Abstract
IgG anti-DNA antibodies are both diagnostic and pathogenic for systemic lupus erythematosus (SLE). They contribute to tissue inflammation through direct tissue binding and to systemic inflammation through activation of Toll-like receptors by nucleic acid-containing immune complexes.
IgG DNA-reactive antibodies originate when B cell tolerance mechanisms are impaired. The heterogeneous immune perturbations in SLE lead to the survival and activation of DNA-reactive B cells in various B cell subsets at distinct stages of B cell maturation and differentiation. We propose that the spectrum of B cell alterations and failed tolerance mechanisms for DNA-reactive B cells in lupus patients is best understood by studying genetic risk alleles.
This implies that the B cells producing anti-DNA IgG antibodies and the failed tolerance mechanisms(s) will differ across patients. A better understanding of these differences should lead to better patient stratification, improved outcomes of clinical trials, and the identification of novel therapeutic targets.
Introduction
IgG anti-DNA antibodies are one of the hallmarks of SLE. The pathogenicity of IgG autoantibodies lies in their ability to bind tissue and engage Fc receptor (FcR) mediated mechanisms of myeloid cell activation. IgG anti-DNA antibodies bound to DNA or cross-reactive antigens in tissue will activate complement or engage activated FcRs to mediate antibody dependent cell cytotoxicity (ADCC) and initiate inflammation [1,2]. In addition, DNA containing IgG immune complexes can be internalized through FcRs and transported to endosomal compartments where they activate Toll like receptors (TLRs), leading to inflammatory responses by myeloid cells [3–5]. Because of the potency of these inflammatory responses, tolerance checkpoints are in place in healthy individuals to prevent production of IgG anti-DNA antibodies.
In this review, we provide an overview of the B cell subsets that can produce IgG anti-DNA antibodies in SLE and the tolerance checkpoints that usually prevent their differentiation into plasma cells. We address the tolerance checkpoints that are breached in SLE patients, and how they are influenced by genetic risk factors.
Tolerance checkpoints of DNA-reactive B cells in mice
In the bone marrow (BM), B cells mature from pro-B to pre-B (heavy chain expression) and from pre-B to immature B cells (heavy and light chain expression). Beginning when surface IgM is expressed in association with surrogate light chain or with a kappa/ lambda light chain, a variety of mechanisms occur to prevent an autoreactive B cell from achieving immunocompetence (Table 1). Anti-DNA Ig transgene or knock-in mouse models on non autoimmune backgrounds have been useful in identifying clonal deletion, receptor editing and anergy among these mechanisms [6–9]. Undefined differences in the affinity, fine specificity or polyreactivity of the anti-DNA antibodies in each model have disclosed this diversity of cell fates. In some, deletion appears to be driven by a strong signal delivered through the BCR. In others, receptor editing occurs. In others, anergy serves as a tolerance mechanism perhaps representing a pathway regulating B cells with lower affinity BCRs. Anergic B cells themselves present an array of phenotypes, including follicular exclusion which bars them from participating in germinal center (GC) reactions in which B cells undergo class switch recombination and somatic mutation, altering the affinity or specificity of the BCR [10]. These models have been extremely useful, but are limited by the focus on a single BCR. Moreover, they are studied on genetic backgrounds that do not necessarily mimic the various genotypes that confer susceptibility to the human disease.
Table 1.
Tolerance mechanisms for DNA-reactive B cells
| B cell subset | Tolerance mechanism | References |
|---|---|---|
| Pro-B cells | Heavy chain receptor editing* |
[6,7] |
| Immature B cells | Clonal deletion | [6,7,9] |
| Light chain receptor editing |
[6,7] | |
| Allelic inclusion* | [6,7] | |
| Transitional B cells | Anergy | [10] |
| Light chain receptor editing |
[8] | |
| GC / post-GC | Deletion | [14,15,17] |
| Receptor editing | [17,18] |
Although heavy chain receptor editing, allelic inclusion and light chain receptor editing in transitional cells have been shown in some studies, their exact contribution to development of tolerance, especially in the context of a normal immune repertoire is not completely established.
Marginal zone (MZ) B cells and B-1 cells have an increased frequency of autoreactive BCRs. These B cells produce mainly IgM antibodies that are important as the first line of defense against pathogens. IgM anti-DNA antibodies are present in healthy individuals and help prevent SLE by assisting in the elimination of cellular debris in a non-inflammatory fashion [11]. These cell types are therefore often considered to be “innate” antibody-producing cells. They primarily produce IgM and IgA (B-1 cells) antibodies, but can also switch to IgG and undergo limited somatic mutation, and can potentially be a source of pathogenic anti-DNA antibodies [12,13].
