Abstract
Background
Accelerometers can objectively measure steps taken per day in individuals without gait deficits, but accelerometers also have the ability to estimate frequency, intensity, and duration of physical activity. However, thresholds to distinguish varying levels of activity intensity using the Actical brand accelerometer are standardized only for the general population and may underestimate intensity in stroke.
Objective
To derive Actical activity count thresholds specific to stroke disability for use in more accurately gauging time spent at differing activity levels.
Methods
Men (n=18) and women (n=10) with chronic hemiparetic gait (4±2 years latency, 43% Caucasian, 56% African American, ages of 47–83 yrs, BMI 19 – 48 kg/m2) participated in the study. Actical accelerometers were placed on the non-paretic hip to obtain accelerometry counts during eight activities of varying intensity: 1) watching TV; 2) seated stretching; 3) standing stretching; 4) floor sweeping; 5) stepping in place; 6) over-ground walking; 7) lower speed treadmill walking (1.0 mph at 4% incline); and 8) higher speed treadmill walking (2.0 mph at 4% incline). Simultaneous portable monitoring (Cosmed K4b2) enabled quantification of energy cost for each activity in metabolic equivalents (METs, or oxygen consumption in multiples of resting level). Measurements were obtained for 10 min of standard rest and 5 minutes during each of the eight activities.
Results
Regression analysis yielded the following new stroke-specific Actical minimum thresholds: 125 counts per minute (cpm) for sedentary/light activity, 667 cpm for light/moderate activity, and 1546 cpm for moderate/vigorous activity.
Conclusion
Our revised cut-points better reflect activity levels after stroke and suggest significantly lower thresholds relative to those observed for the general population of healthy individuals. We conclude that the standard, commonly applied Actical thresholds are inappropriate for this unique population.
Keywords: Accelerometry, Physical Activity, Chronic Stroke
Regular physical activity (PA) results in decreased stroke incidence1, 2 and prevents physical deconditioning common post-stroke.3 However, efforts to enhance both the amount and quality/intensity of PA after stroke are often confounded by physical disability, with other factors such as intrinsic motivation, mood, adaptability, coping skill, cognition, learning ability, comorbidities, and exposure to clinical rehabilitation training also playing a role.4 Since nearly three-quarters of strokes occur in adults over the age of 65,5 aging also adds to the complexity of PA promotion. Older adults engage in different PA patterns than younger adults6 and may have more difficulty accurately recalling PA participation and patterns.7 Stroke is the leading cause of serious long-term disability in aging Americans,8 and the challenge of altering PA patterns in this special population is daunting. The first and most important step in this process is arriving at a method that accurately portrays PA characteristics among stroke patients both in their natural state and in response to any intervention that may be applied in this context. Only then are we well positioned to test approaches towards promoting PA in stroke.
Previous studies suggest that self-report methods of PA quantification drastically overestimate actual levels compared to more objective tools such as pedometers or accelerometers.9 Accelerometers are electromechanical device that measure acceleration forces. Similar to pedometers, accelerometers can objectively and accurately measure steps taken per day in individuals with and without gait deficits10, but accelerometers also have the ability to estimate frequency, intensity, and duration of PA. However, accelerometers are only reliable at profiling the time spent at various PA levels/intensities if carefully calibrated and validated in the specific population of interest. A “one-size-fits-all” approach to characterizing PA patterns with accelerometers has potential for causing misperceptions about PA patterns, particularly in stroke and other age-related disability conditions.
Actical accelerometry thresholds exist for healthy adults, estimating time spent on PA across a range of intensities.11, 12 However, stroke-specific Actical thresholds have not been established, despite the clear need.13 Gait asymmetry and poor gait biomechanics confound the use of healthy adult Actical thesholds, as shown with other accelerometer brands.14 Hence, the purpose of the present study was to define Actical accelerometry thresholds for the first time in those with mild-moderate stroke disability.
