Abstract
Objective
The purpose of this study is to investigate if structural brain phenotypes can be used to predict weight loss success following behavioral interventions in older adults that are overweight or obese and have cardiometabolic dysfunction.
Methods
A support vector machine (SVM) with a repeated random subsampling validation approach was used to classify participants into the upper and lower halves of the weight loss distribution following 18 months of a weight loss intervention. Predictions were based on baseline brain gray matter (GM) and white matter (WM) volume from 52 individuals that completed the intervention and a magnetic resonance imaging session.
Results
The SVM resulted in an average classification accuracy of 72.62 % based on GM and WM volume. A receiver operating characteristic analysis indicated that classification performance was robust based on an area under the curve of 0.82.
Conclusions
Our findings suggest that baseline brain structure is able to predict weight loss success following 18 months of treatment. The identification of brain structure as a predictor of successful weight loss is an innovative approach to identifying phenotypes for responsiveness to intensive lifestyle interventions. This phenotype could prove useful in future research focusing on the tailoring of treatment for weight loss.
Keywords: Weight Loss Prediction, Gray- and White Matter Volume, Structural Brain Imaging, Elderly, Classification
Introduction
Obesity is a leading cause of morbidity and mortality and is associated with most chronic diseases and with physical disability (1). Notwithstanding recent surgical and pharmaceutical treatments, intensive lifestyle interventions (ILIs) remain the most popular and least invasive methods for losing weight. The conundrum is that responsiveness to ILIs is highly variable; moreover, the majority of people who lose weight regain it after the intensive phase of treatment (2, 3). As evident by the recent work sponsored by the National Institutes of Health (http://1.usa.gov/1VCI7pA), an important direction of future research is to identify phenotypes for and underlying mechanisms of treatment responsiveness. This would enable clinicians to better target treatment regimens using personalized medicine and to reduce patient burden and healthcare costs.
There has been considerable interest in understanding the variability in weight loss that is observed with ILIs. Whereas genetic factors (4) and circulating hormones (5, 6) each influence eating behavior, the capacity to self-regulate lifestyle behaviors that influence energy balance has been identified as important to treatment success (7). For example, favorable changes in scores on the weight efficacy lifestyle questionnaire (WEL), a measure that assesses confidence in regulating eating behavior under a variety of internal and external demands, has been found to mediate change in weight loss among older adults during the first 6-month of treatment (3). Also, deficits in executive function may underlie poor self-regulation with eating behavior (8) and influence the ability of participants to sustain changes both in exercise and dietary regimens that are the foundation of ILIs (9, 10). It is also important to note that weight loss is affected by activity in functional brain networks that reside below the level of conscious awareness (11, 12, 13).
With aging there is cerebral atrophy in both gray matter (GM) and white matter (WM) (14). Among the elderly, high body mass index (BMI) has been associated with lower GM volume in the frontal and temporal lobes, thalamus, caudate, putamen, and precuneus (15, 16). Furthermore dietary restraint, which has been linked to increases in sensitivity to food cues (17), has been found to be correlated with higher GM volume in brain reward circuitry and lower GM volume in inhibitory circuitry (18). While a number of studies have investigated how obesity (19) or weight loss (20, 21) is associated with brain structure, less studied is the predictive role that brain structure may have in successful weight loss, particularly as people age.
Traditionally, most studies of brain structure in the obesity and weight loss literature have focused attention on specific regions of interest and univariate statistical modeling to explore weight loss phenotypes (22). Clearly however, multiple regions of the brain interact in complex patterns when attempting to understand obesity and the regulation of weight (23). Thus, in the current study, we took advantage of support vector machine (SVM) analysis, a multivariate statistical model, to discover whether there was a multivariate phenotype within baseline brain anatomy that predicts weight loss success among older adults that are overweight or obese and have cardiometabolic dysfunction. We hypothesized that a pattern of baseline GM and WM volume exists that constitutes a biomarker or phenotype for classification into either the upper or lower halves of the weight loss distribution following 18 months of a lifestyle intervention.
