Abstract
The development of the hematopoietic system during early embryonic stages occurs in spatially and temporally distinct waves. Hematopoietic stem cells (HSC), the most potent and self‐renewing cells of this system, are produced in the final ‘definitive’ wave of hematopoietic cell generation. In contrast to HSCs in the adult, which differentiate via intermediate progenitor populations to produce functional blood cells, the generation of hematopoietic cells in the embryo prior to HSC generation occurs in the early waves by producing blood cells without intermediate progenitors (such as the ‘primitive’ hematopoietic cells). The lineage relationship between the early hematopoietic cells and the cells giving rise to HSCs, the genetic networks controlling their emergence, and the precise temporal determination of HSC fate remain topics of intense research and debate. This Review article discusses the current knowledge on the step‐wise embryonic establishment of the adult hematopoietic system, examines the roles of pivotal intrinsic regulators in this process, and raises questions concerning the temporal onset of HSC fate determination.
Keywords: embryo, endothelial‐to‐hematopoietic cell transition, ES cell differentiation, Gata2, hematopoietic development, hematopoietic stem cells, hematopoietic progenitor cells, HSC fate, Runx1
Abbreviations
AGM, aorta‐gonad‐mesonephros
BL‐CFC, blast colony‐forming progenitor cells
CFU‐C, colony‐forming unit‐cell
CFU‐S, colony‐forming unit‐spleen
EHT, endothelial‐to‐hematopoietic cell transition
EMP, erythroid–myeloid progenitors
ES, embryonic stem
FL, fetal liver
Gpr56, G protein‐coupled receptor 56
HE, hemogenic endothelial
HSC, hematopoietic stem cells
IAHC, intra‐aortic hematopoietic cluster cells
UA, umbilical arteries
VA, vitelline arteries
Vec, vascular endothelial‐cadherin
YS, yolk sac
The adult hematopoietic system consists of a hierarchy of cells that progress from a stem cell state to the terminally differentiated cells of over 10 blood cell lineages. While hematopoietic stem cells (HSC) are rare, long‐lived and self‐renewing, there are many intermediate progenitor cell types that in a stepwise manner, lose their multi‐lineage and self‐renewing potency before becoming mature functioning blood cells. Much is known about the adult hematopoietic hierarchy, but only recently do we begin to know the variety of hematopoietic cells in the embryo and to understand how they relate to the establishment of the adult hierarchy and HSCs. In this review we discuss the current knowledge on the embryonic development of the adult hematopoietic system focusing on endothelial‐to‐hematopoietic cell transition (EHT), and on some of the pivotal transcriptional regulators and their targets involved in this process, and in the generation of HSCs.
Hematopoiesis is initiated by transient generation of primitive cells
Blood cells are one of the first differentiated cell/tissue lineages generated in the vertebrate embryo. Surprisingly, they are produced even before the circulation is established 1. Transient waves of hematopoietic cell production are first initiated extraembryonically in the yolk sac (YS) blood islands (Fig. 1) 2 which are derived from mesodermal cells that migrate to the YS at neural plate stage. At embryonic day 7 (E7), the mesodermal aggregates generate the first blood cells 2, 3. The emergence of blood cells in the YS is in close relationship with the appearance of endothelial cells that form the first vascular structures. This spatiotemporal association between the emergence of hematopoietic and endothelial cells has led to the hypothesis that they arise from a common bipotential ancestor, which is termed the hemangioblast 1.
Figure 1.
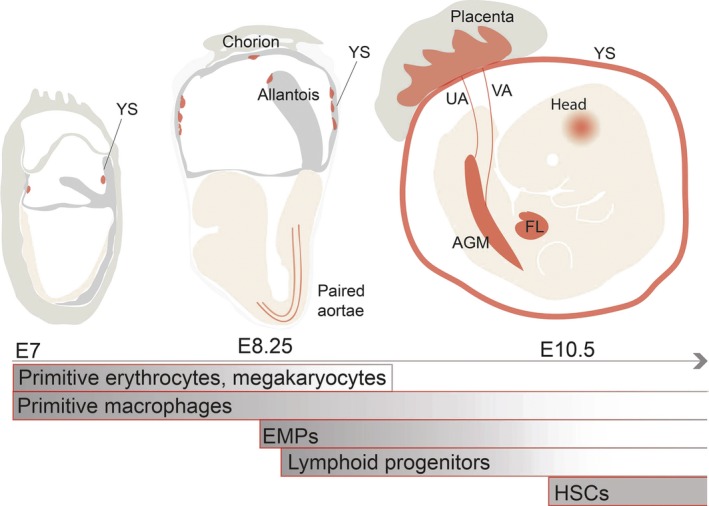
Sites and times of blood cell generation in the mouse embryo. Blood generation in the mouse embryo starts in the blood islands of extraembryonic yolk sac (YS) at embryonic day 7 (E7) with a transient wave of ‘primitive’ erythrocyte, megakaryocyte and macrophage production. The erythrocytes and megakaryocytes of that stage are short‐lived and disappear by E9. Primitive macrophages are hypothesized to be the source of tissue resident macrophages in the adult brain. The second wave of blood generation gives rise to bipotential erythroid‐myeloid progenitors (EMPs) that emerge in the YS from E8.25. Shortly thereafter, lymphoid potential in detected. The paired dorsal aortae contain lymphoid potential as do the allantois/chorion. In the third hematopoietic wave, long‐lived transplantable hematopoietic stem cells (HSCs) are generated beginning at E10.5 in the aorta‐gonad mesonephros (AGM) region. HSCs are also detected in the vitelline (VA) and umbilical (UA) arteries, YS, placenta and in embryonic head. HSCs and EMPs migrate to the fetal liver (FL) where they expand and reside before migrating to the bone marrow niches.
The first genetic evidence supporting a common precursor for hematopoietic and endothelial lineages came from deletion of the Flk1 receptor tyrosine kinase gene in the mouse. Flk1 expression is detected as early as at E7 in the YS mesoderm 4. Embryos lacking Flk1 are not viable and interestingly, show a complete absence of mesodermal cell aggregates in the YS. It was concluded that Flk1 is required for mesodermal cell migration to form YS blood islands and for making hematopoietic and endothelial cells, 5 thus suggesting that a bipotential hemangioblast generates hematopoietic and endothelial cells. Intriguingly, lineage marking/tracing experiments have shown that there is little/no overlap in the mesodermal precursors that are forming the endothelial and hematopoietic cells in individual blood islands, suggesting a segregation in fate early before migration to the YS 6.
Mouse embryonic stem (ES) cell hematopoietic differentiation studies facilitated the search for putative hemangioblast‐like cells. ES cells are pluripotent cells derived from the inner cell mass of the blastocyst 7. They are characterized by self‐renewal ability and the capacity to recapitulate early embryonic development by differentiating into cell derivatives of all three embryonic germ‐cell layers 8.