Many anti-DNA antibodies arise from follicular B cells being recruited into GC responses. As GC reactions lead to B cell differentiation into long-lived memory and plasma cells, this compartment is critically important in protection against autoimmunity. Importantly, somatic hypermutation can lead to de novo autoreactivity arising during the GC reaction. Therefore, mechanisms are in place to prevent the survival of autoreactive B cells during the GC response (Table 1). Since the GC response requires cognate T cell help, a lack of recognition of DNA by T cells might explain why anti-DNA transgenic BCR mice generally do not develop anti-DNA IgG antibodies. However, for cross-reactive B cell receptors, however, that recognize DNA as well as an eliciting foreign antigen, tolerance must be mediated through different mechanisms, as these cells can receive positive signals in the GC from the T cells that recognize the foreign antigen. Mouse models suggest that tolerance induction in post-GC B cells occurs by exposure to soluble antigen as B cells exit the GC. Engagement of the BCR at this stage initiates both receptor editing and apoptosis [14–18].
Together, the data demonstrate that several B cell subsets may produce anti-DNA antibodies and numerous mechanisms exist to prevent the secretion of high affinity IgG anti-DNA antibodies. At a minimum these include reducing the emergence of autoreactive B cells from the BM and their recruitment into follicles early in B cell development, blocking the differentiation of plasma cells from autoreactive GC B cells, and limiting class switching and somatic hypermutation in “innate” B cells.
Amplification loop of autoimmunity through B cell-intrinsic and –extrinsic pathways
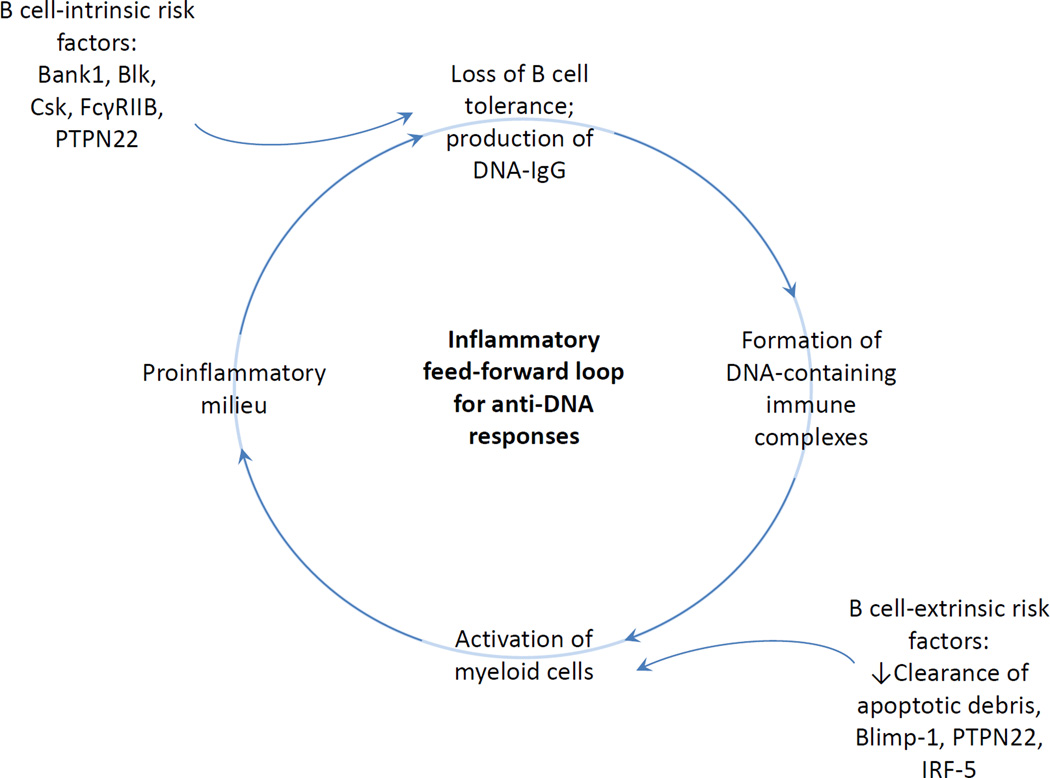
Anti-DNA responses clearly arise through B cell intrinsic or B cell extrinsic pathways (Figure 1). In B cell-intrinsic pathways, tolerance is breached through an aberrant function of molecules in the B cell itself. Once anti-DNA antibodies are in the circulation, DNA-containing immune complexes form and activate myeloid cells through Fc receptor or TLR engagement [19]. Myeloid cells producing increased levels of BAFF, IL-10, type I IFN, or other cytokines, further modulate B cell selection [20–22].
