Abstract
In activated B cells, increased production of phosphatidylcholine (PtdCho), the most abundant cellular phospholipid, is handled primarily by the CDP-choline pathway. B cell-specific deletion of CTP:phosphocholine cytidylyltransferase α (CCTα), the rate-limiting enzyme in the CDP-choline pathway, led to augmented IgM secretion and reduced IgG production, suggesting that PtdCho synthesis is required for germinal center reactions. To specifically assess whether PtdCho influences B cell fate during germinal center responses, we examined immune responses in mice whereby PtdCho synthesis is disrupted in B cells that have undergone class switch recombination to IgG1 (referred to as either Cγ1wt/wt, Cγ1Cre/wt or Cγ1Cre/Cre based on Cre copy number). Serum IgG1 was markedly reduced in naïve Cγ1Cre/wt and Cγ1Cre/Cre mice, while levels of IgM and other IgG subclasses were similar between Cγ1Cre/wt and Cγ1wt/wt control mice. Serum IgG2b titers were notably reduced and IgG3 titers were increased in Cγ1Cre/Cre mice compared with controls. Following immunization with T cell-dependent antigen NP-KLH, control mice generated high titer IgG anti-NP while IgG anti-NP titers were markedly reduced in both immunized Cγ1Cre/wt and Cγ1Cre/Cre mice. Correspondingly, the frequency of NP-specific IgG antibody-secreting cells was also reduced in spleens and bone marrow of Cγ1Cre/wt and Cγ1Cre/Cre mice compared to control mice. Interestingly, though antigen-specific IgM B cells were comparable between Cγ1Cre/wt, Cγ1Cre/Cre and control mice, the frequency and number of IgG1 NP-specific B cells was reduced only in Cγ1Cre/Cre mice. These data indicate that PtdCho is required for the generation of both germinal center-derived B cells and antibody-secreting cells. Further, the reduction in class-switched ASC but not B cells in Cγ1Cre/wt mice suggests that ASC have a greater demand for PtdCho compared to germinal center B cells.
Keywords: CCTα, antibody-secreting cell, antibody, germinal centers, T cell-dependent antigen, unfolded protein response, phospholipid biosynthesis
INTRODUCTION
B cell differentiation into antibody-secreting cells (ASC) invokes the unfolded protein response (UPR), a tightly organized, largely transcriptionally-controlled process resulting in enhanced secretory capacity. A distinctive feature of UPR transcriptional programming licenses B cells to enhance endoplasmic reticulum (ER) biogenesis [1], including components required for expanding the rough ER and Golgi to facilitate the marked demand for antibody secretion following activation and differentiation.
Spliced X-box binding protein 1 (XBP1S) via IRE1 is essential for transcription of UPR genes[2], and consequently for the production of antibody by ASC. One key factor that can be regulated by XBP1S is choline cytidylyltransferase α (CCTα)[3], the predominant isoform of the rate-limiting enzyme in the cytidine phosphocholine (CDP-choline) pathway for phosphatidylcholine synthesis [4, 5]. Indeed, enforced expression of XBP1S in fibroblasts is sufficient to drive induction of phosphatidylcholine (PtdCho) by a mechanism involving regulation of CCTα [6]. The production of CCTα, in turn, increases the supply of CDP-choline for synthesis of phosphatidylcholine (PtdCho) that can be further processed into phosphatidylserine (PtdSer) and phosphatidylethanolamine (PtdEtn)[7, 8]. PtdCho, PtdSer and PtdEtn are all major lipid constituents for cellular membranes, with PtdCho the most abundant [9]. Thus, XBP1S can regulate the supply of membrane lipids, an ability that fits well with its essential function in ER biogenesis in numerous types of dedicated secretory cells such as ASC [10, 11], pancreatic acinar cells [12], Paneth cells [13] and salivary gland cells [14] that possess and utilize large quantities of rough ER for their functions.
In vivo activation of B cells by either T cell-independent (TI) or –dependent (TD) antigens leads to differentiation of B cells into either short-lived plasmablasts [15] or to development of germinal centers that ultimately generate both long-lived ASC and memory B cells [16]. B cells stimulated with bacterial lipopolysaccharide (LPS), a TLR4-dependent model for T cell-independent responses, upregulate CCT activity approximately 2-fold while PtdCho production increases approximately 7-fold [9]. Similarly, LPS stimulation of CH12 lymphoma cells resulted in increased CCTα levels, though this was attributed to reduced protein turnover rather than transcriptional activation [5]. Importantly, CCTα-deficient B cells fail to upregulate PtdCho synthesis after LPS stimulation [17]. Thus, CCTα appears integral for B cell differentiation into ASC in response to T cell-independent stimuli.
Interestingly, mice harboring B cells rendered CCTα-deficient following lineage commitment via CD19-Cre-induced gene deletion generated markedly reduced IgG and increased IgM in response to immunization with TD antigen [17]. IgM production was similarly increased in primary CCTα-deficient B cells in vitro upon stimulation with LPS, despite a corresponding reduction in B cell proliferation. However, reduced frequencies of splenic and peritoneal B cells were also noted in B cell-CCTα-deficient mice [17]. Both splenic marginal zones and the peritoneum contain B-1 cells [18], and B-1 cell-derived IgM is required for normal responses to TD-antigens [19]. This raises the possibility that a reduction of B-1 cells contributed to the impaired antibody responses observed in B cell-CCTα-deficient mice. Moreover, neither germinal center nor antigen-specific antibody levels were measured in those studies. Therefore, the significance of increased PtdCho production in antigen-specific B cell responses remains unknown.
To resolve whether PtdCho production is required for B cell responses to TD antigens, humoral immunity was examined in conditional IgG1 B cell-CCTα-deficient (Cγ1-CCTα) mice in which CCTα is selectively eliminated in B cells that have undergone class switch recombination from IgM to IgG1. Importantly, B cell development appeared normal in all CCTαflox (Cγ1wt/wt, Cγ1Cre/wt, and Cγ1Cre/Cre) mice, and serum immunoglobulin (Ig) levels were similar between Cγ1Cre/wt and wild-type mice, with the exception of selective reduction in IgG1. Serum IgG1 levels in Cγ1Cre/Cre mice were also reduced, while these mice also unexpectedly exhibited decreased IgG2b and increased IgG3 titers as compared to control mice. In response to immunization with NP-KLH emulsified in alum, which generates an IgG1-dominant antibody response to NP, both antigen-specific IgM and IgG primary responses were impaired in Cγ1Cre-expressing mice as compared to CCTα-sufficient control mice. The reduced response was not due to failure of Cγ1-Cre-expressing mice to generate germinal centers since the frequency and number of GC was comparable between each of the three strains examined. Rather, the diminished antigen-specific IgG in Cγ1-Cre-expressing mice correlated with reductions in hapten-specific antibody-secreting cells (ASC). Examination of germinal center B cell populations revealed that, while the frequency and number of NP-specific IgM B cells in Cγ1-Cre-expressing mice was comparable to control mice, the frequency and number of NP-specific IgG1 germinal center B cells was significantly reduced in Cγ1Cre/Cre CCTα mice. Notably, though class-switched, hapten-specific ASC were reduced in Cg1Cre/wt mice, the frequency and number of class-switched hapten-specific germinal center B cells was not, suggesting a differential demand for PtdCho. No differences were observed in the affinity of NP-specific IgG after immunization, suggesting that increased PtdCho synthesis is not required for selection of antigen-specific B cells. In summary, these studies reveal that PtdCho is required for the generation of class switched B cells in germinal centers as well as the production of both IgG1 memory B cells and ASC.
