and CLBP specifically avoid flexion of the lumbar spine. Accordingly, we developed a virtual dodgeball intervention designed to elicit graded increases in lumbar spine flexion while reducing expectations of fear and harm by engaging participants in a competitive game that is both entertaining and distracting. We recruited 52 participants (48% female) with CLBP and high fear of movement and randomized them to either a game group (n=26) or a control group (n=26). All participants completed a pregame baseline and a follow up assessment (4–6 days later) of lumbar spine motion and expectations of pain and harm during standardized reaches to high (easier), middle, and low (hardest to reach) targets. For three consecutive days, participants in the game group completed 15 minutes of virtual dodgeball between baseline and follow up. For the standardized reaching tests, there were no significant effects of group on changes in lumbar spine flexion, expected pain, or expected harm. However, virtual dodgeball was effective at increasing lumbar flexion within and across gameplay sessions. Participants reported strong positive endorsement of the game, no increases in medication use, pain, or disability, and no adverse events. Although these findings indicate that very brief exposure to this game did not translate to significant changes outside the game environment, this was not surprising given that graded exposure therapy for fear of movement among individuals with low back pain typically last 8–12 sessions. Given the demonstration of safety, feasibility and ability to encourage lumbar flexion within gameplay, these findings provide support for a clinical trial wherein the treatment dose is more consistent with traditional graded-exposure approaches to CLBP.
Keywords: Virtual reality, intervention, chronic back pain, fear
INTRODUCTION
Low back pain is one of the most common reasons for seeking medical care and accounts for over 3.7 million physician visits per year in the United States alone.18 It is the leading cause of pain in the U.S., affecting 28.1% of adults each year34 and 90% in their lifetime.1, 5, 10, 17, 20, 59 Although most episodes of low back pain will resolve within a few days or weeks with little more than symptomatic treatment, up to 10% of individuals develop a chronic pain condition.17 These cases of chronic low back pain (CLBP) last from months to years, are associated with significant individual pain, suffering, and disability, and are estimated to cost society more than $300 billion per year in lost wages and productivity.7, 11, 17, 34 Recent reports suggest that the prevalence of CLBP is increasing while the quality of care is decreasing.11, 25 According to Foster (2011), “Despite decades of research and improved quality of clinical trials, the reality is that the treatments we have to offer patients tend to produce small effects, often only in the short term and none appear to effectively change the longer-term prognostic paths or trajectories for patients.”10
Fear-Avoidance Model of Chronic Low Back Pain
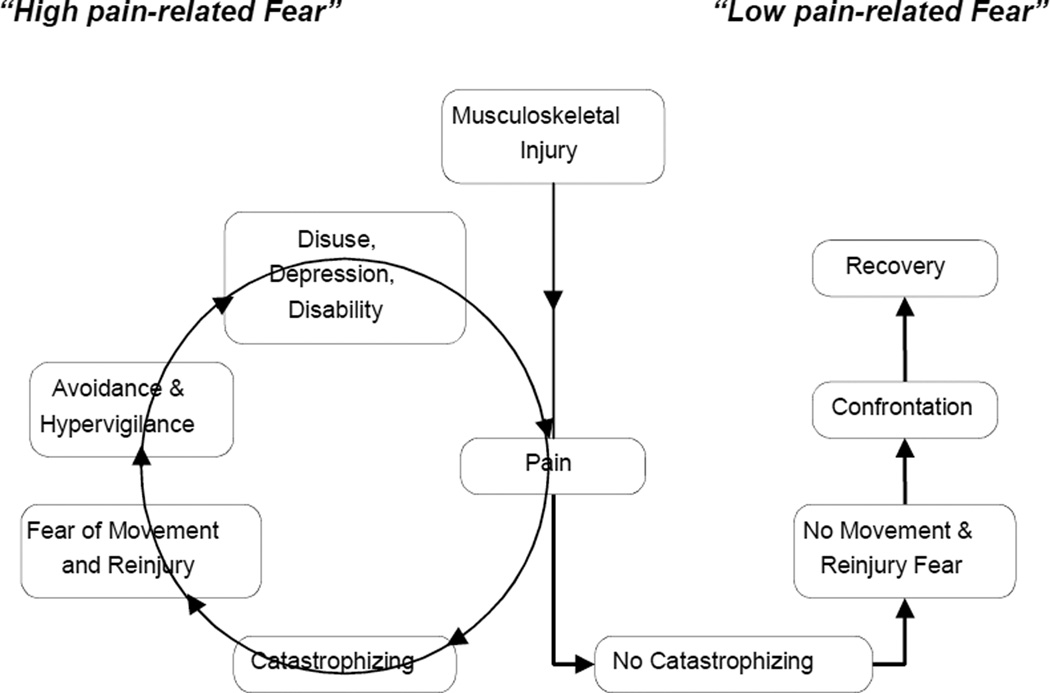
The fear-avoidance model of CLBP is a prominent cognitive-behavioral explanation for the development of chronic pain and disability following acute back injury.21, 57, 58 A fundamental assumption of this model is that individuals who experience acute low back pain will differ with respect to their pre-existing levels of pain-related fear. Those who have high fear are more likely to interpret pain as a sign of serious tissue damage, and consequently they tend to avoid a range of activities that may increase pain in an effort to prevent anticipated injury. More specifically, as shown in the middle and left side of Figure 1, individuals with high pain-related fear are prone to catastrophic thoughts in response to pain (e.g., “The pain will get worse!”), are more likely to experience greater fear of movement and injury as a result of these catastrophic cognitions, and ultimately will engage in behavioral adaptations to avoid or escape pain (e.g., excessively guarded movement patterns). In turn, avoidance behaviors are considered to reinforce the pain experience through physical deconditioning, increased irritability, frustration, and depression due to a loss of essential reinforcers, leading to greater disability. In contrast, as shown in the right side of Figure 1, individuals who are not prone to catastrophize in the face of pain have little fear of movement and injury and therefore are more likely to confront potential or actual pain-provoking situations that are needed to progress towards recovery (e.g., return to daily activities, work, rehabilitation). Consistent with the fear-avoidance model, pain-related fear and avoidance are among the strongest predictors of the transition from acute to CLBP.6, 13, 26, 32, 60 Whereas the fear-avoidance model posits a generic avoidance of all forms of movement that are perceived as threatening, we have repeatedly shown that individuals with high fear and low back pain specifically avoid flexion of the lumbar spine.45, 47, 48 Avoidance of lumbar spine motion may serve to increase subsequent risk for back injury as inactivity can contribute to shortening of peri-articular connective tissues and changes in the surrounding musculature.14, 15, 23 In turn, these changes may increase the risk of injury when the individual is exposed to common, unexpected environmental challenges such slipping on a wet floor.
Figure 1.
The Fear-avoidance model of chronic low back pain.
Applying Virtual Reality Gaming as a Novel Therapeutic Approach
Graded in vivo exposure is a traditional therapeutic approach to fear-related avoidance. In the context of CLBP, graded exposure therapy begins with patient education regarding the role of activity avoidance in perpetuating pain and disability.55 Patients are then acquainted with the therapeutic rationale for enhancing movement despite pain and are encouraged to develop an individualized hierarchy of avoided movements. Finally, patients are gradually exposed to each of the movements in their hierarchy, beginning with the least-feared and working toward the most-feared, with the goal being that direct experience will correct misperceptions that pain is inevitably associated with harm or damage to the back.55 Randomized controlled trials have demonstrated that graded exposure produces significant reductions in fear of movement and perceived harmfulness in CLBP sufferers.22, 61 Unfortunately, in these studies, the observed psychological changes were not accompanied by reductions in pain-related disability when compared to either graded activity (i.e., positive reinforcement of predefined functional activity quotas) or a wait-list control group. We hypothesize that failure to reduce disability can occur in graded exposure and graded activity interventions if patients can complete the prescribed tasks with restricted lumbar spine motion by simply increasing motion at the ankles, knees, and hips. As noted above, restriction of lumbar spine flexion may be a key contributor to continued disability as we have consistently demonstrated that pain-related fear is associated with restricted lumbar flexion among: 1) individuals with subacute LBP,45, 47 2) individuals with CLBP,50 3) asymptomatic individuals who have recently recovered from low back pain,48 and 4) healthy individuals with experimentally-induced low back pain.53 To address this issue, we designed a virtual dodgeball intervention for CLBP sufferers with fear of pain that focuses specifically on enhancing lumbar spine flexion. Although virtual reality gaming has been shown to reduce pain during burn debridement, wound dressings, painful activity, and uncomfortable medical procedures,12, 19 to date such interventions have not been adapted to address CLBP12, 19 nor have they taken advantage of virtual reality interface features such as full-body control of avatars and real time feedback/reinforcement of participant motion.
In this proof-of-concept study, the primary outcomes were changes in lumbar spine flexion and expectations of pain and harm during a standardized reaching task performed before and after three sessions of virtual dodgeball. The secondary outcomes were the changes in lumbar spine flexion as a function of changes in gain across days. Finally, we examined the feasibility and safety of a virtual dodgeball intervention for individuals with CLBP and pain-related fear.