Figure 1.
Inflammatory feed-forward loop for anti-DNA responses.
In B cell-extrinsic pathways, activation of myeloid cells through defects in clearance of apoptotic debris, or genetic alterations in thresholds for activation, can initiate an inflammatory milieu with a loss of tolerance of DNA-specific B cells.
In either case, an amplification loop of autoimmunity develops (Figure 1).
Regulation of DNA-reactive B cells in healthy individuals
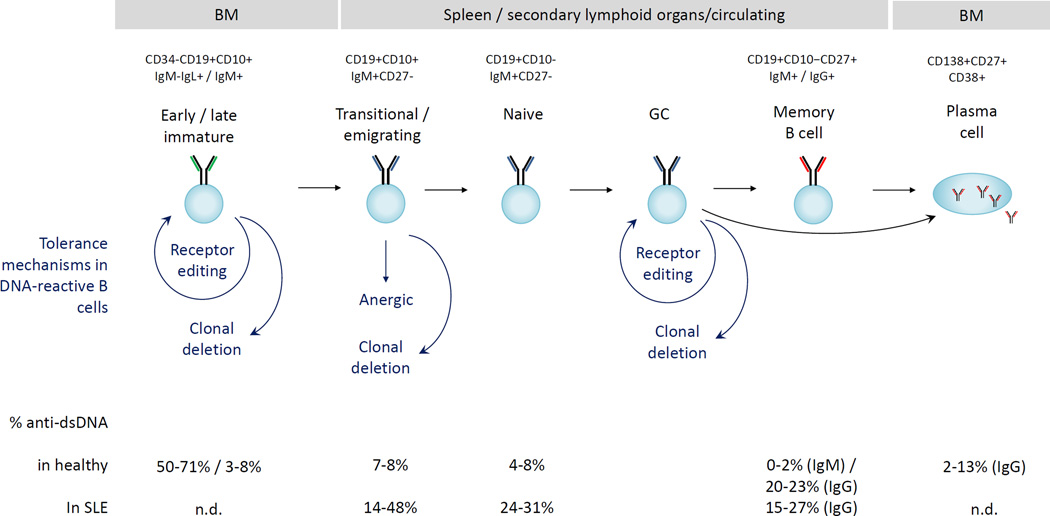
Our insight into tolerance checkpoints in humans has been derived from single-cell cloning of immunoglobulins, a methodology developed a decade ago. These studies have revealed the tolerance checkpoints of polyreactive and autoreactive B cells, including dsDNA-reactive B cells. Whereas B cells exhibit a gradual decrease in anti-nuclear reactivity throughout their development to mature naïve B cells, reactivity towards dsDNA seems to follow a more stringent pattern in healthy individuals, with a large decrease in reactivity as B cells develop from early immature to immature B cells (Figure 2) [23–26]. The degree of autoreactivity in the memory compartment of healthy individuals remains controversial [25,27]. Moreover, some rare populations are enriched for DNA-reactivity. For example, IgD+ λ B cells which are enriched for autoreactivity, are permitted to mature [28]. Why tolerance mechanisms are not operative in these cells is unknown.
Figure 2.
Tolerance checkpoints for DNA-reactive B cells in humans. The percentages of DNA-reactive B cells in each subset are derived from references 17–20, 23, 25. Markers to identify the different populations in these studies are indicated in the top of the figure. N.d. not determined.
Tolerance breaches of DNA-reactive B cells in lupus patients
As shown in Figure 2, lupus patients exhibit increased frequencies of dsDNA-reactive B cells, both in recently emigrating and mature naïve B cell subsets, demonstrating a breach in early B cell tolerance pathways in these individuals [29]. It does not follow, however, that serum anti-DNA antibodies derive from these B cells. Indeed, individuals with deficiencies in MyD88 or IRAK-4 signaling molecules have increased numbers of autoreactive naïve B cells in the absence of serum autoantibodies [30]. Likewise, dsDNA antibodies may be produced by IgG+ memory cells which mostly derive from non-autoreactive precursor cells, suggesting that the loss of tolerance in naïve B cells may not explain all DNA-reactivity [25,31]. Interestingly, in one study similar numbers of autoreactive IgG+ memory cells were observed in healthy individuals and in SLE patients.[31] A different assay for DNA-reactive B cells showed an increased number of DNA-reactive B cells in total B cells in SLE patients. When patients with active and inactive disease were compared, the number of DNA-binding cells in an antigen-experienced population was increased in those with active disease [32]. A single histologic study of tonsils demonstrated follicular exclusion of autoreactive B cells in healthy individuals but not in lupus patients [33]. Using a flow cytometry based assay, we recently found that anergy induction of autoreactive B cells was impaired in lupus patients [27]. These data together suggest that DNA-reactive B cells are more likely to mature, participate in GC reactions and undergo plasma cell differentiation in lupus patients.
