Abstract
Objective
To evaluate the relationship between birth weight (along with a variety of pre- and perinatal characteristics) and the risk of pediatric Hodgkin lymphoma (HL) diagnosed at age <20 years.
Method
We linked California statewide birth records from 1978-2009 and cancer diagnosis data from 1988-2011 to conduct a population-based case-control study with 1,216 cases and 4,485 controls (matched on birth month and year, sex, and race/ethnicity). Conditional logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (95% CI) of pediatric HL overall and by age of diagnosis, controlling for other perinatal factors.
Results
Compared to children with a normal birth weight (2,500-3,999 g), those who had a high birth weight (≥ 4000 g) had an increased risk of pediatric HL overall (OR=1.23, 95% CI: 1.02-1.48) after adjusting for birth order, maternal age at the time of delivery, and paternal age at the time of delivery. The magnitude of association appeared larger for subgroups of children whose age of diagnosis was 0-10 years (OR=1.56, 95% CI: 1.04-2.24) or 15-19 years (OR=1.43, 95% CI: 1.11-1.83), while no association was observed in 11-14 year olds. Compared to firstborn children, those who were third or higher in birth order had a reduced risk of pediatric HL overall (OR=0.80, 95% CI: 0.67-0.95), and this association also varied by age of diagnosis.
Conclusions
In this study with the largest number of pediatric HL cases, high birth weight was associated with an increased disease risk for most but not all ages of diagnosis. The different findings by age of diagnosis regarding both birth weight and birth order underscore the importance to stratify pediatric HL by age at diagnosis in future etiological investigations.
Introduction
In the United States (US), Hodgkin lymphoma (HL) is the third most common malignancy diagnosed in the pediatric population (age 0-19 years), with an estimated number of 1,140 new cases in 2014.1 While HL accounts for only 4% of cancer diagnosed in children 0-14 years of age (with an incidence of 0.6 cases per 100,000 person-years), it represents about 15% of cancer diagnoses in adolescents 15-19 years, with an incidence of 3.0 cases per 100,000 person-years.1,2 Although survival rates are now extremely high due to improved treatments, pediatric HL survivors retain elevated risks of treatment-related morbidities over the long term, which can severely impact quality of life.3 Although infection with Epstein-Barr virus is an established risk factors for HL,4,5 much of the etiology of pediatric HL is still obscure, and research in this area remains a priority.
Recent epidemiologic investigations have evaluated various perinatal characteristics for possible associations with pediatric HL. In particular, birth weight has been assessed in several of these studies as a potential marker for intrauterine growth, with inconsistent results. Multiple studies6-13 and a meta-analysis published in 201214 found no association between birth weight and risk of pediatric HL. However, a large birth cohort study from Sweden observed a significant positive association between increasing birth weight and risk of HL diagnosed at the age of 0-37 years and reported no heterogeneity by age of diagnosis (0-14 years versus 15-37 years).15
Due to the rarity of pediatric HL, most existing studies included a relatively small number of cases and likely had suboptimal statistical power. The number of HL cases in the studies included in the meta-analysis14 ranged from 84 to 474, while the Swedish birth cohort study included 943 HL cases. In addition, different studies focused on different age ranges, with many restricted to HL cases diagnosed at the age of 0-14 years.6-8,10,12,13
Given the high incidence of HL at the age of 15-19 years1 and the lack of consensus on the relation of birth weight to HL risk, we conducted a population-based case-control study with an unprecedented sample size to clarify the role of birth weight in the etiology of HL over the entire pediatric age range (i.e., 0-19 years).
Method
Study Design
This study was based on a population-based linkage of California statewide birth records from 1978-2009 (maintained by the Vital Statistics Division of the California Department of Public Health) and cancer diagnosis data from 1988-2011 (collected by the California Cancer Registry). Histologically confirmed cases of pediatric HL (aged 0-19 years at the time of diagnosis) were identified using information reported to the California Cancer Registry. For each case, up to four controls were randomly sampled from statewide birth records and individually matched to each case on birth month and year, sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander, and other). Record linkage and the selection of cases and controls were performed by research staff at the California Cancer Registry. The study protocol was approved by the Institutional Review Boards of California Health and Human Services and all participating academic institutions (Yale University, University of California, Berkeley, and University of California, San Francisco).
Study Population
The initial study population consisted of 1,323 pediatric HL cases and 5,292 matched controls. In order to preserve data quality, we excluded individuals with missing or unknown values for at least one of the following variables: birth weight, birth order, maternal age at the time of delivery, maternal birthplace, and delivery method (vaginal or cesarean). As a result, a total of 7 cases and 78 controls were excluded. We further excluded one case and one control whose birth weight was outside a plausible range (350g - 5,999g), as well as 98 cases and 377 controls whose gestational age was outside a plausible range (20 - 44 weeks). Finally, one case without matched controls was excluded, so were 315 controls without matched cases, resulting in a final study population of 1,216 pediatric HL cases and 4,485 matched controls.