RESULTS
Conditional Pycta1 mice were generated by crossing a mouse strain containing loxP-flanked Pycta1 alleles (Pycta1flox)[17] with the Cγ1-Cre strain [20] whereby Cre recombinase is expressed when B cells undergo class switch recombination from IgM to IgG1. Progeny from the mouse cross therefore generate B cells that selectively delete Pycta1 upon commitment to expressing IgG1. Pcyta1 encodes CTP:phosphocoline cytidylyltransferase α (CCTα), the rate-limiting in the CDP-choline pathway for synthesis of phosphatidylcholine (PtdCho) [4], the most abundant phospholipid component in cell membranes [9]. Because IgG1 B cells conventionally derive from immune responses, the requirement for phospholipid synthesis for the generation of antibody-secreting and memory B cells was measured following immunization with the well-characterized T cell-dependent (TD) hapten-carrier antigen NP-KLH.
Conditional deletion of CCTα in IgG1 B cells does not alter B cell development
To determine whether conditional deletion of Pycta1 alters B cell development prior to immunization, naïve littermate control mice (Cγ1-wt Pycta1flox/flox, referred to as wild-type) and Cγ1-Cre-expressing Pycta1flox/flox (referred to as Cγ1Cre/wt and Cγ1Cre/Cre) mice were compared. Developing bone marrow B cells were identified as B220+ CD23neg and were further distinguished phenotypically by CD24 and CD43 levels. As shown in Figure 1a, the frequencies of pre-pro (CD24neg, CD43+), pro (CD24int CD43+) and pre B (CD24+ CD43+/−) cells were comparable between control and Cγ1-Cre-expressing mice. Moreover, the frequencies of all developing B cells as well as mature recirculating (B220+ CD23+) B cells were also equivalent between control and experimental groups of mice. Peripheral B cell populations were similarly assessed, and the frequencies of total transitional (B220+ CD93+) and mature (B220+ CD93neg) B cells were again comparable between each of the strains (Figure 1b). Finally, the frequencies of follicular (CD21lo CD23+) and marginal zone (CD21+ CD23int) B cells were measured, and again both CCTα-sufficient and -deficient mice were indistinguishable. These data indicate that B cell development is normal in Cγ1-Cre-expressing mice.
Figure 1. B cell development occurs normally in Cγ1-Cre-expressing mice.
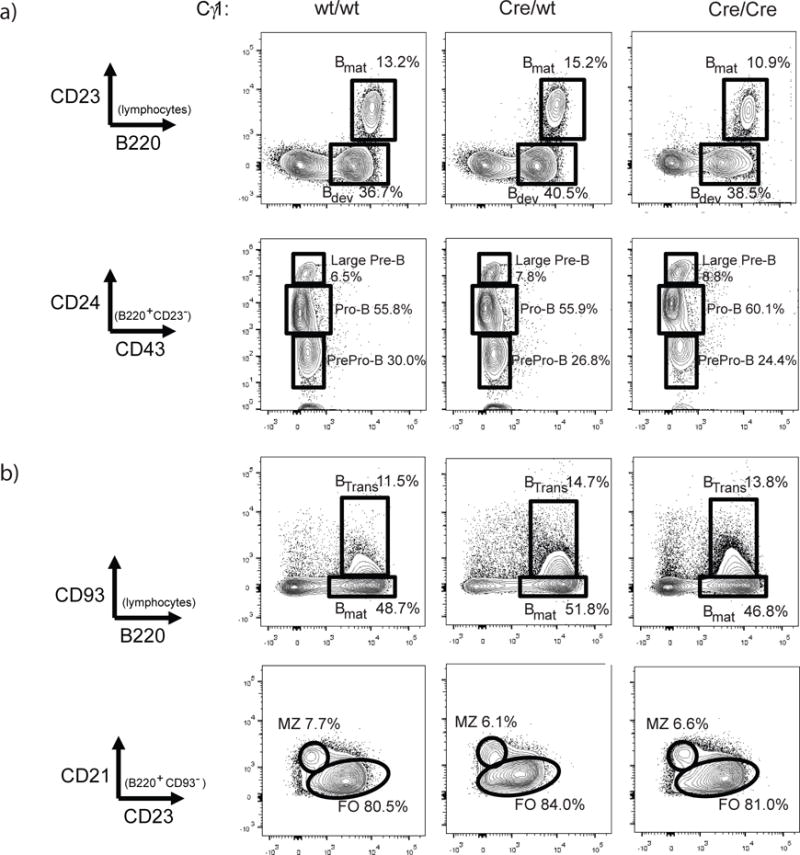
B cell development in representative naïve 2 month-old wild-type (Cγ1wt/wt CCTαflox/flox, left panels) and Cγ1Cre/wt CCTαflox/flox (middle) and Cγ1Cre/Cre CCTαflox/flox (right) mice was assessed by flow cytometry. In (a), developing (B220+ CD23−) and mature (B220+ CD23+) bone marrow B cells were distinguished (top panels). Developing B cells were further assessed using CD43 and CD24 levels. Splenic B cell analyses are shown in (b): transitional B cells were defined as B220+ CD93+ (top panels); marginal zone (MZ) and follicular (FO) were distinguished by CD21 and CD23 (bottom panels).
Impaired antibody-secreting cell (ASC) differentiation would most notably manifest as a reduction in serum immunoglobulin (Ig). Serum levels of IgM, IgG1, IgG2a, IgG2b, and IgG3 were therefore quantified in naïve 10 week-old mice. Serum titers of IgM, IgG2a, IgG2b and IgG3 were similar between wild-type and Cγ1Cre/wt mice (Figure 2). Interestingly, though Cγ1Cre/Cre mice also had comparable titers of IgM and IgG2a antibodies, IgG2b titers were significantly reduced and IgG3 titers were elevated compared to wild-type control mice. While serum IgG1 was detectable up to 1 to 10,000 dilution from control mice, IgG1 was detectable only at 1 to 100 serum dilution from Cγ1-Cre-expressing mice, indicating that CCTα, and PtdCho production, is necessary for the generation of normal serum IgG1.
Figure 2. Antibody levels in naïve Cγ1-Cre-expressing mice.
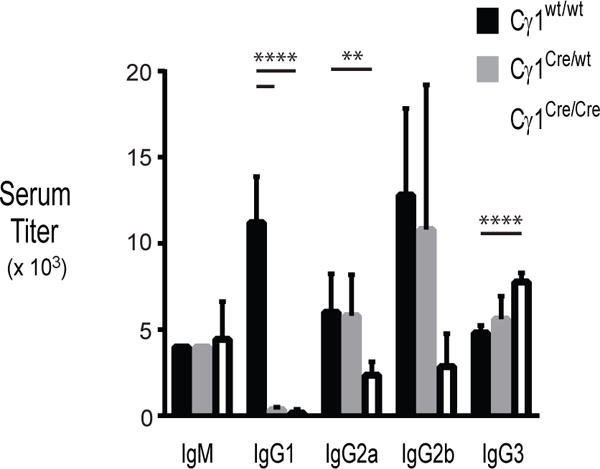
Ig titers for IgM and IgG subclasses in serum from naïve 2 month-old wild-type (n=5, black bars), Cγ1Cre/wt (n=5, gray) and Cγ1Cre/Cre (n=5, white bars) mice were measured by ELISA. Data shown are means, with standard error indicated (**p<0.003, ****p<2 × 10−5, unpaired Student’s t test with Welch’s correction).
CCTα is required for humoral response to TD antigen
To evaluate whether conditional deletion of Pycta1 in IgG1 B cells causes defects in Ig production upon antigen stimulation, mice were immunized intraperitoneally (i.p.) using the TD antigen NP-KLH emulsified in alum and examined three weeks later. As shown in Figure 3a, while wild-type mice generated mean NP-specific IgM and IgG titers of 1,673 and 56,000, Cγ1Cre/wt and Cγ1Cre/Cre mice had mean NP-specific IgM titers of 698 and 643, and mean NP-specific IgG titers of 5,929 and 5,000, respectively. Therefore, Cγ1-Cre-expressing mice exhibit an impaired IgM and IgG primary response to TD antigen.
Figure 3. Impaired antigen-specific IgG response and ASC formation in Cγ1-Cre-expressing mice.