Embryonic stem cells differentiated in hematopoietic culture conditions for 2.5 days generated blast colony‐forming progenitor cells (BL‐CFC), that were able to give rise to both, hematopoietic and endothelial cells 9. The BL‐CFC (putative hemangioblast) represents a transient population that persists for a very short time in the differentiation culture. It expresses genes common to both hematopoietic and endothelial lineage, including Flk1 10. More recently it has been shown that the BL‐CFC have an additional differentiation potential to cardiomyocyte lineage 11 and thus, the physical isolation of the hemangioblast remains difficult. Nonetheless, to better understand embryonic hematopoiesis in vitro ES cell hematopoietic differentiation models have been widely used, as they recapitulate the early stages of hematopoietic cell development and differentiate to almost all hematopoietic lineages, thus facilitating biochemical analyses of transcription factors and other regulatory molecules involved in development.
The earliest blood cells detected in the embryo are primitive erythrocytes, macrophages, and megakaryocytes
Blood cells that emerge in the first wave of hematopoietic cell generation are ‘primitive’ erythrocytes, macrophages and rare megakaryocyte progenitors 2, 12. This developmental wave is categorized as ‘primitive’ due to the distinctive characteristics of the erythrocytes and erythrocyte colony‐forming unit cells (EryP‐CFU‐Cs). ‘Primitive’ red blood cells are nucleated and are three times larger than fetal and six times larger than adult erythrocytes 13, 14. Moreover, they produce a developmentally distinct embryonic (βH1) globin, which is not detected in adult erythrocytes. ‘Primitive’ erythrocytes peak in numbers at E8.25 and disappear rapidly by E9 2, 12. The short developmental time of these cells resembles the transient nature of hemangioblast‐like cells, thus supporting the hypothesis that they originate from a short‐lived precursor.
Concurrently, rare macrophage progenitors are detected in the YS 2, 15. ‘Primitive’ macrophages from this first YS hematopoietic wave (E7–7.5) are directly derived from the blood islands and do not go through a monocyte intermediate 16, 17, 18 that characterizes the macrophages generated from HSCs in the adult bone marrow. Once the bloodstream is established at E8.25–8.5 19 the YS‐derived macrophages migrate to the developing tissues where they become ‘tissue resident’ macrophages expressing high levels of F4/80 macrophage surface marker. These include macrophages in the skin, microglia in the brain, Kupffer cells in the liver, and Langerhans cells in the epidermis. Recent lineage‐tracing studies suggest that ‘tissue resident’ macrophages in the skin, liver, and lung are replaced before birth by ‘monocyte derived’ macrophages generated in later waves of hematopoietic development 20. In contrast, the labeled brain microglia cells are retained throughout adult life. Unique to these macrophages, as compared to those in the adult, are high F4/80 expression, c‐Myb transcription factor independence and PU.1 transcription factor dependence 20, 21, 22, 23. By E9.5, the quantitative abundance of phenotypic ‘primitive’ macrophages and megakaryocytes in the embryo further suggests that these cells are directly generated in the first hematopoietic wave and not from the later waves of hematopoietic progenitor (HPC) and stem cell generation 15, 24.
The need for these early blood cells in the embryo before the circulation is established is puzzling. ‘Primitive’ erythrocytes may be necessary for providing the rapidly growing embryo with oxygen, macrophages for phagocytosis of cells during tissue remodeling and for lymphatic development but the role of megakaryocytes is uncertain, although they are closely associated with red blood cells.
Multipotent progenitors are generated in the YS during a second wave of blood cell generation
After the generation of ‘primitive’ erythrocytes, macrophages, and megakaryocytes, another wave of hematopoietic cell production begins at E8.25 in the YS (Fig. 1). It overlaps temporally with the first wave 2, but produces functionally more complex bipotential erythroid–myeloid progenitors (EMP). EMP cells express high levels of tyrosine receptor kinase ckit (CD117) and CD41, and by E9.5 are positive for granulocyte–monocyte marker CD16/32 expression 24. EMP‐derived erythrocytes are distinguished from their earlier, ‘primitive’ counterpart by the expression of adult (βmajor) globin 2 and by undergoing enucleation. Thus, based in this complexity and the generation of adult‐like cells this wave is termed ‘definitive’ 25. However, ckithiCD41+CD16/32+ EMPs lack lymphoid cell potential, and are able to provide only short‐term in vivo reconstitution, giving rise to mainly circulating red blood cells 24. Hence, EMPs are distinct from HSCs.
Study of Ncx1 null which lack circulation show that EMPs are generated in the YS and not in the embryo proper through E9.5 24, 26. They appear to emerge from ckit+ cell clusters found in the venous and arterial vessels of the YS 27. These cells then colonize the newly forming liver around late E9 25 give rise to the large numbers of erythrocytes, macrophages, granulocytes, and monocytes found before the establishment of a permanent hematopoietic system 20.
Other hematopoietic cells generated in the second wave are rare cells with lymphoid potential, B‐1 B cell progenitors. They are detected at E8.5/9.5 in the YS and aorta 28, 29, 30. Mast cells are also found in the YS from E9.5 onwards 31. Taken together, this ‘definitive’ wave of hematopoietic cell generation yields more adult‐like functionally competent blood cell types. Also, there is growing evidence that these cells may play an interactive role in promoting the third wave of hemogenesis and HSC generation 32, 33, 34.
HSCs and HPCs emerge by EHT
Adult‐type HSCs are defined by their robust ability to repopulate long term all blood lineages upon transplantation into irradiated adult recipients. In the mouse embryo, the first adult HSCs appear and are autonomously generated in the aorta‐gonad‐mesonephros (AGM) region at E10.5 (Fig. 1) 35, 36. They are also found in the vitelline and umbilical arteries (VA, UA) and in the head 37, 38. Shortly thereafter, HSCs are detected in the YS, placenta, circulation and fetal liver (FL) 35, 36, 39, 40. Althoughthe YS and placenta may be capable of autonomously generating HSCs, the FL serves only as a niche for the expansion of HSCs (and EMPs) made in the other tissues 39, 41, 42. Just before birth, HSCs migrate to the bone marrow where they reside throughout mammalian adult life in specialized niches 43.
Hematopoietic stem cells are generated from a subset of embryonic endothelial cells that possess hemogenic potential—the hemogenic endothelial cells (Fig. 2A) 44, 45. They are detected at the time when clusters of hematopoietic cells appear on the ventral wall of the dorsal aorta. These intra‐aortic hematopoietic cluster cells (IAHC) are ckit+ and at E10.5, approximately 600 IAHCs (1–19 ckit+ cells per cluster) were found along the length of the embryo by whole‐mount embryo imaging 46. Figure 2B shows Gata2 mouse model where Gata2 marks the IAHCs. The clusters are also found in the YS vasculature (Fig. 2C). Vital imaging of the mouse embryonic aorta at the time of HSC generation revealed the transition of morphologically flat endothelial cells to cells that bulge out of the vascular wall and form round hematopoietic cells in the lumen of the aorta. This process was visualized in Ly6a (Sca1) GFP fluorescent reporter transgenic embryos. GFP is expressed in all embryonic and adult HSCs in the mouse 37, 38, 40, 47, 48 and hence, is an excellent reporter for observing the emergence of HSC. To visualize EHT in the aorta by confocal time‐lapse imaging, thick sections of Ly6aGFP E10.5 embryos were stained with a combination of antibodies against hematopoietic and endothelial cell surface markers 49. Hemogenic endothelial cells that give rise to HSCs could be distinguished from other aortic endothelial cells by the expression of GFP. Rare GFP+ckit+CD41+ cells were observed bulging into the lumen of the aorta directly from GFP+CD31+ ventral aortic endothelial cells, thus facilitating the tracking of single cells as they transition from an endothelial cell to a HSC/HPC. This process is generally known as the EHT.