METHODS
Participants
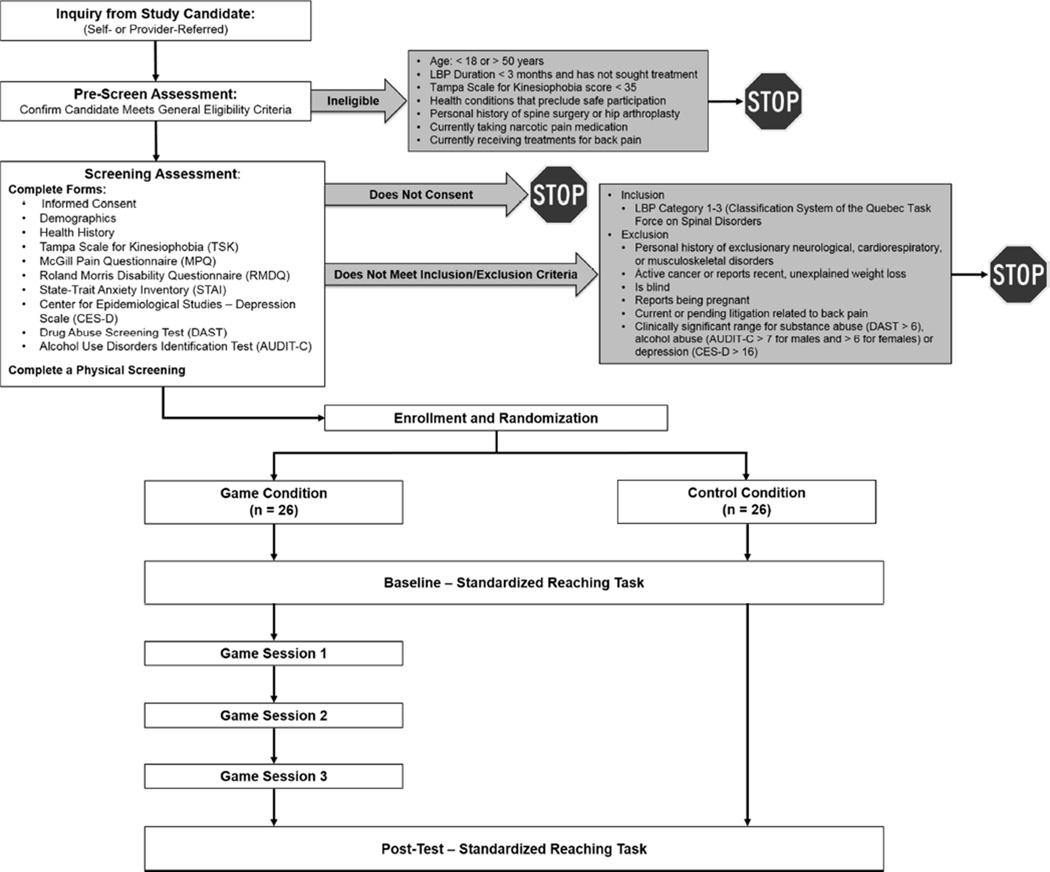
We recruited participants between the ages of 18–50 with CLBP (symptom duration > 3 months) and kinesiophobia (≥ 35 on the Tampa Scale for Kinesiophobia; TSK)57 who reported no health conditions that would restrict movement or preclude safe participation. The inclusion/exclusion criteria and the experimental flow chart are illustrated in Figure 2. In brief, individuals who met all of the inclusion/exclusion requirements were randomized into either the Control group or the Game group. Specifically, using randomizer.org, the statistician block randomized using 6 sets of 10 unique, randomly-generated numbers ranging from 1–10 (odd numbers = Control group; even numbers = Game group) to ensure a 1:1 allocation ratio and a final sample of 26 participants per group. The statistician then created individual, sealed envelopes for each unique participant number (i.e., ID01 to ID60), and a master log that identified the participants’ unique ID numbers and assigned groups was maintained. The study coordinator communicated with participants to schedule the screening examination. Those who met the study criteria and provided informed consent were then assigned to one of two parallel groups based on the contents of the sealed randomization envelope by the study coordinator. All participants completed two laboratory sessions separated by 4 days (± 1) in which they performed a series of reaches to targets located in the mid-sagittal plane using a modified version of our standardized reaching paradigm.42–44, 46, 49, 52 Participants in the Control group had no intervention between visits, while those in the Game group returned each day for three consecutive days to play virtual dodgeball. Neither the participants nor the individuals collecting data during gaming sessions could be masked with respect to group assignment. All outcome measures were completed on a tablet using online survey methods. The analyses were conducted after enrollment and testing was completed on all participants by the principal investigators. Participants received $25 at the end of the baseline testing session and an additional $50 at the end of the last testing session. In addition, those assigned to the game condition were able to receive additional cash bonuses based on their individual performance (see below).
Figure 2.
Experimental flow chart
Procedures
After completing the informed consent process, a medication use and medical history report was collected, followed by a physical screening to verify that the participant did not present with neurological signs or symptoms and met the criteria for classification as low back pain categories 1–3 using the Classification System of the Quebec Task Force on Spinal Disorders.39 The participant then completed a series of self-report instruments to further assess eligibility, including drug abuse (Drug Abuse Screening Test – DAST; eligible if score was ≤ 6),62 alcohol abuse (Alcohol Use Disorders Identification Test – AUDIT-C; eligible if score was ≤ 6 for females and ≤ 7 for males),37 and depression (Center for Epidemiological Studies - Depression – CES-D; eligible if score was ≤ 16).2, 35 If eligibility was confirmed, the participant completed a series of self-report instruments to assess disability (Roland-Morris Disability Questionnaire – RMDQ),36, 40 pain (McGill Pain Questionnaire – MPQ),30, 31 fear of movement (Tampa Scale of Kinesiophobia - TSK),56, 58 and anxiety (State-Trait Anxiety Inventory – STAI).27, 41 The participant was then randomized into the Game or Control group and was provided with black cotton shorts, shirt, and athletic shoes without any reflective materials to facilitate motion capture.
Instrumentation
Movement of light-reflective marker clusters attached to the head, upper arms, forearms, hands, trunk, pelvis, thighs, shanks, and feet were tracked using a 10 camera Vicon Bonita system sampled at 100Hz using TheMotion-Monitor software (Innovative Sports Training, Inc., Chicago, IL). This optoelectric-based kinematic system tracked the three-dimensional coordinates of light reflective marker clusters attached to the participant with a spatial resolution of 0.1 mm.
Standardized Reaching Test
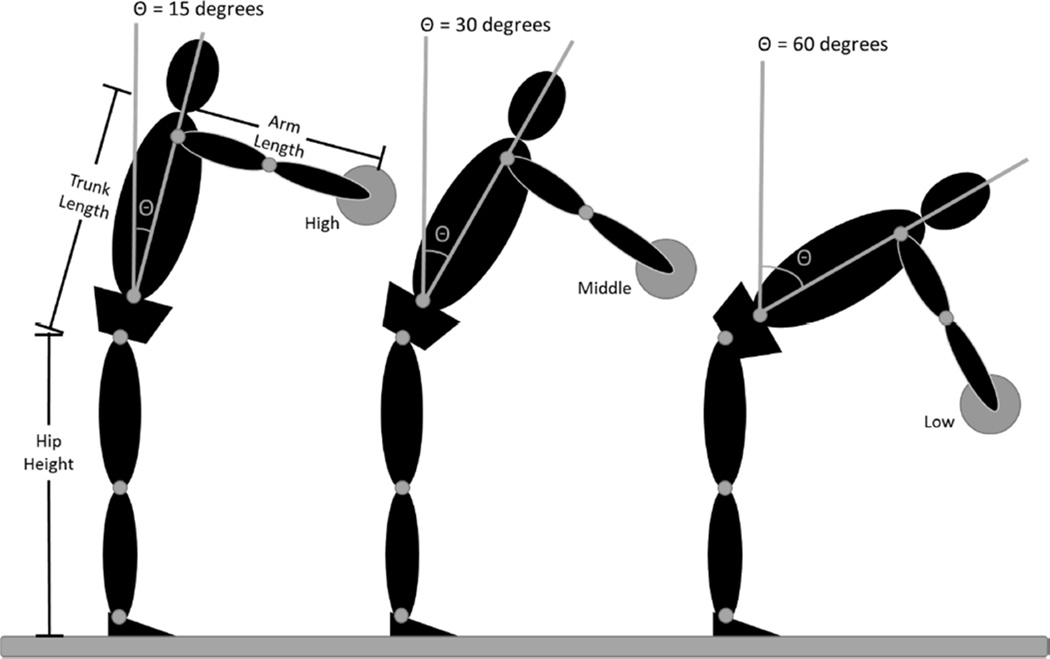
Participants reached to a static target at a comfortable speed holding a regulation dodge ball (24 cm diameter) in both hands. Participants performed five reaching trials to each target location in a set order (5 high, 5 middle, 5 low). They paused at the target for 2 seconds at target contact, and then returned to an upright posture. There was a rest time of approximately 30 seconds provided between each reaching trial. Target locations were determined for each subject based on their hip height, trunk length, and arm length (Figure 3). The high target was located such that the subject could, in theory, reach the target by flexing the hips 15° with shoulders flexed to 90° and the elbows extended. The middle and low targets could be reached by flexing the hips 30° and 60°, respectively. It should be noted that the target locations were determined mathematically and subjects were not actually placed in the positions illustrated in Figure 3. This standardized method allowed for several distinct advantages: 1) compared movement patterns across individuals, 2) challenged participants with tasks that required progressively more lumbar spine flexion, and 3) was sensitive to changes in CLBP patients.45, 47, 48, 50, 51
Figure 3.