Methods
Stroke participants with residual hemiparetic gait abnormalities (>45 years old, >6 months latency) were recruited for an ongoing exercise intervention study from neurology clinics in the Baltimore Veterans Affairs Medical Center, the University of Maryland Medical System, and surrounding Baltimore region. After written informed consent was obtained, participants underwent medical screening with a comprehensive history and physical/neurological examination and a graded exercise treadmill test to determine eligibility for participation. Exclusion criteria included congestive heart failure, unstable angina, peripheral vascular disease, orthopedic conditions, and other medical or neuropsychiatric conditions (e.g. significant dementia) limiting participation in aerobic exercise to assure participant safety during testing and training procedures. This study was approved by the Institutional Review Board and all participants gave informed consent.
Body Composition
Height and body weight were measured and body mass index (BMI, weight [kg]/height [m2]) calculated. Total body fat percentage, fat mass, and lean body mass were determined by dual-energy X-ray absorptiometry (iDXA, LUNAR Radiation Corp., Madison, WI).
Test of Physical Performance
VO2peak was measured to assess cardiorespiratory fitness using a graded treadmill test as previously described.15 Resting (HRrest) and peak (HRpeak) heart rates were recorded and the Karvonen formula used to calculate heart rate reserve (HRR: HRpeak- HRrest)).16 Standard procedures as previously reported were followed for determination of walking capacity (6-minute walking test [6MWT]).17 To increase safety, all participants wore a gait belt and used the same assistive devices and/or orthoses that they used when walking at home.
Participants also performed various activities of daily living (ADLs) with simultaneous indirect calorimetry assessments, as described below. The eight ADLs were chosen to capture a range of activity level from sedentary to vigorous and included 10 minutes of standard rest and 5 minutes of the following: 1) watching tv, 2) seated stretching, 3) standing stretching, 4) floor sweeping, 5) stepping in place, 6) walking around a track, and 7) walking on a treadmill at 4% incline at 1 mph (lower intensity) and 8) walking on a treadmill at 4% incline at 2 mph (higher intensity). Verbal and visual instructions were provided from a script to standardize the pacing and performance of each ADL task. For the seated and standing stretches, participants stretched their hamstrings (instructed to reached toward toes) and trunk (instructed to twist sternum to look over each shoulder), respectively. Participants alternated stretching to each side for 30 seconds. For floor sweeping, small pieces of paper were scattered on the gym floor and participants were asked to sweep it into a pile, which was repeated for the five minute duration. For stepping in place, participants were instructed to keep pace with a metronome. For walking around the track, participants were instructed to walk at their self-selected/normal walking speed around a 100 m track. All activities were tested during a single visit in the same order across subjects. Four participants did not perform the treadmill walking tasks as these tests were deemed above the safe functional capacity level of the participant by study personnel (i.e. the participant was unable to safely walk unassisted for 5 minutes at a particular speed and/or incline and assistance would have affected the intensity of the activity). Tasks were separated by at least two minutes of sitting. Actical accelerometer monitoring devices (Mini-Mitter Co, Bend, OR) were worn during each of the eight ADL tasks to capture activity counts across the continuum of activity levels. Monitors were secured over the non-paretic anterior-superior iliac spine with elastic belts as the hip is recognized as more reliable than other sites (i.e. wrist and ankle) at predicting activity intensity thresholds.18, 19 Further, there is evidence that similar results are observed if accelerometers are worn on the paretic vs. non-paretic hip13; however, we chose the non-paretic hip for consistency and to ensure that movement was captured by the device. The Acticals were initialized with 60 sec epoch lengths to determine counts per minute (cpm).
Indirect Calorimetry
Open circuit spirometry (K4b2; COSMED USA) was used to provide breath-by-breath cardiopulmonary data during all ADL activities, using methods previously described.20 In brief, the device was warmed up for 45 minutes and calibrated using a 3 liter syringe prior to testing. Following this, the O2 and CO2 analyzers were calibrated using a reference gas of known concentrations. Testing was initiated within 10 minute of completing calibration of the metabolic system. A snug fitting face mask was placed on participants and they underwent a period of acclimatization with the portable gear prior to performing the designated activities. Each participant’s mean oxygen consumption (VO2) for the eight activity types was determined and converted to Metabolic Equivalents of Task (METs; 1 MET=3.5 ml/kg/min). In addition, continuous heart rate monitoring provided a mean steady-state heart rate for each test.