Figure 2.
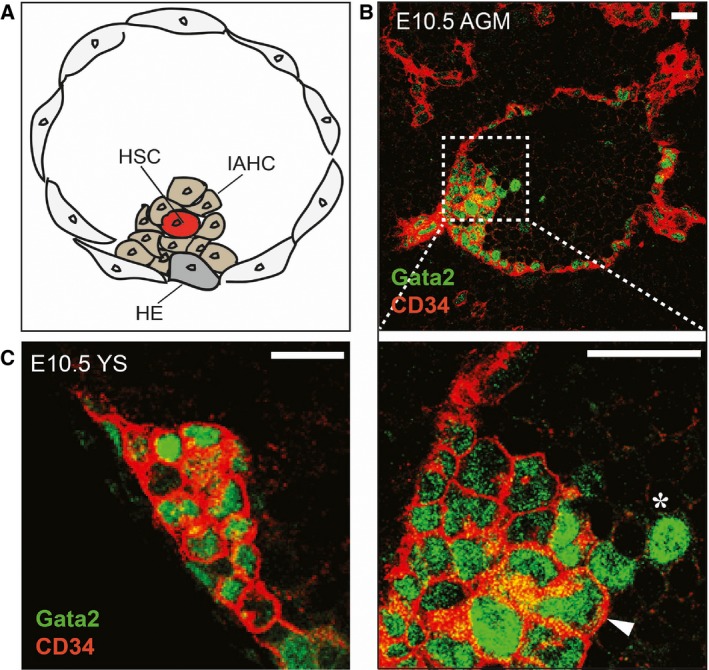
Emergence of HSCs in the AGM and in the YS. (A) A subset of aortic endothelial cells, the hemogenic endothelial (HE) cells transdifferentiate to form intra‐aortic hematopoietic cluster cells (IAHC) and hematopoietic stem cells (HSC). A schematic diagram of a transverse section through an E10.5 mouse aorta indicates HE, IAHC, and emerging HSC. (B) Transverse section through an E10.5 Gata2Venus mouse embryo. Gata2 is one of the pivotal transcription factors expressed in HE, IAHC and emerging HSCs. Thus, Gata2Venus reporter can be exploited to visualize the emergence of HSCs. Section of an E10.5 aorta‐gonad‐mesonephros (AGM) shows Gata2 (green) in most of the CD34+ (red) IAHC (arrowhead) and some endothelial cells. Gata2 is also detected in a few round CD34− cells (asterisk) that are closely associated with IAHC suggesting that they arose from clusters. (C) CD34+Gata2+ clusters are also found in the YS vasculature. Size bars = 20 μm.
Endothelial‐to‐hematopoietic cell transition has also been imaged in zebrafish embryos, however, the process is slightly different than that observed in mouse embryos. The hemogenic endothelial cells in zebrafish bulge ablumenally, and emerge as hematopoietic cells in the interstitial region between the aorta and axial vein. Moreover, multicell clusters do not form. Emerging zebrafish HS/PCs are marked by c‐Myb expression 50. Vital time‐lapse imaging of compound transgenic c‐Myb‐GFP:Kdr1 (Flk1 endothelial marker)‐mCherry zebrafish embryos demonstrates that hematopoietic cells acquiring CD41 expression emerge directly from endothelium in the ventral side of the dorsal aorta 51, 52, 53. They move quickly to extravasate into the lumen of the axial vein where they enter the circulation. They are next found to enter specific niches in caudal hematopoietic tissue, the equivalent of the mouse fetal liver 54.
Endothelial‐to‐hematopoietic cell transition has also been recapitulated in vitro and detected by time‐lapse imaging of ES cell hematopoietic differentiation cultures. ES cell‐derived cells expressing the endothelial marker Tie2 and ckit, when exposed to hematopoietic culture conditions, give rise to CD41+ hematopoietic cells that downregulate Tie2 55, 56. Together these data provide in vitro and in vivo morphological and phenotypical evidence of HSC/HPC emergence via EHT—a process that is conserved in human ESCs 57 and all vertebrate embryos 44 thus far examined.
Pivotal regulators of EHT and HSC generation
Several hematopoietic transcription factors have been found to play an essential role in the generation of ‘definitive’ hematopoietic cells in the mouse embryo. As two of the most frequently studied, Gata2 and Runx1 are the focus of this section. The importance of these factors in the process of HS/PC generation was first highlighted by the creation of germline knockout mice. Gata2 and Runx1 homozygous deletions resulted in embryonic lethality at E10.5 and E12.5, respectively, accompanied by severe fetal liver anemia 58, 59. Functional studies revealed that although Runx1 −/− mice make ‘primitive’ hematopoietic cells, they completely lack ‘definitive’ hematopoietic progenitors in the YS and fetal liver and importantly, no HSCs are generated in the AGM 59, 60. Similarly, the Gata2 −/− embryos are defective for ‘definitive’ hematopoiesis, as demonstrated by greatly reduced progenitor numbers 58, 61. Recently it was demonstrated by using a Gata2Venus reporter mouse model that Gata2 is expressed in all functional HSCs, and in most HPCs 62. In vitro hematopoietic differentiation experiments with Gata2 −/− and Runx1 −/− ES cells show that they retain the ability to undergo ‘primitive’ erythroid differentiation, however, at reduced levels. ‘Definitive’ hematopoietic progenitor generation is profoundly impaired. Analysis of ES cell‐generated Gata2 −/− and Runx1 −/− chimeric mice revealed a lack of knockout cell contribution to any of the hematopoietic organs 58, 59. Thus, Gata2 and Runx1 play pivotal roles in hematopoietic development, affecting mainly the ‘definitive’ stage in which HPCs and HSCs are generated.
The temporal and spatial expression patterns of Runx1 and Gata2 in the embryo (as determined by in situ hybridization, immunostaining, and/or knockin/transgenic reporters) support their important cell‐intrinsic role in HSC and HPC generation. These factors are expressed at E8.0 in the YS, which at that time is the main site of hematopoietic cell (EMP) generation 62, 63, 64, 65. Slightly thereafter, from E8.5 to E11.5 Runx1 marks the endothelial cells on the ventral side of the aorta, umbilical and vitelline arteries, placenta, and head 38, 63, 66, 67. Although Gata2 is expressed in the endothelial cells lining the aorta already at E8.5, the frequency of cells expressing Gata2 increases in the AGM and FL concurrent with the emergence of IAHC and the first HSCs 62. It is also expressed in the vitelline and umbilical arteries and the placenta. Moreover, both Gata2 and Runx1 are expressed in IAHCs in the embryonic arteries and all such hematopoietic clusters are absent in the aortae and other major arteries of Gata2 −/− and Runx1 −/− embryos 63, 68, 69, 70, 71.