Target locations are standardized based on an individual’s hip height, trunk length, and arm length such that the high target could be reached, with the shoulder flexed to 90 degrees and the elbow extended, simply by flexing the hips 15 degrees. The middle and low targets could, in theory, be reached by flexing the hips 30 and 60 degrees respectively. It should be noted that participants were not positioned as shown; rather this illustration shows how the target locations were normalized to each individual.
Participants were instructed to reach for the targets in a way that was “natural and comfortable for them” so as not to bias participants with a perceived correct way to move. Because forward excursions of the trunk require a counterbalanced backward movement of the lower extremities, the targets were located such that they did not require an individual to move anywhere near the limits of the available range of motion of the lumbar spine, pelvis, knee, and ankle. Thus, participants were able reach these targets using an infinite combination of joint excursions.
Primary Outcome - Pain and harm expectancy
Prior to each set of reaches, the participant rated their expectations of pain and harm on a 10 cm visual analog scale displayed digitally on a tablet. The scale consisted of a horizontal line with no numbers, marks, or descriptive vocabulary along its length. For expected pain ratings, the scale was anchored with the descriptors “No pain” and “Worst pain imaginable”, respectively, at each end of the line. For expected harm, the scale was anchored with “Not at all concerned” and “Extremely concerned” regarding potential harm to the back during task performance.
Primary Outcome – Lumbar spine flexion
The time series joint angle data were derived from the 3-D segment coordinate data using an Euler angle sequence of: 1) flexion-extension, 2) lateral bending, and 3) axial rotation28 using TheMotion-Monitor software. Joint excursions, defined as the change in joint angle from initial standing posture to target contact, were extracted using custom software written using Matlab (Mathworks™). The lumbar spine flexion excursion used to complete the reaching tasks was averaged across trials for the high, middle, and low targets. The lumbar spine flexion used to reach the high, middle, and low targets was then used to compute the lowest impact height for the launched virtual dodgeballs for levels 1–3 of gameplay, respectively.
Virtual Dodgeball Intervention
Participants assigned to the Game group returned to the lab on three days, separated by no more than 48 hours, for gameplay sessions. Each session was scheduled at approximately the same time of day, when possible. Prior to gameplay, medication use changes were recorded and the participants rated their current pain using the MPQ. Participants were instrumented as described above and positioned 1.5 m in front of a 60 inch high definition 3D-TV (Samsung 1080p 240Hz 3D Smart LED TV) wall mounted at approximately eye level. The participant wore Samsung 3D shutter glasses, which are required to produce the 3D effect with this TV.
The virtual dodgeball intervention, developed using Vizard software (WorldViz™), was displayed on the 3D-TV (see APPENDIX A: Electronic Supplementary Materials for a video of gameplay). The game environment was a basketball arena in which the participant played dodgeball against four virtual opponents. In the virtual environment, the participant’s avatar, viewed in the 3rd person perspective, was located on one free-throw line and the four opponents were located opposite the free throw line. The opposing players moved 3 m fore-aft and 3 m left-right in a random order during gameplay. Virtual balls were launched every 3.3 ± 0.3 seconds from one of four virtual opponents. The opponent that was about to launch a virtual ball turned green or red 300 ms prior to launch to alert the participant. If the opponent lit green and the launched ball was green, the participant had to attempt to block the ball with the ball held in their hand (co-located with the virtual ball held by the avatar). If the opponent lit red and the launched ball was orange, the participant had to attempt to duck to avoid the ball. There was a large scoreboard within the virtual gym that tracked participant performance and cash rewards earned during gameplay. Finally, 3D sound was incorporated into the game in a number of ways including crowd cheering, buzzers and referee whistles, and a duck quacking sound that occurred when ever an orange ball was launched.
Secondary Outcome – Lumbar spine flexion during gameplay
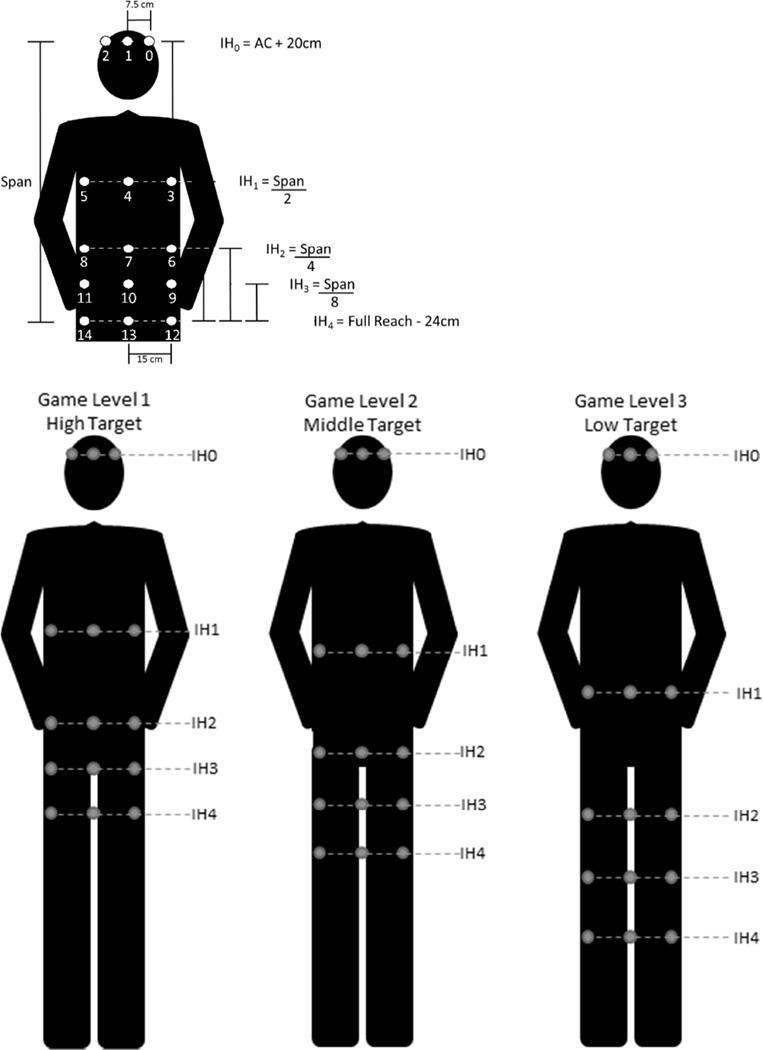
A round of gameplay consisted of a basic practice level to introduce the scoring metrics and 3 game levels, each lasting approximately 2 minutes. There were 2 sets of 15 launched balls within each game level. The intended impact locations of the 15 launched balls were distributed to 5 impact heights (IH) which were determined by the participant’s height and the amount of lumbar flexion they used during the baseline standardized reaching tasks. For example, during Level 1 of gameplay, the participant could successfully block the virtual ball launched to IH4 (i.e., the lowest impact height) simply by using the identical amount of lumbar flexion used in the standardized reaching task to the high target performed at baseline, whereas during Level 3 of gameplay, the participant could successfully block the virtual ball launched to IH4 (i.e., the lowest impact height) simply by using the identical amount of lumbar flexion used in the standardized reaching task to the low target performed at baseline. For each level of gameplay (i.e., levels 1–3), five impact heights of the launched virtual balls were scaled to impact from the height of participant’s eyes (IH0- highest impact) and approximately their shins (IH4- lowest impact) across the three levels of gameplay. Figure 4 (bottom) illustrates an example of the 5 impact heights for each of the 3 levels of gameplay in a single testing session. Three balls were launched at each IH to intersect the participant at their mid-sagittal plane, and 20 cm left or right of this plane. It is important to note that the trajectory of the virtual launched balls was permutated at each round of play (i.e., randomized to ensure 3 impacts at each of 5 target heights) to make the game exciting, challenging, and to some extent unpredictable. The participant was instructed to return to an upright posture between each launched virtual ball. Lumbar flexion was defined as the change in joint angle from initial posture prior to the launch of each ball to the maximal joint angle during the trial.
Figure 4.
The methods for computing location of the impact heights (IH0-IH4) of the launched virtual balls for a single game level (top). The lumbar spine flexion used to reach the high, middle, and low targets during the baseline standardized reaching tasks was used to compute the lowest impact height (IH4). The distribution of launched balls across the three levels of a single gameplay session (bottom).
After each set, the participant was presented with a static virtual ball and instructed to reach out and touch the ball with the ball held in their hands. The location of the virtual ball was co-located to individualized target locations used during the standardized reaching task performed in real-word at pre-treatment baseline such that the locations of the virtual ball during Levels 1, 2, and 3 were co-located to the real-world location of the high target, middle target, and low target, respectively.