Statistical Analyses
Occasional equipment malfunction resulted in some missing cardiopulmonary data points, but all participants had representative values for resting and at least six of the eight ADL tasks. Therefore, all available data were used in the analysis. The %VO2peak (VO2peak/mean VO2 during each designated ADL activity), %HRpeak (HRpeak/mean HR during each designated ADL activity), and %HRR (HR during each designated ADL activity/[HRpeak – HRrest) + HRrest]) achieved for each individual during each task was calculated and utilized towards developing new Actical cutpoints. We utilized regression analysis to find the Actical cpm that corresponded with differentiating levels of METs, %VO2peak, %HRpeak, and %HRR stemming from each activity. Data was analyzed using SPSS (PAWS Statistics, Version 18, Chicago IL). Data are reported as mean±SEM and significance was set at P<0.05.
Results
Twenty eight individuals with residual stroke deficits were included in the analyses. Participant characteristics are presented in Table 1. On average, participants were obese by BMI and deconditioned, as evidenced by poor cardiorespiratory fitness per ACSM guidelines.21 Similarly, 6MWT distances among our stroke participants equated to only ~55% of that normally achieved by healthy adults.22 Maximal speed and incline achieved during the VO2peak test ranged from 0.4–2.5 mph (mean: 1.7±0.3 mph) and 2–18% (mean: 11±1.6%), respectively. Usual medications and lifestyle were maintained throughout the testing period.
Table 1.
Participant Characteristics
| Mean±SEM | Range | |
|---|---|---|
| Age (years) | 60.4±1.6 | 47–83 |
| Latency After Stroke (years) | 4.2±1.8 | 1–11 |
| BMI (kg/m2) | 31.5±1.1 | 19–48 |
| Total Body Fat Mass (%) | 37.1±2.4 | 11–50 |
| Total Body Fat Mass (kg) | 39.9±5.3 | 8–102 |
| Total Body Lean Mass (kg) | 57.5±2.9 | 44–80 |
| VO2peak (ml/kg/min) | 19.0±0.98 | 10–28 |
| HRpeak (bpm) | 124±4 | 74–167 |
| 6MWT distance (m) | 299±30 | 27–457 |
HRpeak: peak heart rate; MWT: minute walk test
Based upon intensity thresholds adapted from ACSM guidelines,21 our set of tested activities ranged from sedentary to vigorous. Mean range values for all variables across the continuum of selected activities (TV watching to higher speed treadmill walking) were as follows: %VO2peak: 17–65, METs: 1.0–4.2, %HRpeak: 57–78, %HRR: 15–50 (data shown for %VO2peak in Figure 1). Using each predictor variable, in a regression model, we determined a curvilinear relationship between physiological intensity variables and Actical cpm. Then, because METs represented the strongest predictor variable for Actical cpm (METs: r=0.65, %VO2peak: r=0.49, %HRpeak: r=0.33, %HRR: r=0.32; all P’s<0.01), we used METs to calculate the new cpm thresholds as follows: 125 cpm for sedentary/light, 667 cpm for light/moderate, and 1546 cpm for moderate/vigorous PA. The graph and predicative equation depicting METs vs. Actical cpm is provided in Figure 2.
Figure 1.
