Abstract
Objective
The objective of this pilot study was to assess the safety and efficacy of short-term meal replacement therapy followed by topiramate for body mass index (BMI) reduction in adolescents with severe obesity.
Methods
Adolescents (ages 12-18 years) with severe obesity (BMI ≥1.2 times the 95th percentile or BMI ≥35 kg/m2) were recruited for this double-blind, randomized, placebo-controlled trial. Participants completed 4 weeks of meal replacement therapy followed by randomization (1:1) to either 24 weeks of topiramate 75 mg/day or placebo. Mean changes were compared between groups.
Results
Thirty adolescents (mean age 15.2 ± 1.7 years, mean BMI 40.3 ± 4.6 kg/m2) completed the meal replacement phase and were randomized; 21 completed the study. The difference in mean percent change in BMI between the topiramate and placebo groups was not significant (−1.9% [95% CI (−5.2%, +1.5%); P=0.291]). Significant improvements in visceral fat and VLDL-c were observed in the topiramate compared to the placebo group. There were no concerning changes in neurocognitive function or bone health.
Conclusion
In this pilot study, 4 weeks of meal replacement therapy followed by 24 weeks of low-dose topiramate compared to meal replacement therapy alone did not result in significant BMI reduction for adolescents with severe obesity.
Keywords: pharmacotherapy, adolescents, obesity, topiramate, meal replacement
Introduction
Severe pediatric obesity, defined as an age- and gender-specific body mass index (BMI) ≥1.2 times the 95th percentile or BMI ≥ 35 kg/m2,1,2 affects approximately 6% of children and adolescents in the United States and the prevalence is rising.1,3,4 Unfortunately, adolescents with severe obesity often reap little benefit from lifestyle modification therapy, the cornerstone of obesity treatment.5-9 Thus, novel treatment strategies are desperately needed for this high risk population. Meal replacement therapy, as an adjunct to lifestyle modification therapy, is one such alternative strategy. Specifically, meal replacement therapy consisting of liquid shakes, meal bars, and frozen entrees of a fixed caloric content, can result in meaningful short-term BMI reduction (approximately 6%), yet the effect tends to wane over time.10 In contrast, meal replacement therapy followed by obesity pharmacotherapy may prove to be a more effective longer term treatment for achieving sustained BMI reduction.
Currently, the only obesity pharmacotherapy approved by the U.S. Food and Drug Administration (FDA) in the pediatric population (age ≥ 12 years) is orlistat. Yet, the use of orlistat is limited by an adverse side effect profile and marginal efficacy.11 Therefore, identification and evaluation of other pharmacotherapies that have the potential to reduce adiposity and improve cardiometabolic outcomes in youth with severe obesity are needed. Topiramate, an antiepileptic agent FDA-approved for the treatment of seizures in children, may be a candidate medication. Our group conducted a retrospective chart review of patients treated with topiramate in a pediatric weight management clinic and found a 6% average reduction in BMI at 6 months.12 Although never studied prospectively for the indication of weight loss in the pediatric population to our knowledge, several large randomized, controlled trials examining topiramate for obesity in adults have demonstrated weight reduction of 5-10% over 24-60 weeks.13-16
The purpose of this pilot study was to assess the safety and efficacy of short-term meal replacement therapy followed by topiramate for BMI reduction in adolescents with severe obesity in order to inform decisions about a larger clinical trial. Specifically, the primary objective of this randomized, placebo-controlled pilot clinical trial was to compare the percent change in BMI from baseline among participants assigned to meal replacement therapy followed by topiramate versus meal replacement therapy followed by placebo. Our secondary objectives were to a) characterize the safety profile of topiramate for obesity treatment in adolescents, b) evaluate the effect of the intervention on cardiometabolic risk factors, and c) investigate whether baseline binge eating behavior influences treatment response, given that some studies indicate that topiramate decreases binge eating frequency and weight in people with binge eating disorder.17-19 We hypothesized that following short-term meal replacement therapy, 24 weeks of topiramate compared to placebo would demonstrate superior reduction in BMI, total body- and visceral fat, systolic blood pressure, fasting triglycerides and insulin. Also, we hypothesized that topiramate would be well tolerated at 75 mg daily and that the presence of binge eating disorder characteristics at baseline would be associated with a greater reduction in BMI with topiramate treatment.
Methods
Trial Design and Eligibility Criteria
This was a 28-week double-blind, randomized, placebo-controlled pilot clinical trial that included 4-weeks of meal replacement therapy followed by 24-weeks of topiramate or placebo without meal replacements. Adolescents 12-18 years old with severe obesity (BMI ≥1.2 times the 95th percentile or BMI ≥ 35 kg/m2) were recruited from four sites comprising the Minnesota Pediatric Obesity Consortium: University of Minnesota Masonic Children's Hospital Pediatric Weight Management Clinic (Minneapolis, MN), McNeely Pediatric Diabetes Center and Endocrinology Clinic at Children's Hospitals and Clinics of Minnesota (St. Paul, MN), International Diabetes Center at Park Nicollet (St. Louis Park, MN), and Mayo Clinic (Rochester, MN). Exclusion criteria were Tanner stage I, II, or III; type 1 or 2 diabetes mellitus; previous (within 6-months) or current use of medication(s) prescribed primarily for weight loss; if currently using weight altering drug(s), any change in drug(s) or dose within the previous 6 months; previous bariatric surgery; recent initiation (within 3-months) of anti-hypertensive or lipid medication; major psychiatric disorder; females who were pregnant, planning to become pregnant, or unwilling to use 2 or more acceptable methods of contraception when engaging in sexual activity throughout the study; tobacco use; liver/renal dysfunction; glaucoma; obesity associated with genetic disorder (monogenetic obesity); hyperthyroidism or uncontrolled hypothyroidism; medically-documented history of suicidal thoughts/attempts; history of nephrocalcinosis or cholelithiasis; and current use of other carbonic anhydrase inhibitor. The protocol was approved by the University of Minnesota institutional review board. Consent and assent were obtained from parents and participants, respectively. An investigational new drug exemption was obtained from the FDA prior to study initiation and the study was registered on the clinicaltrials.gov website (NCT01859013).