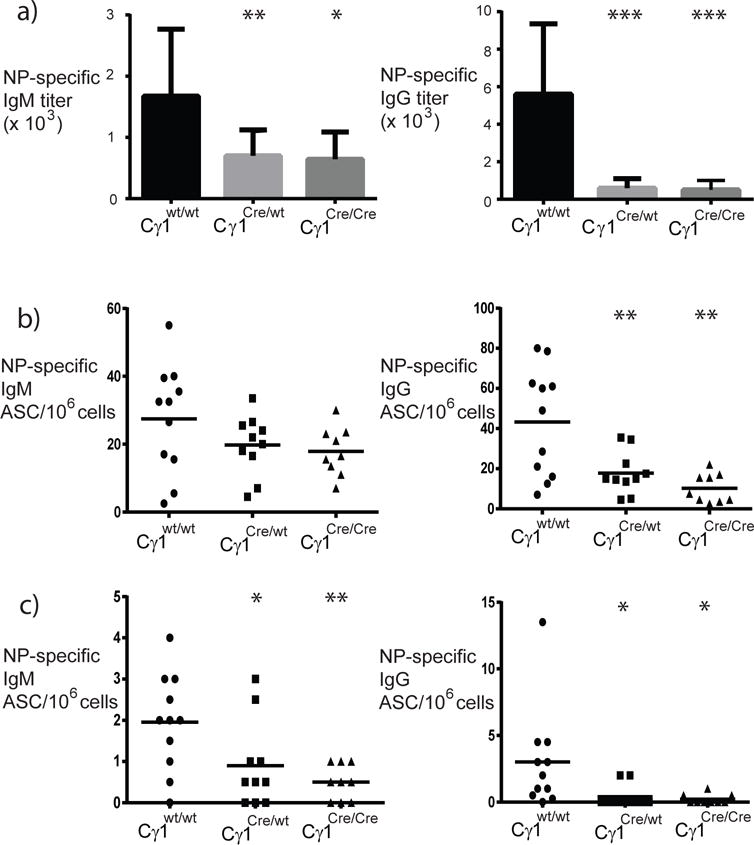
Mice were immunized i.p. using 50 μg NP5-KLH emulsified in alum. Three weeks after immunization, ELISA (a) and ELIspot assays (b and c) were used to measure antigen-specific responses. In (a), IgM and IgG primary antibody responses (mean + standard deviation) in wild-type mice (black bars) and Cγ1-Cre-expressing mice (gray bars). NP-specific IgM (left panels) and IgG (total, right panels) antibody-secreting cells (ASC) in spleen (b) and bone marrow (c); filled circles represent data from individual wild-type mice, filled squares are data from Cγ1Cre/wt mice, and filled triangles are data from Cγ1Cre/Cre mice (all mice were CCTαflox/flox). Statistics were calculated using 1-way ANOVA with Tukey’s multiple comparison test (*p<0.02; **p<0.007; ***p<0.0002).
Serum IgG derives primarily from ASC in the bone marrow, and these bone marrow ASC derive from germinal center responses. To examine whether the production of germinal center-derived ASC was affected in Cγ1-Cre-expressing mice, NP-specific ASC were enumerated by ELIspot assay. Three weeks following immunization with NP-KLH in alum, both wild-type control and Cγ1-CCTα mice generated comparable frequencies of NP-specific IgM-secreting cells in the spleen (Figure 3b left panel, 26.7 ± 5.8, wt vs. 20.8 ± 4.1, Cγ1Cre/wt, and 16.0 ± 4.1, Cγ1Cre/Cre). Consistent with the reduced hapten-specific IgM antibody titers, both Cγ1Cre/wt and Cγ1Cre/Cre mice had reduced NP-specific IgM+ ASC in bone marrow compared to control mice (Figure 3c left panel, 1.6 ± 0.3, wt, 1.0 ± 0.5, Cγ1Cre/wt and 0.4 + 0.2, Cγ1Cre/Cre). By comparison, the production of NP-specific IgG secreting cells was reduced in Cγ1-Cre-expressing mice relative to wild-type control mice in both spleen (Figure 3b right panel, 39.2 ± 9.5, wt vs. 16.9 ± 5.7, Cγ1Cre/wt and 6.5 ± 3.0, Cγ1Cre/Cre) and bone marrow (Figure 3c right panel, 2.7 ± 1.4, wt vs. 1.0 ± 0.4, Cγ1Cre/wt and 0.3 ± 0.1, Cγ1Cre/Cre). Collectively, these results suggest that PtdCho production is required for the generation of class-switched ASC.
Reduced NP-specific IgM during the primary response in Cγ1-CCTα mice was unexpected. To determine whether this could be due to impaired germinal centers, the frequency of splenic germinal centers was quantified. Using flow cytometry, the frequency of PNAhi CD95+ (germinal center) B cells was determined two weeks after immunization with TD antigen NP-KLH (Figure 4a, 4b). The frequency (5.1 ± 0.5, wt vs. 4.1 ± 0.7, Cγ1Cre/wt and 3.8 + 0.5, Cγ1Cre/Cre) and number (241,523 ± 36,718, wt vs. 193,479 ± 45,573, Cγ1Cre/wt and 200,235 ± 27,427, Cγ1Cre/Cre) of germinal center B cells was similar between immunized control and Cγ1-Cre-expressing mice. To complement these analyses, histological analysis was also performed. Approximately 36% of splenic follicles from immunized wild-type mice contained germinal centers, while approximately 39% and 44% of splenic follicles in immunized Cγ1Cre/wt and Cγ1Cre/Cre mice had germinal centers, respectively (Figure 4c). Thus, Cγ1-Cre-expressing mice were as capable as control mice of forming germinal centers in response to TD antigen challenge.
Figure 4. Development of germinal centers occurs normally in Cγ1-Cre-expressing mice.
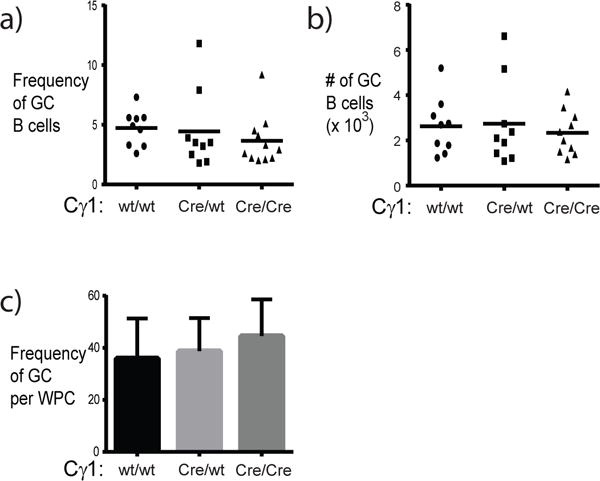
The frequency and number of germinal center B cells in spleen were determined by flow cytometry. The frequency of splenic germinal center B cells (a) identified as the PNAhi CD95+ fraction of the B220+ population. The absolute number of germinal center B cells (b) was calculated by multiplying the frequency by total splenocyte counts. Filled circles represent data from individual wild-type mice, filled squares are data from Cγ1Cre/wt mice, and filled triangles are data from Cγ1Cre/Cre mice (all mice were CCTαflox/flox). In (c), the frequency of germinal centers was determined by immunohistochemical analysis of splenic cryosections. Up to 4 sections from wild-type mice (n=6), Cγ1Cre/wt (n=6), and Cγ1Cre/Cre (n=6) mice were assessed.