Performance was updated in real-time and displayed on a virtual scoreboard in the basketball arena, and the participant was awarded progressively more cash rewards for each successful dodge or block at each level of play (Practice Level = 1¢, Level 1=2¢, Level 2=5¢, Level 3=10¢). Successful contact for each highlighted ball presented between each set resulted in a bonus 25¢ reward. Conversely, the participant lost cash rewards for each failure to block or duck the launched ball. Each player started the game with a cash balance on the scoreboard such that if they failed on every launched or presented ball, their cash balance would be zero. In fact, participants earned an average of $8.42 (SD = 1.36) at each gameplay session, which as paid to them at the end of session 5. The average gameplay session lasted approximately 15 minutes.
Across the three days of gameplay, the impact heights of the launched balls for the 3 levels of gameplay were adjusted such that participants needed to use progressively larger excursions of the lumbar spine to make contact with the launched balls. Specifically, for each level of gameplay the IH of the launched balls was adjusted by 5% during the second game session (gain = 1.05) and 10% during the third game session (gain = 1.10). Thus, in theory, the participant needed to use an additional 5 and 10% of lumbar spine motion, respectively, to successfully block and dodge the virtual balls as compared to game session one.
Feasibility and safety
Following game session three, the participants rated their overall impressions of the game using a survey that was adapted from an existing measure of online health game acceptability.38 Specifically, the Game Experience Survey asked the participants to provide 1 (strongly disagree) to 5 (strongly agree) ratings of their experience along 14 dimensions (e.g., the game was fun; playing the game made me worry about injuring my back; I would recommend the game to other people with back pain). In addition, they were invited to provide written responses to four open-ended questions, including “What did you like most about the game?”, “What did you like least about the game?”, “What would make the game better?”, and “What would make the game easier to learn?” Finally, all participants returned on session five to repeat the standardized reaching task, provide information on current pain medication use, and to complete RMDQ and MPQ measures.
All procedures were reviewed and approved by the Ohio University Institutional Review Board (#13F035). Data were collected in the Motor Control Lab at Ohio University, Athens, Ohio. This study was registered with ClinicalTrials.gov (NCT02301741) prior to data collection. There were no changes to the study design or trial outcomes once enrollment began.
Sample Size
Prior studies that have used high tech visual distraction in a gaming environment for the treatment of pain have shown effect sizes ranging from d=1.00 to 1.98.8, 16, 33 More recently, performance of a competitive computerized task for small monetary incentives has been shown to significantly reduce fear of pain and pain-related avoidance behavior (d = 0.66).54 Using the most conservative of these effect sizes (i.e., d=0.66), G*Power9 was used to conduct a priori power analyses for our primary outcomes. Results indicated that 52 participants (26 per group) would be required to ensure power=.80 with alpha=.05 and a correlation of 0.50 among repeated measures. Because there are no relevant prior studies of gain manipulation, estimating a medium effect size (f=0.3) for the secondary outcome, a total of 21 participants would be required for a repeated measures ANOVA across the three game sessions with alpha=.05, power=.80, and a correlation of 0.50 among repeated measures. In sum, a minimum of 52 participants were needed to meet the power requirements for the proposed outcomes.
Statistical Analyses
To examine the primary outcomes of the effect of virtual dodgeball on lumbar spine flexion and pain/harm expectancies during the standardized reaching task, a series of 2 Group (Game, Control) by 2 Day (Baseline, Post-Test) ANOVAs were conducted. To examine the secondary outcome of the effects of virtual dodgeball on lumbar spine flexion, a 3 Day x 3 Game Level x 5 Impact Height repeated measures ANOVA was conducted. To examine group differences in pain and disability ratings from baseline to post-test, 2 Time (Session 1, Session 5) x 2 Group (Game, Control) repeated measures ANOVAs were conducted on McGill Pain Questionnaire and Roland-Morris Disability Questionnaire scores. To examine day-to-day changes in back pain ratings among those who played the game, repeated measures ANOVAs of McGill Pain Questionnaire subscale scores (Visual Analog Scale rating, Present Pain Intensity, and Pain Rating Index) were conducted across the five study visits.
RESULTS
Participant Recruitment and Characteristics
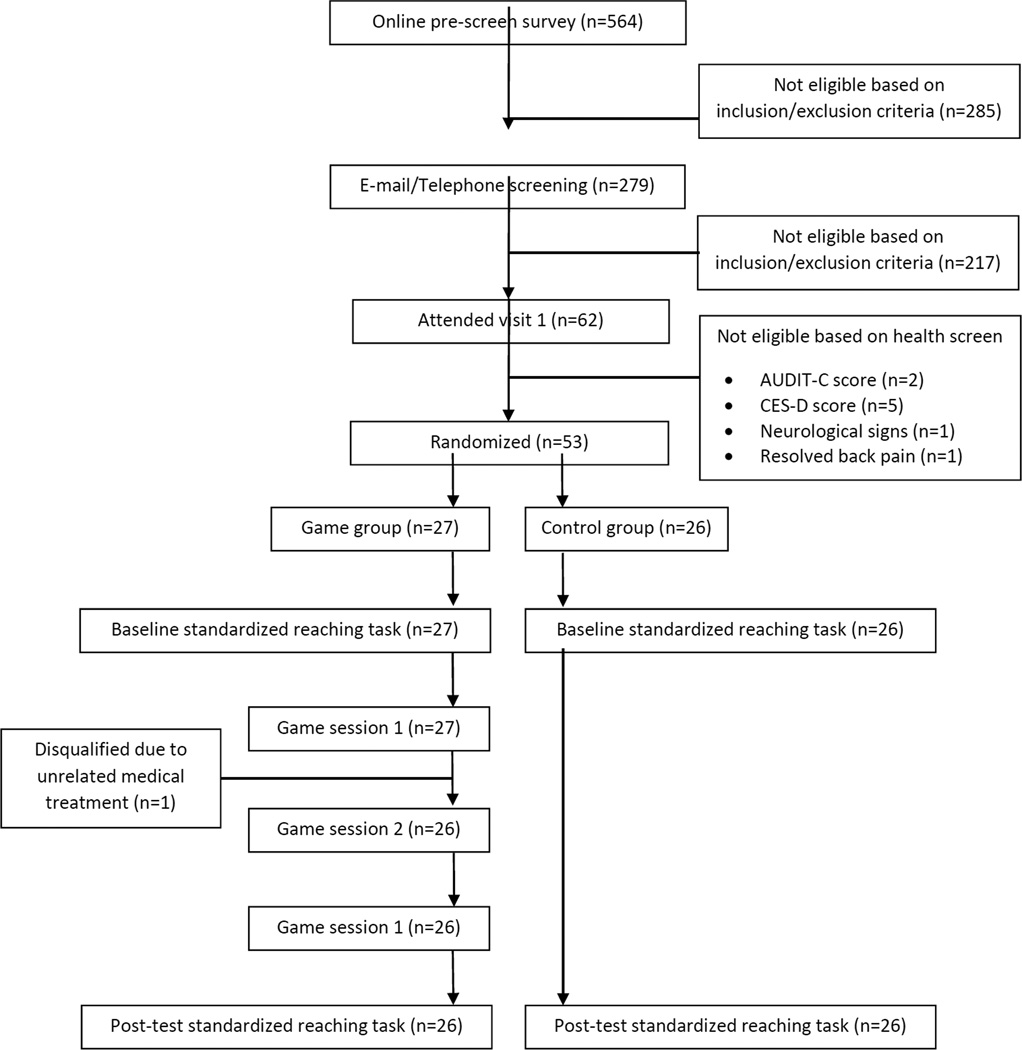
As shown in Figure 5, a total of 564 individuals completed the online screening survey to assess their eligibility for the study based on general inclusion and exclusion criteria (e.g., age, back pain duration, Tampa Scale for Kinesiophobia, etc.). Of these, 285 received an immediate reply that they were ineligible and 279 received follow-up emails to confirm eligibility and receive additional information about the study. An additional 217 were deemed ineligible based on the follow-up, and 62 continued to meet eligibility and were scheduled for an appointment to complete an in-person health screening. An additional 9 were then excluded due to AUDIT-C score (n=2), CES-D score (n=5), neurological signs/symptoms (n=1), and resolved back pain symptoms (n=1). The remaining 53 individuals were randomly assigned to either the Game group (n=27) or the Control group (n=26). Only one participant, who was assigned to the Game group, failed to complete the remainder of their assigned sessions. This participant was dismissed from the study after reporting medical deemed unrelated to study participation by the IRB. Participants in this study were recruited and tested from January 2015 to March 2016, at which point the trial ended.
Figure 5.
Participant flow chart.
Table 1 provides the participant characteristics for the final sample of 52 (26/group) who completed the study. As shown in the table, there were no significant differences between the groups (all p>0.10) with respect to sex, race, ethnicity, height, weight, age, or baseline measures of kinesiophobia, depression, disability, or pain.
Table 1.