Meal Replacement Intervention
Prior to randomization to topiramate or placebo, all participants engaged in meal replacement therapy for 4 weeks. The regimen was adapted from a previously-published protocol used in adolescents with severe obesity.10 During the meal replacement period, participants were counseled to strictly follow the prescribed diet, which included three Slim-Fast® shakes (one for breakfast and two for lunch or vice-versa), two pre-packaged frozen entrée meals for dinner (Weight Watchers, Smart Ones®), two servings of fruit, and three servings of vegetables per day (total caloric intake was approximately 1,400 kcals per day). All shakes and frozen entrees were provided to the participants. Participants were encouraged (but not required) to meet a goal of at least 5% BMI reduction during the meal replacement phase.
Lifestyle Modification/Behavioral Counseling
All participants received lifestyle/behavioral modification counseling throughout the entire study. The curriculum was adapted from the TODAY study lifestyle modification program materials.20 Study staff delivered the lifestyle modification counseling, which focused on small, successive changes in dietary and physical activity behaviors through the use of evidence-based behavior change strategies such as self-monitoring, goal setting, reinforcement for goal achievement, stimulus control, social support, problem solving, and motivational techniques. Counseling was provided on the transition from meal replacements (initial 4 weeks) to regular dietary habits, per the TODAY program. The educational materials were reviewed and discussed at each face-to-face study visit except week 28 (baseline, weeks 2, 4, 12, and 16) and additionally by phone at weeks 8, 20, and 24.
Topiramate and Placebo Intervention
Following the meal replacement period, participants were randomized (1:1) to either topiramate or placebo capsules, which were identical in appearance. Topiramate was initiated at a dose of 25 mg (taken orally once daily in the evening), escalated to 50 mg (taken orally once daily in the evening) after 1 week, and further escalated to 75 mg (taken orally 25 mg in the morning and 50 mg in the evening) after 2 weeks. The randomization scheme was generated based on randomly permuted blocks of size 2, 4, and 6 and maintained by the University of Minnesota – Fairview Investigational Drug Service Pharmacy.
Measurement of Clinical Variables
All study visits and data collection occurred at a single center (University of Minnesota). Height and weight were obtained with participants in light clothes and without shoes using the same standardized stadiometer and electronic scale, respectively. Three consecutive height and weight measurements were averaged. BMI was calculated as the body weight in kilograms divided by the height in meters, squared. Percent total body- and visceral fat were determined using dual-energy x-ray absorptiometry (iDXA, GE Healthcare, Waukesha, WI, USA). Visceral fat measured with this technique has been previously validated.21 Seated blood pressure was obtained after five minutes of quiet rest, on the right arm using an automatic sphygmomanometer and appropriately-fitted cuff. The average of three independent blood pressure measurements was used. Tanner stage (pubertal development) determinations were performed by trained registered nurses. Fasting (≥12 hours) blood samples were analyzed for glucose, insulin, and lipids using standard procedures. Binge eating behaviors were measured with the Loss of Control – Eating Disorder Questionnaire and the Eating Disorder Examination Questionnaire − 6.2.22
Because of concerns regarding associations between antiepileptic medications and both cognitive effects23 and bone mineral density,24 safety outcomes included measures of neurocognitive function and bone health. Changes in neurocognitive function were ascertained with questionnaires and computer-based assessments including the Behavior Rating Inventory of Executive Function – Self Report (BRIEF-SR), Cambridge Neuropsychological Test Automated Battery (CANTAB) (a computerized test of motor speed, memory, and attention), and the Connors Continuous Performance Test II (CPT II) (a computerized measure of attention and impulsivity). Bone density, geometry, and strength were assessed with peripheral quantitative computed tomography (pQCT) (XCT-3000, Stratec Medizintechnik GmbH, Pforzheim, Germany). Images were taken at the distal 4% and along the midshaft (33%) of the non-dominant radius and at the distal 4% and 50% of the tibia. The reference line for both radius and tibia was placed at the proximal end of the distal growth plate using a scout view. Image processing and calculation of bone parameters were completed using the manufacturer's software (version 6.0). Bone outcome measures included volumetric bone mineral density (vBMD, mg/cm3), trabecular cross-sectional area (CSA, mm2), total bone strength index (BSI, mg2/mm4), cortical thickness (mm), non-weighted polar section modulus (Zp, mm3), and strength strain index (SSI, mm3).25
Anthropometrics, blood pressure, heart rate, complete metabolic panel, urine pregnancy test and questionnaires were measured at all face-to-face visits (baseline, weeks 2, 4, 12, 16, and 28). Additionally, iDXA, pQCT, glucose, lipids, and insulin were measured at baseline and at week 28. CANTAB, BRIEF-SR, and CPT II were measured at randomization and at week 28.