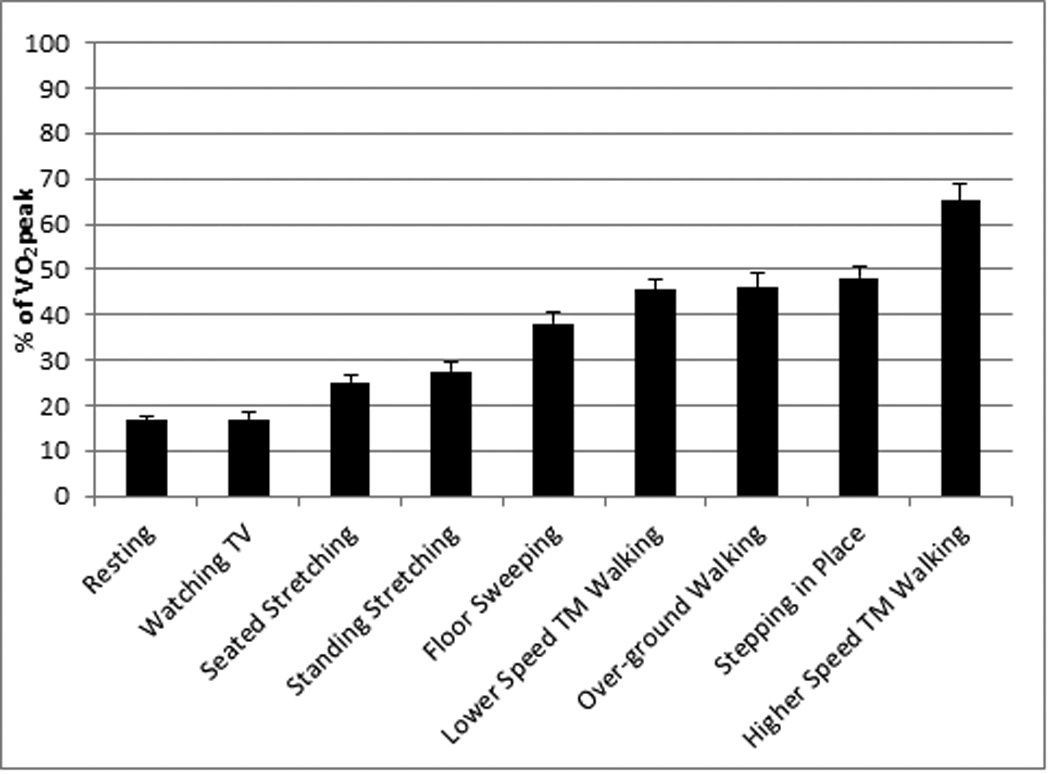
This figure provides evidence that the activities performed range from sedentary to vigorous.
Figure 2.
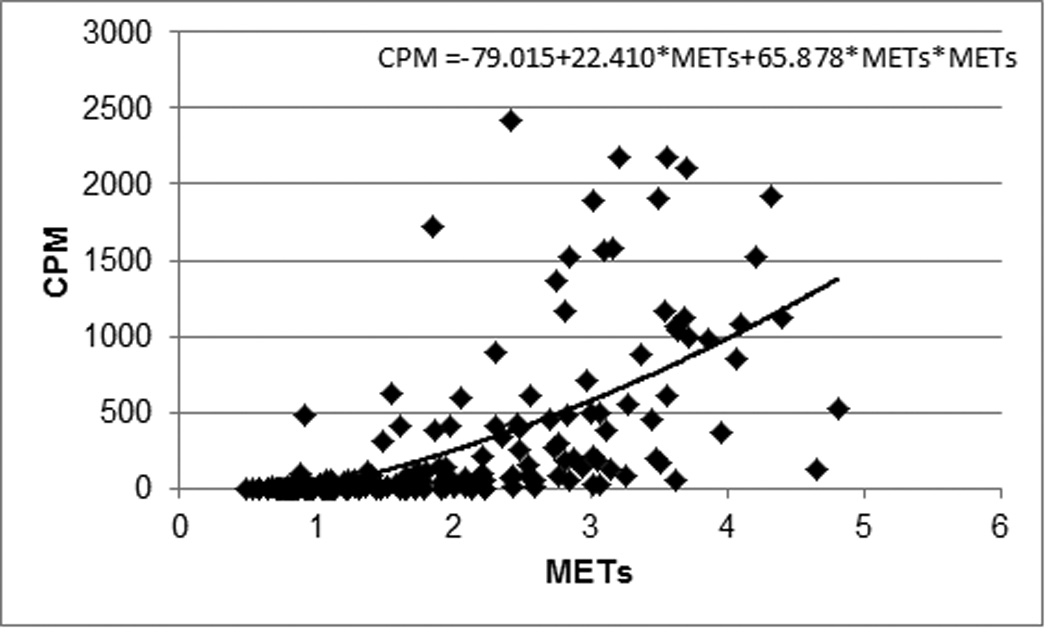
Regression demonstrating a significant curvilinear relationship between METs achieved with indirect calorimetry and counts per minute by Actical accelerometry.
