Abstract
Somatic awareness (SA) refers to heightened sensitivity to a variety of physical sensations and symptoms. Few attempts have been made to dissociate the relationship of SA and affective symptoms with pain outcomes. We used a validated measure of mood and anxiety symptoms that includes questions related to SA to predict the number of tender points found on physical examination in a large cross-sectional community sample (the Midlife in the United States [MIDUS] Biomarker study). General distress, positive affect, and SA, which were all significantly associated with tender point number in bivariate analyses, were used as predictors of the number of tender points in a multivariate negative binomial regression model. In this model a greater number of tender points was associated with higher levels of SA (p = .02) but not general distress (p = .13) or positive affect (p = .50). Follow-up mediation analyses indicated that the relationship between general distress and tender points was partially mediated by levels of SA. Our primary finding is that SA is strongly related to the number of tender points in a community sample. Mechanisms linking SA to the spatial distribution of pain sensitivity should be investigated further.
Perspective
This article presents an analysis of three overlapping psychological constructs and their relationship to widespread pain sensitivity on palpation. The findings suggest that somatic awareness is most strongly related to the spatial distribution of pain sensitivity and that further assessing it may improve our understanding of the relationship between psychological factors and pain.
Keywords: Somatic Awareness, Tender Points, Positive Affect, Distress, Pain
1. Introduction
Heightened Somatic Awareness (SA) refers to a greater than average awareness for a variety of physical sensations and symptoms. Individuals high in SA have a tendency to notice and report non-specific symptoms, such as feeling shortness of breath, faint or having the sensation that one’s muscles are trembling. Higher levels of SA have been linked to the presence of a variety of chronic pain conditions including fibromyalgia (FM)26, 47, irritable bowel syndrome (IBS)48, and temporomandibular disorder (TMD)23. Within pain conditions, higher levels of SA have also been linked to greater painful symptom severity1, 41, 43 and experimental pain testing outcomes such as more tender facial areas on palpation in TMD patients,58 lower heat pain thresholds in women with provoked vestibulodynia,61 and higher pressure sensitivity in FM and TMD.28 This has led to speculation that SA reflects some combination of psychological and neurobiological vulnerability to pain.47
However, the nature of this vulnerability continues to be debated, in part because SA is strongly associated with negative affect (i.e., depressive and anxious symptoms)68 which is also strongly associated with pain9, 10, 15, 22, 27, 33, 65. Complicating matters, the relationship between different types of affective processes and pain outcomes differs substantially across studies. A study of patients with Complex Regional Pain Syndrome found that the previous day’s level of depressed mood, but not anxiety, predicted self-reported clinical pain22 and a recent systematic review found that depression but not anxiety was related to knee pain55. Conversely, preoperative anxiety predicts postoperative pain71 and experimentally induced anxiety produces pronounced increases in pain reports62. Positive affect has also been linked to lower levels of self-reported pain75 and increased tolerance when induced by pleasant images16. Despite its potential relevance, many pain studies do not attempt to differentiate the impact of affect and SA on measures of pain sensitivity. This could be important for researchers interested in the neurobiological basis of pain disorders, given recent neuroimaging investigations suggest that the neural underpinnings of SA may differ from those associated with general depressive and anxious symptoms21, 35. For instance, one functional connectivity study found that higher levels of SA were associated with greater functional connectivity between elements of the so-called pain matrix53, and SA also has been shown to be characterized in part by cognitive biases such as greater attention to and recall of bodily symptoms.72
Tender points are discrete areas of the body where moderate palpation produces pain in some individuals. Tender points have been used to classify individuals with FM for research purposes for many years74, though it has been known for some time that the number of positive tender points demonstrates a linear relationship with measures of distress and disability.14, 19, 73 These findings suggest that tender points measure a continuum of the “widespreadness” of pain sensitivity and possibly a vulnerability to develop clinical pain disorders.
Disentangling the unique contributions of SA and affective states on pain outcomes has the potential to improve the measurement of clinical pain and address potential risk factors for developing pain disorders. To determine the relationship between SA, affect, and the diffuseness of pain sensitivity we used established subscales for SA and affective constructs to predict tender points in a community sample. We hypothesized that variance in the number of tender points would be most strongly explained by SA followed by negative and positive affect. We further hypothesized that part of the association between affective symptoms and the spatial distribution of pain sensitivity would be mediated by comorbid levels of SA, indicating a more proximal role for SA in predicting tender points..
2. Methods
2.1. Sample
We performed a retrospective analysis of the Midlife in the United States (MIDUS) biomarker study. Between 1995 and 1996, 7189 non-institutionalized adults were recruited by random-digit dial to take part in a study of health and aging (MIDUS I)5. Of these, 4963 were re-contacted between 2004 and 2005 to take part in a follow-up study. The Biomarker Project17 represented a subset of these participants who underwent a physical examination, additional questionnaires, and provided blood and urine for analysis of a variety of physiological measures, including markers of inflammation and sympathetic nervous system (SNS) activity. All participants in the second wave (MIDUS II) who completed the phone interview were eligible for this biomarker study. 1255 agreed to participate and were provided compensation to cover travel expenses to one of the three sites (the University of Wisconsin, Madison; the University of California, Los Angeles; Georgetown University, Washington, D.C.). At the University of Wisconsin a long form physical evaluation was conducted that included a tender point examination on 522 participants. Of these, 15 were missing data on a variable of interest and were excluded. Therefore, the final sample consisted of 507 participants. All participants provided informed consent and all procedures were approved by the respective institutional review boards.
2.2. Measures
2.2.1. Mood and Somatic Awareness
The Mood and Anxiety Symptom Questionnaire (MASQ) is a self-report measure of symptoms of anxiety and depression68, 69. In the MIDUS sample a 64-item version was administered. Participants are asked how much they experienced each item in the last week on a 5-point scale (1= not at all, 5 = extremely). These items were used in a principal components analysis to identify mood and SA constructs. A three-part (tripartite) structure of the MASQ has been observed in multiple samples consisting of a) general psychological distress, b) positive affect, and c) somatic awareness factors32, 68 primarily through the use of Principal Components Analysis (PCA). The resulting subscales demonstrate excellent convergent validity when compared to other measures of depression and SA.66 This tripartite structure has also been observed in at least one sample of chronic pain patients25. Others have questioned this model via the use of confirmatory factor analysis4 or by proposing alternative models that employ first and second order factors of depression and anxiety8. To differentiate the items Bedford (1997) has argued for a two part approach to item reduction, retaining items only if they load on an individual factor at .30 or greater and show a .20 higher loading than on any other factor2. Using this approach the tripartite structure of the MASQ provides a good fit to the data in large samples of men and women, healthy controls, patients in primary care, and patients in mental health care settings66. Supplementary Table 1 shows each of the 64 MASQ items.