Statistical methods
The sample size was based primarily on the preliminary nature of the trial (a pilot study), along with limitations of the funding and recruitment timeline associated with the grant support. Baseline characteristics were tabulated with respect to randomized treatment groups using mean (SD) for continuous variables and frequency (%) for categorical variables. The primary endpoint was the mean percent change in BMI from baseline (week 0) to 28 weeks. Secondary efficacy endpoints were defined similarly while secondary safety endpoints were examined from randomization (after 4 weeks meal replacement) to 28 weeks. Analyses of primary and secondary endpoints were based on generalized linear models, adjusting for baseline measurements for added precision.26,27 Confidence intervals and P-values were evaluated based on robust variance estimation. Due to incomplete follow-up on all participants who entered the study, some final values were imputed based on the most recent prior measurement during follow-up to preserve the pre-specified intent-to-treat analysis. For example, when measurements were available from the week 16 visit but not the final 28 week visit, values from the week 16 visit were carried forward and used. A pre-specified sub-group analysis to examine differential treatment effect based on eating behavior (binge eating) was ultimately not conducted due to insufficient number meeting the criteria at baseline. Data were managed in REDCap28 and all statistical analyses were performed using R v3.2.0.29
Results
Recruitment occurred between July 2013 and May 2015. Thirty-seven participants were assessed for eligibility and 30 were enrolled in the trial (Figure 1). All 30 participants completed the 4-week meal replacement phase. Twelve from the topiramate group and nine from the placebo group completed the 24-week randomized phase. The intervention was stopped by the study physician for one participant from the topiramate group (see details in “Safety” section, below) but the participant attended all follow-up visits and remained blinded until trial completion.
Figure 1.
CONSORT Diagram.
Baseline (week 0) demographic characteristics, cardiometabolic risk factors, and binge eating score between the topiramate and placebo groups were similar (Table 1a). The mean age of participants was 15.2 years, mean BMI was 40.3 kg/m2 and 63% were female. Only 3 participants endorsed binge eating, defined as overeating with loss of control at least 4 times in the past month. Randomization visit (week 4) safety measures, including standard scores of learning and memory (CANTAB), executive function (BRIEF), attention, impulsivity, and reaction time (CPT II) were all in the average range (Table 1b). See Supplemental Table 1a for pQCT measures at randomization.
Table 1a.
Participant characteristics at baseline. Values expressed are mean (SD) or N (%) where indicated.
| Covariate | Overall | Placebo | Topiramate |
|---|---|---|---|
| (N=30) | (N=14) | (N=16) | |
| Female | 19 (63.3%) | 9 (64.3%) | 10 (62.5%) |
| African American/Black | 4 (13.3%) | 1 (7.1%) | 3 (18.8%) |
| White | 18 (60.0%) | 8 (57.1%) | 10 (62.5%) |
| Other | 8 (26.7%) | 5 (35.7%) | 3 (18.8%) |
| Age (years) | 15.2 (1.7) | 15.7 (1.8) | 14.9 (1.6) |
| Height (cm) | 168 (8.0) | 169 (7.5) | 168 (8.7) |
| Weight (kg) | 115 (19.9) | 112 (15.3) | 117 (23.5) |
| BMI (kg/m2) | 40.3 (4.6) | 39.5 (4.0) | 41.0 (5.0) |
| BMI z-score | 2.5 (0.26) | 2.5 (0.24) | 2.6 (0.26) |
| Percent of the 95th BMI Percentile | 146 (17.1) | 141 (15.6) | 150 (17.7) |
| Total Fat Mass (kg) | 54.8 (12.0) | 51.2 (10.3) | 57.9 (12.9) |
| Percent Body Fat (%) | 49.0 (4.8) | 47.0 (5.6) | 50.8 (3.2) |
| Visceral Fat Mass (g) | 1320 (626.9) | 1189 (680.0) | 1434 (573.5) |
| Lean Body Mass (kg) | 56.7 (10.4) | 57.5 (9.2) | 56.0 (11.7) |
| Systolic BP (mmHg) | 121 (12.3) | 120 (11.3) | 122 (13.6) |
| Diastolic BP (mmHg) | 67.9 (8.6) | 67.3 (6.2) | 68.4 (10.4) |
| Heart Rate (bpm) | 75.9 (9.4) | 74.2 (8.0) | 77.4 (10.5) |
| Glucose (mg/dL) | 80.7 (10.2) | 79.4 (8.9) | 81.9 (11.3) |
| Insulin (mg/dL) | 21.6 (11.4) | 18.6 (9.2) | 24.3 (12.6) |
| Total cholesterol (mg/dL) | 162 (31.7) | 167 (40.3) | 157 (22.0) |
| LDL-c (mg/dL) | 95.2 (27.1) | 100 (34.8) | 90.3 (17.0) |
| HDL-c (mg/dL) | 42.2 (11.0) | 44.7 (13.4) | 40.0 (8.2) |
| VLDL-c (mg/dL) | 23.1 (11.3) | 21.8 (10.6) | 24.3 (12.1) |
| Triglycerides (mg/dL) | 129 (92.8) | 109 (53.1) | 147 (116.1) |
| ALT (mg/dL) | 37.7 (19.1) | 41.6 (24.1) | 34.2 (13.3) |
| AST (mg/dL) | 24.5 (8.9) | 26.6 (10.0) | 22.7 (7.8) |
| Binge eating ≥ 4 times in past month | 3 (10.0%) | 0 (0.0%) | 3 (18.8%) |
Table 1b.