Sources of anti-DNA in lupus patients
The development of IgG secreting plasma cells can arise as a consequence of class switching of naïve, MZ B cells or B-1 cells, leading to the generation of short-lived plasma cells, as well as through GC-dependent reactions, leading to the generation of memory B cells and long-lived plasma cells. Because lupus is a chronic condition, and serum autoantibodies are present years before disease onset [34], long-lived plasma cells are one likely source of anti-DNA antibodies. A recent study looking specifically at a subset of autoreactive B cells expressing a VH 4–34 encoded heavy chain suggested that autoreactive (including DNA-reactive) plasmablasts arising during lupus flares were derived primarily from memory B cells but also from activated naïve cells [35], perhaps explaining increases in anti-DNA titers during flare, and suggesting that short lived plasma cells contribute to disease.
Genetic risk factors for lupus and their effect on tolerance of DNA-reactive B cells
Over 50 genes have been identified in genome-wide association studies (GWAS) for SLE [36], most of which associate with the production of anti-DNA antibodies in SLE; however, the functionality of most of these is still not known. Recent studies investigating the cell-type specific expression of these risk alleles has revealed that these genes are most highly expressed in B cells, monocytes, and plasmacytoid dendritic cells [37,38]. This suggests that genetic risk factors can contribute to both B cell-intrinsic and B cell-extrinsic triggering of the disease (Figure 2). Genes with well-defined roles of risk alleles and which are associated with the production of anti-DNA antibodies are discussed below and summarized in Table 2.
Table 2.
SLE and DNA autoantibody-associated genes with functional data on risk alleles
| Gene | Full name | SNPs,* mutation type |
Functional consequences |
B cell- intrinsic or extrinsic |
References |
|---|---|---|---|---|---|
| BANK1 | B cell scaffold protein with ankyrin repeats 1 |
rs10516487 (C → T), missense |
Increased BCR- mediated activation (association with Lyn and IPR3, release of intracellular Ca2+) |
intrinsic | [41–43] |
| BLK | B lymphocyte kinase |
rs922483 (G → A), noncoding, 5’UTR |
decreased expression, increased B cell activation, increased switched memory B cells |
intrinsic | [44–47] |
| CSK | C-terminal Src kinase |
rs34933034 (G → A), noncoding, intronic |
increased B cell activation, (Lyn phosphorylation, Ca2+ mobilization), increased transitional B cells |
intrinsic | [48] |
| FCGR2B | Fc-gamma receptor IIb |
rs1050501 (T → C), point mutation |
impairment of receptor mobility, lipid rafts and inhibitory signaling |
Intrinsic, maybe extrinsic? |
[49–51] |
| IRF5 | interferon regulatory factor 5 |
rs10488631 (T → C), noncoding, intergenic |
increased expression and activation in myeloid cells, high serum IFN (SLE only) |
likely extrinsic | [52–57] |
| rs77571059 (3× CGGGG → 4× CGGGG), noncoding, promoter indel |
|||||
| PRDM1 | B lymphocyte- induced maturation protein 1 |
rs548234 (A → G), noncoding, intergenic |
decreased expression, increased TLR- mediated IL6 secretion |
extrinsic | [58,59] |
| PTPN22 | protein tyrosine phosphatase N22, Lyp |
rs2476601 (C → T), point mutation |
decreased B cell activation, proliferation & signaling; impaired T cell responses |
both | [60–62] |
Polymorphisms are shown that are proxies for other risk alleles that make up the risk haplotype. Nucleotides are shown as nonrisk allele → risk allele.