Reduced antigen-specific B cell memory in Cγ1Cre/Cre mice
Germinal center responses generate both antigen-specific ASC and memory B cells. To measure whether the production of memory B cells was also impaired in immunized Cγ1-Cre-expressing mice, the frequency and number of NP-specific germinal center B cells was measured (Figure 5). The frequency and number of IgM+ NP-specific germinal center B cells (Figure 5b and 5d) was similar between CCTα-sufficient and Cγ1-Cre-expressing mice (1.3 ± 0.4 and 2,659 ± 762, wt; 1.0 ± 1.0 and 1,754 ± 480, Cγ1Cre/wt; 1.7 ± 0.4 and 2,751 ± 788, Cγ1Cre/Cre). In contrast, while approximately 2% of germinal center B cells in wild-type mice were NP-specific IgG1+, the frequency was markedly reduced in Cγ1Cre/Cre mice (2.1 ± 0.5, wt vs. 0.3% ± 0.1, Cγ1Cre/Cre) (Figure 5c). The number of NP-specific IgG1 germinal center B cells was also reduced approximately 4-fold in Cγ1Cre/Cre compared to the number in wild-type mice (4,521 ± 1,313, wt vs. 796 ± 295, Cγ1Cre/Cre) (Figure 5e). These differences between wild-type and Cγ1Cre/Cre mice were evident in both CD38+ and CD38lo/neg germinal center cells (data not shown), suggesting that both light and dark zone responses were involved [21]. Surprisingly, the frequency and number of NP-specific IgG1 germinal center B cells did not differ statistically between Cγ1Cre/wt (1.4 ± 0.4 and 2,477 ± 740) and wild-type mice. From these data, we conclude that the generation of antigen-specific class-switched germinal B cells requires de novo PtdCho synthesis, but that this developmental process can proceed under conditions of reduced PtdCho production.
Figure 5. The frequency of germinal center-resident NP-specific IgG1+ B cells is reduced in Cγ1Cre/Cre mice.
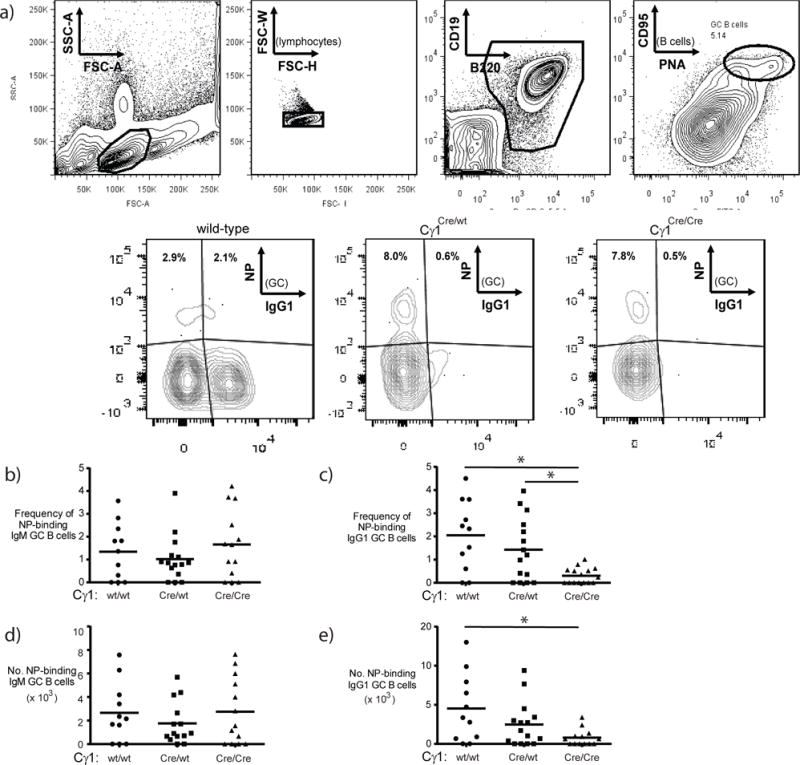
NP-specific germinal center B cells were identified using flow cytometry. Briefly, in (a), single lymphocytes (upper left and middle-left plots) were gated on B cells (middle-right plot) in germinal centers (far right plot), then assessed for frequency of NP-binding IgG1 B cells (lower plots). Minus 1 staining negative controls (not shown) were used to establish gates for NP-binding. Representative data from multiple wild-type, Cγ1Cre/wt and Cγ1Cre/Cre mice are shown. The frequency and number of IgM+ (b and d) and IgG1+ (c and e) NP-binding splenic germinal center B cells. Statistical differences were determined with 1-way ANOVA using Tukey’s multiple comparison test (*p<0.03).
To test whether the reduced antigen-specific IgG1 B cells and ASC had a functional consequence, mice were challenged and secondary responses were measured. As was observed in the primary response, the antigen-specific IgM response after challenge was comparable between wild-type and Cγ1-Cre-expressing mice (Figure 6). Thus, no differences in NP-specific IgM titers (1,300 ± 272, wt; 1,116 ± 210, Cγ1Cre/wt; 2,050 ± 665, Cγ1Cre/Cre) (Figure 6a left panel) nor frequency of NP-specific IgM ASC in bone marrow (5.5 ± 1.6, wt; 2.3 ± 1.3, Cγ1Cre/wt; 4.5 ± 3.7, Cγ1Cre/Cre) (Figure 6c left panel) were observed. There was a trend toward an increased frequency of NP-specific IgM ASC in spleen in Cγ1-Cre-expressing mice compared to wild-type mice, though these data were not statistically significant (8.7 ± 0.9, wt; 261.5 ± 121.3, Cγ1Cre/wt; 101.3 ± 67.2, Cγ1Cre/Cre) (Figure 6b left panel). In contrast to the IgM response, while wild-type mice produced 1,725 NP-specific IgG splenic ASC, the frequency was reduced at least 3-fold in Cγ1-Cre-expressing mice (Figure 6b right panel: 572 ± 83.7, Cγ1Cre/wt; 373.8 ± 83.2, Cγ1Cre/Cre). The frequency of NP-specific IgG bone marrow ASC was also reduced in Cγ1-Cre-expressing mice (12.2 ± 1.6, Cγ1Cre/wt; 9.2 + 2.3, Cγ1Cre/Cre) compared to wild-type mice (45.1 ± 5.8) (Figure 6c right panel). Therefore, PtdCho production is required for the generation of antigen-specific, class-switched ASC.
Figure 6. CCTα is required for IgG but not IgM antibody and ASC during secondary immune responses.
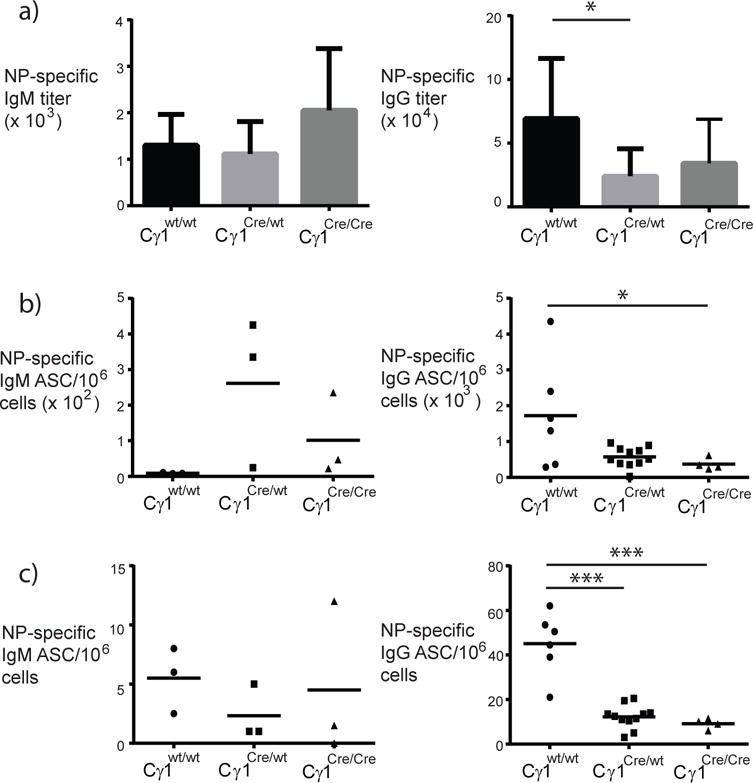
Mice were immunized i.p. using 50 μg NP5-KLH emulsified in alum. Five days after booster immunization, ELISA (a) and ELIspot assays (b) were used to measure antigen-specific responses. In (a), IgM (left panel) and IgG (right panel) primary antibody responses (mean + standard deviation) in wild-type (black bars) and Cγ1-Cre-expressing mice (gray bars). In (b and c), NP-specific IgM (left panels) and IgG (right panels) antibody-secreting cells (ASC) in spleen (b) and bone marrow (c); filled circles represent data from individual wild-type mice, filled squares are data from Cγ1Cre/wt mice, and filled triangles are data from Cγ1Cre/Cre mice (all mice were CCTαflox/flox). Statistical differences were determined with 1-way ANOVA using Tukey’s multiple comparison test (*p<0.03; ***p<0.0001).