Methods
Participants
Sixty-six elderly individuals (ages: 60-79) were recruited for this study in the first year of the Cooperative Lifestyle Interventions Programs II project (CLIP-II). Participants with either overweight or obesity (BMI ≥ 28 kg/m2 but < 42 kg/m2) and with a documented history of either cardiovascular disease (CVD) or metabolic syndrome (MetS) were recruited for this study. A final sample size of 52 participants was used. From the original 66 participants that had MRI scans, 2 individuals did not meet eligibility to be randomized in the trial, 2 individuals withdrew from the study because of claustrophobia during magnetic resonance imaging (MRI), and 4 participants withdrew during the first 6-month of intervention for health reasons. Six additional individuals withdrew from the study after the 6 months intensive phase of intervention. The Institutional Review Board (IRB) at Wake Forest University School of Medicine approved the study protocol and all individuals signed an informed consent.
Details of the CLIP-II study intervention, inclusion and exclusion criteria, and outcomes have been previously described (24). Participants were randomly assigned to a weight loss intervention, including a diet only weight loss condition (WL), WL plus aerobic exercise training (WL+AT), or WL plus resistance exercise training (WL+RT). The weight loss goal of the ILI in CLIP-II was to elicit a 0.3 kg/week weight loss in the intensive phase with a total weight loss of 7-10% of body mass. Greater details are available in the Supporting Information and in (24).
The participants in this brain imaging study represent a subset of the total study sample (N=249) and were drawn from the early waves of recruitment to accommodate funding. This resulted in a random error of a relatively higher number of individuals from the WL+RT intervention subgroup. To address this potential confound, secondary analyses were performed with an intervention group covariate in the classification model. The results of this secondary analysis mirrored the primary analysis. For more details of this analysis, please see Supporting Information.
The distribution of percent weight loss [(18-month values – baseline)/baseline] was then used to split the sample into the upper (high weight loss group) and lower (low weight loss group) halves of weight loss success. The Obesity Society (TOS) guidelines recommend a decrease of 5% as a meaningful weight loss (25). Although we used a median split to create balanced weight loss groups (low versus high), the mean (95%CI) weight loss of the two group was 2.87% (95%CI = 1.41, 4.33) and 13.95% (95%CI = 11.86, 16.05), respectively.
Image Acquisition and Preprocessing
Image acquisition and processing details are presented in the Supporting Information. Briefly, all participants included in this analysis participated in a baseline MRI session. High-resolution structural T1-weighted brain images were collected on a Siemens 3T MRI scanner. Images were visually inspected for quality assurance and preprocessed to generate GM and WM tissue probability maps. Images were first segmented into GM and WM images using statistical parametric mapping toolbox version 12 (SPM12). Advanced normalization tools (ANTS) was then used to warp the segmented images to standard space (26). The warped images were modulated with the Jacobian deformation field and were masked using GM/WM masks provided by the International Consortium for Brain Mapping (http://www.loni.usc.edu/atlases). Finally, the images were smoothed using ANTS median filter with a radius of 1.5 mm. To compute the total GM/WM for each subject, the GM/WM probability maps were summed. The probability maps in the subjects' native space were used.
Classification
The GM and WM images generated from image preprocessing were used in a linear SVM classifier to determine if brain tissue volume was able to predict weight loss performance following 18 months of the intervention. SVM is a supervised machine learning algorithm. It includes a training phase to build a margin that is as broad as possible between two groups (27, 28). This maximum-margin separation can potentially be used to predict group membership of future test samples. For more information see Supporting Information.
A repeated random sub-sampling validation approach, known as Monte Carlo cross-validation, was used to estimate the generalizability of the training model. The dataset was split into training and test subsets, each including 50% of participants from the low and high weight loss groups. Following training, the accuracy of the trained model was determined using the test subjects. This validation approach was performed with 100 permutations of the subject grouping (train and test) in order to obtain an average estimation of the classifier performance. The training and testing procedures were performed independently for the GM and WM images. The SVM was built using the LIBSVM toolbox for MATLAB (29).
In addition to using the GM and WM images independently, a combined score using the classification decision scores from the GM and WM classifiers was generated. For the individual GM and WM classifiers, each participant was assigned a score to classify him/her into either the high (positive scores) or the low weight loss group (negative scores). Larger scores (positive or negative) are associated with higher confidence in the classification. The scores assigned by the GM and WM classifiers were summed to combine the results from the two analyses. Individuals with positive scores were assigned to the high weight loss group and those with negative scores to the low weight loss group. For more information see Supporting Information.