The continuum of expression during the transition from endothelial cells to hematopoietic cluster cells in static images of the aorta implicates these factors in the process of EHT. Indeed, conditional deletion of Gata2 and Runx1 in hemogenic endothelium marked by vascular endothelial‐cadherin (Vec) expression or Tie2 expression demonstrates that these factors are essential in the hemogenic endothelial cells for the formation of hematopoietic clusters and importantly, for the generation of functional HPCs and HSCs 47, 71, 72. Moreover, Runx1 is required for HSC generation between E10.5 and E11.5, as shown by tamoxifen‐induced deletion in Vec‐expressing cells 73. The vital imaging of Runx1 morphant zebrafish embryos provided an interesting insight into its role. In the absence of Runx1, aortic endothelial cells undergo sudden death as they attempt transition to hematopoietic cells, thus suggesting that Runx1 is required during EHT for the survival of emerging hematopoietic cells 51.
To test whether Runx1 and Gata2 are required in hematopoietic cells after they are generated in the mouse embryo, conditional deletion was performed in cells marked by Vav expression. Although Runx1 is not required 70, Gata2 continues to be essential in the HSCs after they are made 71. Therefore, Gata2 and Runx1 are pivotal to HSC and HPC emergence in EHT during embryonic development, but are differentially required as hematopoietic development proceeds.
Gata2 and Runx1 levels are strictly controlled in EHT
It is of importance to note that HSC and HPC development is highly dependent on the levels of Runx1 and Gata2 expression. Gata2 +/− embryos have profoundly reduced numbers of AGM HSCs, HPCs and IAHCs. The bone marrow of Gata2 +/− adult mice contains normal quantities of HSCs, but these are qualitatively impaired, as observed in competitive transplantation assays 61, 71, 74. Overexpression of Gata2 also results in abnormal hematopoiesis: it reduces bone marrow colony‐forming unit‐cell (CFU‐C) and colony‐forming unit‐spleen (CFU‐S) activity and results in a failure of multilineage reconstitution 75. Hematopoietic differentiation of ES cells overexpressing Gata2 suggests that abnormally high Gata2 expression blocks T‐ and B‐cell generation, resulting in myeloid‐biased cell production 76, 77. Thus, Gata2 expression levels are likely to be involved in controlling cell fate decisions. Recent transcriptome analysis of placental cells suggests that Gata2 is continuously expressed in hemogenic and hematopoietic progenitors, but downregulated during commitment to blood lineages 78. Also, Gata2 expression is downregulated during ES cell‐derived hemangioblast differentiation into blast cells 79, thus indicating that levels of Gata2 may play a role in HSC and HPC expansion and potency.
Runx1 also functions in a dose‐dependent manner. Runx1 +/− embryos generate fewer HPCs and HSCs 60, 80, 81, 82. Fascinatingly, Runx1 +/− embryos experience a temporal shift in the emergence of HSCs. HSCs are detected earlier than normal: at E10 in the AGM and YS, and HSC activity is prematurely terminated in the E11 AGM 60. The E10.5/11.5 aorta in Runx1 +/− embryos has fewer IAHCs, suggesting that Runx1 haploinsufficiency reduces HSC generation, maintenance, and/or proliferation. Thus, normal diploid levels of Runx1 are essential during development for the timely emergence of HSCs and HPCs.
Gata2 and Runx1 function synergistically to regulate their downstream targets
Although deletion of a single Gata2 or Runx1 allele disrupts HSC and HPC development, it does not result in embryonic lethality 58, 60, 63. Strikingly, the analysis of Gata2 +/−: Runx1 +/− compound embryos showed a trend toward fewer hematopoietic progenitors and the absence of double haploinsufficient offspring due to embryonic lethality 83. These data suggest that Gata2 and Runx1 function together in the same cells to control the expression of hematopoietic genes involved in HSC and progenitor cell generation.
Further evidence for combinatorial function of Gata2 and Runx1 comes from an extensive ChIP‐seq and bioinformatics analysis revealing interaction complexes between a heptad of hematopoietic cell‐specific transcription factors that includes Runx1 and Gata2. The vast majority of heptad‐bound promoter and enhancer regions of hematopoietic genes contain a GATA consensus binding sequence. Only approximately 40% of them contain a Runx consensus binding motif, suggesting that Runx1 recruitment to the regulatory elements within the complex is mediated by Gata2 83. Combinatorial interactions within the heptad complex result in hematopoietic cell type‐specific chromatin binding and downstream gene expression. How exactly the complex functions in cell fate specification, is yet unknown. Whether the factors act sequentially or all at the same time, whether they regulate each other and how individual factor levels affect complex formation is a matter of debate.
RNA sequencing of endothelial cells, hemogenic endothelial cells, HPCs, and HSCs in the AGM show that heptad transcription factor expression is increasing during EHT, and is accompanied by transcriptional activation of several downstream target genes. One such target gene is the G protein‐coupled receptor 56 (Gpr56) that is significantly upregulated (38‐fold) in HSCs as compared with hemogenic endothelial cells 84. Notably, Gpr56 expression is downregulated as a result of Gata2 (regulatory element) deletion, which is accompanied by severe disruption of hematopoiesis and embryonic lethality 75. Moreover, ChIP experiments reveal direct binding of Gata2 to the Gpr56 +37 enhancer, 84, 85 thus indicating that Gpr56 is a direct target of Gata2. The precise function of Gpr56 in hematopoiesis is as yet unknown. It has been suggested to play a role in the maintenance of self‐renewal 84, and it is essential for HSC repopulation potential in mice 86. In zebrafish, Gpr56 is required for the emergence of hematopoietic cells in the dorsal aorta, 84 thereby supporting its functional involvement in EHT.
Another means by which the heptad complex may regulate HSC and HPC emergence is by inducing a stepwise expression of transcriptional suppressors Gfi1 and Gfi1b—the direct targets of Runx1. The expression of these transcription factors in hemogenic endothelium is pivotal for the normal EHT transition. Time‐lapse imaging of Gfi1+ cells show that they acquire Gfi1b expression in the IAHCs followed by upregulation of ckit and CD41 expression, indicating hematopoietic commitment. Gfi1: Gfi1b double knockout embryos lack IAHCs, and ckit+ cells stay embedded in the endothelial lining of the dorsal aorta sustaining their endothelial program (Vec and Tie2 expression). Thus, Runx1 together with Gfi1 factors promote EHT by suppressing endothelial program, thereby allowing hematopoietic cells to emerge 87, 88, 89.
Do HSCs establish their fate prior to or during EHT?