Safety variables at randomization.
| Covariate | Overall | Placebo | Topiramate |
|---|---|---|---|
| (N=30) | (N=14) | (N=16) | |
| CANTAB | |||
| Standard PAL | 0.51 (0.58) | 0.61 (0.46) | 0.43 (0.67) |
| Standard PAL Shapes | 0.43 (0.81) | 0.60 (0.34) | 0.28 (1.00) |
| Standard PRM | 0.54 (0.62) | 0.44 (0.78) | 0.62 (0.46) |
| Standard SSP* | 0.62 (1.10)2 | 0.71 (0.99)1 | 0.54 (1.30)1 |
|
CPT-II* | |||
| Omissions % | 54.9 (23.6)3 | 59.8 (31.0)1 | 50.4 (13.4)2 |
| Commissions % | 47.3 (14.2)3 | 47.8 (15.7)1 | 46.8 (13.3)2 |
| Hit Reaction Time | 51.9 (14.4)3 | 52.2 (15.2)1 | 51.7 (14.3)2 |
|
BRIEF-SR* | |||
| BRI T-score | 47.5 (15.1)10 | 43.4 (19.0)5 | 50.7 (10.8)5 |
| MI T-score | 54.2 (12.8)12 | 55.3 (15.3)5 | 53.0 (10.4)7 |
| GEC T-score | 51.8 (12.2)12 | 52.1 (14.2)5 | 51.6 (10.8)7 |
superscript denotes number of subjects missing given observation
PAL, Paired Associates Learning; PRM, Pattern Recognition Memory; SSP, Spatial Span; BRI, Behavior Regulation Index; MI, Metacognition Index; GEC, Global Executive Composite.
Change in BMI and Cardiometabolic Risk Factors
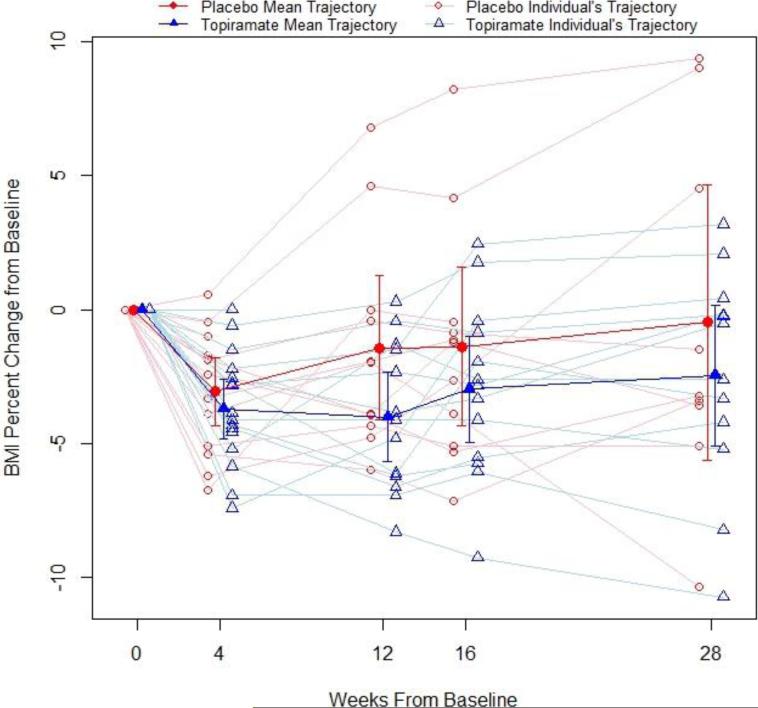
The primary efficacy endpoint, percent change in BMI from baseline, for all participants is illustrated in Figure 2. At 28-weeks in the intention-to-treat analysis, the difference in the percent change in BMI between the topiramate group and placebo group was −1.85% [95% CI (−5.21% - 1.52%); P=0.291]. Fourteen percent of the placebo group and 25% of the topiramate group experienced ≥5% BMI reduction: difference of 11% [(−45%, 24%); P=0.784]. Each group had 1 participant that experienced ≥ 10% BMI reduction.
Figure 2.
Percentage change in BMI from baseline for the topiramate and placebo groups, unadjusted analysis.
Table 2 provides results for the primary efficacy endpoint and secondary outcomes. Visceral fat mass and VLDL-c decreased significantly more in the topiramate group compared to the placebo group and there were trends toward lower triglycerides, insulin and glucose in the topiramate group. The other cardiometabolic risk factors were largely unchanged.
Table 2.
Primary and secondary outcomes from baseline, with differences adjusted for baseline values using intention-to-treat analyses with last observation carried forward.