Characteristics of the final sample, expressed as percentages or means (standard deviations).
| Game | Control | p | |
|---|---|---|---|
| N | 26 | 26 | |
| Sex (% female) | 46.2 | 50.0 | 0.78 |
| Race | |||
| • % White | 80.8 | 88.5 | |
| • % Black or African American | 15.4 | 7.7 | 0.68 |
| • % More than one race | 3.8 | 3.8 | |
| Ethnicity (% Hispanic or Latino) | 3.8 | 15.4 | 0.35 |
| Height (m) | 1.77 (0.1) | 1.78 (0.1) | 0.64 |
| Weight (kg) | 75.6 (17.8) | 79.7 (17.9) | 0.41 |
| Age (years) | 23.9 (6.8) | 26.7 (8.5) | 0.18 |
| Tampa Scale for Kinesiophobia | 38.9 (4.1) | 39.3 (4.6) | 0.75 |
| Center for Epidemiologic Studies - Depression | 5.4 (4.2) | 4.4 (3.8) | 0.38 |
| Roland-Morris Disability Questionnaire | 4.8 (3.0) | 5.3 (3.9) | 0.58 |
| McGill Pain Questionnaire | |||
| • Visual Analog Scale (0–100) | 21.1 (10.3) | 25.2 (16.7) | 0.29 |
| • Present Pain Intensity | 2.69 (0.55) | 2.62 (0.75) | 0.67 |
| • Pain Rating Index | 20.1 (2.2) | 21.5 (6.20 | 0.28 |
Primary Outcome Measures
Results of the 2 Group (Game, Control) by 2 Day (Baseline, Post-Test) of lumbar spine flexion revealed no significant effects of Group, F(1,50)=1.96, p=0.16, Day, F(1,50)=2.24, p=0.14, or Group by Day, F(1,50)=0.21, p=0.64. The analysis of expected pain revealed a significant effect of Day, F(1,50)=11.91, p=0.001, ηp2 = 0.19, which reflected a decrease in pain expectations during the post-test standardized reaches as compared to baseline. However, there were no significant effects of Group, F(1,50)=1.25, p=0.26, or Group by Day, F(1,50)=0.16, p=0.68. Finally, the analysis of expected harm revealed no significant effects of Group, F(1,50)=0.01, p=0.95, Day, F(1,50)=0.03, p=0.85, or Group by Day, F(1,50)=2.38, p=0.12.
Secondary Outcome Measures
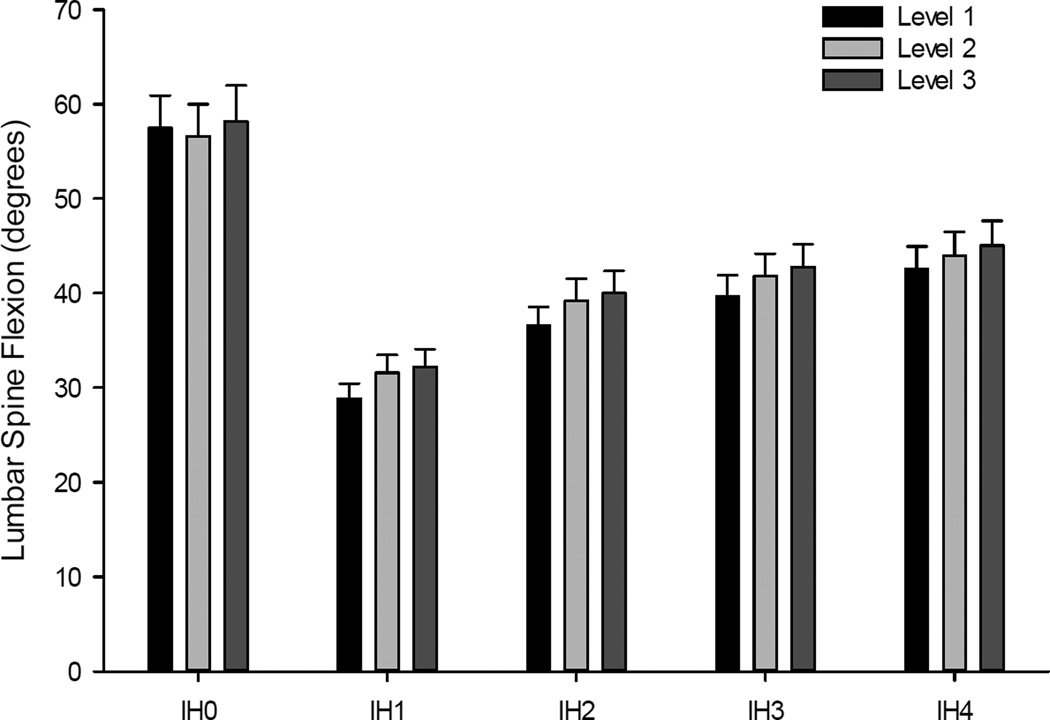
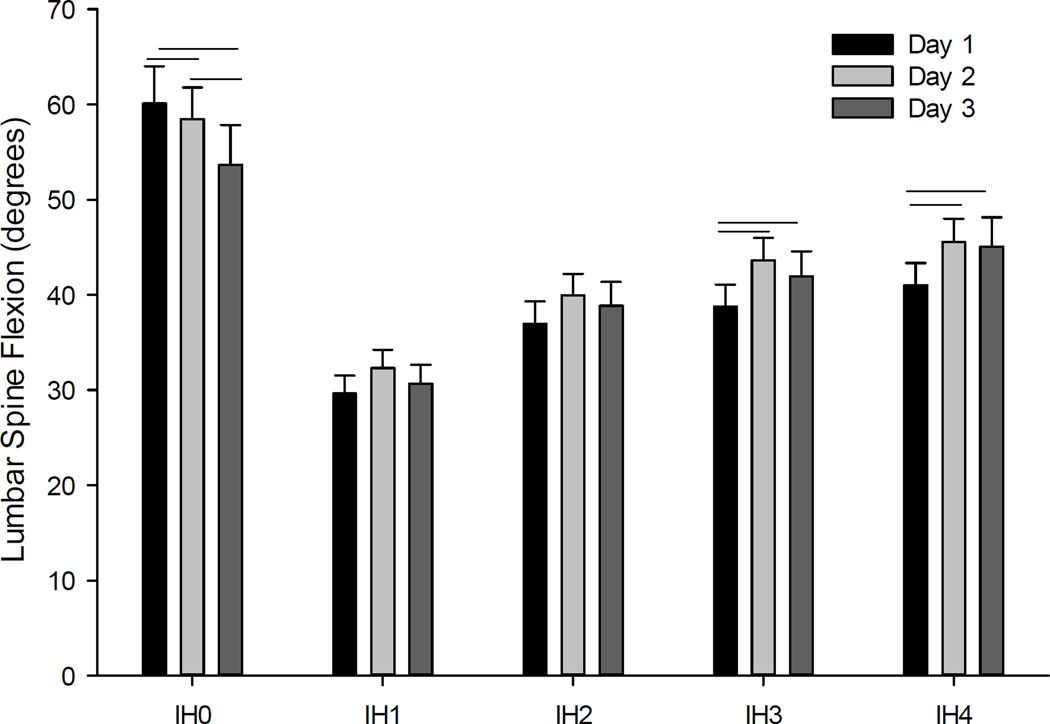
To examine the effects of gameplay on lumbar spine flexion, we conducted a 3 Day x 3 Game Level x 5 Impact Height repeated measures ANOVA. Results revealed significant main effects of Game Level, F (2,22) = 6.54, p<0.01, ηp2 = 0.37, and Impact Height, F (4,20) = 33.3, p<0.001, ηp2 = 0.87, but not Day, F (2,22) = 1.45, p=0.25. The only significant interaction was between Day and Impact Height, F (8,16) = 7.56, p<0.001, ηp2 = 0.79. As illustrated in Figure 6, follow-up analyses of the game level effect revealed that lumbar flexion significantly increased between each level of gameplay. With respect to Impact Height, lumbar flexion was greatest for IH0 (i.e., balls thrown at the participants head which required them to duck instead of block) and progressively increased from IH1-IH4. Follow-up analyses of the Day by Impact Height interaction (See Figure 7), reveal a significant decrease in lumbar spine flexion (p<0.05) across gameplay days for IH0 (p<0.05), but a significant increase in lumbar spine flexion for IH3 (p<0.05) and IH4 (p<0.05). However, there was no effect of Day on lumbar spine flexion for IH1 and IH2.
Figure 6.
The effects of game level at each impact height on lumbar spine flexion is illustrated. While there was no effect of game level on spine flexion for Impact Height 0 (IH0), there were significant increases in spine flexion as a function of game level for Impact Heights 1 through 4 (IH1 to IH4).
Figure 7.
The interaction of days of gameplay by impact height (IH) of the launched virtual balls. Horizontal lines indicate significant pairwise differences in lumbar spine flexion (p<0.05).
Feasibility
The current study was designed to provide initial indicators of feasibility in the form of perceived acceptability and demand for participation among individuals with low back pain and pain-related fear.