B cell-intrinsic risk alleles
B cell intrinsic risk alleles exist for BANK1, BLK, CSK, FCGR2B, and PTPN22. Each of these genes modulates BCR signaling. All of these risk alleles, except for PTPN22, lead to hyper-responsiveness to BCR engagement and enhanced B cell activation. Such hyper-responsiveness to BCR triggering can contribute to defects in tolerance at the late transitional, GC or post-GC stage. Healthy carriers of the CSK risk allele have increased numbers of late transitional B cells. Carriers of the BLK risk allele have increased numbers of switched memory cells and IgD λ B cells which are enriched for autoreactivity.
The PTPN22 risk allele results in decreased phosphorylation of proteins in the BCR signaling pathway and has been shown to diminish tolerance in immature B cells. As PTPN22 is also expressed by T cells and myeloid cells, there appear to be B cell-extrinsic effects of this risk allele as well.
B cell-extrinsic risk alleles
Risk alleles with defined effects on myeloid cells are PRDM1, IRF5, and as stated above, PTPN22. Although they have clear B cell-extrinsic effects, an additional role for direct effects on B cells cannot yet be excluded.
The risk allele for PRDM1 (Blimp-1), a transcriptional repressor, exhibits decreased expression and decreased repression of several inflammatory cytokines in dendritic cells (DCs). The proinflammatory environment created by Blimp-1 deficient DCs leads to increased Tfh activation, increased GC responses and production of GC derived DNA-reactivity. While Blimp-1 is critical in plasma cell differentiation, an allele-specific function in B cells has not been demonstrated. Like PRDM1, the IRF5 risk allele, which is most highly expressed by monocytes and DCs, leads to increased production of cytokines that impair B cell selection and modulate differentiation.
B cell targeted therapy
Because antibodies contribute to both tissue damage and systemic inflammation in SLE, B cell targeted therapies are of great interest. B cell depletion failed in randomized placebo-controlled double blind studies [39,40]; it has been speculated that the high levels of BAFF that are present following B cell depletion impair early B cell tolerance checkpoints. Anti-BAFF antibody (belimumab) was approved by the FDA for moderate SLE in 2011, and appears to restore anergy induction of naïve autoreactive B cells. While it is useful, it has not replaced more classic cytotoxic therapy. There is interest in plasma cell depletion in SLE, but great concern that the approach may be too immunosuppressive. B cell signaling pathways are also potential therapeutic targets, especially in light of the hyperresponsiveness of the BCR on SLE B cells.
Conclusions
Our understanding of which B cells express DNA-reactive BCRs and which of these become plasma cells comes for the most part from studies of mice that express monoclonal DNA-reactive BCRs and have genetic backgrounds that do not mimic the known SLE risk alleles. Moving forward, the study of the B cell intrinsic or extrinsic functionality of SLE risk alleles may help delineate the pathogenic B cell subset(s) in each patient and thereby provide a rational stratification of patients for clinical trials and elucidate new mechanisms of B cell tolerance.
Acknowledgments
Grants that funded this review: NIH R01AR057084; Alliance for Lupus Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaynor B, Putterman C, Valadon P, Spatz L, Scharff MD, Diamond B. Peptide inhibition of glomerular deposition of an anti-DNA antibody. Proc Natl Acad Sci U S A. 1997;94:1955–1960. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raz E, Brezis M, Rosenmann E, Eilat D. Anti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidney. J Immunol. 1989;142:3076–3082. [PubMed] [Google Scholar]
- 3.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 4.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 5.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fields ML, Erikson J. The regulation of lupus-associated autoantibodies: immunoglobulin transgenic models. Curr Opin Immunol. 2003;15:709–717. doi: 10.1016/j.coi.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Kumar KR, Mohan C. Understanding B-cell tolerance through the use of immunoglobulin transgenic models. Immunol Res. 2008;40:208–223. doi: 10.1007/s12026-007-8008-7. [DOI] [PubMed] [Google Scholar]