Variables of Interest
Data on birth weight, as well as various other infant, maternal, and paternal characteristics were obtained from birth records. Based on data available in birth records, we considered the following variables as potential confounders of the relation between birth weight and risk of pediatric HL: gestational age, birth order, plurality, maternal age at delivery, maternal birthplace, maternal education level, paternal age at delivery, paternal education level, delivery method, maternal history of diabetes, maternal history of pregnancy loss (defined as history of miscarriage and/or stillbirth), and proportion of the population living in poverty in the zip code of the mother's residence at time of delivery (as a proxy for socioeconomic status). Additional information about the diagnosis of pediatric HL, including the age at diagnosis and histological subtype, was obtained from the California Cancer Registry.
Statistical Analysis
Infant and parental characteristics were categorized and compared between cases and controls using Pearson's Chi-square test. To evaluate the association between birth weight and risk of pediatric HL, multivariable conditional logistic regression models (conditioned on matching factors of birth month and year, sex, and race/ethnicity) were used to estimate odds ratios 8 and 95% confidence intervals 16. We examined birth weight, the primary exposure of interest, as both a categorical and continuous variable. For categorical analysis, birth weights were defined as low birth weight (<2,500g), normal birth weight (2,500g - 3,999g), and high birth weight (≥4,000g). For the multivariable analysis, the initial model included gestational age (20-36 weeks, 37-41 weeks, or 42-44 weeks), birth order (1st, 2nd, or 3rd and higher), plurality (singleton versus multiple birth), maternal age the time of delivery (<20, 20-24, 25-29, 30-34, or ≥35 years), maternal birthplace (foreign- versus US-born), maternal education level (12th grade or less, more than 12th grade, or unknown), paternal age at the time of delivery (<20, 20-24, 25-29, 30-34, ≥35 years, or unknown), paternal education level (12th grade or less, more than 12th grade, or unknown), delivery method (vaginal versus cesarean), maternal history of diabetes (yes versus no), maternal history of pregnancy loss (yes versus no), and the proportion of the population living in poverty within the zip code of the mother's residence at the time of delivery (<5%, 5-9%, 10-19%, ≥20%, or unknown). After a stepwise selection using SAS (version 9.4, SAS Institute, Inc., Cary, North Carolina), the final model evaluating the association between birth weight and risk of overall pediatric HL adjusted for birth order, maternal age at the time of delivery, and paternal age at the time of delivery.
We further evaluated the relationship between birth weight and risk of pediatric HL stratified by age at diagnosis (0-10, 11-14, and 15-19 years), sex, histological subtype (nodular sclerosis and mixed cellularity), and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and Asian/Pacific Islander). The cut-off points for age at diagnosis were chosen based on two considerations: (1) age-specific incidence of HL in California; and (2) number of cases available in each subgroup. The histological subtype of HL was classified using the International Classification of Diseases for Oncology (3rd edition, ICD-O-3) codes17: nodular sclerosis (ICD-O-3 codes: 9661, 9663-7), mixed cellularity (ICD-O-3 code 9652), and other (ICD-O-3 codes: 9650, 9651, 9653-5, 9659). Within each subgroup analysis, covariates in the final model were also obtained using stepwise selection procedures. Therefore, the sets of covariates included in the final models for different subgroup analyses varied.
We also conducted a confirmatory analysis in which unconditional logistic regression models were performed that included the matching factors as well as all other covariates described above. All significance tests were two-sided with an α-level of 0.05. All analyses were conducted using SAS (version 9.4, SAS Institute, Inc.).
Results
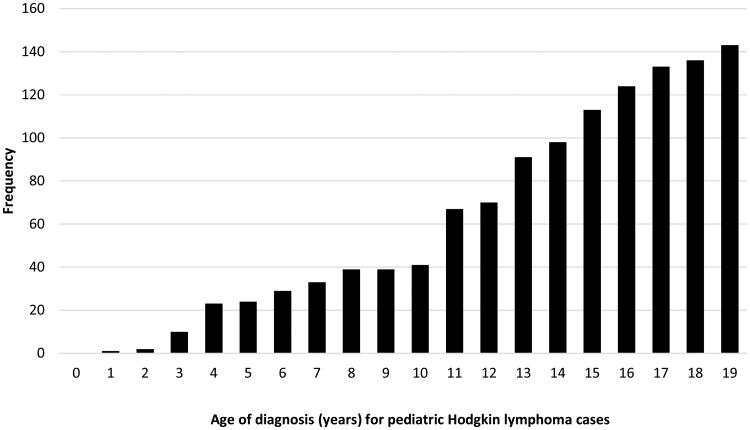
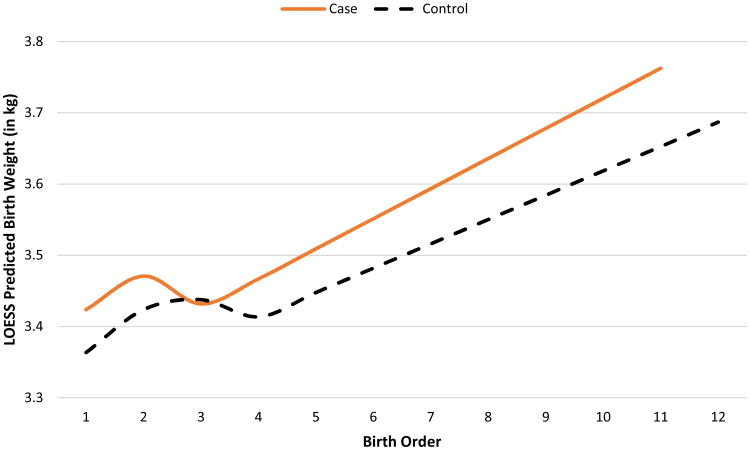
Of the 1,216 pediatric HL cases included in the study, the majority (56%) were male, and 53% were diagnosed at 15-19 years of age (Table 1, Figure 1a). Based on an unadjusted bivariate analysis, cases were significantly heavier at birth than controls, with 14.8% of cases having a high birth weight compared to 12.1% of controls. When modeled as a function of birth order via LOESS (local polynomial regression), cases were also generally expected to be heavier than controls (Figure 1b). Maternal and paternal ages were also significantly higher for cases than controls. (p<0.01 for both) (Table 1). In addition, cases were more likely to be delivered by cesarean section (25% for cases compared to 22% for controls; p=0.04). The remaining characteristics were similar between cases and controls (Table 1).