Results
Table 1 provides descriptive data and weight loss information for the entire sample as well as for the two weight loss groups. The mean (SD) age was 67.62 (0.73) years, 75% were women and on average they had a BMI of 34.11 (0.52). As a group, their self-reported physical function—physical composite scale of the SF-12 (30)—was roughly 1 SD below the national average of 50; however, they were ∼0.5 of a SD higher on the mental health composite score of the SF-12 (30). As expected, there were substantially and statistically significant differences in percent weight loss between the low and high groups: low = 2.87% (3.62) versus high = 13.96% (5.18); p < 0.001. Of interest is the fact that the low and high groups were almost identical in distribution by sex, age, baseline BMI, scores on the Montreal cognitive assessment test (31), executive function as defined by the trail making test B (32) and symbol digit modalities test (Western Psychological Services, www.wpspublish.com), SF-12 scores, scores on the weight efficacy lifestyle questionnaire (33), physical function as defined by performance on a 400 m walk test (34), and total GM/WM volume.
Table 1. Descriptive Data, Mean ± SD [range], on Entire Sample and Weight Loss Groups, Mean ± SD.
| Variable | Entire Sample (N = 52) | Low Weight Loss Group (N = 26) | High Weight Loss Group (N = 26) | P-Value |
|---|---|---|---|---|
| Sex | ||||
| Women (n) | 39 | 19 | 20 | 0.75 |
| Men (n) | 13 | 7 | 6 | |
| Age | 67.62 ± 0.73 [60.70, 79.80] |
66.73 ± 3.88 | 68.43 ± 6.08 | 0.23 |
| BMI (Kg/m2) | 34.11 ± 0.52 [28.14, 41.98] |
34.48 ± 3.63 | 33.88 ± 3.72 | 0.56 |
| Baseline Weight (Kg) | 93.28 ± 12.80 [71.76, 129.09] |
95.56 ±14.58 | 91.00 ± 10.53 | 0.20 |
| Weight Loss (Kg) | 7.69 ± 6.66 [-2.81, 23.41] |
2.64 ± 3.42 | 12.74 ± 5.07 | <0.001 |
| Weight Loss (%) | 8.41 ± 7.14 [-2.84, 26.44] |
2.87 ± 3.62 | 13.96± 5.18 | <0.001 |
| SBP (mm Hg)* | 130.21 ± 2.12 [101, 162] |
130.46 ± 15.30 | 131.29 ± 15.96 | 0.84 |
| DBP (mm Hg)* | 76.55 ± 0.98 [60, 90] |
77.11 ± 8.05 | 76.75 ± 5.72 | 0.85 |
| MOCA* | 26.24 ± 0.31 [19, 30] |
26.11 ± 2.28 | 25.92 ± 2.56 | 0.78 |
| Trail making test B | 90.30 ± 36.70 [41.50, 252.97] |
92.51 ± 42.90 | 88.01 ± 29.63 | 0.45 |
| SDMT | -0.33 ±0.90 [-2.50, 2.50] |
-0.28 ± 1.032 | -0.39 ± 0.78 | 0.43 |
| SF-12 PCS* | 41.68 ± 1.49 [13.10, 66.27] |
40.95 ± 11.16 | 42.40 ± 10.07 | 0.63 |
| SF-12 MCS* | 54.28 ± 1.24 [32.55, 69.17] |
50.89 ± 17.02 | 51.52 ± 13.50 | 0.88 |
| 400 m Walk Time (sec) | 334.78 ± 8.97 [ 241, 643] |
348.74 ± 75.39 | 318.77 ± 41.90 | 0.80 |
| WEL Total Score* | 101.80 ± 6.12 [17, 180] |
108.96 ± 35.51 | 91.54 ± 49.01 | 0.14 |
| Total GM volume | 0.62 ± 0.06 [0.50, 0.75] |
0.62 ± 0.06 | 0.62 ± 0.06 | 0.97 |
| Total WM volume | 0.39 ± 0.07 [0.28, 0.79] |
0.39 ± 0.09 | 0.36 ± 0.04 | 0.96 |
Notes: SBP = systolic blood pressure; DBP = diastolic blood pressure; MOCA = Montreal Cognitive Assessment Test; SDMT = symbol digit modalities test; SF-12 PCS = SF-12 physical health composite score; MCS = SF-12 mental health composite score; WEL total = total score for the weight efficacy lifestyle questionnaire.