2.2.2. Health Information and Current Medication Data
The following were collected by self-report: engaging in regular exercise (20 minutes or more at least 3 times/week), smoking status (current/former, never) age, gender, and presence of chronic conditions/symptoms (heart disease, high blood pressure, circulation problems, blood clots, heart murmur, transient ischemic attack or stroke, anemia or other blood disease, cholesterol problems, diabetes, asthma, emphysema/chronic obstructive pulmonary disease, tuberculosis (TB), positive TB skin test, thyroid disease, peptic ulcer disease, cancer, colon polyp, arthritis, glaucoma, cirrhosis/liver disease, alcoholism, depression, blood transfusion before 1993). Sleep efficiency was calculated from participants (n=409) who wore an activity monitor for seven consecutive days. Sleep efficiency was calculated from the percentage of scored total sleep time (from the device) divided by the interval duration and averaged over the seven sleep periods.
Participants were instructed to bring all medication, in original bottles to the University of Wisconsin-Madison site at the time of the evaluation. Codes were applied to each medication based on medication name, route of administration, and purpose, following the American Hospital Formulary System (AHFS) Pharmacologic-Therapeutic classification system. For the current analyses, medications were coded into categories based on common pharmacologic effects that might impact pain and/or mood. These were antidepressants (e.g., selective serotonin/norepinephrine reuptake inhibitors, tricyclic antidepressants), corticosteroids, opiates, nonsteroidal anti-inflammatory drugs, and anxiolytics/sedatives (e.g. benzodiazepines).
2.2.3 Physical Examination
The physical examination was conducted by a credentialed clinician (i.e., advanced practice nurse, nurse practitioner, physician assistant, medical doctor). Height and weight were measured for calculation of body mass index. Joints were examined for deformities, crepitation, limited range of motion, swelling, heat, and redness. Muscles were examined for tremor, atrophy, and fasciculation. Participants were then coded as having either normal or abnormal joint/musculature findings. A neurological sensation examination was conducted on the right and left upper and lower extremities (light touch, pinprick, temperature, vibration, and limb position) and participants were coded as having either normal (i.e., sensation detected) or abnormal (i.e., sensation not detected) responses to each stimulus.
A tender point examination was conducted on eighteen distinct areas of the body using the tender point examination portion of the American College of Rheumatology 1990 criteria for fibromyalgia.74 Examiners were initially trained by the same experienced clinician to ensure consistent and proper technique. Further training was conducted by the most experienced clinician available. These were tested bilaterally at the occiput: suboccipital muscle insertions, trapezius: midpoint of the upper border, supraspinatus: above the medial border of the scapular spine, gluteal: upper and outer quadrants of the buttocks, greater trochanter: posterior to the trochanteric prominence, low cervical: anterior aspects of the intertransverse spaces at C5–C7, second rib: second costochondral junction, lateral epicondyle: 2 cm distal to the epicondyles, and knee: medial fat pad proximal to the joint line. A tender point was determined by applying either the thumb or first two fingers at a pressure of approximately 4 kg.
2.2.4 C-Reactive Protein (CRP)
Fasting blood samples were obtained from participants prior to breakfast. Samples were stored at −80°C. CRP was assayed with an immunonepholometric assay using a BNII nephelometer (Dade Behring Inc). The inter-assay coefficient of variation (CV) is 2.1 to 5.7%.
2.2.5. Norepinephrine & Creatinine
A 12-hour overnight (7:00 pm – 7:00 am) urine sample was obtained from each participant in a container with 25 mL of 50% acetic acid. These were stored at −80°C. High-Pressure Liquid Chromatography (HPLC) was used to measure norepinephrine31. The inter-assay CV is 6.7–6.9%. Creatinine was measured using an Enzymatic Colorimetric Assay. The inter-assay CV is 0.85%. Norepinephrine levels were then adjusted to levels of creatinine.
2.3 Statistical Analyses
Analyses were conducted in SPSS version 22.0 and R version 3.2.2 (Package ‘MASS’).
2.3.1 Affect and Somatic Awareness – Mood and Anxiety Symptom Questionnaire
We used the available items from a three factor solution using the full 90-item MASQ questionnaire in a large sample (n=534) that resulted from this item reduction approach (Keogh and Reidy, 2000; sixteen items for general psychological distress, thirteen items for positive mood, sixteen items for SA).32 These subscales were correlated with other measures of mood/affect administered in the MIDUS Biomarker project (Perceived Stress Scale [PSS]13, Center for Epidemiological Studies – Depression [CES-D]56, Spielberger Trait Anxiety Inventory [STAI]60). Because the MASQ version administered in the MIDUS Biomarker subsample used 64 items rather than the 90 items frequently used in other samples, we also opted to confirm the tripartite structure in this sample via PCA (Supplementary Table 1).
2.3.2. Bivariate Analyses
Associations between potential covariates and number of tender points were examined using non-parametric methods: Spearman’s rank correlations for continuous variables and Mann-Whitney U tests for categorical variables. Covariates that were significantly associated with the number of tender points (p < .05) were retained in multivariate models. Additionally, associations between the measures of interest (general psychological distress, positive affect, SA) and number of tender points were examined by Spearman’s rank correlation.
2.3.3. Multivariate Model
To determine which measures of SA and mood were most strongly associated with the number of tender points, we used negative binomial regression in a model including each of the measures (general psychological distress, positive mood, SA) and significant covariates. Negative binomial regression is a similar approach to Poisson regression with one additional parameter to account for the overdispersion of the data – relevant here because of the large number of participants without any tender points (76%). This model was then compared to Poisson regression, and zero-inflated negative binomial models using the same data by Bayesian Information Criteria (BIC). By these metrics the negative binomial model provided the best fit to the data (negative binomial model: 981 < zero-inflated Poisson model: 997 < Poisson regression model: 1256). Fixed effects from negative binomial models are interpreted in the same way as results from Poisson regression models.
2.3.4 Secondary Analysis
To determine if observed relationships between SA and tender points were driven by those participants with the highest levels of SA, we conducted a secondary analysis identical to the multivariate model but excluding participants whose SA levels were one standard deviation above the mean (SA ≥ 28; n = 63).
2.3.5. Mediation Models
To determine if the relationship between general psychological distress and positive affect with tender points is mediated by somatic awareness, we conducted causal mediation analyses using the framework advocated by Imai, Keele and Tingley (2010)29, implemented in Python Statsmodels. The mediation is between: a) positive affect and SA, and b) general psychological distress and SA. These models provide estimates of average mediated and direct effects while accounting for covariates, and also estimate both types of effects for different levels of the independent variable. Standardized values of general psychological distress, positive affect, and SA were used for these models.