Discussion
Determining stroke-specific Actical accelerometry intensity thresholds is of paramount importance towards better understanding activity behavior patterns and the impact of physical activity interventions in this special population. Although Actical accelerometers are typically viewed as the gold standard in PA assessment, having proven more reliable than other PA quantification methods,23 associated activity intensity thresholds are only established in healthy adults.11, 12 We are the first to determine activity intensity thresholds in individuals with neurological gait following stroke.
In younger adults without neurological deficits or mobility impairment, Actical intensity thresholds are set as follows: 100 cpm for sedentary/light, 1535 cpm for light/moderate, and 3960 cpm for moderate/vigorous PA.11, 12 In older adults (>45 years), the cutpoint for light/moderate is moved slightly lower, with studies suggesting about a 1,070 cpm to demarcate that threshold.24, 25 Our data in stroke indicate that the previously established thresholds from healthy, older adults, while somewhat adequate for distinguishing sedentary/light activity from higher intensity activity, drastically underestimates energy expenditure during more intense activities (~2 fold). Additionally, our findings suggest that the threshold for moderate/vigorous activity in stroke survivors with hemiparetic gait is equivalent to the threshold for light/moderate activity for adults without gait deficits.
Since stroke survivors report activity limitations extending years into the chronic phase of stroke recover, 26 it is important that we consider stroke appropriate tasks in our assessment of PA. Thus, several activities that typically span the range of “low” activity were selected for the current study. This decision also was influenced by literature suggesting that accelerometers are less accurate at estimating low level activities 27. A self-selected walking speed of 2.0 mph is often used as a speed that reflects transition from light to moderate activity due to prior reports that healthy, older adults (40+ years) typically chose a self-selected walking speed of 1.5–2.5 mph and 2.0–3.0 mph when asked to walk at a slow/strolling or normal/“walking for exercise” pace, respectively.25, 28 However, due to the increased energy cost of hemiparetic ambulation post-stroke,29 a pace of 2.0 mph is more vigorous in individuals with a gait deficit. Indeed, our data suggests that when stroke survivors walk at an intensity of 2.0 mph (at only 4% grade) they are exercising at a vigorous intensity. This point further emphasizes that energy cost for any given task is substantively higher in stroke compared with the general population of healthy, non-disabled adults. This higher energy cost of activity confirms the need for stroke-specific Actical thresholds to differentiate time spent on differing activity levels when a gait deficit is present. By design, our sequence of activity testing progressed gradually, with no more than a 10–15% increase in %VO2peak, %HRpeak, and %HRR observed between activity intensity levels, thereby enabling full characterization of each step in the range from low to high after stroke.
Our newly proposed Actical activity thresholds for stroke have several limitations. First, both racial30 and gender31 differences have been observed with regard to self-selected walking speed and may indicate the need for subgroup analyses. However, we were unable to perform these secondary analyses due to the relatively small sample size. Although our objective cardiopulmonary measurements confirm that activities tested spanned the full range of intensity, from sedentary to vigorous, we did not include assessment of subjective ratings of perceived exertion, which might have allowed for even more confidence by accounting for perception of effort. Thus, future studies should aim for larger sample size, inclusion of both subjective and objective measures of exercise intensity, and consideration of gender/race subgroup analyses. Our current study was strengthened by inclusion of a wide range of ages, BMIs, and functional abilities. We have previously shown relationships between lean tissue mass, functional impairments, and VO2peak,32 indicating that including individuals across a spectrum of body composition and functional ability is beneficial in terms of generalizability. Similar body composition and functional impairments in chronic stroke are reported by other groups.13, 33
In summary, our new Actical thresholds provide cut points that are more relevant in the context of stroke and neurological deficits. This is especially true for higher intensity activities. These stroke-specific thresholds will allow researchers to more accurately assess PA patterns and monitor PA changes in stroke patients prone to sedentary behavior and poor metabolic health.