| Outcome | N | Placebo Δ [mean (SD)] | Topiramate Δ [mean (SD)] | Mean Difference [Topiramate-Placebo] (95% CI) | P-value |
|---|---|---|---|---|---|
| Percentage Change in BMI | 30 | −0.85 (5.31) | −2.74 (3.74) | −1.85 (−5.21, 1.52) | 0.291 |
| Change in BMI | 30 | −0.30 (2.05) | −1.16 (1.58) | −0.81 (−2.15, 0.53) | 0.244 |
| Change in BMI z-score | 30 | −0.04 (0.11) | −0.07 (0.07) | −0.04 (−0.11, 0.02) | 0.206 |
| Change in Percentage of the 95th BMI Percentile | 30 | −2.55 (6.99) | −6.20 (5.65) | −3.78 (−8.55, 0.99) | 0.132 |
| Change in Weight (kg) | 30 | −0.46 (5.63) | −2.89 (4.92) | −2.07 (−5.80, 1.65) | 0.285 |
| Percentage Change in Weight | 30 | −0.37 (5.45) | −2.18 (4.00) | −1.54 (−4.94, 1.85) | 0.380 |
| Change in Total Body Fat Mass (kg) | 30 | 0.32 (4.07) | −1.51 (3.81) | −1.30 (−4.21, 1.61) | 0.388 |
| Change in % Body Fat | 30 | 0.32 (1.82) | −0.63 (1.46) | −0.57 (−1.83, 0.68) | 0.377 |
| Change in Visceral Fat Mass (g) | 30 | 70.57 (168.36) | −95.75 (197.02) | −160.09 (−296.93, −23.25) | 0.030 |
| Change in Lean Body Mass (kg) | 30 | −0.36 (1.80) | −0.06 (2.19) | 0.22 (−1.38, 1.83) | 0.787 |
| Change in Systolic BP (mmHg) | 30 | −1.86 (8.70) | −2.63 (10.71) | −0.18 (−5.83, 5.47) | 0.951 |
| Change in Diastolic BP (mmHg) | 30 | 0.00 (9.32) | 0.94 (6.87) | 1.45 (−3.76, 6.66) | 0.590 |
| Change in Heart Rate (bpm) | 30 | 3.71 (9.62) | 2.25 (10.53) | 0.09 (−6.60, 6.79) | 0.978 |
| Change in Glucose (mg/dL) | 30 | 3.07 (12.55) | −4.69 (12.35) | −6.23 (−14.09, 1.62) | 0.131 |
| Change in Insulin (mg/dL) | 30 | 7.36 (18.02) | −3.94 (10.28) | −10.16 (−20.91, 0.59) | 0.075 |
| Change in Total Cholesterol (mg/dL) | 30 | −10.79 (17.65) | −7.94 (17.78) | 1.25 (−11.33, 13.83) | 0.847 |
| Change in LDL-c (mg/dL) | 29* | −12.36 (14.60) | −4.60 (13.60) | 7.27 (−3.33, 17.88) | 0.190 |
| Change in HDL-c (mg/dL) | 30 | −1.29 (6.46) | 1.38 (5.99) | 1.76 (−2.62, 6.14) | 0.438 |
| Change in VLDL-c (mg/dL) | 29 | 6.21 (14.02) | −3.53 (8.16) | −9.40 (−17.81, −0.99) | 0.038 |
| Change in Triglycerides (mg/dL) | 30 | 13.64 (56.14) | −28.88 (51.19) | −29.01 (−60.67, 2.65) | 0.084 |
| Change in ALT (mg/dL) | 30 | −3.21 (12.72) | −2.69 (12.28) | −0.61 (−9.65, 8.43) | 0.896 |
| Change in AST (mg/dL) | 30 | −2.29 (9.14) | −1.81 (6.27) | −0.66 (−6.14, 4.81) | 0.814 |
unable to measure one participant due to high triglycerides
Safety
The most common adverse event was paresthesia, reported by 25% in the topiramate group and none in the placebo group (Table 3). One participant from each group experienced worsening depressive symptoms during the trial. The participant randomized to the topiramate group had a history of mild depression, which was stable prior to enrollment in the study but re-emerged 2 days after randomization. For this reason, the intervention was stopped but this participant completed all study visits, blinded. The participant randomized to the placebo group reported transient depressed mood, which resolved on its own. Two participants in the topiramate group experienced concussions; one was hit in the head with a softball and the other fell while skiing. There were no significant differences between the topiramate and placebo group on any of the CANTAB subscales or the BRIEF subscales. For the CPT II, only the hit reaction time was different indicating a faster reaction time in the topiramate group compared to placebo (Table 4). No significant changes in bone density, strength, or geometry were observed between the groups (Supplemental Table 1b). None of the participants withdrew from the trial due to adverse events.
Table 3.
Adverse event summary.
| Adverse Event | Placebo (N=14) | Topiramate (N=16) |
|---|---|---|
| Amenorrhea | 0 (0%) | 1 (6.2%) |
| Concussion | 0 (0%) | 2 (12.5%) |
| Dizziness | 0 (0%) | 1 (6.3%) |
| Drowsiness | 2 (14.3%) | 3 (18.8%) |
| Dysgeusia | 0 (0%) | 1 (6.2%) |
| Insomnia | 1 (7.1%) | 0 (0%) |
| Mood problems | 1 (7.1%) | 1 (6.2%) |
| Paresthesia | 0 (0%) | 4 (25.0%) |
| Pharyngitis | 2 (14.3%) | 1 (6.2%) |
| Sinusitis | 1 (7.1%) | 1 (6.2%) |
| Fever due to pharyngitis | 0 (0%) | 1 (6.2%) |
| Tendon injury | 0 (0%) | 1 (6.2%) |
| Diarrhea | 1 (7.1%) | 2 (12.5%) |
| Nausea | 0 (0%) | 1 (6.2%) |
| Vomiting | 0 (0%) | 1 (6.2%) |
Table 4.
Change in safety variables from randomization using completers only.