3. Results
3.1. Sample Demographic and Health Characteristics
Participants were approximately 53 years old on average. A majority were female (59%) and married or living with a partner (56%). On average, participants were using approximately three prescription medications and had approximately four chronic conditions. See Table 1 for demographic and health characteristics of the complete sample including more detailed information regarding medication use, and comparisons of participants with and without tender points.
Table 1.
Participant characteristics and a comparison of those with and without tender points.
| Characteristic Mean (SD) |
All (n=507) |
0 TPts (n=385) |
≥ 1 TPts (n =122) |
F1,505 | p |
|---|---|---|---|---|---|
| Age | 53.38 (11.74) | 52.71 (11.60) | 55.48 (11.99) | 5.20 | .023 |
| Somatic Awareness | 21.64 (6.02) | 20.46 (4.51) | 25.37 (8.28) | 69.86 | < .01 |
| Positive Affect | 40.72 (9.95) | 41.17 (9.62) | 39.30 (10.84) | 3.31 | .069 |
| General Psychological Distress |
24.41 (8.39) | 23.39 (7.12) | 27.62 (10.75) | 25.01 | < .01 |
| Body Mass Index | 30.70 (7.20) | 30.06 (6.90) | 32.70 (7.76) | 12.79 | < .01 |
| Number of Chronic Conditions |
4.17 (2.97) | 3.72 (2.73) | 5.58 (3.26) | 38.87 | < .01 |
| Sleep Efficiency (%) | 79.48 (10.37) | 79.73 (10.63) | 78.71 (9.55) | .74 | .39 |
| Blood C-Reactive Protein, µg/mLa |
0.25 (.51) | .20 (.50) | .40 (.50) | 14.35 | < .01 |
| Urine Norepinephrine, µg/gb |
1.37 (.20) | 1.35 (.19) | 1.42 (.19) | 11.14 | < .01 |
| Number of Rx Medications | 2.62 (2.87) | 2.24 (2.64) | 3.84 (3.22) | 30.49 | <.01 |
| Number of Tender Points | 0.80 (2.12) | NA | 3.34 (3.20) | NA | NA |
| % |
Pearson χ2 |
p | |||
| Gender (Female) | 59 | 52 | 81 | 32.65 | < .01 |
| Using NSAID | 42 | 40 | 49 | 3.58 | .058 |
| Using Anxiolytic/Sedative | 5 | 2 | 14 | 25.61 | < .01 |
| Using Opiate/Opioid | 8 | 5 | 16 | 18.35 | < .01 |
| Using Corticosteroids | 6 | 6 | 8 | .97 | .33 |
| Using Antidepressants | 13 | 11 | 21 | 7.42 | < .01 |
| Abnormal Joint/Musculature |
38 | 32 | 55 | 20.86 | < .01 |
| Abnormal Sensation | 58 | 59 | 57 | .18 | .68 |
| Regular Exercise | 72 | 74 | 66 | 3.50 | .061 |
| Smokerc | 52 | 53 | 50 | .28 | .60 |
log-transformed;
log-transformed, adjusted to urine creatinine;
current or former
3.2 MASQ subscales
The subscales derived from Keogh & Reidy’s three factor solution32 were correlated with other measures of emotionality administered in the MIDUS Biomarker subsample in the manner expected (e.g., MASQ-general psychological distress was associated with measures of negative mood, MASQ-positive mood was associated with positive affect). See Table 2. The PCA conducted on the 64 items resulted in a three factor solution very similar to those previously reported (See Supplementary Table 1 for methods and results.) The subscales used in subsequent analyses were highly correlated with the subscales derived from the PCA (General Psychological Distress subscales, r = .96; Positive Mood subscales, r = .994; SA subscales, r = .98; all p < .001). These results are unsurprising as eleven items were common to both general psychological distress subscales, thirteen items were common to both positive mood subscales, and thirteen were common to both SA subscales.
Table 2.
Correlation table showing associations between the MASQ subscales and other negative emotionality measures administered in the MIDUS biomarker study.
| Perceived Stress Scale13 |
Spielberger Trait Anxiety58 |
Social Anxiety Scale24 |
CESD total54 |
CESD anhedonic/ vegetative subscale |
CESD negative mood |
CESD positive mood |
|
|---|---|---|---|---|---|---|---|
| MASQ Somatic Arousal |
.460 | .497 | .274 | .574 | .533 | .550 | −.254 |
| MASQ General Distress |
.676 | .697 | .353 | .775 | .594 | .786 | −.455 |
| MASQ Positive Affect |
−.528 | −.561 | −.288 | −.587 | −.380 | −.444 | .676 |
All correlations are significant, (p <.01). Correlations above .6 are in bold.
3.3. Bivariate Analyses
Using non-parametric correlations, age, BMI, number of chronic conditions, CRP, and norepinephrine were associated with the number of tender points (all p <.05) while sleep efficiency was not (p =.10). Using Mann-Whitney U tests, female gender, abnormal joints/musculature, use of NSAIDs, use of opioids/analgesics, use of sedatives/anxiolytics, and use of antidepressants were associated with more tender points (all p < .05) while use of corticosteroids (p = .40), regular exercise (p = .07), smoking status (p = .65), and abnormal neurological examination results (p = .40) were not. General psychological distress (Spearman’s rho = .189, p < .01), positive affect (Spearman’s rho = − .101, p = .019) and SA (Spearman’s rho = .295, p < .01) were each associated with the number of tender points in bivariate analyses.