Vital imaging demonstrates that HSCs and HPCs are generated by morphological transdifferentiation of specialized endothelial cells, and genetic tracing studies show that functional HSCs/HPCs descend from cells expressing endothelial markers. But when is hematopoietic fate, and more precisely, when is HSC fate established? Current research interests are addressing the issue of whether HSC fate and function is determined in the endothelium during EHT, or primed earlier or later in development.
A Runx1 + 23 enhancer GFP (+23GFP) reporter mouse was used to explore this issue. Runx1 expression in vast majority of mouse hematopoietic stem and progenitor cells and aortic endothelial cells is controlled by a Runx1 + 23 enhancer, thus the +23GFP mouse model allows specific isolation of hemogenic endothelial cells 90, 91. Transcription analysis (Fluidigm) with a panel of endothelial and hematopoietic genes demonstrated that at E8.5 the +23GFP expressing aortic hemogenic endothelium is distinguished from +23GFP negative endothelium by higher expression of hematopoietic regulators such as Meis1, Gata2, Gata3 and SCL. Single‐cell transcriptome analysis showed in approximately 50% of +23GFP hemogenic endothelial cells that higher Meis1 expression is accompanied by downregulation of the endothelial marker Etv2, thus arguing for hematopoietic fate establishment earlier than previously recognized 91. At a later developmental time (at E10.5) in the Ly6a GFP model, the transcriptome of the aortic hemogenic endothelial fraction (CD31+ckit−GFP+) showed differences to the endothelial fraction (CD31+ckit−GFP−), with heptad transcription factor and Notch gene expression increased 84. Few indications of hematopoietic gene expression were found in the hemogenic endothelial fraction as compared to the HPC/HSC fraction (CD31+ckit+GFP+). However, these experiments were performed with populations of sorted cells and await single‐cell transcriptomic analysis. Importantly, the expression of the heptad factors is the first and pivotal step directing a hematopoietic program, and as such Runx1 +23GFP is an excellent indicator showing that the hemogenic and hematopoietic programs are established already in a subset of endothelial cells at the beginning stage of ‘definitive’ hematopoietic cell development.
If hematopoietic and HSC commitment occurs earlier than functional HSCs emerge, the aortic endothelium may harbor immature cells that in the proper microenvironment are able to mature into functional HSCs. To test this, an OP9 stromal cell coaggregation culture was established that facilitates the ex vivo maturation of hematopoietic/endothelial cells obtained by multisurface marker phenotypic sorting 92. Using this approach it was shown that E9.5 dorsal aorta contains a VEC+CD41+CD45−CD43− cell population (termed pro‐HSC) that lacks repopulating activity in direct in vivo transplantation assays. However, when co‐aggregated with OP9 and ex vivo cultured for 7 days, this population is able to reconstitute the hematopoietic system of the recipient 93. These pro‐HSCs are almost devoid of endothelial cells and it is thought that they may represent a stage directly downstream of the Runx1 +23GFP+ hemogenic endothelium present in the E8.5 AGM 91.
Also, the E10.5 and E11.5 AGM is thought to contain immature HSCs—this VEC+CD41low CD45−CD43+ population (termed pre‐HSC) upregulates CD45 expression when coaggregated with OP9 and mature into functional, repopulating HSCs 92. Interestingly, it has been proposed that the pre‐HSCs may be generated independently of Runx1, as a developmental block is not observed before the transition of CD41+ cells to CD45+ in Runx1‐deficient mice 94. Cells with a pre‐HSC phenotype are present also in the E11.5 YS and FL, but they are not able to mature into engrafting HSCs. Thus, functional pre‐HSCs are thought to be present mainly in the AGM region and in the extraembryonic arteries 92, 93, 95, 96.
These data propose that definitive HSCs may be primed for a hematopoietic gene expression program very early in development, making the precise temporal onset of the HSC program debatable. However, it should be taken into account that ex vivo manipulations, such as stromal cell and explant (co‐)cultures, consequently introduce new variables into the model, that might not be present in in vivo. Advances in in vivo lineage and vital imaging tracing tools and single cell transcriptomics will assist in further investigations of such cells under more physiologic conditions representative of the in vivo embryonic milieu.
Recent studies have suggested that mouse embryonic head produces adult HSCs and HPCs independently from other hematopoietic organs and circulation. Lineage‐tracing experiments show that embryonic head‐derived HSC progeny contribute to the adult HSC population 38. However, to date, it has not been demonstrated that the head HSCs are emerging via an EHT in a similar manner to those in AGM. Moreover, the head vasculature lacks IAHCs 66, 97. Also, head HSCs do not seem to go through the putative pre‐HSC state since no/few pre‐HCSs have been reported in the head as demonstrated by OP9 coaggregation culture of E11.5 head region 98. These studies suggest that there may be an alternative way by which functional HSCs are generated, and could include generation in different spatial and temporal frameworks, and different regulatory programs and networks. Defining such mechanisms could contribute to answering questions currently arising in the field of hematopoiesis: for instance, such information may clarify the source of heterogeneity among HSCs—BMP‐activated and BMP‐nonactivated, myeloid or lymphoid biased 99, 100. Also, it may provide insight into why there are many more HPCs in the IAHCs than HSCs 96, and explain the source of the large cohort of FL HSCs that appears within 24 h following the generation of the first HSCs in the AGM and the rapid decrease in the pre‐HSC numbers in the AGM 98.
Concluding remarks
The adult hematopoietic system is established through the progressive generation of hematopoietic cells with increasing functional complexity, culminating in the de novo generation of long‐lived self‐renewing HSCs that provide the adult organism with all functional blood cells. Although the precise temporal onset of expression and of the HSC program and initiation of function is debatable, it is certain that it is directed by the expression of a small set of pivotal hematopoietic transcription factors. In combination, these factors create a highly complex network that, depending on the time and levels of expression, drive the determination of hematopoietic progenitors and stem cell fate. It remains a future challenge to determine all the players in this process, to examine all the precursors to HSCs, as well as to cells along the process of endothelial‐to‐hematopoietic transition on the single‐cell level to converge transcriptomics with cell biology and function. This would ultimately enable the recapitulation of physiologic HSC development in vitro for the de novo production of transplantable HSCs for therapeutic strategies.
Acknowledgements
The authors acknowledge funding support from the Landsteiner Society for Bloodtransfusion Research (1109), ZonMM – Netherlands Scientific Research Council – TOP (103127), and National Institutes of Health – NIDDK (R37 DK 54077). The authors acknowledge the grant support of the Landsteiner Society for Blood Research (LSBR 1344‐1) and the NIH NIDDKK (R37 DK 054077).