| Outcome | N | Placebo Δ [mean (SD)] | Topiramate Δ [mean (SD)] | Mean Difference [Topiramate-Placebo] (95% CI) | P-value |
|---|---|---|---|---|---|
| CANTAB | |||||
| Standard PAL | 21 | −0.24 (0.23) | 0.04 (0.60) | 0.26 (−0.16, 0.69) | 0.238 |
| Standard PAL Shapes | 21 | −0.24 (0.53) | 0.11 (1.62) | 0.07 (−0.74, 0.88) | 0.863 |
| Standard PRM | 21 | 0.24 (0.80) | −0.64 (2.00) | −0.70 (−2.13, 0.73) | 0.348 |
| Standard SSP | 19 | 0.11 (0.79) | 0.36 (1.21) | 0.16 (−0.80, 1.11) | 0.750 |
|
CPT-II | |||||
| Omissions % | 20 | −0.09 (18.09) | 0.34 (4.62) | −3.81 (−13.72, 6.09) | 0.461 |
| Commissions % | 20 | 2.20 (7.29) | 1.55 (11.04) | −0.30 (−8.01, 7.42) | 0.941 |
| Hit Reaction Time | 20 | 6.37 (10.38) | −2.67 (7.44) | −11.13 (−18.32, −3.93) | 0.008 |
|
BRIEF-SR | |||||
| BRI T-score | 15 | 1.17 (10.96) | −1.00 (8.80) | −1.97 (−10.57, 6.62) | 0.661 |
| MI T-score | 14 | 0.33 (13.38) | 0.00 (5.32) | −0.64 (−10.50, 9.21) | 0.900 |
| GEC T-score | 14 | 1.00 (12.81) | −1.00 (7.52) | −2.02 (−11.74, 7.71) | 0.692 |
PAL, Paired Associates Learning; PRM, Pattern Recognition Memory; SSP, Spatial Span; BRI, Behavior Regulation Index; MI, Metacognition Index; GEC, Global Executive Composite
Discussion
In this double-blind, randomized, placebo-controlled pilot clinical trial, we observed a marginal and statistically insignificant effect on percent BMI reduction from meal replacement therapy followed by topiramate compared to meal replacement alone for adolescents with severe obesity. However, there were reductions in visceral fat mass and VLDL-c favoring the treatment arm, and though the other changes in cardiometabolic disease risk factors were not statistically significant, the magnitude of improvement in triglycerides, insulin and glucose were notable and clinically meaningful. Importantly, topiramate at 75 mg daily had an acceptable safety profile compared to placebo and was generally well-tolerated.
At least ten randomized controlled clinical trials have examined topiramate for weight reduction in adults.30 The first was a dose-ranging study which found that at 24 weeks, the placebo-subtracted mean weight reduction from topiramate was 2.4%, 2.2%, 3.7%, and 3.7% at doses of 64, 96, 192, and 384 mg/day, respectively.14 The longest trial was a 60-week randomized, double-blind placebo-controlled study which found that compared to placebo, the mean weight reduction from topiramate was 5.3%, 7.4%, and 8.0% at topiramate doses of 96, 192, and 256 mg/day, respectively.16 Our findings are not directly comparable to these adult trials given that our study design utilized meal replacement therapy for the initial period of treatment before topiramate/placebo was started. However, if we consider only our 4 to 28 week randomization period, after the meal replacement therapy ended, we showed a 2.4% (95% CI: −5.4%, +0.6%) difference in BMI favoring topiramate, which is comparable to the aforementioned adult dose ranging study outcomes.
Although the adult randomized controlled trials suggested that the weight loss efficacy of topiramate improves with increasing dose, up to 400 mg/day,30 we had two primary reasons for utilizing a 75 mg/day dose in this pilot trial. First, we previously reported significant BMI reduction (about 6%) among adolescent patients treated with topiramate for severe obesity in a weight management clinic based on retrospective chart review (80% were treated with a dose of 75 mg/day).12 Second, the studies in adults identified that adverse events, primarily neurologic, were dose-dependent, and that these in turn led to study withdrawal.30 Nevertheless, if we used a higher dose, we may have observed greater BMI reduction, though possibly at the risk of prompting neurologic (albeit reversible) side effects.
The duration of the adult topiramate trials was also a predictor of response, with study durations longer than 28 weeks eliciting more weight loss than shorter studies.30 For this pilot study, we used a 28 week duration (including 4 weeks of the meal replacement therapy before randomization) in order to minimize subject drop out. We recognize that the FDA recommends that obesity pharmacotherapy studies include at least 1 year duration, yet 28 weeks may be considered reasonable for this pilot study.
Few other medications have been studied for the indication of weight loss in the pediatric population using randomized controlled trials.31 Orlistat, a gastrointestinal lipase inhibitor, is currently the only FDA approved medication for weight reduction in youth ≥ 12 years old. In the largest randomized, controlled study of orlistat, the placebo subtracted mean BMI decrease was 0.8 kg/m2 (less than 3% BMI reduction) favoring orlistat at 52 weeks.11 Metformin, although only FDA approved for diabetes in adolescents, has also been widely studied for the indication of obesity in the pediatric population. In a systematic review of 14 randomized clinical trials, metformin resulted in a BMI reduction of 1.38 kg/m2 (95% CI, −1.93 to −0.82 kg/m2) from baseline (equating to approximately 3% BMI reduction) compared to controls at 6 months. However, the pooled estimate from studies, which included one year of treatment, was not statistically significant.32 Our group has also examined exenatide, a glucagon like peptide-1 agonist, for BMI reduction in adolescents with severe obesity. In a small randomized controlled crossover trial we observed a 4.9% (95% CI, −8.61% to −1.23%) decrease in BMI with 3 months of treatment with exenatide.33 In a separate randomized placebo controlled pilot study we observed a 2.7% (95% CI, −5.02% to −0.37%) decrease in BMI at 3 months.34 Again, it should be noted that the results of the current pilot trial may not be directly comparable to these other pediatric outcomes because we utilized a meal replacement initial period. Yet, the degree of change that we observed with topiramate was relatively comparable to these other medications.
The most common side effects observed in the adult studies of topiramate for weight reduction were related to the peripheral and central nervous systems. These included paresthesia, difficulty with concentration/attention, psychomotor slowing, difficulty with memory, and mood problems such as depression. Most of these adverse events were dose dependent.16 In our study, the most common adverse event was paresthesia, reported by 25% of the topiramate group and none in the placebo group. We found no concerning changes in memory, motor speed or attention, which is consistent with other reports of topiramate use in adolescents for migraine up to a dose of 100 mg/day.35 Further, there was no meaningful difference in executive function or impulsivity between treatment groups. The relevance of the participant who developed a re-emergence of his depression symptoms 2 days into the topiramate treatment arm is unclear but warrants discussion. All antiepileptic medications increase the risk of depression symptoms in patients taking these medications for any indication, and should be monitored for the emergence or worsening of such symptoms. Nevertheless, in interpreting this outcome, it is important to note that one participant in the placebo group also experienced transient depression symptoms.