3.4. Multivariate Model
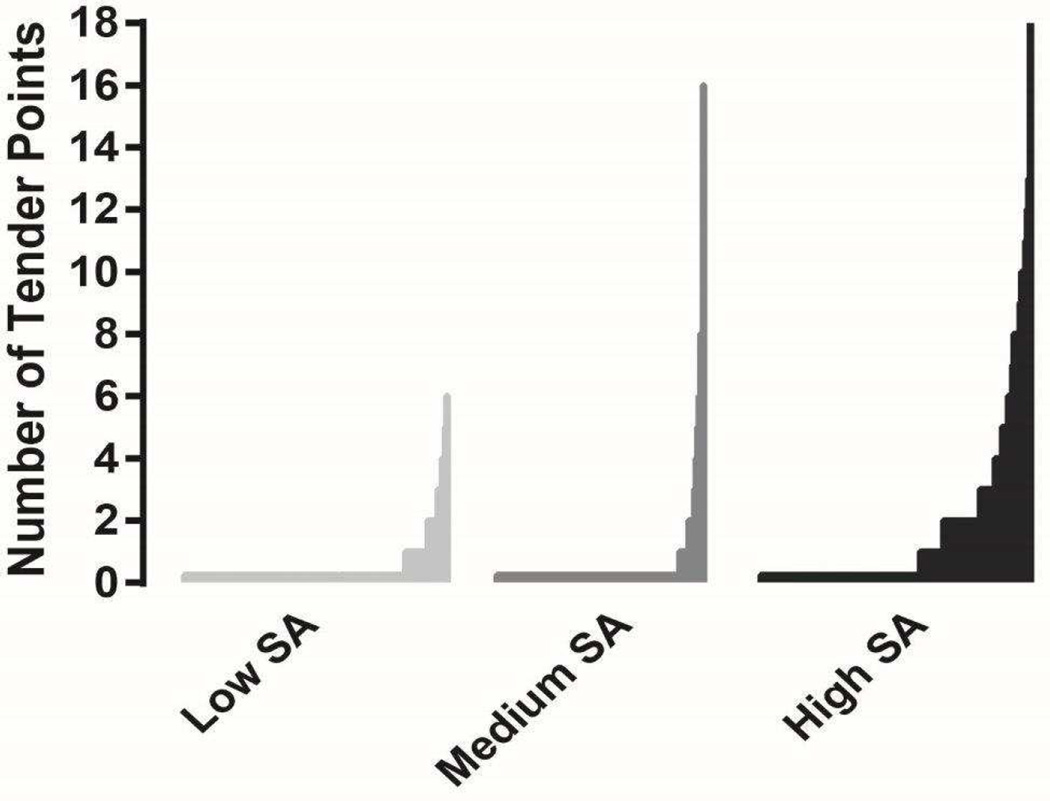
Higher levels of SA were associated with a greater number of tender points on physical examination (p = .019) while levels of general negative mood (p = .13) and positive affect (p =.50) were not, controlling for age, gender, BMI, use of antidepressants, use of sedatives/anxiolytics, use of opioids, CRP and norepinephrine, physical examination results and the number of chronic conditions present. Older age (p = .014), female gender (p <.01), use of sedatives/anxiolytics (p =.035), use of opioids (p =.040), and abnormal joints/musculature on physical examination (p =.020) were also associated with a greater number of tender points. Each one point increase on the SA subscale (range 16–51) was associated with an approximately 5% higher estimated likelihood of finding an additional tender point during the examination (Estimated likelihood=1.051, 95% CI = 1.008, 1.098. See Table 3 for full parameters of the model including estimates for each covariate and Figure 1 showing the distribution of tender points by low, medium and high levels of SA (for illustrative purposes).
Table 3.
Multivariate negative binomial regression model for number of tender points.
| Estimate | S.E. | Z value | p | exp | 95% CI, ll | ul | |
|---|---|---|---|---|---|---|---|
| (Intercept) | −6.749 | 1.233 | −5.476 | <.001 | 0.001 | 0.000 | 0.014 |
| Somatic Awareness | 0.050 | 0.021 | 2.355 | 0.019 | 1.051 | 1.008 | 1.098 |
| General Distress | 0.025 | 0.017 | 1.519 | 0.129 | 1.026 | 0.991 | 1.062 |
| Positive Affect | −0.008 | 0.012 | −0.680 | 0.496 | 0.992 | 0.968 | 1.016 |
| Age | 0.028 | 0.011 | 2.455 | 0.014 | 1.028 | 1.005 | 1.052 |
| Female | 1.507 | 0.258 | 5.829 | <.001 | 4.511 | 2.719 | 7.613 |
| C-reactive protein | 0.130 | 0.236 | 0.551 | 0.582 | 1.139 | 0.696 | 1.866 |
| Norepinephrine | 0.618 | 0.564 | 1.096 | 0.273 | 1.855 | 0.546 | 6.334 |
| Using Sedative | 0.942 | 0.448 | 2.105 | 0.035 | 2.565 | 1.068 | 6.652 |
| Using Anti-depressant | 0.567 | 0.300 | 1.890 | 0.059 | 1.764 | 0.966 | 3.296 |
| Using NSAID | −0.152 | 0.229 | −0.663 | 0.508 | 0.859 | 0.529 | 1.384 |
| Using Opioid | 0.731 | 0.355 | 2.059 | 0.040 | 2.077 | 1.015 | 4.492 |
| # of Chronic Conditions | 0.013 | 0.043 | 0.296 | 0.767 | 1.013 | 0.928 | 1.106 |
| Abnormal Joint/muscle | 0.526 | 0.227 | 2.318 | 0.020 | 1.692 | 1.070 | 2.685 |
| Body Mass Index | 0.027 | 0.015 | 1.768 | 0.077 | 1.027 | 0.995 | 1.062 |
Significant variables are in bold.
Figure 1.
Distribution of tender points by low, medium and high levels of somatic awareness (divided into tertiles by SA rank). Low group SA mean (SD) = 16.98 (.83), medium group SA = 19.85 (.78), high group SA= 27.56 (6.30).. Low group SA mean (SD) = 16.98 (.83), medium group SA = 19.85 (.78), high group SA= 27.56 (6.30).
3.5. Secondary Analysis
Excluding high SA participants resulted in no substantial differences from the results of the multivariate model including all participants. SA was associated with the number of tender points found on physical examination (IR= 1.096, 95% CI =1.001, 1.202, p = .047) while neither general distress (p = .20) nor positive affect (p = .76) were associated with the number of tender points.
3.6. Mediation Analyses
SA was a significant mediator of the effect of general psychological distress on tender points, accounting for approximately 55% of the total effect (Est. = .561, 95% CI = .076, 1.46, p = .016). In this model the direct effect of general psychological distress on tender points was not significant (Est. = .170, 95% CI = −.090, .499, p = .21) while the indirect (mediated) effect was significantly associated with tender points (Est. = .202, 95% CI = .038, .388, p = .014).
Conversely, SA did not mediate the effect of positive affect on tender points (mediated effect Est. = −.044, 95% CI = −.151, .027, p = .21) nor was there a significant direct effect of positive affect on tender points (Est. = −.068, 95% CI = −.239, .115, p = .41).