Table 2 summarizes the average classification accuracy, sensitivity, and specificity across all 100 permutations of the data obtained using the GM, WM, and combined classifiers (see Supporting Information for definitions). Classifications based on GM consistently outperformed those based on WM. Taking advantage of both GM and WM patterns in the combined classifier enhanced discrimination performance above either GM or WM alone. The combined classifier correctly assigned the individuals to the appropriate categories with sensitivity and specificity of 80.00% and 76.92%, respectively.
Table 2. Average classification performance within all permutations obtained using GM, WM and combined classifiers.
| Classifiers | Accuracy (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| GM | 76.73 ± 7.16 | 78.23 ± 12.85 | 75.23 ± 12.87 |
| WM | 73.81 ± 6.84 | 74.00 ± 11.87 | 73.62 ± 14.18 |
| Combined | 78.46 ± 6.30 | 80.00 ± 10.99 | 76.92 ± 12.98 |
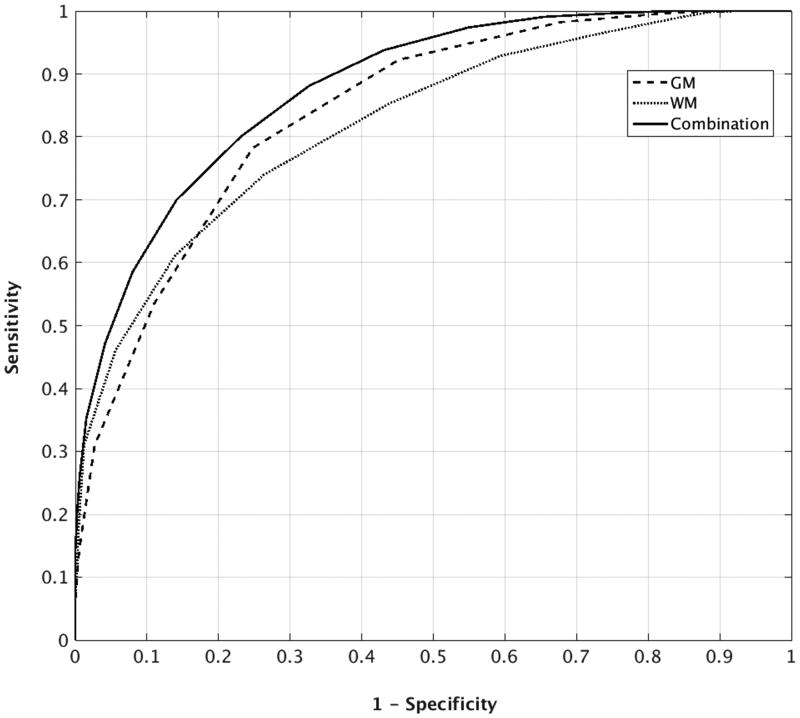
Figure 1 shows average ROC curves across all permutations for the 3 classifiers based on 18 months of treatment. The results illustrate the higher discriminant power of GM compared to WM and the superior performance of the combined GM+WM classifier. An area under the curve (AUC) analysis further supported the finding that the combined classifier achieved the best prediction. For each permutation of data, GM, WM and combined classifications were performed and an AUC value was estimated. A one-way repeated measure analysis of variance (ANOVA) revealed a significant difference for AUC between the 3 classifiers, F(2,297) = 28.78, p<0.001. The average AUC values were 0.84, 0.82, and 0.88 for GM, WM, and combined classifications, respectively. Post-hoc paired t-tests showed that the combined classifier significantly outperformed both individual classifiers (p<0.001 when compared to both GM and WM) and that GM outperformed WM (p<0.01). According to a conventional categorization for AUC values, an AUC of above 80% is considered a good classification performance (35).
Figure 1.
Average ROC curves for GM, WM, and combined classifiers. The curves each represent the average from 100 repetitions of the analysis. To make the figure legible, error bars have not been included.