Edited by Wilhelm Just
References
- 1. Palis J, Chan RJ, Koniski A, Patel R, Starr M and Yoder MC (2001) Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc Natl Acad Sci USA 98, 4528–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palis J, Robertson S, Kennedy M, Wall C and Keller G (1999) Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126, 5073–5084. [DOI] [PubMed] [Google Scholar]
- 3. Haar JL and Ackerman GA (1971) A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse. Anat Rec 170, 199–223. [DOI] [PubMed] [Google Scholar]
- 4. Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML and Rossant J (1993) flk‐1, an flt‐related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 118, 489–498. [DOI] [PubMed] [Google Scholar]
- 5. Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML and Schuh AC (1995) Failure of blood‐island formation and vasculogenesis in Flk‐1‐deficient mice. Nature 376, 62–66. [DOI] [PubMed] [Google Scholar]
- 6. Ueno H and Weissman IL (2006) Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell 11, 519–533. [DOI] [PubMed] [Google Scholar]
- 7. Evans MJ and Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156. [DOI] [PubMed] [Google Scholar]
- 8. Bradley A, Evans M, Kaufman MH and Robertson E (1984) Formation of germ‐line chimaeras from embryo‐derived teratocarcinoma cell lines. Nature 309, 255–256. [DOI] [PubMed] [Google Scholar]
- 9. Choi K, Kennedy M, Kazarov A, Papadimitriou JC and Keller G (1998) A common precursor for hematopoietic and endothelial cells. Development 125, 725–732. [DOI] [PubMed] [Google Scholar]
- 10. Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N and Keller G (1997) A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature 386, 488–493. [DOI] [PubMed] [Google Scholar]
- 11. Kouskoff V, Lacaud G, Schwantz S, Fehling HJ and Keller G (2005) Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc Natl Acad Sci USA 102, 13170–13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, de Mesy‐Bentley KK, Waugh R and Palis J (2007) The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood 109, 1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kingsley PD, Malik J, Emerson RL, Bushnell TP, McGrath KE, Bloedorn LA, Bulger M and Palis J (2006) “Maturational” globin switching in primary primitive erythroid cells. Blood 107, 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kingsley PD, Malik J, Fantauzzo KA and Palis J (2004) Yolk sac‐derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood 104, 19–25. [DOI] [PubMed] [Google Scholar]
- 15. Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A and Godin I (2005) Three pathways to mature macrophages in the early mouse yolk sac. Blood 106, 3004–3011. [DOI] [PubMed] [Google Scholar]
- 16. Naito M, Umeda S, Yamamoto T, Moriyama H, Umezu H, Hasegawa G, Usuda H, Shultz LD and Takahashi K (1996) Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. J Leukoc Biol 59, 133–138. [DOI] [PubMed] [Google Scholar]
- 17. Naito M, Takahashi K and Nishikawa S (1990) Development, differentiation, and maturation of macrophages in the fetal mouse liver. J Leukoc Biol 48, 27–37. [DOI] [PubMed] [Google Scholar]
- 18. Takahashi K, Yamamura F and Naito M (1989) Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light‐microscopic, enzyme‐cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol 45, 87–96. [DOI] [PubMed] [Google Scholar]
- 19. McGrath KE, Koniski AD, Malik J and Palis J (2003) Circulation is established in a stepwise pattern in the mammalian embryo. Blood 101, 1669–1676. [DOI] [PubMed] [Google Scholar]
- 20. Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F et al (2015) Tissue‐resident macrophages originate from yolk‐sac‐derived erythro‐myeloid progenitors. Nature 518, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C et al (2013) Microglia emerge from erythromyeloid precursors via Pu.1‐ and Irf8‐dependent pathways. Nat Neurosci 16, 273–280. [DOI] [PubMed] [Google Scholar]
- 22. Schulz C, Gomez Perdiguero E, Chorro L, Szabo‐Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW et al (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science, 336, 86–90. [DOI] [PubMed] [Google Scholar]
- 23. Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC et al (2015) C‐Myb(+) erythro‐myeloid progenitor‐derived fetal monocytes give rise to adult tissue‐resident macrophages. Immunity 42, 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGrath KE, Frame JM, Fegan KH, Bowen JR, Conway SJ, Catherman SC, Kingsley PD, Koniski AD and Palis J (2015) Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep 11, 1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frame JM, McGrath KE and Palis J (2013) Erythro‐myeloid progenitors: “definitive” hematopoiesis in the conceptus prior to the emergence of hematopoietic stem cells. Blood Cells Mol Dis 51, 220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J and Yoder MC (2008) All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood 111, 3435–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frame JM, Fegan KH, Conway SJ, McGrath KE and Palis J (2016) Definitive hematopoiesis in the yolk sac emerges from Wnt‐responsive hemogenic endothelium independently of circulation and arterial identity. Stem Cells 34, 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yokota T, Huang J, Tavian M, Nagai Y, Hirose J, Zuniga‐Pflucker JC, Peault B and Kincade PW (2006) Tracing the first waves of lymphopoiesis in mice. Development 133, 2041–2051. [DOI] [PubMed] [Google Scholar]
- 29. Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, Cardoso AA, Kaplan MH and Yoder MC (2012) Autonomous murine T‐cell progenitor production in the extra‐embryonic yolk sac before HSC emergence. Blood 119, 5706–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Godin I, Dieterlen‐Lievre F and Cumano A (1995) Emergence of multipotent hemopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proc Natl Acad Sci USA 92, 773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sonoda T, Hayashi C and Kitamura Y (1983) Presence of mast cell precursors in the yolk sac of mice. Dev Biol 97, 89–94. [DOI] [PubMed] [Google Scholar]
- 32. Travnickova J, Tran Chau V, Julien E, Mateos‐Langerak J, Gonzalez C, Lelievre E, Lutfalla G, Tavian M and Kissa K (2015) Primitive macrophages control HSPC mobilization and definitive haematopoiesis. Nat Commun 6, 6227. [DOI] [PubMed] [Google Scholar]
- 33. Li Y, Esain V, Teng L, Xu J, Kwan W, Frost IM, Yzaguirre AD, Cai X, Cortes M, Maijenburg MW et al (2014) Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev 28, 2597–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Espin‐Palazon R, Stachura DL, Campbell CA, Garcia‐Moreno D, Del Cid N, Kim AD, Candel S, Meseguer J, Mulero V and Traver D (2014) Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell 159, 1070–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muller AM, Medvinsky A, Strouboulis J, Grosveld F and Dzierzak E (1994) Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1, 291–301. [DOI] [PubMed] [Google Scholar]
- 36. Medvinsky A and Dzierzak E (1996) Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86, 897–906. [DOI] [PubMed] [Google Scholar]