Acknowledgments
Our appreciation is extended to the volunteers who participated in this study. We are grateful to the medical team and exercise physiologists of the University of Maryland School of Medicine Division of Gerontology and Geriatric Medicine and Baltimore VA GRECC and MERCE for their assistance in this project.
Sources of Funding: This study was supported by Veterans Affairs (VA) Merit, Senior Research Career Scientist, and CDA-2 Awards, NIH R01-AG030075, NIH 5T35AG036679, the National Institute on Aging (NIA) Claude D. Pepper Older Americans Independence Center (P30-AG028747), Baltimore VA Research Service, Geriatric Research, Education and Clinical Center (GRECC), Maryland Exercise and Robotics Center of Excellence (MERCE).
Footnotes
Clinical Trial Registration: Clinical Trial Registration-URL: http://www.clinicaltrials.gov. Unique identifier: NCT00891514
Conflicts of Interest/Disclosures: None.
References
- 1.Sacco RL, Gan R, Boden-Albala B, et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;29(2):380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 2.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 3.Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke. 1997;28(2):326–330. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 4.Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–2553. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 5.The Internet Stroke Center. [Accessed April 10, 2015];Stroke Statistics. http://www.strokecenter.org/patients/about-stroke/stroke-statistics/
- 6.Murphy SL. Review of physical activity measurement using accelerometers in older adults: considerations for research design and conduct. Preventive Medicine. 2009;48(2):108–114. doi: 10.1016/j.ypmed.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kowalski K, Rhodes R, Naylor PJ, Tuokko H, MacDonald S. Direct and indirect measurement of physical activity in older adults: a systematic review of the literature. The International Journal of Behavioral Nutrition and Physical Activity. 2012;9:148. doi: 10.1186/1479-5868-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 9.Hagstromer M, Oja P, Sjostrom M. Physical activity and inactivity in an adult population assessed by accelerometry. Medicine and Science in Sports and Exercise. 2007;39(9):1502–1508. doi: 10.1249/mss.0b013e3180a76de5. [DOI] [PubMed] [Google Scholar]
- 10.Motl RW, Snook EM, Agiovlasitis S. Does an accelerometer accurately measure steps taken under controlled conditions in adults with mild multiple sclerosis? Disabil Health J. 2011;4(1):52–57. doi: 10.1016/j.dhjo.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Wong SL, Colley R, Connor Gorber S, Tremblay M. Actical accelerometer sedentary activity thresholds for adults. Journal of Physical Activity & Health. 2011;8(4):587–591. doi: 10.1123/jpah.8.4.587. [DOI] [PubMed] [Google Scholar]
- 12.Colley RC, Tremblay MS. Moderate and vigorous physical activity intensity cut-points for the Actical accelerometer. Journal of Sports Sciences. 2011;29(8):783–789. doi: 10.1080/02640414.2011.557744. [DOI] [PubMed] [Google Scholar]
- 13.Rand D, Eng JJ, Tang PF, Jeng JS, Hung C. How active are people with stroke?: use of accelerometers to assess physical activity. Stroke. 2009;40(1):163–168. doi: 10.1161/STROKEAHA.108.523621. [DOI] [PubMed] [Google Scholar]
- 14.Haeuber E, Shaughnessy M, Forrester LW, Coleman KL, Macko RF. Accelerometer monitoring of home- and community-based ambulatory activity after stroke. Arch Phys Med Rehabil. 2004;85(12):1997–2001. doi: 10.1016/j.apmr.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Macko RF, Katzel LI, Yataco A, et al. Low-velocity graded treadmill stress testing in hemiparetic stroke patients. Stroke. 1997;28(5):988–992. doi: 10.1161/01.str.28.5.988. [DOI] [PubMed] [Google Scholar]
- 16.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate a longitudinal study. Annales Medicinae Experimentalis et Biologiae Fenniae. 1957;35(3):307–315. [PubMed] [Google Scholar]