Table 1. Characteristics of the Study Population.
| Cases | Controls | ||||
|---|---|---|---|---|---|
| Characteristics | n | (%) | n | (%) | p-value* |
| Total | 1216 | 4485 | |||
| Sex | 0.84 | ||||
| Female | 537 | (44) | 1966 | (44) | |
| Male | 679 | (56) | 2519 | (56) | |
| Race/ethnicity | 1.00 | ||||
| Non-Hispanic white | 562 | (46) | 2089 | (47) | |
| Non-Hispanic black | 99 | (8.1) | 362 | (8.1) | |
| Hispanic | 467 | (38) | 1713 | (38) | |
| Asian/Pacific Islander | 70 | (5.8) | 259 | (5.8) | |
| Other | 18 | (1.5) | 62 | (1.4) | |
| Year of birth | 0.72 | ||||
| 1978-1982 | 245 | (20) | 840 | (19) | |
| 1983-1987 | 310 | (25) | 1145 | (26) | |
| 1988-1992 | 418 | (34) | 1579 | (35) | |
| 1993-2009 | 243 | (20) | 921 | (20) | |
| Birth weight (in grams) | 0.01 | ||||
| Low (<2500) | 52 | (4.3) | 249 | (5.6) | |
| Normal (2500-3999) | 984 | (81) | 3694 | (82) | |
| High (≥4000) | 180 | (15) | 542 | (12) | |
| Gestational age (in weeks) | 0.51 | ||||
| 20-36 | 101 | (8.3) | 416 | (9.3) | |
| 37-41 | 984 | (81) | 3568 | (80) | |
| 42-44 | 131 | (10.8) | 501 | (11.2) | |
| Birth order | 0.19 | ||||
| 1st | 483 | (40) | 1851 | (41) | |
| 2nd | 409 | (34) | 1386 | (31) | |
| 3rd or higher | 324 | (27) | 1248 | (28) | |
| Plurality | 0.17 | ||||
| Singleton | 1186 | (98) | 4402 | (98) | |
| Multiple | 30 | (2.5) | 83 | (1.9) | |
| Maternal age at delivery (in years) | <.01 | ||||
| <20 | 107 | (8.8) | 534 | (11.9) | |
| 20-24 | 265 | (22) | 1240 | (28) | |
| 25-29 | 400 | (33) | 1309 | (29) | |
| 30-34 | 303 | (25) | 951 | (21) | |
| ≥35 | 141 | (11.6) | 451 | (10.1) | |
| Maternal education level | 0.81 | ||||
| 12th grade or less | 375 | (31) | 1424 | (32) | |
| More than 12th grade | 242 | (20) | 892 | (20) | |
| Unknown | 599 | (49) | 2169 | (48) | |
| Paternal age at delivery (in years) | <.01 | ||||
| <20 | 43 | (3.5) | 184 | (4.1) | |
| 20-24 | 170 | (14.0) | 903 | (20) | |
| 25-29 | 343 | (28) | 1208 | (27) | |
| 30-34 | 311 | (26) | 1122 | (25) | |
| ≥35 | 302 | (25) | 865 | (19) | |
| Unknown | 47 | (3.9) | 203 | (4.5) | |
| Paternal education level | 0.65 | ||||
| 12th grade or less | 341 | (28) | 1319 | (29) | |
| More than 12th grade | 242 | (20) | 873 | (20) | |
| Unknown | 633 | (52) | 2293 | (51) | |
| Maternal birthplace | 0.25 | ||||
| United States | 799 | (66) | 3025 | (67) | |
| Foreign | 417 | (34) | 1460 | (33) | |
| Delivery method | 0.04 | ||||
| Vaginal | 913 | (75) | 3494 | (78) | |
| Cesarean | 303 | (25) | 991 | (22) | |
| Maternal diabetes | 0.61 | ||||
| No | 1207 | (99) | 4445 | (99) | |
| Yes | 9 | (0.74) | 40 | (0.89) | |
| History of pregnancy loss | 0.23 | ||||
| No | 994 | (82) | 3719 | (83) | |
| Yes | 221 | (18) | 753 | (17) | |
| Unknown | 1 | (0.08) | 13 | (0.29) | |
| % population living in poverty (by zip code) | 0.53 | ||||
| <5% | 154 | (12.7) | 519 | (11.6) | |
| 5-9% | 259 | (21) | 1035 | (23) | |
| 10-19% | 372 | (31) | 1327 | (30) | |
| ≥20% | 215 | (18) | 832 | (19) | |
| Unknown | 216 | (18) | 772 | (17) | |
P-values were calculated using Pearson's Chi-square test
Column percent totals may not sum to 100% due to rounding
Figure 1a. Age of cases at time of pediatric HL diagnosis.