- 8.Kiefer K, Nakajima PB, Oshinsky J, Seeholzer SH, Radic M, Bosma GC, Bosma MJ. Antigen receptor editing in anti-DNA transitional B cells deficient for surface IgM. J Immunol. 2008;180:6094–6106. doi: 10.4049/jimmunol.180.9.6094. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Solal J, Diamond B. Lessons from an anti-DNA autoantibody. Mol Immunol. 2011;48:1328–1331. doi: 10.1016/j.molimm.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vas J, Gronwall C, Marshak-Rothstein A, Silverman GJ. Natural antibody to apoptotic cell membranes inhibits the proinflammatory properties of lupus autoantibody immune complexes. Arthritis Rheum. 2012;64:3388–3398. doi: 10.1002/art.34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy B, Shukla S, Lyszkiewicz M, Krey M, Viegas N, Duber S, Weiss S. Somatic hypermutation in peritoneal B1b cells. Mol Immunol. 2009;46:1613–1619. doi: 10.1016/j.molimm.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Hande S, Notidis E, Manser T. Bcl-2 obstructs negative selection of autoreactive, hypermutated antibody V regions during memory B cell development. Immunity. 1998;8:189–198. doi: 10.1016/s1074-7613(00)80471-9. [DOI] [PubMed] [Google Scholar]
- 15.Notidis E, Heltemes L, Manser T. Dominant, hierarchical induction of peripheral tolerance during foreign antigen-driven B cell development. Immunity. 2002;17:317–327. doi: 10.1016/s1074-7613(02)00392-8. [DOI] [PubMed] [Google Scholar]
- 16.Alabyev B, Rahman ZS, Manser T. Quantitatively reduced participation of anti-nuclear antigen B cells that down-regulate B cell receptor during primary development in the germinal center/memory B cell response to foreign antigen. J Immunol. 2007;178:5623–5634. doi: 10.4049/jimmunol.178.9.5623. [DOI] [PubMed] [Google Scholar]
- 17.Rice JS, Newman J, Wang C, Michael DJ, Diamond B. Receptor editing in peripheral B cell tolerance. Proc Natl Acad Sci U S A. 2005;102:1608–1613. doi: 10.1073/pnas.0409217102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YH, Diamond B. B cell receptor revision diminishes the autoreactive B cell response after antigen activation in mice. J Clin Invest. 2008;118:2896–2907. doi: 10.1172/JCI35618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vogelpoel LT, Hansen IS, Rispens T, Muller FJ, van Capel TM, Turina MC, Vos JB, Baeten DL, Kapsenberg ML, de Jong EC, et al. Fc gamma receptor-TLR cross-talk elicits pro-inflammatory cytokine production by human M2 macrophages. Nat Commun. 2014;5:5444. doi: 10.1038/ncomms6444. ** This study shows that the presence of autoantibody-TLR ligand immune complexes leads to skewing of M2 macrophages towards a proinflammatory phenotype and can thus contribute to an inflammatory feedforward loop we describe in this manuscript.
- 20. Goenka R, Matthews AH, Zhang B, O'Neill PJ, Scholz JL, Migone TS, Leonard WJ, Stohl W, Hershberg U, Cancro MP. Local BLyS production by T follicular cells mediates retention of high affinity B cells during affinity maturation. J Exp Med. 2014;211:45–56. doi: 10.1084/jem.20130505. * The authors demonstrate that TFH can produce BAFF (BLyS), and by using T cell-specific BAFF deletion in BM chimeras, they show that locally produced and T cell-derived BAFF can regulate selection of B cells in the germinal center.
- 21.Ota M, Duong BH, Torkamani A, Doyle CM, Gavin AL, Ota T, Nemazee D. Regulation of the B cell receptor repertoire and self-reactivity by BAFF. J Immunol. 2010;185:4128–4136. doi: 10.4049/jimmunol.1002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moisini I, Huang W, Bethunaickan R, Sahu R, Ricketts PG, Akerman M, Marion T, Lesser M, Davidson A. The Yaa locus and IFN-alpha fine-tune germinal center B cell selection in murine systemic lupus erythematosus. J Immunol. 2012;189:4305–4312. doi: 10.4049/jimmunol.1200745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 24.Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H. A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheid JF, Mouquet H, Kofer J, Yurasov S, Nussenzweig MC, Wardemann H. Differential regulation of self-reactivity discriminates between IgG+ human circulating memory B cells and bone marrow plasma cells. Proc Natl Acad Sci U S A. 2011;108:18044–18048. doi: 10.1073/pnas.1113395108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malkiel S, Jeganathan V, Wolfson S, Orduno NM, Marasco E, Aranow C, Mackay M, Gregersen PK, Diamond B. Checkpoints for Autoreactive B Cells in Peripheral Blood of Lupus Patients Assessed By Flow Cytometry. Arthritis Rheumatol. 2016 doi: 10.1002/art.39710. ** The authors developed a flow cytometry assay to easily identify autoreactive B cells recognizing nuclear antigens (ANA+ B cells). Both lupus patients and healthy individuals displayed transitional/naïve and naïve/memory B cell tolerance checkpoints of ANA+ B cells; however, anergy induction was impaired in lupus patients.