Discriminatory Patterns Associated with Weight Loss Prediction
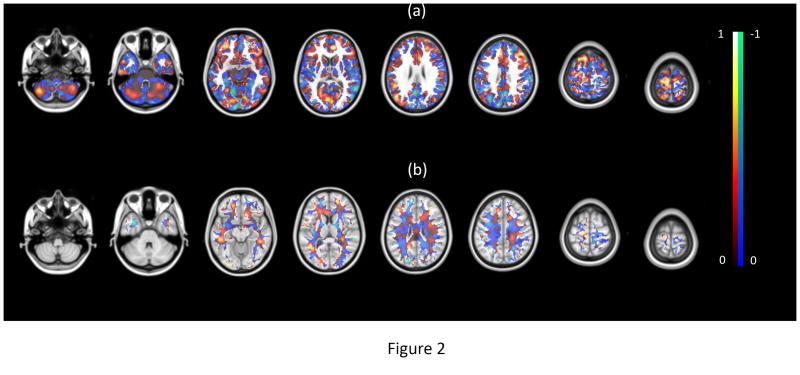
During the classifier training phase, a vector is estimated with each element representing the importance of a voxel in distinguishing between the weight loss groups. These voxel values were mapped back into brain space to generate a discriminatory map for each analysis. The maps shown in Figure 2 are average discriminatory maps across all permutations for GM and WM classifiers. Voxels with positive values were stronger contributors to recognizing individuals in the high weight loss group and the voxels with negative values were stronger contributors to recognizing individuals in the low weight loss group. Although voxels with a higher value have a higher discriminant power between groups, it should be noted that one cannot readily interpret each region individually. This is because the SVM is a multivariate analysis method that examines relationships between the voxels to discover this discriminatory pattern. In other words, the maps show a network of distributed regions across the entire brain whose interactions with each other contributed to discriminate between participants who fell in the lower and upper halves of the weight loss distribution.
Figure 2.
The discriminatory map for (a) GM and (b) WM classifier. The red and blue color maps indicate voxels with a corresponding positive and negative value, respectively. According to MNI template (2mm), the z coordination of the axial slices is -50, -32, -12, 10, 26, 40, 62 and 74, respectively.
Discussion
Success in losing weight through an ILI is complex and influenced by a variety of factors including genetic, hormonal, psychological, behavioral, social, and environmental. The current findings illustrate that baseline structural brain images can be used to predict weight loss after 18 months of participating in an ILI among older adults that are overweight or obese with cardiometabolic dysfunction. The use of a machine learning technique allowed us to perform predictions for a complex problem that may defy simple mechanistic explanations. We found that brain gray matter and white matter tissue volume at baseline was predictive of 18 month weight loss. The performance of a combined classifier using both GM and WM resulted in predictions that significantly surpassed GM or WM alone. Although the combined improvement was modest, each percentage point improvement in prediction is critical to limit false positive and negative cases if this technology is to ultimately become clinically efficacious. To our knowledge, this is the first study to demonstrate that the combination of a machine learning technology and structural brain phenotype can be used to predict weight loss success in an ILI.
Structural brain organization has been previously shown to be related to weight loss and eating behavior. For example, self-regulated eating behavior has been linked to the higher GM volume in specific regions within the insula (22), frontal, and temporal lobe areas (18). However, it is important to note that the complex relationship between brain anatomy and eating behavior is likely governed by what has been called many-to-many mapping (36). What this means is that there is no simple univariate mapping between brain structure and complex behavior. Thus, traditional studies that attempt to map a specific behavior (eating behavior or weight loss) to a specific brain region are likely challenged, because such a 1:1 relationship between behavior and anatomy is unlikely to exist.
We applied a multivariate prediction model to determine if brain tissue volume could predict weight loss. The discriminatory maps shown in Figure 2 reveal a distributed spatial pattern of brain areas that contributed to between-group discrimination. These maps do not support a direct (1:1) relationship between brain tissue volume and weight loss success. These maps should only be interpreted as an anatomical circuit that contributes to the resulting separation between groups, not as individual regions responsible for the degree of success with weight loss. The GM/WM volume in each voxel was not in itself predictive of successful weight loss. Rather, the GM/WM volume in each voxel as related to every other voxel was important in predicting weight loss success. In addition, the positive weights do not indicate that higher GM/WM in that region was associated with greater weight loss. Positive weights indicate that the tissue volume in that voxel, as related to other brain voxels, was a strong predictor of the high weight loss group. Negative values indicate a strong prediction for the low weight loss group. The predictive value of each voxel could be due to either higher or lower tissue volume as related to the other brain voxels.