4. Discussion
The primary finding of this study is that a higher tender point count is most strongly associated with SA, controlling for measures of general psychological distress and positive mood. This is the first study to our knowledge to attempt to disentangle the association of SA from affective states in relation to the spatial distribution of pain sensitivity in a large community sample. These findings are similar to well-established associations between SA and other pain-related outcomes18, 46 and extend previous work by demonstrating the primacy of SA even when accounting for affective processes frequently found to be comorbid with more tender points. While both general psychological distress and positive mood were associated with tender points in bivariate analyses, these associations were no longer significant in multivariate models including SA. The results of the mediation analyses confirm that some of the association between general psychological distress and the diffuseness of pain sensitivity on physical examination is due to levels of SA.
Evaluations of the association of a variety of pain outcomes with measures of affect and SA have revealed inconsistent results. Self-reported pain was not associated with SA scores in one study of 280 chronic pain patients when affective measures were also used in the analyses52; this may be because negative affective measures are more strongly associated with clinical pain reports than the distribution of pain sensitivity as measured by tender points, or because that study used raw scores, rather than extracted components. In contrast, a mediational analysis evaluating the association of SA and negative affect measures with clinical abdominal pain in schoolchildren outcomes found that SA was strongly associated with this measure, and that it mediated the association of depression with painful abdominal symptoms36; this finding is echoed by a study of adults with functional gastrointestinal symptoms in which SA was associated with all types of GI symptoms while depression was only moderately associated with non-painful symptoms12. A recent study of healthy individuals employing a factor analytic approach found that SA, but not negative or positive mood, was related to the qualitative evaluation of evoked pain37. The most comparable study to date examined patients with psychological distress and found that SA was associated with the likelihood of having a high number of tender points (>5),45 though this study did not attempt to psychometrically isolate components of SA and psychological distress. Prospective analyses have demonstrated that SA is a strong predictor of the development of painful disorders, including TMD and widespread fibromyalgia-like pain/tenderness23, 46; these are critical findings as they suggest that SA is a risk factor for, rather than a consequence of, the development of chronic pain conditions.
The breadth of the MIDUS Biomarker study allowed for a variety of important controls that might contribute to peripheral nociceptive input. The physical examination and medical history allowed for assessment of chronic conditions that are characterized by musculoskeletal pain (e.g., arthritis) and abnormal joints or musculature that could might result in increased numbers of tender points. Elevated peripheral inflammation has previously been associated with increased pain sensitivity both under basal conditions38, and during experimentally induced inflammation with immunogenic challenges70 an effect that may be mediated by increases in negative affective processes34. Sympathetic nervous system activation measured by urinary catecholamine (as in the MIDUS study) has also been linked to increased reports of musculoskeletal pain.20 Various medications can also modulate pain responses. For instance, long-term use of opioids is suspected of promoting hyperalgesia in some patients,67 antidepressants may be effective in pain relief for some conditions but not others57, 63 and the chronic use of benzodiazepines is associated with chronic pain, though the association is not yet well understood.40, 51 In these analyses the use of opioids, sedatives and antidepressants were all associated with a greater number of tender points, perhaps indicating neural modulation of pain or psychopathology, though determining the nature of the association is not possible in a cross-sectional study. However, controlling for all of the above factors did not eliminate the association of SA with tender points.
That SA was still strongly associated with tender point counts in the presence of a comprehensive set of covariates suggests that it is a construct of primary importance when evaluating widespread pain sensitivity by physical examination. One possibility is that SA is related to abnormal pain-evoked brain activity. While few neuroimaging studies have used SA as a construct in relation to imaging outcomes those that have seem to reveal distinct activity in pain networks associated with the construct. In patients with mood disorders, SA scores derived from the MASQ were associated particularly with resting state functional connectivity between the rostral anterior cingulate cortex (rACC) and the ventral striatum53; this is of interest to pain researchers because the rACC plays a central role in descending inhibitory pain networks,3 and such networks have been found to be dysfunctional in chronic pain patients50. In diverticular disease patients, high SA is associated with less deactivation of ascending pain structures such as the ventral posterolateral thalamus and posterior insula, and less deactivation in pain affect structures such as the hippocampus and amygdala in anticipation of painful heat59. Taken together, these results suggest that SA may be associated with impaired inhibition of pain.
SA has also been associated with abnormal responses to experimental pain testing and sensory tasks. Higher SA is associated with lower heat pain tolerance in women with provoked vestibulodynia61, greater soreness in the trapezius after rapid needle insertion withdrawal44, a greater number of masticatory sites rated as painful by TMD patients58, and lower orofacial pressure pain thresholds11. These evoked-pain outcomes are echoed by studies of sensation and interoceptive processes. SA is associated with increases in the false-alarm rate on the somatic signal detection task -- an experience of illusory touch – in healthy individuals, as well as those with both medically explained and medically unexplained chronic abdominal pain6. Similarly, SA is associated with a worse performance on a heartbeat detection ability task42 and longer ERP latency following auditory cues49. These findings suggest that SA is not simply an increased awareness of or sensitivity to somatic sensations, but involves a distortion of attentional processes as well. There is also evidence that SA is associated with the gain control for other sensory modalities, as it has been shown to correlate with perceived unpleasantness of auditory tones.28 It is worth noting that most of these studies used populations with clinical pain or mood disorders. Our findings suggest that SA is an important construct in relation to the distribution of pain sensitivity found on palpation in a community sample, and our secondary analysis indicated that this is so even when those with the highest levels of SA were removed.
4.1. Limitations
A significant limitation of this study is the absence of clinical pain measures. Previous research has revealed moderate associations between clinical pain measures and tender point counts (i.e., Pearson correlations between .4 and .6).54, 73 These findings suggest that tender point counts, while related to clinical pain, are not a proxy for self-report measures. Our findings relate to the diffuseness of pain-sensitivity on manual palpation only – tender points – and it is possible that other measures of experimental pain evaluations might reveal substantial differences in the relationship between affective symptoms, SA, and other evoked-pain outcomes. For instance, the so-called “medial” pain system has been differentially associated with pain affect rather than sensory qualities of pain64, and it is possible that general psychological distress or positive affect are associated with affective qualities of pain including clinical pain reports, while SA is associated more with lower thresholds or tolerance in experimental pain paradigms. The overall low prevalence of tender points in this sample limits inferences about those at the extreme end of this spectrum -- those with tenderness and pain across the body characteristic of fibromyalgia or related chronic pain conditions. Other important psychological constructs such as pain catastrophizing were not measured in the MIDUS Biomarker study, and we therefore cannot draw conclusions about their association with pain sensitivity on physical examination in this sample.