To gauge whether the production of antigen-experienced B cells was also affected in Cγ1-Cre-expressing mice, germinal centers were interrogated (Figure 7). As expected, the frequency and number of NP-binding IgG1+ germinal center B cells increased relative to the frequency observed in primary germinal center responses (Figure 7a, c, e). Notably, the mean level of NP binding (MFI) appeared greater in IgG1 versus IgM B cells (Figure 7a). In contrast to the recall response of CCTα-sufficient control mice, the frequency and number of IgG1+ NP-specific germinal center B cells was reduced in Cγ1-Cre-expressing mice (IgG1, mean ± SEM: 7.7 ± 1.0 and 97,651 ± 26,303, wt; 3.7 ± 0.4 and 47,977 ± 6,426, Cγ1Cre/wt; 2.3 ± 0.7, 22,238 ± 4,404, Cγ1Cre/Cre). Thus, reduced primary responses of Cγ1-CCTα mice manifested as reduced recall germinal center responses.
Figure 7. Germinal center recall responses are impaired in Cγ1-Cre-expressing mice.
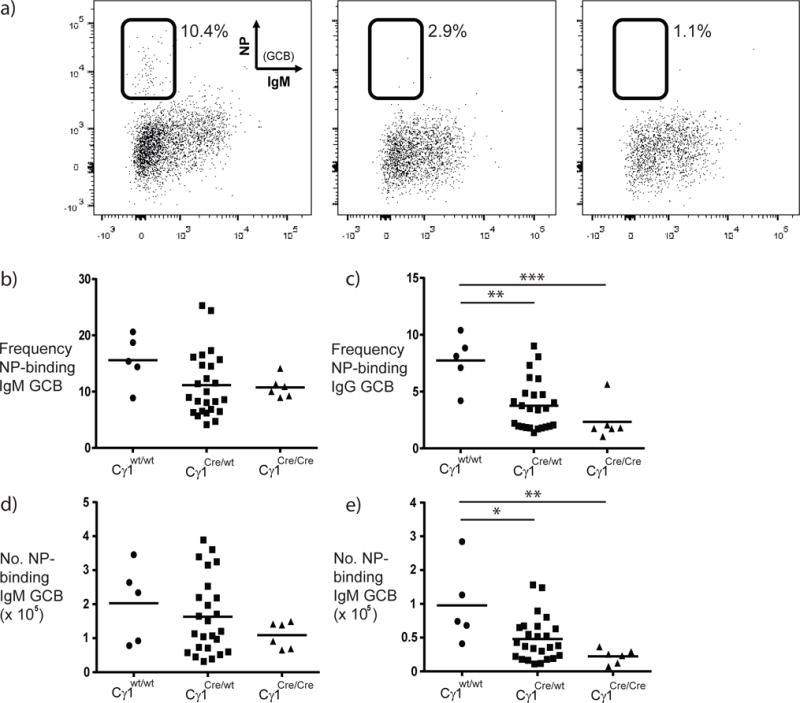
NP-specific germinal center B cells were identified using flow cytometry as described earlier. Representative data from multiple wild-type, Cγ1Cre/wt and Cγ1Cre/Cre mice are shown in (a). The frequency (b, c) and number (d, e) of IgM+ (b, d) and IgG1+ (c, e) NP-binding splenic germinal center B cells for multiple mice are shown. Statistical differences are indicated (*p<0.02, **p≤0.002, ***p=0.0006, 1-way ANOVA with Tukey’s multiple comparisons).
Antigen-specific IgG affinity is comparable in wild-type and Cγ1-Cre-expressing mice
Reducing competition within germinal centers by loss of high-affinity B cells can allow lower-affinity B cells to be selected [22, 23]. To determine whether elimination of CCTα in B cells that class switch to IgG1 affects germinal center selection, relative changes in antigen-specific serum IgG antibody affinity were measured by determining the ratio of antibody titers binding low (NP2) to highly (NP20) haptenated BSA. The ratio of NP2/NP20 IgG titers from wild-type mice increased from approximately 0.5 to approximately 0.7 (Figure 8). Comparatively, the NP2/NP20 IgG titer ratio was lower after primary immunization in Cγ1Cre/wt mice, but increased to approximately 0.5 following secondary challenge. Cγ1Cre/Cre mice exhibited a trend toward more increased affinity following secondary challenge with antigen, however the increase was not statistically different from affinity changes in wild-type mice. Thus, no significant differences were observed in the relative serum affinity of IgG antibodies between wild-type and Cγ1-Cre-expressing mice.
Figure 8. Changes in relative antibody affinity in immunized Cγ1-CCTα mice.
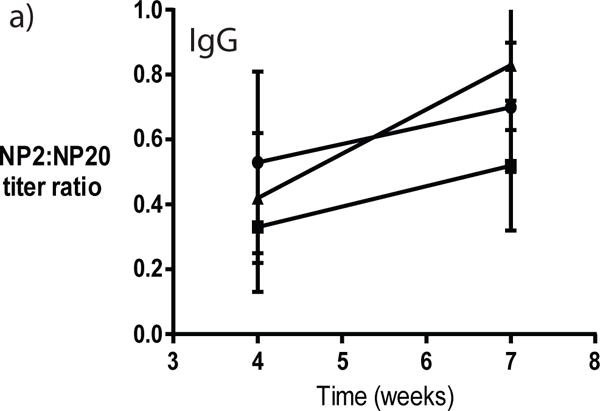
Sera from individual mice were titrated in ELISA to determine the amount of high-affinity (NP2-BSA) and total (NP20-BSA) NP-specific IgG (a) and IgM (b) for wild-type (filled circles), Cγ1Cre/wt (filled squares) and Cγ1Cre/Cre (filled triangles) mice. The ratio of NP2:NP20 titers is expressed as mean ratios ± SEM and are compiled from three independent experiments.
DISCUSSION
Among the key events in B cells undergoing differentiation into ASC, upregulation of phospholipid biosynthesis facilitates the expansion of the ER and Golgi to accommodate an increased demand for Ig synthesis, assembly and secretion. Previous studies detailed that CCTα is the rate-limiting enzyme for PtdCho synthesis in B cells and that it was necessary to direct class switch recombination of B cells to the TI stimulus LPS in a proliferation-independent manner [17]. To address whether B cells responding to TD antigen in germinal centers differed in their dependence for CCTα, we utilized Cγ1-Cre CCTαflox/flox mice whereby CCTα would be deleted upon class switch recombination of B cells from IgM to IgG1. We observed that B cell development and germinal center development is normal in Cγ1-Cre-expressing mice; however, these mice had reduced serum IgG1 levels and reduced antigen-specific IgG1 antibody and ASC upon antigen challenge. Interestingly, class-switched hapten-specific germinal center B cells, though reduced in Cγ1Cre/Cre mice, were similar between wild-type and Cγ1Cre/wt mice, suggesting that proliferating germinal center B cells may demand less PtdCho than ASC. Surprisingly, the IgM antibody response in immunized Cγ1-Cre-expressing mice was also reduced compared to wild-type mice, though the frequency and number of hapten-specific IgM germinal center B cells was similar in all three strains of mice. These studies indicate that CCTα is required for the production of germinal center-derived class-switched ASC and memory cells. Further, these results are consistent with a model whereby PtdCho is rate-limiting for both cell proliferation and differentiation of germinal center B cells (Figure 9).