As shown in Figure 2, discriminant voxels are distributed throughout the entire brain rather than being localized to a few brain regions. This finding is consistent with between-group differences that are distributed rather than localized to a single brain region (36). In other words, a very complex interaction between brain regions is associated with weight loss in this population as opposed to a specific region of interest or a simple circuit. Nevertheless, the highly-weighted GM voxels were more densely distributed within frontal, visual, and cerebellar areas (positive values) and in the temporal and occipital areas, and precuneus (negative values), whereas high (positive and negative) values of WM maps were distributed throughout the white matter tracts. The mechanistic explanation describing why these voxels are key to discriminating between the high and low weight loss groups remains to be determined. However, it is interesting to note that in the behavioral neuroscience literature the developmentally later-evolving areas of the cerebellar lobes are involved in the organization and coordination of language, memory, and reasoning (37), whereas the frontal cortex play a key role in conscious self-regulation of behavior (38). These are intriguing topics to explore in future research and serve to illustrate the hypothesis generating potential of machine learning algorithms as a way of understanding underlying mechanisms.
As evident in Table 1, the low and high weight loss groups did not differ on a number of potentially relevant variables that one may argue contribute to the success of our classification algorithm. These included sex, age, baseline BMI, a global measure of scores of cognitive function, executive function and processing speed, total GM and WM volume, self-reported physical and mental health, scores on a measure of self-efficacy related to control with eating, and physical function as defined by performance on a 400 m walk test. Hence, these variables likely did not yield a confounding effect on the prediction performance. Our results suggest that the discrimination between the groups resulted from the complex and multivariate pattern associated with brain anatomical characteristics.
The ultimate goal of many studies which apply machine learning to biological data is to improve diagnostic or prognostic information, in order to improve clinical decision making. A test that can predict intentional weight loss success using structural brain characteristics could ultimately be used to tailor treatment. For example, individuals identified at high risk for failure in ILIs might well benefit from highly structured meal plans, supportive self-monitoring by others, or supplemental pharmacological therapies to control appetite. Mindfulness-based practices are another alternative for the individuals identified with lower chance of success. Of interest is a recent analysis of a large dataset revealing that people who engaged in mind-body practices had lower BMIs than those who do not (39) and a review by O'Reilly which suggests that mindfulness-based interventions are particularly effective in managing binges, emotional eating, and hypersensitivity to environmental cues (40). Finally, another possibility is that individuals who are classified as having a high probability for success with ILIs might well respond favorably to less intensive treatment. Such a finding would have tremendous public health significance and contribute significantly to the area of personalized medicine.
While we have provided exciting initial results regarding a structural brain phenotype that predicts weight loss success in an ILI among older adults that are overweight or obese adults and have cardiometabolic dysfunction, this study is not without limitations. Our analyses were based on a relatively small sample size and we were not able to perform analyses based on the 3 different clinical interventions. However, it is important to note that controlling for the intervention effect did not alter the results of the SVM algorithm (see Supplemental Information). Although the randomized groups were balanced according to sex, the small sample size prevented us from independently predicting weight loss in males and females.
Future larger cohorts will allow training more specific classifiers to determine group-specific classification performance. Such studies will move these methods from the research laboratory to the clinic with the goal of achieving personalized medicine. Given that the current findings are limited to the specific study population and lifestyle interventions used in this paper, future studies would be required to explore the generalizability of our findings. Finally, the lack of prior studies using similar methods prevented us from making more detailed interpretations concerning the anatomical circuits revealed by the SVM algorithm. However, the present results establish a foundation for future studies that will focus on the relationship between weight loss and brain networks that are involved in regulation of eating behavior during ILIs (38).
In summary, we successfully predicted weight loss success within an 18-month ILI among older adults that were overweight or obese and had cardiometabolic dysfunction. Brain GM volume provided higher prediction accuracy compared to WM and the combination of the two outperformed either one alone. The findings presented are a first step in personalized medicine for the treatment of obesity. Specifically, if we are able to accurately identify those that will, or will not, succeed in weight loss programs we can direct clinical effort and funding appropriately. Those people with a high probability of failure would benefit from intensive treatment and close guidance. Such efforts are clearly costly and the most effective targeting of the high-cost interventions could result in the best care at the best price. We also believe that machine learning possesses scientific value as a hypothesis-generating methodology. Future studies will investigate whether functional brain networks in association with patterns of brain anatomy may improve prediction, as our recent research has demonstrated that fMRI brain circuits are associated with food craving and the self-regulation of eating behavior (11).