In line with one of our secondary aims, we attempted to examine the effect of baseline binge eating behavior on the treatment efficacy of topiramate. Binge eating is characterized by consuming a large amount of food in a discrete time period accompanied by a sense of loss of control over the eating episode,36 and prior randomized controlled trials suggested that topiramate decreases binge eating episodes in adults with this disorder.17-19 Because only three participants in our pilot study endorsed binge eating, we were unable to appropriately evaluate this question. Therefore, future trials of topiramate could attempt to address this important issue.
Strengths of this study include the double-blind, randomized, placebo-controlled design and the practical and translatable nature of the intervention. Limitations include the relatively small number of participants and the short duration of treatment (28 weeks). Further, we did not report on participant fidelity to the lifestyle modification therapy and introduction of a medication may influence adherence.
Conclusion
In summary, this randomized placebo-controlled pilot clinical trial of 4 weeks of meal replacement therapy followed by 24 weeks of topiramate at a dose of 75 mg/day demonstrated limited efficacy for BMI reduction in adolescents with severe obesity compared to meal replacement followed by placebo. Importantly, topiramate at 75 mg/day appeared to be well-tolerated, had an acceptable safety profile, and had a beneficial impact on visceral adiposity. Adult studies suggest that higher doses of topiramate may be effective for weight reduction, though at the expense of the potential for neurocognitive side effects. For adolescents, this still needs to be explored. As with any intervention, the risks and benefits have to be measured against each other. Since severe obesity in adolescents is associated with considerable physiological, psychological, and social morbidity, perhaps relative risk in treatment is warranted. This pilot study suggests that studies using higher doses of topiramate may be justified to examine the risk/benefit profile of this medication for use in severe adolescent obesity, an otherwise recalcitrant disease.
Supplementary Material
What is Already Known About this Subject
Meal replacement therapy results in modest, albeit transient, weight reduction in adolescents with severe obesity.
Topiramate promotes weight reduction in adults with obesity, but has not yet been studied prospectively for weight reduction in adolescents with obesity.
What this Study Adds
In this double-blind, randomized, placebo-controlled pilot clinical trial, four weeks of meal replacement therapy followed by 24 weeks of low-dose (75 mg/day) topiramate compared to meal replacement therapy alone did not produce significantly better body mass index (BMI) reduction.
Low-dose topiramate for weight reduction in adolescents was safe and well-tolerated.
Significant improvements in visceral fat and VLDL-c were observed in the topiramate compared to the placebo group.
Studies using higher doses of topiramate for obesity in adolescents may be warranted.
Acknowledgements
We thank the participants in this study as well as the extraordinary study coordination by Cameron Naughton, Andrea Metzig, and Anne Norris.
Funding: Funding for this study was provided by grants from the University of Minnesota Clinical and Translational Science Institute and the Vikings Children's Fund, the Minnesota Obesity Center (NIH Grant P30DK050456 NORC), the National Center for Advancing Translational Sciences (Award Number UL1TR000114), and an individual training grant from the NIH/NHLBI (F32-HL127851 to J.R.R). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Disclosures: Dr. Abuzzahab receives research grant support from: Astra Zeneca, Grifols, Genentech, Medtronic, Novo Nordisk, Roche, Versartis, and Zafgen. She serves on the Speakers Bureau for Novo Nordisk. Dr. Kelly serves as an unpaid consultant for Novo Nordisk and receives research support in the form of drug and placebo from Astra Zeneca for an NIDDK-funded clinical trial. Dr. Billington is a consultant for Novo Nordisk, EnteroMedics, and Optum Health and has research grant from Covidien. All the other authors declare no conflicts of interest.
Author Contributions: CF, ASK, KR, BN, MA, BS, SK and CB conceived the study and design. CF, ASK, MS, BN, MA, BS, SK recruited patients and executed study. AMK and KR conducted statistical analysis. AP oversaw pQCT data collection and analysis. All authors participated in data interpretation and in writing the paper, and reviewed and approved the final submitted version of the manuscript.