Figure 9. XBP1S and PtdCho in the humoral immune response.
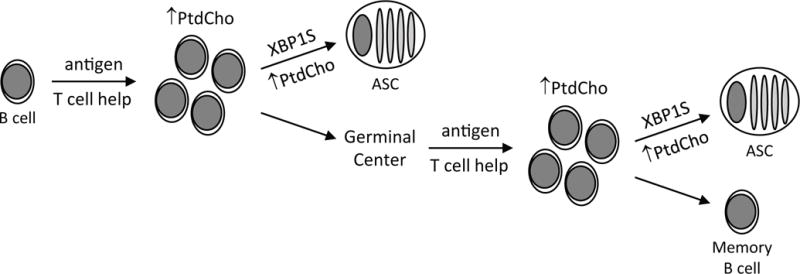
When B cells are activated by antigen and T cell help (as well as by T cell-independent stimuli), synthesis of phosphatidylcholine (PtdCho) and other membrane lipids increases to meet the needs of membrane biogenesis in dividing cells. At this point, activated B cells can differentiate into short-lived antibody-secreting cells (ASC), a developmental process that is coordinated by the XBP1S transcription factor and that requires an increased supply of membrane lipids like PtdCho to fuel expansion of the secretory apparatus. Alternatively, in the case of B cells activated with T cell help, entry into the germinal center reaction ushers in additional rounds of cell division that require more membrane lipids, followed by the potential of either memory B cell development or XBP1S-dependent, lipid-demanding differentiation of long-lived ASC.
Gene-targeted deletion of Pcyt1a is embryonically lethal (day 3.5)[24], whereas deletion of other CCT isoforms such as CCTβ have less profound effects [25], supporting that CCTα is the dominant isoform required for PtdCho synthesis in multiple tissues. The requirement for CCTα in B cells was assessed using CD19-mediated conditional deletion, and resulted in reduced numbers of peritoneal and splenic B cells, as well as significantly reduced serum IgG levels [17]. Upon challenge with TD antigen, CD19-CCTα mice notably failed to form germinal centers, likely due to the requirement for CCTα in the oligoclonal proliferative burst that initiates the germinal center response [26]. Both reduced B cell numbers and the failure to form germinal centers were likely responsible for impaired IgG production in CD19-CCTα mice following TD antigen challenge. Use of Cγ1-Cre-expressing mice in the current work offered an approach that does not affect B cell development or the formation of germinal centers, thereby allowing the requirement of CCTα and PtdCho synthesis in germinal center responses to be directly assessed. Therefore, antigen-specific B cells retain the ability to synthesize PtdCho to allow for expansion and generation of germinal centers.
Differentiation of B cells into antibody-secreting cells requires the UPR transcription factor XBP1S. This requirement extends to both responses to TI antigens by marginal zone B cells and to TD antigens that occur through germinal centers. The link between XBP1S and PtdCho is 3-fold: XBP1 is sufficient to upregulate CCTα and PtdCho synthesis (3, 6), deletion of XBP1 impairs PtdCho synthesis in activated B cells [27] and blocking PtdCho synthesis in B cells through selective deletion of CCTα induces XBP1(S) (18). Interestingly, CCTα-deficient B cells stimulated in vitro with LPS fail to class switch Ig and also secrete more IgM compared to CCTα-sufficient B cells [17]. Therefore, disabling new PtdCho production in activated B cells drives them to upregulate XBP1 and secrete IgM. By comparison, conditional elimination of XBP1 in B cells leads to reduced basal Ig levels, as well as reduced specific Ig in response to immunization using TD antigen NP-KLH emulsified in alum. The reduced antigen-specific response was limited to the generation of ASC, as the development of antigen-specific B cells in germinal centers was not affected [28]. We demonstrate that conditional elimination of CCTα in germinal center B cells upon class switch recombination to IgG1 leads to a reduction in both antigen-specific ASC and B cells. Taken together, these separate studies would suggest that limiting PtdCho availability acts ahead of the need for XBP1S, likely due to the requirement for PtdCho in expansion of class-switched antigen-specific B cells prior to differentiation within the germinal center reaction.
Interestingly, levels of serum IgM increase in CD19-CCTα mice following immunization, suggesting that reduced capacity to synthesize PtdCho in B cells may manifest as a type of hyper-IgM syndrome [17]. The expression of CCTα is cell-cycle regulated [4, 29], raising the possibility that inhibiting proliferation by impairing PtdCho synthesis can direct antigen-specific B cells to differentiate into ASC. Consistent with this idea, depletion of PtdCho levels leads to induction of XBP1S [17]. In Cγ1-CCTα mice, while the frequency of antigen-specific IgM germinal center B cells was comparable with those observed in primary-immunized control mice, levels of antigen-specific IgM were significantly reduced. In addition, antigen-specific IgM bone marrow ASC in Cγ1-CCTα mice were reduced compared with control mice. Thus, impairment of PtdCho synthesis in class-switched B cells affected the production of IgM-ASC but not IgM-B cells. The reduction in antigen-specific IgG observed in Cγ1-Cre expressing mice may contribute to a general reduction in germinal center efficiency by limiting IgG immune complexes, though this would not be expected to differentially affect the production of IgM-ASC. IgM B cells in germinal centers generally would not express Cre recombinase, since Cre is translated from Cγ1 transcripts via an internal ribosomal entry site. However, it is possible that a percentage of these germinal center B cells begin, but do not complete, class switch recombination. In this scenario, sterile transcripts could be generated, thereby allowing Cre recombinase to be expressed and CCTα to be subsequently deleted. The effect would be more profound for IgM-ASC than IgM B cells due to increased demands for PtdCho synthesis to expand the endoplasmic reticulum to accommodate enhanced secretory capacity.
Depleting PtdCho in non-lymphoid cells initiates the UPR and leads to apoptosis [30, 31]. A similar phenotype could explain the reduction in antigen-specific IgG B cells and ASC in immunized Cγ1-Cre-expressing mice. Alternatively, the observation that both antigen-specific IgG B cells and ASC were similarly reduced in Cγ1Cre/Cre cohorts may suggest that PtdCho is required at the level of clonal expansion within the germinal center prior to commitment to differentiation into ASC. However, only antigen-specific IgG ASC and not B cells were reduced in Cγ1Cre/wt mice, raising the intriguing possibility that ASC are more sensitive to limited PtdCho supply than proliferating germinal center B cells. Notably, though titers of antigen-specific IgG were reduced in Cγ1-Cre-expressing mice, the affinity of the IgG response was comparable between immunized Cγ1-Cre-expressing mice and control animals. Therefore, impaired PtdCho synthesis had no effect on affinity of the IgG response.
Previous studies examining selection of B cells within germinal centers found that reducing competition of high-affinity B cells allows lower-affinity B cells to be selected [22, 23]. Signaling via membrane IgG is more potent compared to IgM [32], possibly providing a competitive advantage for IgG B cells in the germinal center response. If true, it follows that reducing the number of IgG B cells through conditional deletion of CCTα could manifest as an increase in antigen-specific IgM B cells. This is not the case, as we observed approximately equivalent frequency and numbers of NP-specific IgM B cells within germinal centers of Cγ1-Cre-expressing mice and control mice. Because germinal centers also serve to potentiate affinity maturation, we speculate that in a setting whereby the frequency and number of IgG B cells is limiting, IgM B cells compete for antigen within the germinal centers thereby resulting in an increase in serum antibody affinity. Though affinity of antigen-specific IgM is very difficult to measure, the reduced NP-specific IgM bone marrow ASC observed in Cγ1-Cre-expressing mice would at least be consistent with this hypothesis, though we cannot exclude post-germinal center selection.