- 28.Koelsch K, Zheng NY, Zhang Q, Duty A, Helms C, Mathias MD, Jared M, Smith K, Capra JD, Wilson PC. Mature B cells class switched to IgD are autoreactive in healthy individuals. J Clin Invest. 2007;117:1558–1565. doi: 10.1172/JCI27628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von Bernuth H, Zhang SY, Puel A, Jouanguy E, Picard C, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, Gonzalez JB, Pascual V, Stichweh D, Wardemann H, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci U S A. 2008;105:9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobi AM, Zhang J, Mackay M, Aranow C, Diamond B. Phenotypic characterization of autoreactive B cells--checkpoints of B cell tolerance in patients with systemic lupus erythematosus. PLoS One. 2009;4:e5776. doi: 10.1371/journal.pone.0005776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cappione A, 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 35. Tipton CM, Fucile CF, Darce J, Chida A, Ichikawa T, Gregoretti I, Schieferl S, Hom J, Jenks S, Feldman RJ, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16:755–765. doi: 10.1038/ni.3175. * Through the use of deep sequencing of immunoglobulin heavy chain variable region genes, this group discovered that a considerable portion of plasmablasts during SLE flares derive from a subset they define as activated naïve B cells. Understanding the different types of B cells involved in maintaining an autoreactive response is crucial in the development of effective targeted therapeutics.
- 36.Deng Y, Tsao BP. Advances in lupus genetics and epigenetics. Curr Opin Rheumatol. 2014;26:482–492. doi: 10.1097/BOR.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Yin X, Wen L, Yang C, Sheng Y, Lin Y, Zhu Z, Shen C, Shi Y, Zheng Y, et al. Several Critical Cell Types, Tissues, and Pathways Are Implicated in Genome-Wide Association Studies for Systemic Lupus Erythematosus. G3 (Bethesda) 2016;6:1503–1511. doi: 10.1534/g3.116.027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gutierrez-Arcelus M, Rich SS, Raychaudhuri S. Autoimmune diseases - connecting risk alleles with molecular traits of the immune system. Nat Rev Genet. 2016;17:160–174. doi: 10.1038/nrg.2015.33. * This article discusses methods to study human gene expression data sets and their utilization to infer immune cell subsets that are affected by genetic risk alleles. This methodology represents a novel approach to understand human autoimmunity.
- 39.Mok CC. Update on B-cell targeted therapies for systemic lupus erythematosus. Expert Opin Biol Ther. 2014;14:773–788. doi: 10.1517/14712598.2014.895810. [DOI] [PubMed] [Google Scholar]
- 40.Nakayamada S, Iwata S, Tanaka Y. Relevance of lymphocyte subsets to B cell-targeted therapy in systemic lupus erythematosus. Int J Rheum Dis. 2015;18:208–218. doi: 10.1111/1756-185X.12534. [DOI] [PubMed] [Google Scholar]
- 41.Chung SA, Taylor KE, Graham RR, Nititham J, Lee AT, Ortmann WA, Jacob CO, Alarcon-Riquelme ME, Tsao BP, Harley JB, et al. Differential genetic associations for systemic lupus erythematosus based on anti-dsDNA autoantibody production. PLoS Genet. 2011;7:e1001323. doi: 10.1371/journal.pgen.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozyrev SV, Abelson AK, Wojcik J, Zaghlool A, Linga Reddy MV, Sanchez E, Gunnarsson I, Svenungsson E, Sturfelt G, Jonsen A, et al. Functional variants in the B-cell gene BANK1 are associated with systemic lupus erythematosus. Nat Genet. 2008;40:211–216. doi: 10.1038/ng.79. [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama K, Su Ih IH, Tezuka T, Yasuda T, Mikoshiba K, Tarakhovsky A, Yamamoto T. BANK regulates BCR-induced calcium mobilization by promoting tyrosine phosphorylation of IP(3) receptor. EMBO J. 2002;21:83–92. doi: 10.1093/emboj/21.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tretter T, Ross AE, Dordai DI, Desiderio S. Mimicry of pre-B cell receptor signaling by activation of the tyrosine kinase Blk. J Exp Med. 2003;198:1863–1873. doi: 10.1084/jem.20030729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bewarder N, Weinrich V, Budde P, Hartmann D, Flaswinkel H, Reth M, Frey J. In vivo and in vitro specificity of protein tyrosine kinases for immunoglobulin G receptor (FcgammaRII) phosphorylation. Mol Cell Biol. 1996;16:4735–4743. doi: 10.1128/mcb.16.9.