4.2. Conclusions and Future Directions
The present results indicate that somatic awareness is significantly associated with the distribution of pain sensitivity in the general community and suggest that this psychological construct mediates some of the association between affect and pain. SA should be more frequently assessed in both research and clinical settings; the availability of validated short forms of the MASQ should be helpful toward this end.39, 66 SA’s robust association with evoked pain on palpation, in a manner independent of the influence of mood, could make it particularly useful for gleaning information about the distribution of a patient’s pain sensitivity without requiring attendance at the clinic. Psychosocial interventions in samples characterized by high levels of SA have demonstrated some success in reducing clinical pain levels;24 whether these interventions would also reduce the spatial distribution of pain sensitivity revealed during clinical examinations is an open question. Future studies of pain in community samples might employ measures of SA and affect and relate them to a variety of experimental and self-report pain outcomes. For instance, pain tolerance or pain affect might have different relationships with SA and negative/positive mood than pain threshold and sensory pain ratings. Recent research suggests that simple evaluations of the diffuseness of pain are prospectively useful for predicting pain related outcomes and determining pain treatment responses following common procedures7, 30; therefore, measuring SA may contribute to better prediction of these outcomes and treatment responses.
Supplementary Material
Highlights.
Somatic awareness is an emerging concept in pain research
Somatic awareness is associated with higher tender point count
Somatic awareness mediates the relationship between distress and tender point count
Acknowledgments
This work was funded by the National Institute on Aging [Grant number: P01-AG020166]. The original study was supported, in part, by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. Authors Schrepf and Harper were supported in part by the National Institute of Dental and Craniofacial Research [Grant number: K12 DE023574]. Dr. Harte has received research funding from Cerephex, Forest Laboratories, Eli Lily and Merck; and served as a consultant for Pfizer, Analgesic Solutions, Aptinyx, and deCode Genetics. Dr. Williams is currently the President of the American Pain Society; he has served as a consultant with Pfizer Inc. and with Community Health Focus Inc. Dr. Hassett is a consultant for Precision Health Economics and Happify, Inc. She has also had research funded by Bristol-Myers Squibb and Pfizer, Inc.
The authors wish to thank Kerby Shedden, PhD, and the University of Michigan Consulting for Statistics, Computing & Analytics Research (CSCAR) center for their critical reading and contributions to the data analysis strategy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Conflicts of Interest: The authors declare no conflict of interest and no competing financial interests with this work.
References
- 1.Ailliet L, Rubinstein SM, Knol D, van Tulder MW, de Vet HC. Somatization is associated with worse outcome in a chiropractic patient population with neck pain and low back pain. Manual therapy. 2016;21:170–176. doi: 10.1016/j.math.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Bedford A. On Clark-Watson's tripartite model of anxiety and depression. Psychological Reports. 1997;80:125–126. doi: 10.2466/pr0.1997.80.1.125. [DOI] [PubMed] [Google Scholar]
- 3.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Boschen MJ, Oei TP. Factor structure of the Mood and Anxiety Symptom Questionnaire does not generalize to an anxious/depressed sample. Australian and New Zealand Journal of Psychiatry. 2006;40:1016–1024. doi: 10.1080/j.1440-1614.2006.01926.x. [DOI] [PubMed] [Google Scholar]
- 5.Brim OG, Ryff CD, Kessler RC. The MIDUS National Survey: An Overview. University of Chicago Press; 2004. [Google Scholar]
- 6.Brown RJ, Skehan D, Chapman A, Perry EP, McKenzie KJ, Lloyd DM, Babbs C, Paine P, Poliakoff E. Physical symptom reporting is associated with a tendency to experience somatosensory distortion. Psychosomatic medicine. 2012;74:648–655. doi: 10.1097/PSY.0b013e3182595358. [DOI] [PubMed] [Google Scholar]
- 7.Brummett CM, Janda AM, Schueller CM, Tsodikov A, Morris M, Williams DA, Clauw DJ. Survey Criteria for Fibromyalgia Independently Predict Increased Postoperative Opioid Consumption after Lower Extremity Joint Arthroplasty: A Prospective, Observational Cohort Study. Anesthesiology. 2013;119 doi: 10.1097/ALN.0b013e3182a8eb1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns DD, Eidelson RJ. Why are depression and anxiety correlated? A test of the tripartite model. Journal of Consulting and Clinical Psychology. 1998;66:461–473. doi: 10.1037//0022-006x.66.3.461. [DOI] [PubMed] [Google Scholar]
- 9.Chieng YJS, Chan WCS, Klainin-Yobas P, He HG. Perioperative anxiety and postoperative pain in children and adolescents undergoing elective surgical procedures: a quantitative systematic review. Journal of advanced nursing. 2014;70:243–255. doi: 10.1111/jan.12205. [DOI] [PubMed] [Google Scholar]
- 10.Chou K-L. Reciprocal relationship between pain and depression in older adults: Evidence from the English Longitudinal Study of Ageing. Journal of Affective Disorders. 2007;102:115–123. doi: 10.1016/j.jad.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Cioffi I, Michelotti A, Perrotta S, Chiodini P, Ohrbach R. Effect of somatosensory amplification and trait anxiety on experimentally induced orthodontic pain. European journal of oral sciences. 2016;124:127–134. doi: 10.1111/eos.12258. [DOI] [PubMed] [Google Scholar]
- 12.Clauwaert N, Jones MP, Holvoet L, Vandenberghe J, Vos R, Tack J, Van Oudenhove L. Associations between gastric sensorimotor function, depression, somatization, and symptom-based subgroups in functional gastroduodenal disorders: are all symptoms equal? Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24:e1088–e1565. doi: 10.1111/j.1365-2982.2012.01985.x. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 14.Croft P, Burt J, Schollum J, Thomas E, Macfarlane G, Silman A. More pain, more tender points: is fibromyalgia just one end of a continuous spectrum? Annals of the rheumatic diseases. 1996;55:482–485. doi: 10.1136/ard.55.7.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Heer EW, Gerrits MM, Beekman AT, Dekker J, van Marwijk HW, de Waal MW, Spinhoven P, Penninx BW, van der Feltz-Cornelis CM. The association of depression and anxiety with pain: a study from NESDA. 2014 doi: 10.1371/journal.pone.0106907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Wied M, Verbaten MN. Affective pictures processing, attention, and pain tolerance. Pain. 2001;90:163–172. doi: 10.1016/s0304-3959(00)00400-0. [DOI] [PubMed] [Google Scholar]