Game Experience Survey - Ratings
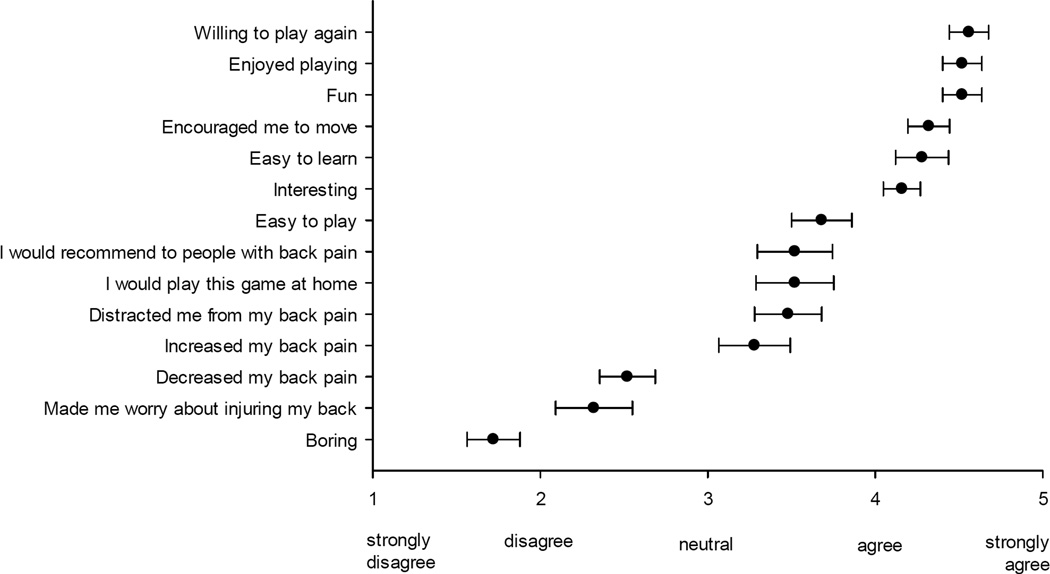
As shown in Figure 8, participant responses on the Game Experience Survey revealed the strongest agreement ratings for 6 of the 14 items, including “I would be willing to play the game again” (4.56), “I enjoyed playing the game” (4.52), “The game was fun” (4.52), “The game encouraged me to move” (4.32), “The game was easy to learn” (4.28), and “The game was interesting” (4.16). Other items that were rated positively, but with less strong agreement (i.e., above “neutral” but below “agree”), included “The game was easy to play” (3.68), “I would recommend the game to other people with back pain” (3.52), “If it was available, I would play this game at home” (3.52), “The game distracted me from my back pain” (3.48), and “Playing the game increased my back pain” (3.28). Lastly, items that were rated in direction of non-agreement included “The game decreased my back pain” (2.52), “The game made me worry about injuring my back” (2.32), and “The game was boring” (1.72). On the whole, these ratings suggest a high level of acceptability for the game with strong ratings of enjoyment combined with a willingness to continue to play the game and to recommend it to others with back pain. Participants reported slight agreement with the notion that playing the game increased their back pain, but at the same time they also noted that it distracted them from their pain during gameplay and disagreed with the notion that playing the game made them worry about injuring their back.
Figure 8.
Participant ratings of gameplay experience.
Game Experience Survey – Written comments
After completing the 14 rating items, participants were asked to provide written responses to four open-ended questions regarding what they liked about the game, what they disliked about the game, what might improve the game, and what might be done to make the game easier to learn. As noted in Table 2, with respect to things that were liked, several participants indicated an appreciation for the game’s ability to engage them in an interactive experience that was not overly challenging, even for non-gamers, and that succeeded in distracting them from their back pain. With respect to aspects of the game that participants did not like, several respondents noted that the game increased their pain or discomfort when they had to reach for lower targets. A number of valuable suggestions were offered to improve the game, and these mostly focused on enhancing variety in the game itself (e.g., different avatars, more color options, different game settings, and greater motion). Finally, most participants indicated that the game was easy to learn as is, but a few indicated that adding an option for more practice, and perhaps more varied practice, could make the game easier to learn.
Table 2.
Participant open-ended response to the Game Experience Survey.
| Question | Sample Response 1 | Sample Response 2 | Sample Response 3 |
|---|---|---|---|
|
What did you like most about the game? |
“It was a fun way to engage my back in exercise and I was most focused on the game than the pain in my body.” |
“The levels increased in difficulty, not too much, but enough to make each level more challenging.” |
“The game was fun and interactive. I do not like video games or anything related to them, but I enjoyed playing this game.” |
|
What did you like least about the game? |
“I didn’t like how low the balls went, but that was only because of my back.” |
“When it threw balls at my feet I had to bend lower, that was not cool. It hurt my back the most.” |
“The bending over to hit the extra points ball. That caused minor pain in my back and seemed difficult for someone with back pain.” |
|
What would make the game better? |
Have a variety of avatars, be able to have a wider range of motion (move around more) |
Possibility of different colors. If someone is red/green color deficient, they would not be able to differentiate between the body throws and head throws aside from sound. |
Having different arenas, more talking from the game would make it feel more interactive |
|
What would make the game easier to learn? |
Practice rounds that incorporate different ball speeds and target ranges (high and low targets) |
I think getting more practice would make it easier to learn. |
Nothing, the practice round did its job and helped to be able to know what to expect. |
With the exception of one participant who was dismissed from the study due to an unrelated medical treatment, all participants completed all three gameplay sessions (i.e., 100% retention). Responses from the Game Experience Survey also support a high level of demand for the game, as indicated by agreement with the question “If it was available, I would play this game at home” (3.52).
Safety
Measures obtained during virtual dodgeball sessions
For those assigned to the game group, measures of participant safety included repeated assessments of pain and medication use at each game session as well as a monitoring of participant attendance rates, study withdrawal, and experience of adverse events.
McGill Pain Questionnaire
ANOVAs of McGill Pain Questionnaire subscale scores across the five study visits revealed significant main effects for the Visual Analog Scale rating, F (4,20) = 6.91, p<0.01, ηp2 = 0.58, and Present Pain Intensity, F (4,20) = 3.61, p<0.05, ηp2 = 0.42, but not for the overall Pain Rating Index, F (4,20) = 1.36, p=0.28, ηp2 = 0.21. Follow-up analyses for both of the significant effects revealed significantly reductions in Visual Analog Scores (M = - 6.7, SD = 7.1) and Present Pain Intensity (M = −0.4, SD = 0.5) on the final study visit relative to the first study visit.
Pain Medication
Five participants in the game group (5/26 = 19.2%) reported taking pain medication at some point during the study. Two participants reported taking medication for back pain at baseline and one reported taking pain medication for something other than back pain. All three of these participants continued to report the same pain medication use at each of the following testing sessions. The other two participants reported taking pain medication at only one of the five study sessions; one reported taking medication for back pain during their second visit (i.e., before playing the game) and the other reported taking pain medication at the final visit, but for something other than back pain.
Adherence and Adverse Events
With the exception of the one person who was dismissed, all participants in the game group completed all three sessions of gameplay, none withdrew from the study, and there were no reported adverse events related to gameplay.
Comparisons between game and control groups
Participants in the game and control groups were compared at sessions 1 and 5 to determine if significant differences in pain, physical function, or medication use emerged as a function of engaging in three session of gameplay.
McGill Pain Questionnaire
The 2 Time (Session 1, Session 5) x 2 Group (Game, Control) repeated measures ANOVAs revealed significant main effects of Time for the Visual Analog Scale, F (1,50) = 24.82, p<0.001, ηp2 = 0.33, and Present Pain Intensity scale, F (1,50) = 10.30, p<0.01, ηp2 = 0.17, and a marginal effect for the overall Pain Rating Index, F (1,50) = 3.74, p=0.06, ηp2 = 0.07. In each case, this reflected lower average pain scores on the final study visit relative to the first study visit, and the absence of any significant Time by Group interactions indicates that these changes did not differ as a function of group.
Roland-Morris Disability Questionnaire
The 2 Time (Session 1, Session 5) x 2 Group (Game, Control) repeated measures ANOVA revealed no significant Time or Group x Time effects. Hence, participants did not report a change in physical disability scores over time in either group.
Pain Medication
One participant in the Control group (1/26 = 3.8%) and two participants in the Game group (2/26 = 7.7%) reported taking medication for back pain at the initial testing session. In each case they also reported taking the same medication at the last testing session.
DISCUSSION
The goal of the virtual dodgeball intervention was to promote graded increases in lumbar spine flexion, with the expectation that repeated exposure to gameplay without adverse events would reduce fear of harm upon movement and encourage generalization of increased spinal motion outside of gameplay. With respect to the primary outcomes, we did not observe any significant effects of group on changes in lumbar spine flexion, expected pain, or expected harm during the standardized reaching test. We found a significant reduction in expected pain ratings from baseline to post-test reaching, but this effect was similar in both groups. These findings indicate that very brief exposure to this intervention (i.e., only three 15 minute sessions) in participants with chronic back pain does not translate to significant changes outside the game environment. This is not surprising given that graded exposure therapy for fear of movement among individuals with low back pain typically last 8–12 sessions.4, 22, 24, 61
Although the virtual dodgeball intervention did not elicit significant group differences in lumbar flexion during post game testing, the data clearly indicate that individuals with chronic low back pain and high fear can be encouraged to increase lumbar spine flexion within gameplay sessions. Specifically, among those who played the game, lumbar flexion was significantly increased by manipulation of the impact height of the launched virtual balls, and this effect was observed both within each game (Figure 6) and across game days (Figure 7). These data provide strong evidence that by manipulating impact heights of the launched balls, we can finely tune spine flexion within gameplay. Specifically, as shown in Figure 6, discrete changes in impact height led to graduated changes in lumbar spine flexion. This implies that in future applications of this intervention we can vary impact height as a continuous variable in order to customize the game to each player’s initial and enhanced spinal flexion performance over time. It is worth noting that launched virtual balls that necessitated the participant to duck to successfully score points resulted in the greatest amount of lumbar spine flexion (See IH0 in Figures 7 & 8). However, for this impact location only participants reduced lumbar flexion across days, presumably because they are learning to calibrate how much movement is required to achieve a successful duck. Nonetheless, it is interesting that for IH0 participants chose a movement strategy that involved significant spine flexion when they could have achieved the same goal by flexing the ankle, knees, and hips (i.e., by squatting) while avoiding spine flexion. It may be that in the context of the virtual environment participants default to engrained movement patterns learned before the onset of CLBP. This serendipitous finding suggests an important factor that can be used to enhance spinal motion in future iterations of this intervention.