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpfendorfer KR, Olsson LM, Manjarrez Orduno N, Khalili H, Simeone AM, Katz MS, Lee AT, Diamond B, Gregersen PK. The autoimmunity-associated BLK haplotype exhibits cis-regulatory effects on mRNA and protein expression that are prominently observed in B cells early in development. Hum Mol Genet. 2012;21:3918–3925. doi: 10.1093/hmg/dds220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpfendorfer KR, Armstead BE, Shih A, Li W, Curran M, Manjarrez-Orduno N, Lee AT, Diamond B, Gregersen PK. Autoimmune disease-associated haplotypes of BLK exhibit lowered thresholds for B cell activation and expansion of Ig class-witched B cells. Arthritis Rheumatol. 2015;67:2866–2876. doi: 10.1002/art.39301. [DOI] [PubMed] [Google Scholar]
- 48.Manjarrez-Orduno N, Marasco E, Chung SA, Katz MS, Kiridly JF, Simpfendorfer KR, Freudenberg J, Ballard DH, Nashi E, Hopkins TJ, et al. CSK regulatory polymorphism is associated with systemic lupus erythematosus and influences B-cell signaling and activation. Nat Genet. 2012;44:1227–1230. doi: 10.1038/ng.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Floto RA, Clatworthy MR, Heilbronn KR, Rosner DR, MacAry PA, Rankin A, Lehner PJ, Ouwehand WH, Allen JM, Watkins NA, et al. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat Med. 2005;11:1056–1058. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 50.Blank MC, Stefanescu RN, Masuda E, Marti F, King PD, Redecha PB, Wurzburger RJ, Peterson MG, Tanaka S, Pricop L. Decreased transcription of the human FCGR2B gene mediated by the −343 G/C promoter polymorphism and association with systemic lupus erythematosus. Hum Genet. 2005;117:220–227. doi: 10.1007/s00439-005-1302-3. [DOI] [PubMed] [Google Scholar]
- 51.Kono H, Kyogoku C, Suzuki T, Tsuchiya N, Honda H, Yamamoto K, Tokunaga K, Honda Z. FcgammaRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum Mol Genet. 2005;14:2881–2892. doi: 10.1093/hmg/ddi320. [DOI] [PubMed] [Google Scholar]
- 52.Lazzari E, Jefferies CA. IRF5-mediated signaling and implications for SLE. Clin Immunol. 2014;153:343–352. doi: 10.1016/j.clim.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Lien C, Fang CM, Huso D, Livak F, Lu R, Pitha PM. Critical role of IRF-5 in regulation of B-cell differentiation. Proc Natl Acad Sci U S A. 2010;107:4664–4668. doi: 10.1073/pnas.0911193107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sigurdsson S, Nordmark G, Garnier S, Grundberg E, Kwan T, Nilsson O, Eloranta ML, Gunnarsson I, Svenungsson E, Sturfelt G, et al. A risk haplotype of STAT4 for systemic lupus erythematosus is over-expressed, correlates with anti-dsDNA and shows additive effects with two risk alleles of IRF5. Hum Mol Genet. 2008;17:2868–2876. doi: 10.1093/hmg/ddn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng D, Stone RC, Eloranta ML, Sangster-Guity N, Nordmark G, Sigurdsson S, Wang C, Alm G, Syvanen AC, Ronnblom L, et al. Genetic variants and disease-associated factors contribute to enhanced interferon regulatory factor 5 expression in blood cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62:562–573. doi: 10.1002/art.27223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stone RC, Feng D, Deng J, Singh S, Yang L, Fitzgerald-Bocarsly P, Eloranta ML, Ronnblom L, Barnes BJ. Interferon regulatory factor 5 activation in monocytes of systemic lupus erythematosus patients is triggered by circulating autoantigens independent of type I interferons. Arthritis Rheum. 2012;64:788–798. doi: 10.1002/art.33395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B. Tolerogenic function of Blimp-1 in dendritic cells. J Exp Med. 2011;208:2193–2199. doi: 10.1084/jem.20110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SJ, Gregersen PK, Diamond B. Regulation of dendritic cell activation by microRNA let-7c and BLIMP1. J Clin Invest. 2013;123:823–833. doi: 10.1172/JCI64712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, Vella A, Nutland S, Rance HE, Maier L, et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 61.Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol. 2007;179:4704–4710. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- 62.Arechiga AF, Habib T, He Y, Zhang X, Zhang ZY, Funk A, Buckner JH. Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J Immunol. 2009;182:3343–3347. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]