In conclusion, these studies support a requirement for PtdCho synthesis in the generation of both memory and ASC during germinal center responses to TD antigens. This dependency likely reflects a dependence for PtdCho in both the proliferative burst of antigen-specific B cells as well as the need for the expansion of the ER, Golgi and plasma membranes required for ASC differentiation. Putting our studies into context with earlier work demonstrating that limiting PtdCho synthesis triggers induction of XBP1S in the B cell response to LPS, it is likely that the fate and/or function of responding B cells employing the UPR is dependent on the degree of proliferation involved in the differentiation process.
MATERIALS AND METHODS
Generation of mice with CCTα-deficient IgG1 B cells
Pcyta1flox/flox mice [31] were crossed with Cγ1-Cre [20] mice to generate Cγ1Cre/Cre Pcta1flox/flox, Cγ1Cre/wt Pcta1flox/flox, and Cγ1wt/wt Pcta1flox/flox littermates. Notably, all examined aspects of B cell development and serum immunoglobulin levels were comparable between Cγ1wt/wt Pcta1flox/flox and non-floxed wild-type mice, and therefore they are collectively referred to as wild-type herein. Mice were housed at the University of South Alabama in an AAALAC-certified specific pathogen-free facility. Maintenance of breeding colonies and all procedures involving mice were performed according to protocols approved by the University of South Alabama Institutional Animal Care and Use Committee.
Immunization and serum analysis
Mice were immunized intraperitoneally (i.p.) with 0.05 mg Imject-precipitated (Thermo Scientific, Grand Island, NY) 4-hydroxy-3-nitrophenyl conjugated to keyhole limpet hemocyanin (NP5-KLH; Biosearch Technologies, Novato, CA). Booster immunizations were administered identically 3 weeks after primary immunization.
Anti-NP Response and Affinity Measurements by ELISA
Serum was collected from individual mice and NP-specific antibody titers were determined by sandwich ELISA. 96-well plates (Nunc; Thermo Scientific) were coated with 5 ug/well NP2-BSA or NP20-BSA. Plates were blocked with the addition of 5% dry milk (Carnation®) in PBS (Blotto). NP-specific IgG serum antibody in serially diluted samples was detected by horse radish peroxidase–conjugated goat anti–mouse IgG (Southern Biotechnology, Birmingham, AL). Between incubations, plates were washed with PBS containing 0.1% Tween. Color was developed using substrates 3, 3′, 5, 5′-tetramethylbenzemidine (TMB, Thermo Scientific) and hydrogen peroxide, and absorbance was measured at 405 nm using Softmax software package (Molecular Devices, Sunnyvale, CA). Antibody titer was determined as the reciprocal of the greatest dilution whose absorbance remained at least two-fold above background. For relative affinity measurements, the ratio of titers to NP2-BSA and NP20-BSA was calculated for individual mice using OD405 in the linear ranges of the assays as previously described [33]. For ELISA measuring IgM and IgG subclass titers in serum, a sandwich method was used by coating wells with goat anti-mouse Ig, and developing as above with subclass-specific IgM, IgG1, IgG2a, IgG2b or IgG3 horseradish peroxidase conjugated secondary antibodies (Southern Biotechnology).
Enzyme-linked Immunospot Assay for NP-specific antibody-secreting cells (ASC)
Frequencies of NP-specific ASCs were quantitated as previously described [33]. In brief, 24-well polystyrene plates (Corning Inc., Corning, NY) were coated with NP5-BSA. After extensive washing, plates were blocked using 1% BSA in PBS for 2 hours. Serial ten-fold diluted splenic mononuclear cells or BM cells (106 to 103 cells/well) were added in DMEM media with 2% fetal bovine serum and incubated overnight at 37 C. Each dilution of cells was assayed in duplicate. Plates were then washed with PBS containing 0.1% Tween and incubated with alkaline phosphatase–conjugated goat anti–mouse IgG antibody (Sigma-Aldrich). Plates were developed using 5-bromo-4-chloro-3-indolyl phosphate at 1 mg/ml in 0.6% agarose to produce blue-colored spots identifying NP-specific ASCs. Spots were counted to determine ASC frequency. As controls, each sample was also plated in wells coated with BSA or KLH. Few BSA-specific ASCs were detected in the BM or spleen and fewer than 100 KLH-specific ASCs were observed per recipient spleen (data not shown).
Flow cytometry and cell sorting
Single cell suspensions of bone marrow and splenic mononuclear cells (MNC) were isolated by density gradient centrifugation using Lympholyte M (Cedarlane Laboratories, Burlington, N.C.). To detect IgG1 B cells, MNCs were stained with biotinylated anti-IgG1 antibody (clone X-56, Miltenyi Biotec, Auburn, CA), followed by streptavidin-PerCP-Cy5.5 conjugate (BD Biosciences, San Jose, CA). Other antibodies used for flow cytometric analyses of B cell subsets in bone marrow, spleen and cervical lymph nodes included the following (BD Biosciences and eBioscience, San Diego, CA): CD19 (1D3), CD21/CD35 (7G6), CD23 (B3B4), CD24 (M1/69), CD38 (90), CD43 (S7), CD45R/B220 (RA3-6B2), CD93 (AA4.1), CD95 (Jo2), CD138 (281–2), IgM (II/41, R6-60.2), IgD (11–26), T and B cell activation antigen (GL-7), and PNA (Vector Laboratories, Burlingame, C.A.). To identify NP-specific B cells, cells were incubated with NP coupled to allophycocyanin (Thermo Scientific). Cells were analyzed by FACSCanto II and sorted using multi-laser FACSAria II-SORP (BD Biosciences) housed in the University of South Alabama College of Medicine Flow Cytometry Core Laboratory. Data were analyzed with FlowJo software (Tree Star Ashland, OR).
Immunohistochemistry
Isolated spleens were preserved in OCT compound on dry ice. 5-μm thick cryosections were examined following staining with biotinylated anti-B220 (BD Biosciences) and FITC-conjugated peanut agglutinin (Vector Laboratories). Streptavidin-alkaline phosphatase (Invitrogen) and HRP-conjugated anti-FITC (Thermo Scientific) were used as secondary staining reagents. Staining was visualized using a Nikon Eclipse microscope (Nikon Instruments Inc., Melville, NY) and images were analyzed using Nikon Elements software.
Statistical analysis
Data comparing three groups were analyzed by a 1-way ANOVA test with Tukey’s multiple comparisons test applied to compare individual group means. Statistical significance was determined by p value as indicated within the figure legends.
Highlights.
CCTα is required for antigen-specific, germinal center-derived antibody-secreting cells
CCTα is required for antigen-specific, germinal center-derived memory B cells
Phosphatidylcholine is required for germinal center B cell responses
Acknowledgments
This work was supported by NIH R01 GM61970. The authors thank Dr. Suzanne Jackowski (St. Jude Children’s Research Hospital, Memphis, TN) for providing CD19-Cre; Pcyta1flox/flox mice and Dr. Stefano Casola (IFOM, Milan, Italy) for providing Cγ1-Cre mice.
Abreviations
- ASC
antibody-secreting cell
- ER
endoplasmic reticulum
- UPR
unfolded protein response
- XBP1
X-box binding protein 1
- XBP1S
spliced X-box binding protein 1
- IRE1
inositol requiring 1
- PtdCho
phosphatidylcholine
- CDP-choline
cytidine diphosphocholine
- CCT
choline cytidylyltransferase
- PtdEtn
phosphatidylethanolamine
- PtdSer
phosphatidylserine
- NP-KLH
nitrophenyl-keyhole limpet hemocyanin
- TD
T cell-dependent
- TI
T cell-independent
- LPS
lipopolysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of the manuscript.
Author contributions: JWB initially conceived the idea of the project, developed the necessary mouse colony and assisted with experimental design and manuscript preparation. VS conducted experiments and analyzed data. IA assisted with ELISA assays and with management of the mouse colony. RAB conceived and performed experiments, analyzed results, prepared data for publication and wrote the manuscript.