- 37. de Bruijn MF, Speck NA, Peeters MC and Dzierzak E (2000) Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J 19, 2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Z, Lan Y, He W, Chen D, Wang J, Zhou F, Wang Y, Sun H, Chen X, Xu C et al (2012) Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell 11, 663–675. [DOI] [PubMed] [Google Scholar]
- 39. Gekas C, Dieterlen‐Lievre F, Orkin SH and Mikkola HK (2005) The placenta is a niche for hematopoietic stem cells. Dev Cell 8, 365–375. [DOI] [PubMed] [Google Scholar]
- 40. Ottersbach K and Dzierzak E (2005) The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell 8, 377–387. [DOI] [PubMed] [Google Scholar]
- 41. Ema H and Nakauchi H (2000) Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 95, 2284–2288. [PubMed] [Google Scholar]
- 42. Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J and Medvinsky A (2002) Quantitative developmental anatomy of definitive haematopoietic stem cells/long‐term repopulating units (HSC/RUs): role of the aorta‐gonad‐mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development 129, 4891–4899. [DOI] [PubMed] [Google Scholar]
- 43. Mendelson A and Frenette PS (2014) Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med 20, 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jaffredo T, Nottingham W, Liddiard K, Bollerot K, Pouget C and de Bruijn M (2005) From hemangioblast to hematopoietic stem cell: an endothelial connection? Exp Hematol 33, 1029–1040. [DOI] [PubMed] [Google Scholar]
- 45. Dzierzak E and Speck NA (2008) Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol 9, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yokomizo T and Dzierzak E (2010) Three‐dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development 137, 3651–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada‐Inagawa T, Vink CS, Bhandoola A, Dzierzak E and Speck NA (2011) Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 9, 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ma X, de Bruijn M, Robin C, Peeters M, Kong ASJ, de Wit T, Snoijs C and Dzierzak E (2002) Expression of the Ly‐6A (Sca‐1) lacZ transgene in mouse haematopoietic stem cells and embryos. Br J Haematol 116, 401–408. [DOI] [PubMed] [Google Scholar]
- 49. Boisset JC, van Cappellen W, Andrieu‐Soler C, Galjart N, Dzierzak E and Robin C (2010) In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120. [DOI] [PubMed] [Google Scholar]
- 50. Jing L and Zon LI (2011) Zebrafish as a model for normal and malignant hematopoiesis. Dis Model Mech 4, 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kissa K and Herbomel P (2010) Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115. [DOI] [PubMed] [Google Scholar]
- 52. Bertrand JY, Kim AD, Teng S and Traver D (2008) CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development 135, 1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY and Traver D (2010) Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tamplin OJ, Durand EM, Carr LA, Childs SJ, Hagedorn EJ, Li P, Yzaguirre AD, Speck NA and Zon LI (2015) Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 160, 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V and Lacaud G (2009) The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457, 892–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eilken HM, Nishikawa S and Schroeder T (2009) Continuous single‐cell imaging of blood generation from haemogenic endothelium. Nature 457, 896–900. [DOI] [PubMed] [Google Scholar]
- 57. Rafii S, Kloss CC, Butler JM, Ginsberg M, Gars E, Lis R, Zhan Q, Josipovic P, Ding BS, Xiang J et al (2013) Human ESC‐derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood 121, 770–780. [DOI] [PubMed] [Google Scholar]
- 58. Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW and Orkin SH (1994) An early haematopoietic defect in mice lacking the transcription factor GATA‐2. Nature 371, 221–226. [DOI] [PubMed] [Google Scholar]
- 59. Okuda T, van Deursen J, Hiebert SW, Grosveld G and Downing JR (1996) AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84, 321–330. [DOI] [PubMed] [Google Scholar]
- 60. Cai Z, de Bruijn M, Ma X, Dortland B, Luteijn T, Downing RJ and Dzierzak E (2000) Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity 13, 423–431. [DOI] [PubMed] [Google Scholar]
- 61. Ling KW, Ottersbach K, van Hamburg JP, Oziemlak A, Tsai FY, Orkin SH, Ploemacher R, Hendriks RW and Dzierzak E (2004) GATA‐2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med 200, 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kaimakis P, de Pater E, Eich C, Solaimani Kartalaei P, Kauts ML, Vink CS, van der Linden R, Jaegle M, Yokomizo T, Meijer D et al (2016) Functional and molecular characterization of mouse Gata2‐independent hematopoietic progenitors. Blood 127, 1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin‐Padilla M and Speck NA (1999) Cbfa2 is required for the formation of intra‐aortic hematopoietic clusters. Development 126, 2563–2575. [DOI] [PubMed] [Google Scholar]
- 64. Minegishi N, Suzuki N, Yokomizo T, Pan X, Fujimoto T, Takahashi S, Hara T, Miyajima A, Nishikawa S and Yamamoto M (2003) Expression and domain‐specific function of GATA‐2 during differentiation of the hematopoietic precursor cells in midgestation mouse embryos. Blood 102, 896–905. [DOI] [PubMed] [Google Scholar]
- 65. Robert‐Moreno A, Espinosa L, de la Pompa JL and Bigas A (2005) RBPjkappa‐dependent Notch function regulates Gata2 and is essential for the formation of intra‐embryonic hematopoietic cells. Development 132, 1117–1126. [DOI] [PubMed] [Google Scholar]
- 66. Iizuka K, Yokomizo T, Watanabe N, Tanaka Y, Osato M, Takaku T and Komatsu N (2016) Lack of phenotypical and morphological evidences of endothelial to hematopoietic transition in the murine embryonic head during hematopoietic stem cell emergence. PLoS One 11, e0156427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC and Mikkola HK (2008) The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2, 252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nardelli J, Thiesson D, Fujiwara Y, Tsai FY and Orkin SH (1999) Expression and genetic interaction of transcription factors GATA‐2 and GATA‐3 during development of the mouse central nervous system. Dev Biol 210, 305–321. [DOI] [PubMed] [Google Scholar]
- 69. Pimanda JE, Ottersbach K, Knezevic K, Kinston S, Chan WY, Wilson NK, Landry JR, Wood AD, Kolb‐Kokocinski A, Green AR et al (2007) Gata2, Fli1, and Scl form a recursively wired gene‐regulatory circuit during early hematopoietic development. Proc Natl Acad Sci USA 104, 17692–17697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E and Speck NA (2009) Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457, 887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Pater E, Kaimakis P, Vink CS, Yokomizo T, Yamada‐Inagawa T, van der Linden R, Kartalaei PS, Camper SA, Speck N and Dzierzak E (2013) Gata2 is required for HSC generation and survival. J Exp Med 210, 2843–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC et al (2008) Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3, 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tober J, Yzaguirre AD, Piwarzyk E and Speck NA (2013) Distinct temporal requirements for Runx1 in hematopoietic progenitors and stem cells. Development 140, 3765–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rodrigues NP, Janzen V, Forkert R, Dombkowski DM, Boyd AS, Orkin SH, Enver T, Vyas P and Scadden DT (2005) Haploinsufficiency of GATA‐2 perturbs adult hematopoietic stem‐cell homeostasis. Blood 106, 477–484. [DOI] [PubMed] [Google Scholar]