- 17.Enright PL. The six-minute walk test. Respiratory Care. 2003;48(8):783–785. [PubMed] [Google Scholar]
- 18.Rosenberger ME, Haskell WL, Albinali F, Mota S, Nawyn J, Intille S. Estimating activity and sedentary behavior from an accelerometer on the hip or wrist. Medicine and Science in Sports andEexercise. 2013;45(5):964–975. doi: 10.1249/MSS.0b013e31827f0d9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleland I, Kikhia B, Nugent C, et al. Optimal placement of accelerometers for the detection of everyday activities. Sensors (Basel) 2013;13(7):9183–9200. doi: 10.3390/s130709183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stookey AD, McCusker MG, Sorkin JD, et al. Test-retest reliability of portable metabolic monitoring after disabling stroke. Neurorehabilitation and Neural Repair. 2013;27(9):872–877. doi: 10.1177/1545968313497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ACSM's Guidelines for Exercise Testing and Prescription. 9th. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 22.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. American Journal of Respiratory and Critical Care Medicine. 1998;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 23.Esliger DW, Tremblay MS. Technical reliability assessment of three accelerometer models in a mechanical setup. Medicine and Science in Sports and Exercise. 2006;38(12):2173–2181. doi: 10.1249/01.mss.0000239394.55461.08. [DOI] [PubMed] [Google Scholar]
- 24.Hooker SP, Feeney A, Hutto B, et al. Validation of the actical activity monitor in middle-aged and older adults. Journal of Physical Activity & Health. 2011;8(3):372–381. doi: 10.1123/jpah.8.3.372. [DOI] [PubMed] [Google Scholar]
- 25.Trumpeter NN, Lawman HG, Wilson DK, Pate RR, Van Horn ML, Tate AK. Accelerometry cut points for physical activity in underserved African Americans. The International Journal of Behavioral Nutrition and Physical Activity. 2012;9:73. doi: 10.1186/1479-5868-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadidi V, Katz-Leurer M, Carmeli E, Bornstein NM. Long-term outcome poststroke: predictors of activity limitation and participation restriction. Arch Phys Med Rehabil. 2011;92(11):1802–1808. doi: 10.1016/j.apmr.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Calabro MA, Lee JM, Saint-Maurice PF, Yoo H, Welk GJ. Validity of physical activity monitors for assessing lower intensity activity in adults. The International Journal of Behavioral Nutrition and Physical Activity. 2014;11:119. doi: 10.1186/s12966-014-0119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Medicine and Science in Sports and Exercise. 1988;20(2):161–166. doi: 10.1249/00005768-198820020-00010. [DOI] [PubMed] [Google Scholar]
- 29.Gersten JW, Orr W. External work of walking in hemiparetic patients. Scand J Rehabil Med. 1971;3(1):85–88. [PubMed] [Google Scholar]
- 30.Sims EL, Keefe FJ, Kraus VB, Guilak F, Queen RM, Schmitt D. Racial differences in gait mechanics associated with knee osteoarthritis. Aging Clinical and Experimental Research. 2009;21(6):463–469. doi: 10.1007/bf03327442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolea MI, Costa PT, Terracciano A, et al. Sex-specific correlates of walking speed in a wide age-ranged population. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2010;65B(2):174–184. doi: 10.1093/geronb/gbp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan AS, Dobrovolny CL, Silver KH, Smith GV, Macko RF. Cardiovascular fitness after stroke: Role of muscle mass and gait deficit severity. J Stroke Cerebrovasc Dis. 2000;9(4):185–191. doi: 10.1053/jscd.2000.7237. [DOI] [PubMed] [Google Scholar]
- 33.Pang MY, Ashe MC, Eng JJ. Tibial bone geometry in chronic stroke patients: influence of sex, cardiovascular health, and muscle mass. J Bone Miner Res. 2008;23(7):1023–1030. doi: 10.1359/jbmr.080224. [DOI] [PMC free article] [PubMed] [Google Scholar]