References
- 1.Brewer JW, Hendershot LM. Building an antibody factory: a job for the unfolded protein response. Nat Immunol. 2005;6:23–29. doi: 10.1038/ni1149. [DOI] [PubMed] [Google Scholar]
- 2.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 3.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackowski S, Fagone P. CTP: Phosphocholine cytidylyltransferase: paving the way from gene to membrane. J Biol Chem. 2005;280:853–856. doi: 10.1074/jbc.R400031200. [DOI] [PubMed] [Google Scholar]
- 5.Fagone P, Sriburi R, Ward-Chapman C, Frank M, Wang J, Gunter C, Brewer JW, Jackowski S. Phospholipid biosynthesis program underlying membrane expansion during B-lymphocyte differentiation. J Biol Chem. 2007;282:7591–7605. doi: 10.1074/jbc.M608175200. [DOI] [PubMed] [Google Scholar]
- 6.Sriburi R, Bommiasamy H, Buldak GL, Robbins GR, Frank M, Jackowski S, Brewer JW. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J Biol Chem. 2007;282:7024–7034. doi: 10.1074/jbc.M609490200. [DOI] [PubMed] [Google Scholar]
- 7.Brewer JW. Phospholipids: “greasing the wheels” of humoral immunity. Biochim Biophys Acta. 2013;1831:642–651. doi: 10.1016/j.bbalip.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kent C. Eukaryotic phospholipid biosynthesis. Annu Rev Biochem. 1995;64:315–343. doi: 10.1146/annurev.bi.64.070195.001531. [DOI] [PubMed] [Google Scholar]
- 9.Rush JS, Sweitzer T, Kent C, Decker GL, Waechter CJ. Biogenesis of the endoplasmic reticulum in activated B lymphocytes: temporal relationships between the induction of protein N-glycosylation activity and the biosynthesis of membrane protein and phospholipid. Arch Biochem Biophys. 1991;284:63–70. doi: 10.1016/0003-9861(91)90264-j. [DOI] [PubMed] [Google Scholar]
- 10.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 11.Wen XY, Stewart AK, Sooknanan RR, Henderson G, Hawley TS, Reimold AM, Glimcher LH, Baumann H, Malek LT, Hawley RG. Identification of c-myc promoter-binding protein and X-box binding protein 1 as interleukin-6 target genes in human multiple myeloma cells. Int J Oncol. 1999;15:173–178. doi: 10.3892/ijo.15.1.173. [DOI] [PubMed] [Google Scholar]
- 12.Hess DA, Humphrey SE, Ishibashi J, Damsz B, Lee AH, Glimcher LH, Konieczny SF. Extensive pancreas regeneration following acinar-specific disruption of Xbp1 in mice. Gastroenterology. 2011;141:1463–1472. doi: 10.1053/j.gastro.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adolph TE, Tomczak MF, Niederreiter L, Ko HJ, Bock J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M, Hartwig J, Hosomi S, Flak MB, Cusick JL, Kohno K, Iwawaki T, Billmann-Born S, Raine T, Bharti R, Lucius R, Kweon MN, Marciniak SJ, Choi A, Hagen SJ, Schreiber S, Rosenstiel P, Kaser A, Blumberg RS. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272–276. doi: 10.1038/nature12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AH, Chu GC, Iwakoshi NN, Glimcher LH. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24:4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 16.McHeyzer-Williams LJ, Driver DJ, McHeyzer-Williams MG. Germinal center reaction. Curr Opin Hematol. 2001;8:52–59. doi: 10.1097/00062752-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Fagone P, Gunter C, Sage CR, Gunn KE, Brewer JW, Jackowski S. CTP:phosphocholine cytidylyltransferase alpha is required for B-cell proliferation and class switch recombination. J Biol Chem. 2009;284:6847–6854. doi: 10.1074/jbc.M807338200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 20.Casola S, Cattoretti G, Uyttersprot N, Koralov SB, Seagal J, Hao Z, Waisman A, Egert A, Ghitza D, Rajewsky K. Tracking germinal center B cells expressing germ-line immunoglobulin gamma1 transcripts by conditional gene targeting. Proc Natl Acad Sci U S A. 2006;103:7396–7401. doi: 10.1073/pnas.0602353103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Carter RH. CD19 regulates B cell maturation, proliferation, and positive selection in the FDC zone of murine splenic germinal centers. Immunity. 2005;22:749–761. doi: 10.1016/j.immuni.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Shih TA, Meffre E, Roederer M, Nussenzweig MC. Role of BCR affinity in T cell dependent antibody responses in vivo. Nat Immunol. 2002;3:570–575. doi: 10.1038/ni803. [DOI] [PubMed] [Google Scholar]
- 23.Dal Porto JM, Haberman AM, Kelsoe G, Shlomchik MJ. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J Exp Med. 2002;195:1215–1221. doi: 10.1084/jem.20011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Magdaleno S, Tabas I, Jackowski S. Early embryonic lethality in mice with targeted deletion of the CTP:phosphocholine cytidylyltransferase alpha gene (Pcyt1a) Mol Cell Biol. 2005;25:3357–3363. doi: 10.1128/MCB.25.8.3357-3363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackowski S, Rehg JE, Zhang YM, Wang J, Miller K, Jackson P, Karim MA. Disruption of CCTbeta2 expression leads to gonadal dysfunction. Mol Cell Biol. 2004;24:4720–4733. doi: 10.1128/MCB.24.11.4720-4733.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroese FG, Wubbena AS, Seijen HG, Nieuwenhuis P. Germinal centers develop oligoclonally. Eur J Immunol. 1987;17:1069–1072. doi: 10.1002/eji.1830170726. [DOI] [PubMed] [Google Scholar]
- 27.McGehee AM, Dougan SK, Klemm EJ, Shui G, Park B, Kim YM, Watson N, Wenk MR, Ploegh HL, Hu CC. XBP-1-deficient plasmablasts show normal protein folding but altered glycosylation and lipid synthesis. J Immunol. 2009;183:3690–3699. doi: 10.4049/jimmunol.0900953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todd DJ, McHeyzer-Williams LJ, Kowal C, Lee AH, Volpe BT, Diamond B, McHeyzer-Williams MG, Glimcher LH. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J Exp Med. 2009;206:2151–2159. doi: 10.1084/jem.20090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackowski S. Cell cycle regulation of membrane phospholipid metabolism. J Biol Chem. 1996;271:20219–20222. doi: 10.1074/jbc.271.34.20219. [DOI] [PubMed] [Google Scholar]
- 30.van der Sanden MH, Houweling M, van Golde LM, Vaandrager AB. Inhibition of phosphatidylcholine synthesis induces expression of the endoplasmic reticulum stress and apoptosis-related protein CCAAT/enhancer-binding protein-homologous protein (CHOP/GADD153) Biochem J. 2003;369:643–650. doi: 10.1042/BJ20020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang D, Tang W, Yao PM, Yang C, Xie B, Jackowski S, Tabas I. Macrophages deficient in CTP:Phosphocholine cytidylyltransferase-alpha are viable under normal culture conditions but are highly susceptible to free cholesterol-induced death. Molecular genetic evidence that the induction of phosphatidylcholine biosynthesis in free cholesterol-loaded macrophages is an adaptive response. J Biol Chem. 2000;275:35368–35376. doi: 10.1074/jbc.M007099200. [DOI] [PubMed] [Google Scholar]
- 32.Horikawa K, Martin SW, Pogue SL, Silver K, Peng K, Takatsu K, Goodnow CC. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J Exp Med. 2007;204:759–769. doi: 10.1084/jem.20061923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrington RA, Pozdnyakova O, Zafari MR, Benjamin CD, Carroll MC. B lymphocyte memory: role of stromal cell complement and FcgammaRIIB receptors. J Exp Med. 2002;196:1189–1199. doi: 10.1084/jem.20021110. [DOI] [PMC free article] [PubMed] [Google Scholar]