With respect to feasibility, results of the game experience survey clearly demonstrate that participants found the game to be engaging, easy to learn, and would recommend it others with low back pain. An important theme emerged from a combined examination of participant ratings and written comments on the game experience survey. On the whole, participants indicated slight agreement with the notion that gameplay increased their back pain and their written comments suggested that this concern was most prominent for the lowest targets (e.g., “When it threw balls at my feet I had to bend lower, that was not cool. It hurt my back the most.”) At the same time, participants tended to agree with the notion that the game distracted them from their back pain and to disagree with the idea that the game made them worry about injuring their back. Combined with the fact that participants voluntarily completed all three gameplay sessions, these responses indicate that even though virtual dodgeball may have increased low back pain for some participants, they were nonetheless willing to repeatedly flex their lumbar spine as part of the game. This clearly indicates the potential to get participants with CLBP to actively engage in movements that may otherwise be avoided due to fear of harm. Indeed, it is noteworthy that the highest average rating on the game experience survey was for participant willingness to play the game again (i.e., Mean = 4.6; SD = 0.6 on a 1”strongly disagree” to 5 “strongly agree” scale). When viewed from the perspective of the fear-avoidance model of CLBP,21, 57, 58 these findings suggest that the game encourages participants with high fear to alter their tendency to avoid spine flexion and instead to repeatedly engage in such movements to successfully navigate the game despite possible pain. In individuals with experimental, acute, sub-acute, or CLBP, we have previously demonstrated that high fear of movement is associated with reduced lumbar spine flexion.45, 47, 48, 53 By specifically focusing on the reduction of avoidance of lumbar spine flexion, virtual dodgeball addresses a key limitation of existing graded exposure and graded activity therapies. That is, existing approaches encourage greater movement but they do not compel patients to complete the prescribed tasks by increasing lumbar spine flexion (i.e., patients may adopt alternative strategies that involve greater motion of the ankles, knees, and hips to achieve target behavior).
We developed a virtual dodgeball intervention that is engaging, but also potentially challenging to play for individuals with low back pain as it necessitates 90 repetitions of lumbar flexion ranging from approximately 25 to 60 degrees. As a result, it is critical to demonstrate that the game is safe to play. Although additional testing in larger samples will be required, in the present study, there were no adverse events and participants who played the game reported no changes in disability, no changes in medication use, and a decrease in pain from baseline to post-gameplay. These findings are highly encouraging in that a concentrated exposure to three consecutive days of repetitive spinal flexion, which is often avoided in this cohort, did not exacerbate existing back pain symptoms on the McGill Pain Questionnaire. Further, the observed decrease in pain ratings suggests that the intervention does not promote delayed onset muscle soreness, which can follow performance of intense or novel motor tasks even among healthy individuals, possibly because it requires both concentric and eccentric muscle contractions.29
As in all studies, the current study is not without its limitations and the most obvious is perhaps the fact that participants were only permitted to play the game on three occasions. Most graded exposure therapies include at least 8 separate sessions; however, in the present case it was critical to first establish feasibility and safety in a smaller exposure dose. Given our findings, we believe that this intervention is well-suited to a Phase II Clinical Trial. Another limitation was that the game was played using a semi-immersive virtual environment (i.e., 3D-TV). We have recently demonstrated that a fully immersive environment with a head mounted display (i.e., Oculus Rift) provides greater distraction, ever stronger positive evaluations of the game, and even larger changes in lumbar flexion as compared to gameplay on a 3D-TV.3 Finally, based on the data obtained from participant feedback and game performance in this initial experiment, we plan to further refine the virtual dodgeball intervention to incorporate a variety of virtual environments, to permit a choice of player avatars, and to allow players to change their location within the virtual environment.
In sum, the results of this proof-of-concept study demonstrate that virtual dodgeball is safe, feasible, and capable of shaping changes in lumbar spine flexion during gameplay. We believe that these findings provide support for a clinical trial wherein the treatment dose is more consistent with traditional graded-exposure approaches to CLBP.
Perspective.
This study of a virtual reality dodgeball intervention provides evidence of feasibility, safety and utility to encourage lumbar spine flexion among individuals with chronic low back pain and high fear of movement.
Highlights.
Virtual dodgeball intervention designed to elicit increased lumbar spine flexion.
Virtual dodgeball increased lumbar flexion within and across gameplay sessions.
Demonstrated safety and feasibility of novel intervention for back pain.
Brief intervention did not translate to movements outside virtual environment.
Provides support for a clinical trial with longer intervention period.
Acknowledgments
Trial Registration: This study was registered with ClinicalTrials.gov (NCT02301741).
APPENDIX A.
Electronic Supplementary Materials for a video of gameplay A video link of a healthy actor playing the virtual dodgeball game. https://youtu.be/We0BrUeYrxo
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: JST, CRF, MEA, STL, and SW have read and approved the final manuscript and certify that they have no conflicts of interest or financial involvement with this manuscript. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21AR064430. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Andersson GBJ. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 3.Applegate ME, Leitkam LT, Cost J, Proctor R, France CR, Thomas JS. Program No. 282.06 Neuroscience Meeting Planner. Society for Neuroscience. Washington, DC: Online., Chicago IL: 2015. Effects of avatar presentation and display environment on game perception and lumbar motion in virtual dodgeball. [Google Scholar]
- 4.Boersma K, Linton S, Overmeer T, Jansson M, Vlaeyen J, de Jong J. Lowering fear-avoidance and enhancing function through exposure in vivo. A multiple baseline study across six patients with back pain. Pain. 2004;108:8–16. doi: 10.1016/j.pain.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Carey TS, Garrett JM, Jackman AM. Beyond the good prognosis Examination of an inception cohort of patients with chronic low back pain. Spine (Phila Pa 1976) 2000;25:115–120. doi: 10.1097/00007632-200001010-00019. [DOI] [PubMed] [Google Scholar]
- 6.Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? Jama. 2010;303:1295–1302. doi: 10.1001/jama.2010.344. [DOI] [PubMed] [Google Scholar]
- 7.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Dahlquist LM, McKenna KD, Jones KK, Dillinger L, Weiss KE, Ackerman CS. Active and passive distraction using a head-mounted display helmet: effects on cold pressor pain in children. Health Psychol. 2007;26:794–801. doi: 10.1037/0278-6133.26.6.794. [DOI] [PubMed] [Google Scholar]
- 9.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 10.Foster NE. Barriers and progress in the treatment of low back pain. BMC Med. 2011;9:108. doi: 10.1186/1741-7015-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, Castel LD, Kalsbeek WD, Carey TS. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrett B, Taverner T, Masinde W, Gromala D, Shaw C, Negraeff M. A rapid evidence assessment of immersive virtual reality as an adjunct therapy in acute pain management in clinical practice. Clin J Pain. 2014;30:1089–1098. doi: 10.1097/AJP.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 13.George SZ, Beneciuk JM. Psychological predictors of recovery from low back pain: a prospective study. BMC Musculoskelet Disord. 2015;16:49. doi: 10.1186/s12891-015-0509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hides JA, Richardson CA, Jull GA. Magnetic resonance imaging and ultrasonography of the lumbar multifidus muscle Comparison of two different modalities. Spine (Phila Pa 1976) 1995;20:54–58. doi: 10.1097/00007632-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine (Phila Pa 1976) 1996;21:2763–2769. doi: 10.1097/00007632-199612010-00011. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman HG, Seibel EJ, Richards TL, Furness TA, Patterson DR, Sharar SR. Virtual reality helmet display quality influences the magnitude of virtual reality analgesia. J Pain. 2006;7:843–850. doi: 10.1016/j.jpain.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Hoy D, Brooks P, Blyth F, Buchbinder R. The Epidemiology of low back pain. Best Pract Res Clin Rheumatol. 2010;24:769–781. doi: 10.1016/j.berh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): The National Academies Press; 2011. [PubMed] [Google Scholar]