- 75. Gao X, Johnson KD, Chang YI, Boyer ME, Dewey CN, Zhang J and Bresnick EH (2013) Gata2 cis‐element is required for hematopoietic stem cell generation in the mammalian embryo. J Exp Med 210, 2833–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nandakumar SK, Johnson K, Throm SL, Pestina TI, Neale G and Persons DA (2015) Low‐level GATA2 overexpression promotes myeloid progenitor self‐renewal and blocks lymphoid differentiation in mice. Exp Hematol 43, 565–577. [DOI] [PubMed] [Google Scholar]
- 77. Ikonomi P, Rivera CE, Riordan M, Washington G, Schechter AN and Noguchi CT (2000) Overexpression of GATA‐2 inhibits erythroid and promotes megakaryocyte differentiation. Exp Hematol 28, 1423–1431. [DOI] [PubMed] [Google Scholar]
- 78. Pereira CF, Chang B, Gomes A, Bernitz J, Papatsenko D, Niu X, Swiers G, Azzoni E, de Bruijn MF, Schaniel C et al (2016) Hematopoietic reprogramming in vitro informs in vivo identification of hemogenic precursors to definitive hematopoietic stem cells. Dev Cell 36, 525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lugus JJ, Chung YS, Mills JC, Kim SI, Grass J, Kyba M, Doherty JM, Bresnick EH and Choi K (2007) GATA2 functions at multiple steps in hemangioblast development and differentiation. Development 134, 393–405. [DOI] [PubMed] [Google Scholar]
- 80. Wang Q, Stacy T, Miller JD, Lewis AF, Gu TL, Huang X, Bushweller JH, Bories JC, Alt FW, Ryan G et al (1996) The CBFbeta subunit is essential for CBFalpha2 (AML1) function in vivo. Cell 87, 697–708. [DOI] [PubMed] [Google Scholar]
- 81. Mukouyama Y, Chiba N, Hara T, Okada H, Ito Y, Kanamaru R, Miyajima A, Satake M and Watanabe T (2000) The AML1 transcription factor functions to develop and maintain hematogenic precursor cells in the embryonic aorta‐gonad‐mesonephros region. Dev Biol 220, 27–36. [DOI] [PubMed] [Google Scholar]
- 82. Robin C, Ottersbach K, Durand C, Peeters M, Vanes L, Tybulewicz V and Dzierzak E (2006) An unexpected role for IL‐3 in the embryonic development of hematopoietic stem cells. Dev Cell 11, 171–180. [DOI] [PubMed] [Google Scholar]
- 83. Wilson NK, Foster SD, Wang X, Knezevic K, Schutte J, Kaimakis P, Chilarska PM, Kinston S, Ouwehand WH, Dzierzak E et al (2010) Combinatorial transcriptional control in blood stem/progenitor cells: genome‐wide analysis of ten major transcriptional regulators. Cell Stem Cell 7, 532–544. [DOI] [PubMed] [Google Scholar]
- 84. Solaimani Kartalaei P, Yamada‐Inagawa T, Vink CS, de Pater E, van der Linden R, Marks‐Bluth J, van der Sloot A, van den Hout M, Yokomizo T, van Schaick‐Solerno ML et al (2015) Whole‐transcriptome analysis of endothelial to hematopoietic stem cell transition reveals a requirement for Gpr56 in HSC generation. J Exp Med 212, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chacon D, Beck D, Perera D, Wong JW and Pimanda JE (2014) BloodChIP: a database of comparative genome‐wide transcription factor binding profiles in human blood cells. Nucleic Acids Res 42 (Database issue), D172–D177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Holmfeldt P, Ganuza M, Marathe H, He B, Hall T, Kang G, Moen J, Pardieck J, Saulsberry AC, Cico A et al (2016) Functional screen identifies regulators of murine hematopoietic stem cell repopulation. J Exp Med 213, 433–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lancrin C, Mazan M, Stefanska M, Patel R, Lichtinger M, Costa G, Vargel O, Wilson NK, Moroy T, Bonifer C et al (2012) GFI1 and GFI1B control the loss of endothelial identity of hemogenic endothelium during hematopoietic commitment. Blood 120, 314–322. [DOI] [PubMed] [Google Scholar]
- 88. Thambyrajah R, Mazan M, Patel R, Moignard V, Stefanska M, Marinopoulou E, Li Y, Lancrin C, Clapes T, Moroy T et al (2016) GFI1 proteins orchestrate the emergence of haematopoietic stem cells through recruitment of LSD1. Nat Cell Biol 18, 21–32. [DOI] [PubMed] [Google Scholar]
- 89. Moignard V, Macaulay IC, Swiers G, Buettner F, Schutte J, Calero‐Nieto FJ, Kinston S, Joshi A, Hannah R, Theis FJ et al (2013) Characterization of transcriptional networks in blood stem and progenitor cells using high‐throughput single‐cell gene expression analysis. Nat Cell Biol 15, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nottingham WT, Jarratt A, Burgess M, Speck CL, Cheng JF, Prabhakar S, Rubin EM, Li PS, Sloane‐Stanley J, Kong ASJ et al (2007) Runx1‐mediated hematopoietic stem‐cell emergence is controlled by a Gata/Ets/SCL‐regulated enhancer. Blood 110, 4188–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Swiers G, Baumann C, O'Rourke J, Giannoulatou E, Taylor S, Joshi A, Moignard V, Pina C, Bee T, Kokkaliaris KD et al (2013) Early dynamic fate changes in haemogenic endothelium characterized at the single‐cell level. Nat Commun 4, 2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rybtsov S, Sobiesiak M, Taoudi S, Souilhol C, Senserrich J, Liakhovitskaia A, Ivanovs A, Frampton J, Zhao S and Medvinsky A (2011) Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J Exp Med 208, 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rybtsov S, Batsivari A, Bilotkach K, Paruzina D, Senserrich J, Nerushev O and Medvinsky A (2014) Tracing the origin of the HSC hierarchy reveals an SCF‐dependent, IL‐3‐independent CD43(‐) embryonic precursor. Stem Cell Reports 3, 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liakhovitskaia A, Rybtsov S, Smith T, Batsivari A, Rybtsova N, Rode C, de Bruijn M, Buchholz F, Gordon‐Keylock S, Zhao S et al (2014) Runx1 is required for progression of CD41+ embryonic precursors into HSCs but not prior to this. Development 141, 3319–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gordon‐Keylock S, Sobiesiak M, Rybtsov S, Moore K and Medvinsky A (2013) Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood 122, 2338–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Boisset JC, Clapes T, Klaus A, Papazian N, Onderwater J, Mommaas‐Kienhuis M, Cupedo T and Robin C (2015) Progressive maturation toward hematopoietic stem cells in the mouse embryo aorta. Blood 125, 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li Z, Vink CS, Mariani SA and Dzierzak E (2016) Subregional localization and characterization of Ly6aGFP‐expressing hematopoietic cells in the mouse embryonic head. Dev Biol 416, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rybtsov S, Ivanovs A, Zhao S and Medvinsky A (2016) Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development 143, 1284–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Crisan M, Kartalaei PS, Vink CS, Yamada‐Inagawa T, Bollerot K, van IJcken W, van der Linden R, de Sousa Lopes SM, Monteiro R, Mummery C et al (2015) BMP signalling differentially regulates distinct haematopoietic stem cell types. Nat Commun 6, 8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M, Rudolph KL, Ema H and Nakauchi H (2013) Clonal analysis unveils self‐renewing lineage‐restricted progenitors generated directly from hematopoietic stem cells. Cell 154, 1112–1126. [DOI] [PubMed] [Google Scholar]