Figure 1b. Local regression (LOESS) predictions for birth weight according to birth order by case/control status.
Based on the final multivariable conditional logistic regression model (Table 2), compared to normal birth weight, high birth weight was positively associated with the risk of pediatric HL (OR=1.23, 95% CI: 1.02-1.48). When birth weight was modeled as a continuous variable, a 1,000g increase in birth weight was associated with a 16% increase in risk of pediatric HL (95% CI: 1.03-1.30). Compared with firstborn children, those who were third or higher in birth order had a significantly reduced risk of pediatric HL (OR=0.80, 95% CI: 0.67-0.95). There was also suggestion that younger parental age was associated with a reduced risk of pediatric HL in the overall study population: compared with the age-group of 25-29 years, a maternal age < 25 years or a paternal age of 20-24 years was associated with a lower risk of HL (Table 2).
Table 2. Factors associated with risk of pediatric Hodgkin lymphoma.
| Characteristics | Odds ratio* | (95% confidence interval) | p-value |
|---|---|---|---|
| Overall; ages 0-19 years (1216 cases/4485 controls) | |||
| Birth weight (in grams) | |||
| Low (<2500) | 0.79 | (0.58, 1.07) | 0.13 |
| Normal (2500-3999) | Reference | ||
| High (≥4000) | 1.23 | (1.02, 1.48) | 0.03 |
| Per 1,000g increase | 1.16 | (1.03, 1.30) | 0.01 |
| Birth Order | |||
| 1st | Reference | ||
| 2nd | 1.02 | (0.87, 1.19) | 0.79 |
| 3rd or higher | 0.80 | (0.67, 0.95) | 0.01 |
| Maternal Age at Delivery (in years) | |||
| <20 | 0.66 | (0.48, 0.89) | <0.01 |
| 20-24 | 0.73 | (0.60, 0.88) | <0.01 |
| 25-29 | Reference | ||
| 30-34 | 1.03 | (0.86, 1.24) | 0.76 |
| ≥35 | 0.94 | (0.73, 1.21) | 0.63 |
| Paternal Age at Delivery (in years) | |||
| <20 | 0.99 | (0.66, 1.51) | 0.98 |
| 20-24 | 0.76 | (0.61, 0.95) | 0.02 |
| 25-29 | Reference | ||
| 30-34 | 0.94 | (0.78, 1.14) | 0.54 |
| ≥35 | 1.22 | (0.98, 1.52) | 0.08 |
| Unknown | 0.90 | (0.63, 1.28) | 0.56 |
| Ages 0-10 years (241 cases/904 controls) | |||
| Birth weight (in grams) | |||
| Low (<2500) | 0.64 | (0.28, 1.44) | 0.28 |
| Normal (2500-3999) | Reference | ||
| High (≥4000) | 1.56 | (1.04, 2.34) | 0.03 |
| Per 1,000g increase | 1.28 | (0.97, 1.69) | 0.08 |
| Maternal education level | |||
| 12th grade or less | Reference | ||
| More than 12th grade | 1.14 | (0.75, 1.71) | 0.54 |
| Unknown | 15.64 | (1.71, 142.6) | 0.01 |
| Ages 11-14 years (326 cases/1196 controls) | |||
| Birth weight (in grams) | |||
| Low (<2500) | 1.40 | (0.83, 2.37) | 0.21 |
| Normal (2500-3999) | Reference | ||
| High (≥4000) | 0.81 | (0.55, 1.20) | 0.30 |
| Per 1,000g increase | 0.87 | (0.70, 1.09) | 0.22 |
| Maternal Birthplace | |||
| United States | 1.00 | (Reference) | |
| Foreign | 1.52 | (1.10, 2.10) | 0.01 |
| % population living in poverty (by zip code) | |||
| <5% | 0.66 | (0.41, 1.06) | 0.08 |
| 5-9% | 0.90 | (0.64, 1.27) | 0.54 |
| 10-19% | Reference | ||
| ≥20% | 0.86 | (0.59, 1.25) | 0.43 |
| Unknown | 0.20 | (0.06, 0.67) | 0.01 |
| Ages 15-19 years (649 cases/2385 controls) | |||
| Birth weight (in grams) | |||
| Low (<2500) | 0.56 | (0.36, 0.89) | 0.01 |
| Normal (2500-3999) | Reference | ||
| High (≥4000) | 1.43 | (1.11, 1.83) | <0.01 |
| Per 1,000g increase | 1.33 | (1.13, 1.57) | <0.01 |
| Birth Order | |||
| 1st | Reference | ||
| 2nd | 0.96 | (0.77, 1.19) | 0.69 |
| 3rd or higher | 0.66 | (0.52, 0.84) | <0.01 |
| Maternal Age at Delivery (in years) | |||
| <20 | 0.48 | (0.30, 0.76) | <0.01 |
| 20-24 | 0.67 | (0.51, 0.87) | <0.01 |
| 25-29 | Reference | ||
| 30-34 | 1.01 | (0.78, 1.31) | 0.93 |
| ≥35 | 0.90 | (0.63, 1.29) | 0.57 |
| Maternal education level | |||
| 12th grade or less | Reference | ||
| More than 12th grade | 0.90 | (0.67, 1.23) | 0.51 |
| Unknown | 1.23 | (0.37, 4.06) | 0.74 |
| Paternal Age at Delivery (in years) | |||
| <20 | 0.99 | (0.51, 1.93) | 0.98 |
| 20-24 | 0.69 | (0.51, 0.95) | 0.02 |
| 25-29 | Reference | ||
| 30-34 | 0.93 | (0.72, 1.20) | 0.57 |
| ≥35 | 1.37 | (1.02, 1.84) | 0.04 |
| Unknown | 0.72 | (0.41, 1.25) | 0.24 |
| % population living in poverty (by zip code) | |||
| <5% | 1.30 | (0.97, 1.75) | 0.08 |
| 5-9% | 0.84 | (0.64, 1.09) | 0.18 |
| 10-19% | Reference | ||
| ≥20% | 1.01 | (0.76, 1.35) | 0.94 |
| Unknown | 0.82 | (0.47, 1.44) | 0.49 |
Odds ratios were derived from multivariable stepwise conditional logistic regression; all variables in the model were mutually adjusted for each other.