- 19.Keefe FJ, Huling DA, Coggins MJ, Keefe DF, Zachary Rosenthal M, Herr NR, Hoffman HG. Virtual reality for persistent pain: a new direction for behavioral pain management. Pain. 2012;153:2163–2166. doi: 10.1016/j.pain.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klenerman L, Slade PD, Stanley IM, Pennie B, Reilly JP, Atchison LE, Troup JD, Rose MJ. The prediction of chronicity in patients with an acute attack of low back pain in a general practice setting. Spine (Phila Pa 1976) 1995;20:478–484. doi: 10.1097/00007632-199502001-00012. [DOI] [PubMed] [Google Scholar]
- 21.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 22.Leeuw M, Goossens ME, van Breukelen GJ, de Jong JR, Heuts PH, Smeets RJ, Koke AJ, Vlaeyen JW. Exposure in vivo versus operant graded activity in chronic low back pain patients: results of a randomized controlled trial. Pain. 2008;138:192–207. doi: 10.1016/j.pain.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Lieber RL. Skeletal muscle structure, function, and plasticity: The physiological basis for rehabilitation. 2nd. Baltimore: Lippincott, Williams &Wilkins; 2002. [Google Scholar]
- 24.Linton SJ, Boersma K, Jansson M, Overmeer T, Lindblom K, Vlaeyen JW. A randomized controlled trial of exposure in vivo for patients with spinal pain reporting fear of work-related activities. Eur J Pain. 2008;12:722–730. doi: 10.1016/j.ejpain.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening trends in the management and treatment of back pain. JAMA Intern Med. 2013;173:1573–1581. doi: 10.1001/jamainternmed.2013.8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Main CJ, George SZ. Psychologically informed practice for management of low back pain: future directions in practice and research. Phys Ther. 2011;91:820–824. doi: 10.2522/ptj.20110060. [DOI] [PubMed] [Google Scholar]
- 27.McCracken LM, Gross RT, Sorg PJ, Edmands TA. Prediction of pain in patients with chronic low back pain: effects of inaccurate prediction and pain-related anxiety. Behav Res Ther. 1993;31:647–652. doi: 10.1016/0005-7967(93)90117-d. [DOI] [PubMed] [Google Scholar]
- 28.McGill SM, Cholewicki J, Peach JP. Methodological considerations for using inductive sensors (3SPACE ISOTRAK) to monitor 3-D orthopaedic joint motion. Clin Biomech (Bristol, Avon) 1997;12:190–194. doi: 10.1016/s0268-0033(97)00063-6. [DOI] [PubMed] [Google Scholar]
- 29.McHugh MP, Connolly DA, Eston RG, Gleim GW. Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Med. 1999;27:157–170. doi: 10.2165/00007256-199927030-00002. [DOI] [PubMed] [Google Scholar]
- 30.Melzack R. The McGill pain questionnaire: Major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 31.Melzack R, Katz J. The McGill Pain Questionnaire: Appraisal and current status. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. New York: Guilford Press; 2001. [Google Scholar]
- 32.Nicholas MK, Linton SJ, Watson PJ, Main CJ Decade of the Flags” Working G. Early identification and management of psychological risk factors (“yellow flags”) in patients with low back pain: a reappraisal. Phys Ther. 2011;91:737–753. doi: 10.2522/ptj.20100224. [DOI] [PubMed] [Google Scholar]
- 33.Patterson DR, Hoffman HG, Palacios AG, Jensen MJ. Analgesic effects of posthypnotic suggestions and virtual reality distraction on thermal pain. J Abnorm Psychol. 2006;115:834–841. doi: 10.1037/0021-843X.115.4.834. [DOI] [PubMed] [Google Scholar]
- 34.Prevention CfDCa. Prevalence and most common causes of disabilty among adults - United States. Hyattsville, MD: 2010. [Google Scholar]
- 35.Radloff L. The CES-D Scale: A Self-report depression scale for research in the general population. Applied Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 36.Roland M, Morris R. A study of the natural history of back pain Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine (Phila Pa 1976) 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 38.Schneider KL, Ferrara J, Lance B, Karetas A, Druker S, Panza E, Olendzki B, Andersen V, Pbert L. Acceptability of an Online Health Videogame to Improve Diet and Physical Activity in Elementary School Students: “Fitter Critters”. Games Health J. 2012;1:262–268. doi: 10.1089/g4h.2012.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spitzer W, LeBlanc F, Dupris M. Scientifice approach to the assessment and management of activity related spinal diorders. A monograph for clinicians Report of the Quebec Task Force on Spinal Disorders. Spine (Phila Pa 1976) 1987;12:S1–S59. [PubMed] [Google Scholar]
- 40.Stratford PW, Binkley J, Solomon P, Finch E, Gill C, Moreland J. Defining the minimum level of detectable change for the Roland-Morris questionnaire. Phys Ther. 1996;76:359–365. doi: 10.1093/ptj/76.4.359. discussion 366–358. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan MJ, Rodgers WM, Wilson PM, Bell GJ, Murray TC, Fraser SN. An experimental investigation of the relation between catastrophizing and activity intolerance. Pain. 2002;100:47–53. doi: 10.1016/s0304-3959(02)00206-3. [DOI] [PubMed] [Google Scholar]
- 42.Thomas JS, Corcos DM, Hasan Z. The effects of movement speed on postural joint kinematics and ground reaction force during a reaching task. In: Synder-Mackler L, editor. APTA Combined Sections. Section on Research Newsletter. Seattle, WA: APTA; 1999. p. 17. [Google Scholar]
- 43.Thomas JS, Corcos DM, Hasan Z. Kinematic rules underlying multi-joint reaching movements. 30th Annual Meeting. Society for Neuroscience; New Orleans, LA. 2000. p. 1719. [Google Scholar]
- 44.Thomas JS, Corcos DM, Hasan Z. Kinematic and kinetic constraints on arm, trunk, and leg segments in target-reaching movements. J Neurophysiol. 2005;93:352–364. doi: 10.1152/jn.00582.2004. [DOI] [PubMed] [Google Scholar]
- 45.Thomas JS, France CR. Pain-related fear is associated with avoidance of spinal motion during recovery from low back pain. Spine (Phila Pa 1976) 2007;32:E460–E466. doi: 10.1097/BRS.0b013e3180bc1f7b. [DOI] [PubMed] [Google Scholar]
- 46.Thomas JS, France CR. The relationship between pain-related fear and lumbar flexion during natural recovery from low back pain. Eur Spine J. 2008;17:97–103. doi: 10.1007/s00586-007-0532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas JS, France CR. The relationship between pain-related fear and lumbar flexion during natural recovery from low back pain. Eur Spine J. 2008;17:97–103. doi: 10.1007/s00586-007-0532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas JS, France CR, Lavender SA, Johnson MR. Effects of fear of movement on spine velocity and acceleration after recovery from low back pain. Spine (Phila Pa 1976) 2008;33:564–570. doi: 10.1097/BRS.0b013e3181657f1a. [DOI] [PubMed] [Google Scholar]
- 49.Thomas JS, France CR, Sha D, Vander Wiele N, Moenter S, Swank K. The effect of chronic low back pain on trunk muscle activations in target reaching movements with various loads. Spine (Phila Pa 1976) 2007;32:E801–E808. doi: 10.1097/BRS.0b013e31815d0003. [DOI] [PubMed] [Google Scholar]
- 50.Thomas JS, France CR, Sha D, Vander Wiele N, Moenter S, Swank K. The effect of chronic low back pain on trunk muscle activations in target reaching movements with various loads. Spine (Phila Pa 1976) 2007;32:E801–E808. doi: 10.1097/BRS.0b013e31815d0003. [DOI] [PubMed] [Google Scholar]
- 51.Thomas JS, France CR, Sha D, Wiele NV. The influence of pain-related fear on peak muscle activity and force generation during maximal isometric trunk exertions. Spine (Phila Pa 1976) 2008;33:E342–E348. doi: 10.1097/BRS.0b013e3181719264. [DOI] [PubMed] [Google Scholar]
- 52.Thomas JS, Gibson GE. Coordination and timing of spine and hip joints during full body reaching tasks. Hum Mov Sci. 2007;26:124–140. doi: 10.1016/j.humov.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trost Z, France CR, Sullivan MJ, Thomas JS. Pain-related fear predicts reduced spinal motion following experimental back injury. Pain. 2012;153:1015–1021. doi: 10.1016/j.pain.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Van Damme S, Van Ryckeghem DM, Wyffels F, Van Hulle L, Crombez G. No pain no gain? Pursuing a competing goal inhibits avoidance behavior. Pain. 2012;153:800–804. doi: 10.1016/j.pain.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 55.Vlaeyen J, Morley SJ, Linton SJ, Boersma K, de Jong J. Pain-related fear: Exposure-based treatment of chronic Pain. Seattle, WA: International Association for the Study of Pain Press; 2012. [Google Scholar]
- 56.Vlaeyen JW, Kole-Snijders AM, Boeren RG, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 57.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 58.Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153:1144–1147. doi: 10.1016/j.pain.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Von Korff M. Studying the natural history of back pain. Spine (Phila Pa 1976) 1994;19:20415–20465. doi: 10.1097/00007632-199409151-00005. [DOI] [PubMed] [Google Scholar]
- 60.Wertli MM, Rasmussen-Barr E, Held U, Weiser S, Bachmann LM, Brunner F. Fear-avoidance beliefs-a moderator of treatment efficacy in patients with low back pain: a systematic review. Spine J. 2014;14:2658–2678. doi: 10.1016/j.spinee.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 61.Woods MP, Asmundson GJ. Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: a randomized controlled clinical trial. Pain. 2008;136:271–280. doi: 10.1016/j.pain.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 62.Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treat. 2007;32:189–198. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]