Supplementary Material
Study Importance Questions.
-
What is already known about this subject?
There has been considerable interest in the variability of weight loss that is observed following intensive lifestyle interventions involving caloric restriction and increased physical activity, although there has been little systemic study of whether baseline brain structure is predictive of successful weight loss.
-
What does this study add?
Using machine learning, the current study demonstrates that baseline brain anatomy may be an important biomarker of potential success with weight loss among older adults that are overweight or obese and have cardiometabolic dysfunction.
The results of this study suggest that an anatomical brain phenotype may prove to be useful in tailoring treatment for weight loss; this finding contributes to the growing interest in personalized medicine.
Acknowledgments
The authors would like to thank Robert Lyday for computer programming support.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Malnick SD, Knobler H. The medical complications of obesity. Qjm. 2006;99:565–579. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- 2.Kramer FM, Jeffery RW, Forster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: patterns of weight regain among men and women. International journal of obesity. 1988;13:123–136. [PubMed] [Google Scholar]
- 3.Rejeski WJ, Mihalko SL, Ambrosius WT, Bearon LB, McClelland JW. Weight loss and self-regulatory eating efficacy in older adults: the cooperative lifestyle intervention program. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66:279–286. doi: 10.1093/geronb/gbq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchard C, Tremblay A. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. The Journal of nutrition. 1997;127:943S–947S. doi: 10.1093/jn/127.5.943S. [DOI] [PubMed] [Google Scholar]
- 5.Verdich C, Toubro S, Buemann B, Holst JJ, Bülow J, Simonsen L, et al. Leptin Levels Are Associated with Fat Oxidation and Dietary-Induced Weight Loss in Obesity. Obesity Research. 2001;9:452–461. doi: 10.1038/oby.2001.59. [DOI] [PubMed] [Google Scholar]
- 6.Dustman RE, Emmerson RY, Ruhling R, Shearer D, Steinhaus L, Johnson S, et al. Age and fitness effects on EEG, ERPs, visual sensitivity, and cognition. Neurobiology of aging. 1990;11:193–200. doi: 10.1016/0197-4580(90)90545-b. [DOI] [PubMed] [Google Scholar]
- 7.Wing RR. Behavioral weight control. Handbook of obesity treatment. 2002;2:301–317. [Google Scholar]
- 8.Manasse SM, Juarascio AS, Forman EM, Berner LA, Butryn ML, Ruocco AC. Executive Functioning in Overweight Individuals with and without Loss-of-Control Eating. European Eating Disorders Review. 2014;22:373–377. doi: 10.1002/erv.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAuley E, Mullen SP, Szabo AN, White SM, Wójcicki TR, Mailey EL, et al. Self-regulatory processes and exercise adherence in older adults: Executive function and self-efficacy effects. American journal of preventive medicine. 2011;41:284–290. doi: 10.1016/j.amepre.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall PA, Fong GT, Epp LJ, Elias LJ. Executive function moderates the intention-behavior link for physical activity and dietary behavior. Psychology and Health. 2008;23:309–326. doi: 10.1080/14768320701212099. [DOI] [PubMed] [Google Scholar]
- 11.Paolini BM, Laurienti PJ, Simpson SL, Burdette JH, Lyday RG, Rejeski WJ. Global integration of the hot-state brain network of appetite predicts short term weight loss in older adult. Frontiers in aging neuroscience. 2015;7 doi: 10.3389/fnagi.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kullmann S, Pape A-A, Heni M, Ketterer C, Schick F, Häring H-U, et al. Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cerebral cortex. 2012 doi: 10.1093/cercor/bhs124. bhs124. [DOI] [PubMed] [Google Scholar]
- 13.Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, Horwitz B. Effective connectivity of a reward network in obese women. Brain research bulletin. 2009;79:388–395. doi: 10.1016/j.brainresbull.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Human brain mapping. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustafson D, Lissner L, Bengtsson C, Björkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 16.Pannacciulli N, Del Parigi A, Chen K, Le DSN, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 17.Stice E, Fisher M, Lowe MR. Are dietary restraint scales valid measures of acute dietary restriction? Unobtrusive observational data suggest not. Psychological assessment. 2004;16:51. doi: 10.1037/1040-3590.16.1.51. [DOI] [PubMed] [Google Scholar]