- 17.Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. Journal of aging and health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dworkin SF, Von Korff M, LeResche L. Multiple pains and psychiatric disturbance: an epidemiologic investigation. Archives of general psychiatry. 1990;47:239–244. doi: 10.1001/archpsyc.1990.01810150039007. [DOI] [PubMed] [Google Scholar]
- 19.Eggermont LH, Shmerling RH, Leveille SG. Tender point count, pain, and mobility in the older population: the mobilize Boston study. The journal of pain : official journal of the American Pain Society. 2010;11:62–70. doi: 10.1016/j.jpain.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elfering A, Grebner S, Gerber H, Semmer NK. Workplace observation of work stressors, catecholamines and musculoskeletal pain among male employees. Scandinavian journal of work, environment & health. 2008;34:337–344. doi: 10.5271/sjweh.1280. [DOI] [PubMed] [Google Scholar]
- 21.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. 2014 doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 22.Feldman SI, Downey G, Schaffer-Neitz R. Pain, negative mood, and perceived support in chronic pain patients: A daily diary study of people with reflex sympathetic dystrophy syndrome. Journal of Consulting and Clinical Psychology. 1999;67:776–785. doi: 10.1037//0022-006x.67.5.776. [DOI] [PubMed] [Google Scholar]
- 23.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14:T75–T90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fjorback LO, Arendt M, Ørnbøl E, Walach H, Rehfeld E, Schröder A, Fink P. Mindfulness therapy for somatization disorder and functional somatic syndromes — Randomized trial with one-year follow-up. Journal of psychosomatic research. 2013;74:31–40. doi: 10.1016/j.jpsychores.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Geisser ME, Cano A, Foran H. Psychometric properties of the mood and anxiety symptom questionnaire in patients with chronic pain. Clin J Pain. 2006;22:1–9. doi: 10.1097/01.ajp.0000146180.55778.4d. [DOI] [PubMed] [Google Scholar]
- 26.Geisser ME, Casey KL, Brucksch CB, Ribbens CM, Appleton BB, Crofford LJ. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: association with mood, somatic focus, and catastrophizing. Pain. 2003;102:243–250. doi: 10.1016/S0304-3959(02)00417-7. [DOI] [PubMed] [Google Scholar]
- 27.Gerrits MM, van Marwijk HW, van Oppen P, van der Horst H, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. Journal of psychosomatic research. 2015;78:64–70. doi: 10.1016/j.jpsychores.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Hollins M, Harper D, Gallagher S, Owings EW, Lim PF, Miller V, Siddiqi MQ, Maixner W. Perceived intensity and unpleasantness of cutaneous and auditory stimuli: an evaluation of the generalized hypervigilance hypothesis. Pain. 2009;141:215–221. doi: 10.1016/j.pain.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychological methods. 2010;15:309. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 30.Janda AM, As-Sanie S, Rajala B, Tsodikov A, Moser SE, Clauw DJ, Brummett CM. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. The Journal of the American Society of Anesthesiologists. 2015;122:1103–1111. doi: 10.1097/ALN.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 31.Jiang N, Machacek D. Measurement of catecholamines in blood and urine by liquid chromatography with amperometric detection. Progress in HPLC-HPCE. 1987;2:397–426. [Google Scholar]
- 32.Keogh E, Reidy J. Exploring the factor structure of the Mood and Anxiety Symptom Questionnaire (MASQ) J Pers Assess. 2000;74:106–125. doi: 10.1207/S15327752JPA740108. [DOI] [PubMed] [Google Scholar]
- 33.Kindler S, Samietz S, Houshmand M, Grabe HJ, Bernhardt O, Biffar R, Kocher T, Meyer G, Völzke H, Metelmann H-R, Schwahn C. Depressive and Anxiety Symptoms as Risk Factors for Temporomandibular Joint Pain: A Prospective Cohort Study in the General Population. The Journal of Pain. 2012;13:1188–1197. doi: 10.1016/j.jpain.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Lacourt TE, Houtveen JH, Veldhuijzen van Zanten JJCS, Bosch JA, Drayson MT, Van Doornen LJP. Negative affectivity predicts decreased pain tolerance during low-grade inflammation in healthy women. Brain, Behavior, and Immunity. 2015;44:32–36. doi: 10.1016/j.bbi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Larson CL, Nitschke JB, Davidson RJ. Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion. 2007;7:182–191. doi: 10.1037/1528-3542.7.1.182. [DOI] [PubMed] [Google Scholar]
- 36.Lavigne JV, Saps M, Bryant FB. Models of anxiety, depression, somatization, and coping as predictors of abdominal pain in a community sample of school-age children. Journal of pediatric psychology. 2014;39:9–22. doi: 10.1093/jpepsy/jst060. [DOI] [PubMed] [Google Scholar]
- 37.Lee JE, Watson D, Frey Law LA. Lower-order pain-related constructs are more predictive of cold pressor pain ratings than higher-order personality traits. The journal of pain : official journal of the American Pain Society. 2010;11:681–691. doi: 10.1016/j.jpain.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, Edwards RR. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis care & research. 2011;63:320–327. doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin A, Yung AR, Wigman JT, Killackey E, Baksheev G, Wardenaar KJ. Validation of a short adaptation of the Mood and Anxiety Symptoms Questionnaire (MASQ) in adolescents and young adults. Psychiatry. Res. 2014;215:778–783. doi: 10.1016/j.psychres.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Luijendijk HJ, Tiemeier H, Hofman A, Heeringa J, Stricker BH. Determinants of chronic benzodiazepine use in the elderly: a longitudinal study. British journal of clinical pharmacology. 2008;65:593–599. doi: 10.1111/j.1365-2125.2007.03060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahrer NE, Montano Z, Gold JI. Relations between anxiety sensitivity, somatization, and health-related quality of life in children with chronic pain. Journal of pediatric psychology. 2012;37:808–816. doi: 10.1093/jpepsy/jss054. [DOI] [PubMed] [Google Scholar]
- 42.Mailloux J, Brener J. Somatosensory amplification and its relationship to heartbeat detection ability. Psychosomatic medicine. 2002;64:353–357. doi: 10.1097/00006842-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 43.Manfredini D, Borella L, Favero L, Ferronato G, Guarda-Nardini L. Chronic pain severity and depression/somatization levels in TMD patients. The International journal of prosthodontics. 2010;23:529–534. [PubMed] [Google Scholar]