In further analyses evaluating the relationship between birth weight and pediatric HL in specific diagnostic age ranges (Tables 2-3), significant positive associations for high birth weight and risk of HL were observed among those diagnosed at 0-10 years (OR=1.56, 95% CI: 1.04-2.34) or 15-19 years (OR=1.43, 95% CI: 1.11-1.83). No significant association was observed for the age group of 11-14 years. Within the age group of 15-19 years, low birth weight was significantly associated with a 44% decrease in odds of HL (OR=0.56, 95% CI: 0.36-0.89), an association which was absent in the overall study population (Tables 2-3). In addition, the association between birth order and risk of HL also varied by age of diagnosis, in that a significant association was observed among those diagnosed at 15-19 years (OR=0.66, 95% CI: 0.52-0.84), but not among those diagnosed at 0-10 years or 11-14 years (Table 2).
Table 3. Birth weight and risk of pediatric Hodgkin lymphoma by age of diagnosis, sex, histological subtype, and race/ethnicity.
| Birth weight categories† | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Low | Normal | High | ||||||
|
|
||||||||
| Group | Cases/Controls | OR* | (95% CI)* | p-value | OR* | (95% CI)* | p-value | |
| Overall (0-19 years)a | 1216/4485 | 0.79 | (0.58, 1.07) | 0.13 | Reference | 1.23 | (1.02, 1.48) | 0.03 |
| Sex | ||||||||
| Maleb | 679/2519 | 0.73 | (0.47, 1.11) | 0.14 | Reference | 1.26 | (1.00, 1.59) | 0.05 |
| Femalec | 537/1966 | 0.87 | (0.55, 1.37) | 0.55 | Reference | 1.17 | (0.86, 1.61) | 0.32 |
| Histological Subtype | ||||||||
| Nodular sclerosisd | 883/3259 | 0.73 | (0.50, 1.06) | 0.10 | Reference | 1.26 | (1.02, 1.57) | 0.03 |
| Mixed cellularitye | 124/464 | 0.88 | (0.33, 2.36) | 0.80 | Reference | 0.85 | (0.46, 1.58) | 0.61 |
| Race/Ethnicity | ||||||||
| Non-Hispanic whitef | 562/2089 | 0.46 | (0.24, 0.89) | 0.02 | Reference | 1.27 | (0.98, 1.64) | 0.07 |
| Non-Hispanic blackg | 99/362 | 0.48 | (0.20, 1.16) | 0.10 | Reference | 1.40 | (0.65, 3.04) | 0.39 |
| Hispanich | 467/1713 | 1.15 | (0.72, 1.83) | 0.56 | Reference | 1.19 | (0.87, 1.63) | 0.27 |
| Asian/Pacific Islanderi | 70/259 | 3.10 | (1.19, 8.11) | 0.02 | Reference | 0.75 | (0.21, 2.64) | 0.65 |
Abbreviations: OR, odds ratio; CI, confidence interval; ref, reference.
Birth weight categories (in grams): Low (<2500), Normal (2500-3999), High (≥4000)
Adjusted for birth order, maternal age at delivery, and paternal age at delivery;
Adjusted for paternal age at delivery;
Adjusted for birth order, maternal age at delivery, maternal education level, paternal age at delivery, and % pop. living in poverty (by zip code);
Adjusted for paternal age at delivery, delivery method, and % pop. living in poverty (by zip code);
Unadjusted (no significant predictors remained after stepwise regression);
Adjusted for gestational age, maternal age at delivery, paternal age at deliver, maternal birthplace and % pop. living in poverty (by zip code);
Unadjusted (no significant predictors remained after stepwise regression);
Adjusted for birth order and paternal age;
Unadjusted (no significant predictors remained after stepwise regression).
Additional stratified analyses revealed that high birth weight was significantly associated with increased disease risk among males (OR=1.26, 95% CI: 1.00-1.59) or the histological subtype of nodular sclerosis (OR=1.26, 95% CI: 1.02-1.57), but not among females or those with mixed cellularity subtype (Table 3). High birth weight was not associated with increased risk of HL in any of the subgroups defined by race/ethnicity. However, low birth weight was associated with decreased risk of HL in non-Hispanic whites (OR=0.46, 95% CI: 0.24-0.89) and increased risk of HL among Asian/Pacific Islanders (OR=3.10, 95% CI: 1.19-8.11) (Table 3).