- 18.van der Laan LN, Charbonnier L, Griffioen-Roose S, Kroese FM, van Rijn I, Smeets PA. Supersize my brain: A cross-sectional voxel-based morphometry study on the association between self-reported dietary restraint and regional grey matter volumes. Biological Psychology. 2016;117:108–116. doi: 10.1016/j.biopsycho.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Driscoll I, Gaussoin SA, Wassertheil-Smoller S, Limacher M, Casanova R, Yaffe K, et al. Obesity and Structural Brain Integrity in Older Women: The Women's Health Initiative Magnetic Resonance Imaging Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2016 doi: 10.1093/gerona/glw023. glw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honea RA, Szabo-Reed AN, Lepping RJ, Perea R, Breslin F, Martin LE, et al. Voxel-based morphometry reveals brain gray matter volume changes in successful dieters. Obesity. 2016 doi: 10.1002/oby.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prehn K, von Schwartzenberg RJ, Mai K, Zeitz U, Witte AV, Hampel D, et al. Caloric Restriction in Older Adults—Differential Effects of Weight Loss and Reduced Weight on Brain Structure and Function. Cerebral Cortex. 2016 doi: 10.1093/cercor/bhw008. bhw008. [DOI] [PubMed] [Google Scholar]
- 22.Smucny J, Cornier M-A, Eichman LC, Thomas EA, Bechtell JL, Tregellas JR. Brain structure predicts risk for obesity. Appetite. 2012;59:859–865. doi: 10.1016/j.appet.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neuroscience & Biobehavioral Reviews. 2013;37:2047–2058. doi: 10.1016/j.neubiorev.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh AP, Janssen JA, Ambrosius WT, Burdette JH, Gaukstern JE, Morgan AR, et al. The Cooperative Lifestyle Intervention Program-II (CLIP-II): Design and methods. Contemporary clinical trials. 2013;36:382–393. doi: 10.1016/j.cct.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American College of Cardiology. 2013;2014;63 doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burges CJ. A tutorial on support vector machines for pattern recognition. Data mining and knowledge discovery. 1998;2:121–167. [Google Scholar]
- 28.Smola AJ, Schölkopf B. Learning with kernels. Citeseer; 1998. [Google Scholar]
- 29.Chang C-C, Lin C-J. LIBSVM: A library for support vector machines. ACM Transactions on Intelligent Systems and Technology (TIST) 2011;2:27. [Google Scholar]
- 30.Ware JE, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 32.Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nature protocols. 2006;1:2277–2281. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- 33.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. Journal of consulting and clinical psychology. 1991;59:739. doi: 10.1037//0022-006x.59.5.739. [DOI] [PubMed] [Google Scholar]
- 34.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. Jama. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 35.Zhu W, Zeng N, Wang N. Sensitivity, specificity, accuracy, associated confidence interval and ROC analysis with practical SAS implementations. NESUG proceedings: health care and life sciences, Baltimore, Maryland. 2010:1–9. [Google Scholar]
- 36.Pessoa L. Understanding brain networks and brain organization. Physics of life reviews. 2014;11:400–435. doi: 10.1016/j.plrev.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmahmann JD. The cerebellum and cognition. Academic Press; San Diego: 1997. [Google Scholar]
- 38.Heatherton TF. Neuroscience of self and self-regulation. Annual review of psychology. 2011;62:363. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camilleri GM, Méjean C, Bellisle F, Hercberg S, Péneau S. Mind– Body Practice and Body Weight Status in a Large Population-Based Sample of Adults. American journal of preventive medicine. 2015 doi: 10.1016/j.amepre.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 40.O'Reilly GA, Cook L, Spruijt-Metz D, Black DS. Mindfulness-based interventions for obesity-related eating behaviours: a literature review. Obesity reviews. 2014;15:453–461. doi: 10.1111/obr.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.