- 44.Martin-Pintado Zugasti A, Rodriguez-Fernandez AL, Garcia-Muro F, Lopez-Lopez A, Mayoral O, Mesa-Jimenez J, Fernandez-Carnero J. Effects of spray and stretch on postneedling soreness and sensitivity after dry needling of a latent myofascial trigger point. Arch Phys Med Rehabil. 2014;95:1925.e1921–1932.e1921. doi: 10.1016/j.apmr.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 45.McBeth J, Macfarlane GJ, Benjamin S, Morris S, Silman AJ. The association between tender points, psychological distress, and adverse childhood experiences: a community-based study. Arthritis and rheumatism. 1999;42:1397–1404. doi: 10.1002/1529-0131(199907)42:7<1397::AID-ANR13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 46.McBeth J, Macfarlane GJ, Benjamin S, Silman AJ. Features of somatization predict the onset of chronic widespread pain: results of a large population-based study. Arthritis and rheumatism. 2001;44:940–946. doi: 10.1002/1529-0131(200104)44:4<940::AID-ANR151>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 47.McDermid AJ, Rollman GB, McCain GA. Generalized hypervigilance in fibromyalgia: Evidence of perceptual amplification. Pain. 1996;66:133–144. doi: 10.1016/0304-3959(96)03059-x. [DOI] [PubMed] [Google Scholar]
- 48.Miller AR, North CS, Clouse RE, Wetzel RD, Spitznagel EL, Alpers DH. The association of irritable bowel syndrome and somatization disorder. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2001;13:25–30. doi: 10.1023/a:1009060731057. [DOI] [PubMed] [Google Scholar]
- 49.Nakao M, Barsky AJ, Nishikitani M, Yano E, Murata K. Somatosensory amplification and its relationship to somatosensory, auditory, and visual evoked and event-related potentials (P300) Neuroscience letters. 2007;415:185–189. doi: 10.1016/j.neulet.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis and rheumatism. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielsen S, Lintzeris N, Bruno R, Campbell G, Larance B, Hall W, Hoban B, Cohen ML, Degenhardt L. Benzodiazepine use among chronic pain patients prescribed opioids: associations with pain, physical and mental health, and health service utilization. Pain medicine. 2015;16:356–366. doi: 10.1111/pme.12594. [DOI] [PubMed] [Google Scholar]
- 52.O'Brien EM, Atchison JW, Gremillion HA, Waxenberg LB, Robinson ME. Somatic focus/awareness: Relationship to negative affect and pain in chronic pain patients. Eur J Pain. 2008;12:104–115. doi: 10.1016/j.ejpain.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oathes DJ, Patenaude B, Schatzberg AF, Etkin A. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biol Psychiatry. 2015;77:385–393. doi: 10.1016/j.biopsych.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petzke F, Gracely RH, Park KM, Ambrose K, Clauw DJ. What do tender points measure? Influence of distress on 4 measures of tenderness. J. Rheumatol. 2003;30:567–574. [PubMed] [Google Scholar]
- 55.Phyomaung PP, Dubowitz J, Cicuttini FM, Fernando S, Wluka AE, Raaijmaakers P, Wang Y, Urquhart DM. Are depression, anxiety and poor mental health risk factors for knee pain? A systematic review. BMC musculoskeletal disorders. 2014;15:10. doi: 10.1186/1471-2474-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1:385–401. [Google Scholar]
- 57.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. The Cochrane database of systematic reviews. 2007:Cd005454. doi: 10.1002/14651858.CD005454.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sherman JJ, LeResche L, Huggins KH, Mancl LA, Sage JC, Dworkin SF. The relationship of somatization and depression to experimental pain response in women with temporomandibular disorders. Psychosomatic medicine. 2004;66:852–860. doi: 10.1097/01.psy.0000140006.48316.80. [DOI] [PubMed] [Google Scholar]
- 59.Smith JK, Marciani L, Humes DJ, Francis ST, Gowland P, Spiller RC. Anticipation of thermal pain in diverticular disease. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2016 doi: 10.1111/nmo.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spielberger CD. State-trait anxiety inventory: a comprehensive bibliography. Consulting Psychologists Press; 1993. [Google Scholar]
- 61.Sutton KS, Pukall CF, Chamberlain S. Pain ratings, sensory thresholds, and psychosocial functioning in women with provoked vestibulodynia. Journal of sex & marital therapy. 2009;35:262–281. doi: 10.1080/00926230902851256. [DOI] [PubMed] [Google Scholar]
- 62.Tang J, Gibson SJ. A psychophysical evaluation of the relationship between trait anxiety, pain perception, and induced state anxiety. The Journal of Pain. 2005;6:612–619. doi: 10.1016/j.jpain.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 63.Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW. Antidepressants for non-specific low back pain. The Cochrane database of systematic reviews. 2008:Cd001703. doi: 10.1002/14651858.CD001703.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward MM. Are patient self-report measures of arthritis activity confounded by mood? A longitudinal study of patients with rheumatoid arthritis. The Journal of rheumatology. 1994;21:1046–1050. [PubMed] [Google Scholar]
- 66.Wardenaar KJ, van Veen T, Giltay EJ, de Beurs E, Penninx BW, Zitman FG. Development and validation of a 30-item short adaptation of the Mood and Anxiety Symptoms Questionnaire (MASQ) Psychiatry Research. 2010;179:101–106. doi: 10.1016/j.psychres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Wasserman RA, Hassett AL, Harte SE, Goesling J, Malinoff HL, Berland DW, Zollars J, Moser SE, Brummett CM. Pressure pain sensitivity in patients with suspected opioid-induced hyperalgesia. Regional anesthesia and pain medicine. 2015;40:687–693. doi: 10.1097/AAP.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- 69.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 70.Wegner A, Elsenbruch S, Maluck J, Grigoleit J-S, Engler H, Jäger M, Spreitzer I, Schedlowski M, Benson S. Inflammation-induced hyperalgesia: Effects of timing, dosage, and negative affect on somatic pain sensitivity in human experimental endotoxemia. Brain, behavior, and immunity. 2014;41:46–54. doi: 10.1016/j.bbi.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Weisensee W, Scheer M, Muller L, Rothamel D, Kistler F, Bayer G, Johren P, Neugebauer J. Impact of anxiety parameters on prospective and experienced pain intensity in implant surgery. Implant Dent. 2012;21:502–506. doi: 10.1097/ID.0b013e3182703a44. [DOI] [PubMed] [Google Scholar]
- 72.Witthoft M, Gerlach AL, Bailer J. Selective attention, memory bias, and symptom perception in idiopathic environmental intolerance and somatoform disorders. J Abnorm Psychol. 2006;115:397–407. doi: 10.1037/0021-843X.115.3.397. [DOI] [PubMed] [Google Scholar]
- 73.Wolfe F. The relation between tender points and fibromyalgia symptom variables: evidence that fibromyalgia is not a discrete disorder in the clinic. Annals of the rheumatic diseases. 1997;56:268–271. doi: 10.1136/ard.56.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Mccain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and rheumatism. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 75.Zautra AJ, Johnson LM, Davis MC. Positive Affect as a Source of Resilience for Women in Chronic Pain. Journal of consulting and clinical psychology. 2005;73:212–220. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.