References
- 1.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nut. 2009;90(5):1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 2.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 3.Koebnick C, Smith N, Coleman KJ, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. J Pediatr. 2010;157(1):26–31. e22. doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999-2012. JAMA Pediatr. 2014;168(6):561–6. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 5.Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolesc Med. 2012;166(12):1103–1108. doi: 10.1001/2013.jamapediatrics.319. [DOI] [PubMed] [Google Scholar]
- 6.Johnston CA, Tyler C, Palcic JL, Stansberry SA, Gallagher MR, Foreyt JP. Smaller weight changes in standardized body mass index in response to treatment as weight classification increases. J Pediatr. 2011;158(4):624–627. doi: 10.1016/j.jpeds.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 7.Kalarchian MA, Levine MD, Arslanian SA, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124(4):1060–1068. doi: 10.1542/peds.2008-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knop C, Singer V, Uysal Y, Schaefer A, Wolters B, Reinehr T. Extremely obese children respond better than extremely obese adolescents to lifestyle interventions. Pediatr Obes. 2013 doi: 10.1111/j.2047-6310.2013.00212.x. 10.1111/j.2047-6310.2013.00212.x. [DOI] [PubMed] [Google Scholar]
- 9.Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Is family-based behavioral weight control appropriate for severe pediatric obesity? Int J Eat Disord. 2001;30(3):318–328. doi: 10.1002/eat.1091. [DOI] [PubMed] [Google Scholar]
- 10.Berkowitz RI, Wadden TA, Gehrman CA, et al. Meal replacements in the treatment of adolescent obesity: a randomized controlled trial. Obesity (Silver Spring) 2011;19(6):1193–1199. doi: 10.1038/oby.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293(23):2873–2883. doi: 10.1001/jama.293.23.2873. [DOI] [PubMed] [Google Scholar]
- 12.Fox CK, Marlatt KL, Rudser KD, Kelly AS. Topiramate for weight reduction in adolescents with severe obesity. Clin Pediatr. 2015;54(1):19–24. doi: 10.1177/0009922814542481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astrup A, Caterson I, Zelissen P, et al. Topiramate: long-term maintenance of weight loss induced by a low-calorie diet in obese subjects. Obesity Res. 2004;12(10):1658–1669. doi: 10.1038/oby.2004.206. [DOI] [PubMed] [Google Scholar]
- 14.Bray GA, Hollander P, Klein S, et al. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obesity Res. 2003;11(6):722–733. doi: 10.1038/oby.2003.102. [DOI] [PubMed] [Google Scholar]
- 15.Tonstad S, Tykarski A, Weissgarten J, et al. Efficacy and safety of topiramate in the treatment of obese subjects with essential hypertension. Am J Cardiol. 2005;96(2):243–251. doi: 10.1016/j.amjcard.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 16.Wilding J, Van Gaal L, Rissanen A, Vercruysse F, Fitchet M, Obesity Study Group A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399–1410. doi: 10.1038/sj.ijo.0802783. [DOI] [PubMed] [Google Scholar]
- 17.McElroy SL, Arnold LM, Shapira NA, et al. Topiramate in the treatment of binge eating disorder associated with obesity: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160(2):255–261. doi: 10.1176/appi.ajp.160.2.255. [DOI] [PubMed] [Google Scholar]
- 18.McElroy SL, Shapira NA, Arnold LM, et al. Topiramate in the long-term treatment of binge-eating disorder associated with obesity. J Clin Psychiatry. 2004;65(11):1463–1469. doi: 10.4088/jcp.v65n1104. [DOI] [PubMed] [Google Scholar]
- 19.McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039–1048. doi: 10.1016/j.biopsych.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 20.TODAY Study Group. Zeitler P, Hirst K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosch TA, Dengel DR, Kelly AS, Sinaiko AR, Moran A, Steinberger J. Visceral adipose tissue measured by DXA correlates with measurement by CT and is associated with cardiometabolic risk factors in children. Pediatr Obes. 2015;10(3):172–179. doi: 10.1111/ijpo.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binford RB, Le Grange D, Jellar CC. Eating Disorders Examination versus Eating Disorders Examination-Questionnaire in adolescents with full and partial-syndrome bulimia nervosa and anorexia nervosa. Int J Eat Disord. 2005;37(1):44–49. doi: 10.1002/eat.20062. [DOI] [PubMed] [Google Scholar]
- 23.Ijff DM, Aldenkamp AP. Cognitive side-effects of antiepileptic drugs in children. Handb Clin Neurol. 2013;111:707–718. doi: 10.1016/B978-0-444-52891-9.00073-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Zheng YX, Zhu JM, Zhang JM, Zheng Z. Effects of antiepileptic drugs on bone mineral density and bone metabolism in children: a meta-analysis. J Zhejiang Univ Sci B. 2015;16(7):611–621. doi: 10.1631/jzus.B1500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zemel B, Bass S, Binkley T, et al. Peripheral quantitative computed tomography in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11(1):59–74. doi: 10.1016/j.jocd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med. 1992;11(13):1685–1704. doi: 10.1002/sim.4780111304. [DOI] [PubMed] [Google Scholar]
- 27.Senn S. Change from baseline and analysis of covariance revisited. Stat Med. 2006;25(24):4334–4344. doi: 10.1002/sim.2682. [DOI] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- 30.Kramer CK, Leitao CB, Pinto LC, Canani LH, Azevedo MJ, Gross JL. Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials. Obes Rev. 2011;12(5):e338–347. doi: 10.1111/j.1467-789X.2010.00846.x. [DOI] [PubMed] [Google Scholar]
- 31.Kelly AS, Fox CK, Rudser KD, Gross AC, Ryder JR. Pediatric obesity pharmacotherapy: current state of the field, review of the literature and clinical trial considerations. Int J Obes (Lond) 2016 May 17; doi: 10.1038/ijo.2016.69. doi: 10.1038/ijo.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonagh MS, Selph S, Ozpinar A, Foley C. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168(2):178–184. doi: 10.1001/jamapediatrics.2013.4200. [DOI] [PubMed] [Google Scholar]
- 33.Kelly AS, Metzig AM, Rudser KD, et al. Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity (Silver Spring) 2012;20(2):364–370. doi: 10.1038/oby.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly AS, Rudser KD, Nathan BM, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167(4):355–360. doi: 10.1001/jamapediatrics.2013.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandina GJ, Ness S, Polverejan E, et al. Cognitive effects of topiramate in migraine patients aged 12 through 17 years. Pediatr Neurol. 2010;42(3):187–195. doi: 10.1016/j.pediatrneurol.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 36.de Zwaan M. Binge eating disorder and obesity. Int J Obes Relat Metab Disord. 2001;25(Suppl 1):S51–55. doi: 10.1038/sj.ijo.0801699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.