Results from the confirmatory analysis using unconditional logistic regression were consistent with the findings from conditional logistic regression models (detailed data not presented).
Discussion
In this large population-based study, we found that high birth weight was associated with an increased risk of pediatric HL. Additionally, having a birth order of third or higher (compared to being the firstborn) conferred a significant protective effect. However, these effects were not consistent across subgroups defined by age of diagnosis, sex, histological subtype, and race/ethnicity.
In our study, the association between birth weight and HL risk was similar in the age groups of 0-10 years and 15-19 years, while the association between birth order and HL risk was only observed in 15-19 year olds. It is possible that HL diagnosed at different ages may have distinct etiologies. HL incidence varies by age, following a bimodal age distribution with peaks at 15-34 years and greater than 60 years.18 It has been proposed that age of diagnosis may be associated with the etiologic profile of HL and that those diagnosed under the age of 10 years should be considered separately from those diagnosed at 15-34 years.19
While the OR for high birth weight was elevated for both males and females, the association only reached statistical significance in males in our study. This was similar to the observation of Milne et al., who utilized proportion of optimal birth weight (POBW) as a metric for appropriateness of fetal growth20 and found that a higher POBW was associated with an increased risk of childhood HL (age 0-14 years) in boys but not girls.21 A majority of cases in our study population were classified as nodular sclerosis based on histology, and the relationship between birth weight and risk of pediatric HL in this subgroup mirrored the results from the overall population. As the histological subtype of mixed cellularity is much rarer, we were unable to assess whether the apparent absence of association between birth weight and this subtype was due to small sample size and limited power, versus any possible difference in etiology. Subgroup analyses by race/ethnicity generally showed an elevated OR for high birth weight (with the exception of Asians/Pacific Islanders), but all confidence intervals included 1. Given the small number of subjects who were non-Hispanic black or Asian/Pacific Islander, results derived from these two groups should be interpreted with caution.
It has been proposed that high birth weight (as marker for accelerated fetal growth) may be related to risk of pediatric cancers via biological mechanisms involving elevated levels of insulin-like growth factors that may be implicated in carcinogenesis.22 Although high birth weight has been implicated as a risk factor for several other types of pediatric cancer, such as leukemia, neuroblastoma, brain tumors (astrocytoma and medulloblastoma), and Wilms' tumor,23-27 the role of birth weight in the etiology of pediatric HL has been inconsistent. The positive association between high birth weight and risk of HL had previously been reported to be similar in cases diagnosed at 0-14 years and 15-37 years.15 Milne et al. found that a higher POBW was associated with an increased risk of childhood HL (age 0-14 years) in boys.21 Aside from these studies, the majority of published studies evaluating birth weight and risk of HL in pediatric populations have reported null findings,6-14 and most of those studies included only cases diagnosed at the age of 0-14 years.6-8,10,12,13 In our study, when we analyzed the age of 0-14 years as one single group, we did not find any significant association between HL and birth weight (measured either categorically or continuously), which was consistent with most previous studies. The differing risk profiles observed in our study between cases diagnosed at ages 0-10 years and those diagnosed at ages 11-14 years suggest that the age grouping of 0-14 years used in many previous studies may be too broad. In future epidemiological research of pediatric HL, it is probably important to consider age of diagnosis as a potential marker for distinct underlying etiology.
Our results pointed to a decreased risk of pediatric HL for those with a birth order of third or higher. A study evaluating HL risk in a young adult population (aged 15-39 years at the time of diagnosis) identified a protective effect for higher birth order relative to those born third or earlier.28 Another study reported significantly different risk patterns for birth order between childhood (<15 years) and young adult (≥15 years) populations, such that higher birth order appeared to decrease risk of HL in the young adult population and (non-significantly) increase risk of HL in children under 15 years.29 These differing risk patterns for birth order and risk of HL between childhood and young adult populations are particularly noteworthy in the context of our results. Subgroup analyses of our data revealed that birth order was only a significant predictor in those aged 15-19 years. As such, the effect of birth order on risk of HL may be modified by age at diagnosis. This observation may be related to infectious origins of HL due to Epstein-Barr virus (EBV) or other agents, under which those with a delayed exposure to infections have a higher risk of HL, and those with earlier exposure to infectious agents via contact with older siblings have a reduced risk.28,30 Alternatively, a higher incidence of HL in the age group of 15-19 years may have simply offered an improved statistical power to detect an association, if one truly exists.
Our study has several important strengths. Population-based record linkage in a state as populous as California gave rise to a large number of pediatric HL cases relative to earlier studies, and presented a significant advantage in terms of statistical power. A record-based study without the need to trace or consent any subjects minimized concerns of selection bias. Additionally, data on exposure of interest, potential confounders, and disease outcome were obtained exclusively from preexisting records, which minimized potential for information bias.
In light of these noted advantages, there were also limitations to our study. In particular, we were limited to data available in existing records, and therefore could not adjust for additional possible risk factors for pediatric HL such as maternal smoking during pregnancy31 and history of -EBV- infection or infectious mononucleosis.32,33 Interestingly, a recent study conducted by the Children's Oncology Group reported that fewer than 24% of childhood HL cases (age 0-14 years) had EBV-positive tumor, and there did not appear to be systematic differences in risk factors by EBV status.34 In addition, we did adjust for a number of other potential confounder based on information contained in birth records, and we excluded individuals with missing or improbable values for important variables in order to ensure high data quality. Approximately 7% of subjects were excluded due to implausible values in birth weight or gestational age. To evaluate the impact of such exclusions, we repeated all analyses without excluding these subjects, and the results were essentially the same (data not shown). Furthermore, despite the fact that our study included more pediatric HL cases than any other previous studies, the sample sizes for some of the subgroup analyses were small, preventing us from drawing more definitive conclusions. The relatively long period for case ascertainment (1988-2011) was also a potential weakness, due to possible temporal changes in classification systems and cancer reporting. On the other hand, the incidence rate of pediatric HL has remained stable in California over the study period. When we conducted additional analyses by including year of diagnosis as a covariate and by stratifying the study population by different eras of diagnosis (1988-1999 versus 2000-2011), there was barely any change in the results. Finally, it is possible that some cases were not captured by the California Cancer Registry as a result of moving out of California or incomplete case ascertainment, and some control patients may have been misclassified. However, under the extreme assumption that all controls were lost to follow-up, based on the age-adjusted incidence rates of pediatric HL (age 0-19 years) in the US over the study period,2 only fewer than two incident cases of HL would have arisen from the 4,485 controls, had they been followed through their 20th birthday. As such, the potential for bias due to misclassification of the disease outcome was minimal.
In conclusion, this study provides evidence for a positive association between high birth weight and risk of pediatric HL. The different findings by age of diagnosis with regard to both birth weight and birth order suggest that it may be important to stratify pediatric HL by age at diagnosis in future etiological investigations.
Highlights.
In this large population-based study, we found that pediatric Hodgkin lymphomas diagnosed at different ages might have distinct etiological profiles.
Acknowledgments
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Funding: This work was supported by research grants from the National Institutes of Health (R01 CA155461, R01 CA175737, T32 CA151022, and P01 ES018172) and the Environmental Protection Agency (RD83451101), United States.
Footnotes
Conflict of Interest Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975 – 2013. Bethesda, MD: National Cancer Institute; 2016. [Google Scholar]
- 3.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–16. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong AA, Alexander FE, Cartwright R, et al. Epstein-Barr virus and Hodgkin's disease: further evidence for the three disease hypothesis. Leukemia. 1998;12:1272–6. doi: 10.1038/sj.leu.2401097. [DOI] [PubMed] [Google Scholar]
- 5.Glaser SL, Lin RJ, Stewart SL, et al. Epstein-Barr virus-associated Hodgkin's disease: epidemiologic characteristics in international data. Int J Cancer. 1997;70:375–82. doi: 10.1002/(sici)1097-0215(19970207)70:4<375::aid-ijc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Spector LG, Puumala SE, Carozza SE, et al. Cancer risk among children with very low birth weights. Pediatrics. 2009;124:96–104. doi: 10.1542/peds.2008-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith A, Lightfoot T, Simpson J, Roman E. Birth weight, sex and childhood cancer: a report from the United Kingdom Childhood Cancer Study. Cancer epidemiology. 2009;33:363–7. doi: 10.1016/j.canep.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Roman E, Simpson J, Ansell P, et al. Perinatal and reproductive factors: a report on haematological malignancies from the UKCCS. European Journal of Cancer. 2005;41:749–59. doi: 10.1016/j.ejca.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Yeazel MW, Ross JA, Buckley JD, Woods WG, Ruccione K, Robison LL. High birth weight and risk of specific childhood cancers: a report from the Children's Cancer Group. The Journal of pediatrics. 1997;131:671–7. doi: 10.1016/s0022-3476(97)70091-x. [DOI] [PubMed] [Google Scholar]
- 10.Petridou ET, Dikalioti SK, Skalkidou A, Andrie E, Dessypris N, Trichopoulos D. Sun exposure, birth weight, and childhood lymphomas: a case control study in Greece. Cancer Causes & Control. 2007;18:1031–7. doi: 10.1007/s10552-007-9044-2. [DOI] [PubMed] [Google Scholar]
- 11.Marcotte EL, Ritz B, Cockburn M, Clarke CA, Heck JE. Birth characteristics and risk of lymphoma in young children. Cancer epidemiology. 2014;38:48–55. doi: 10.1016/j.canep.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petridou ET, Sergentanis TN, Skalkidou A, et al. Maternal and birth anthropometric characteristics in relation to the risk of childhood lymphomas: a Swedish nationwide cohort study. Eur J Cancer Prev. 2015;24:535–41. doi: 10.1097/CEJ.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill KA, Murphy MF, Bunch KJ, et al. Infant birthweight and risk of childhood cancer: international population-based case control studies of 40 000 cases. International journal of epidemiology. 2015;44:153–68. doi: 10.1093/ije/dyu265. [DOI] [PubMed] [Google Scholar]
- 14.Papadopoulou C, Antonopoulos C, Sergentanis T, Panagopoulou P, Belechri M, Petridou E. Is birth weight associated with childhood lymphoma? A meta-analysis. International Journal of Cancer. 2012;130:179–89. doi: 10.1002/ijc.26001. [DOI] [PubMed] [Google Scholar]
- 15.Crump C, Sundquist K, Sieh W, Winkleby MA, Sundquist J. Perinatal and Family Risk Factors for Hodgkin Lymphoma in Childhood Through Young Adulthood. American Journal of Epidemiology. 2012;176:1147–58. doi: 10.1093/aje/kws212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenaux P, Giagounidis A, Selleslag D, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–76. doi: 10.1182/blood-2011-01-330126. [DOI] [PubMed] [Google Scholar]
- 17.Fritz A, editor. International classification of diseases for oncology. 3rd. World Health Organization; 2000. [Google Scholar]
- 18.Nakatsuka Si, Aozasa K. Epidemiology and pathologic features of Hodgkin lymphoma. International journal of hematology. 2006;83:391–7. doi: 10.1532/IJH97.05184. [DOI] [PubMed] [Google Scholar]
- 19.Jarrett R. Viruses and Hodgkin's lymphoma. Annals of Oncology. 2002;13:23–9. doi: 10.1093/annonc/13.s1.23. [DOI] [PubMed] [Google Scholar]
- 20.Blair EM, Liu Y, de Klerk NH, Lawrence DM. Optimal fetal growth for the Caucasian singleton and assessment of appropriateness of fetal growth: an analysis of a total population perinatal database. BMC pediatrics. 2005;5:1. doi: 10.1186/1471-2431-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milne E, Laurvick CL, Blair E, de Klerk N, Charles AK, Bower C. Fetal growth and the risk of childhood CNS tumors and lymphomas in Western Australia. International Journal of Cancer. 2008;123:436–43. doi: 10.1002/ijc.23486. [DOI] [PubMed] [Google Scholar]
- 22.Callan AC, Milne E. Involvement of the IGF system in fetal growth and childhood cancer: an overview of potential mechanisms. Cancer Causes & Control. 2009;20:1783–98. doi: 10.1007/s10552-009-9378-z. [DOI] [PubMed] [Google Scholar]
- 23.Caughey RW, Michels KB. Birth weight and childhood leukemia: A meta-analysis and review of the current evidence. International Journal of Cancer. 2009;124:2658–70. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 24.Daling JR, Starzyk P, Olshan AF, Weiss NS. Birth Weight and the Incidence of Childhood Cancer. Journal of the National Cancer Institute. 1984;72:1039–41. [PubMed] [Google Scholar]
- 25.Harder T, Plagemann A, Harder A. Birth weight and risk of neuroblastoma: a meta-analysis. International journal of epidemiology. 2010;39:746–56. doi: 10.1093/ije/dyq040. [DOI] [PubMed] [Google Scholar]
- 26.Harder T, Plagemann A, Harder A. Birth weight and subsequent risk of childhood primary brain tumors: a meta-analysis. American journal of epidemiology. 2008;168:366–73. doi: 10.1093/aje/kwn144. [DOI] [PubMed] [Google Scholar]
- 27.Chu A, Heck JE, Ribeiro KB, et al. Wilms' tumour: a systematic review of risk factors and meta-analysis. Paediatric and perinatal epidemiology. 2010;24:449–69. doi: 10.1111/j.1365-3016.2010.01133.x. [DOI] [PubMed] [Google Scholar]
- 28.Gutensohn N, Cole P. Childhood social environment and Hodgkin's disease. New England Journal of Medicine. 1981;304:135–40. doi: 10.1056/NEJM198101153040302. [DOI] [PubMed] [Google Scholar]
- 29.Westergaard T, Melbye M, Pedersen JB, Frisch M, Olsen JH, Andersen PK. Birth order, sibship size and risk of Hodgkin's disease in children and young adults: a population-based study of 31 million person-years. International journal of cancer. 1997;72:977–81. doi: 10.1002/(sici)1097-0215(19970917)72:6<977::aid-ijc10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Gutensohn N, Cole P. Epidemiology of Hodgkin's disease in the young. International Journal of Cancer. 1977;19:595–604. doi: 10.1002/ijc.2910190502. [DOI] [PubMed] [Google Scholar]
- 31.Antonopoulos C, Sergentanis T, Papadopoulou C, et al. Maternal smoking during pregnancy and childhood lymphoma: A meta-analysis. International Journal of Cancer. 2011;129:2694–703. doi: 10.1002/ijc.25929. [DOI] [PubMed] [Google Scholar]
- 32.Hjalgrim H, Askling J, Sørensen P, et al. Risk of Hodgkin's Disease and Other Cancers After Infectious Mononucleosis. Journal of the National Cancer Institute. 2000;92:1522–8. doi: 10.1093/jnci/92.18.1522. [DOI] [PubMed] [Google Scholar]
- 33.Hjalgrim H, Askling J, Rostgaard K, et al. Characteristics of Hodgkin's Lymphoma after Infectious Mononucleosis. New England Journal of Medicine. 2003;349:1324–32. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 34.Linabery AM, Erhardt EB, Richardson MR, et al. Family history of cancer and risk of pediatric and adolescent Hodgkin lymphoma: A Children's Oncology Group study. International Journal of Cancer. 2015;137:2163–74. doi: 10.1002/ijc.29589. [DOI] [PMC free article] [PubMed] [Google Scholar]