Abstract
Sphingolipids are bioactive lipids found in cell membranes that exert a critical role in signal transduction. In recent years, it has become apparent that sphingolipids participate in growth, senescence, differentiation and apoptosis. The anabolism and catabolism of sphingolipids occurs in discrete subcellular locations and consist of a strictly regulated and interconnected network, with ceramide as the central hub. Altered sphingolipid metabolism is linked to several human diseases. Hence, an advanced knowledge of how and where sphingolipids are metabolized is of paramount importance in order to understand the role of sphingolipids in cellular functions. In this review, we provide an overview of sphingolipid metabolism. We focus on the distinct pathways of ceramide synthesis, highlighting the mitochondrial ceramide generation, transport of ceramide to mitochondria and its role in the regulation of mitochondrial-mediated apoptosis, mitophagy and implications to disease. We will discuss unanswered questions and exciting future directions.
Keywords: Sphingolipid metabolism, ceramide, mitochondrial apoptosis, mitophagy, cancer
1. Introduction
Sphingolipids are a class of bioactive lipids found in cell membranes that modulate the biophysical properties of biological membranes (1) and exert a critical role in signal transduction (2,3). Multiple studies in the last decades revealed that sphingolipids control critical cellular functions such as cell cycle, senescence, apoptosis, cell migration and inflammation (4). Sphingolipids are composed of an eighteen carbon amino-alcohol backbone, sphingosine, and synthesized in the (ER) (see Figure 1). Sphingosine (2-amino-4-octadecene-1,3-diol) (Sph) and dihydrosphingosine (2-aminooctadecane-1,3-diol) (dhSph), also known as sphinganine, are the basic building blocks of all mammalian sphingolipids. The sphingoid backbone can be modified leading to a large variety of structures and functions of sphingolipids. For example, phosphorylation of the C1-hydroxyl group of sphingosine by sphingosine kinase (SK) produces sphingosine-1-phosphate (S1P), whereas N-acylation of sphingosine by ceramide synthases (CerS) generates ceramide (Cer). Indeed, S1P and ceramide are the most studied sphingolipids and it is well established that they play opposite biological roles in the cell (5). S1P promotes inflammation, cell survival, angiogenesis and cell invasion. On the contrary, ceramide modulates apoptosis, cell cycle arrest and senescence. Furthermore, ceramide serves as a precursor of more complex sphingolipids, such as glycosphingolipids (e.g. glucosylceramide and galactosylceramide) and sphingomyelin (SM), and in turn, ceramide is the breakdown product of glycosphingolipids and sphingomyelin. Therefore, the metabolism of bioactive lipids is branched, interconnected and the numerous enzymes involved are highly regulated as their activities dictate cellular levels of sphingolipids.
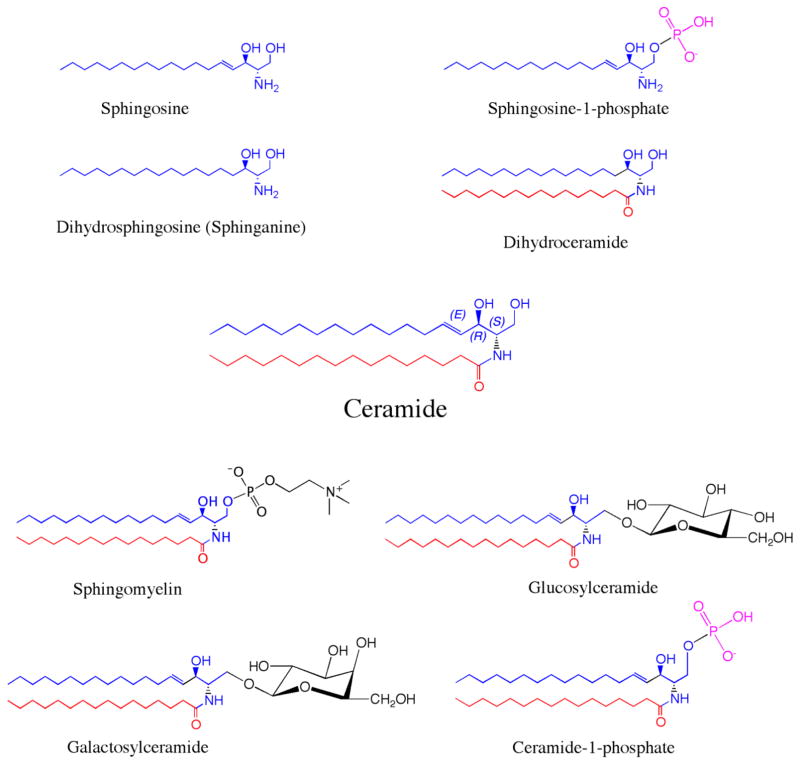
Figure 1. Chemical structures of sphingolipids.
Sphingolipids are composed of a sphingosine or dihrydrosphingosine backbone (marked in blue). N-acylation (red) of the sphingoid backbone generates (dihydro)ceramide and derivatives. Complex sphingolipids are generated by the addition of head groups (black) to ceramide. Sphingomyelin contains a phosphocholine head group; glucosylceramide and galactosylceramide contain a single glucose or galactose molecule, respectively, whereas ceramide-1-phosphate consists of a phosphate head group linked to ceramide.
One interesting feature to consider is that sphingolipid metabolism is highly compartmentalized within the cell. Some of the steps in the biosynthesis of sphingolipids occur only in discrete cellular compartments. In addition, several enzymes of the sphingolipid pathway can be found in different organelles, which restrict their activity to a specific pools of sphingolipids that are hypothesized to have distinct functions.
Another crucial aspect about sphingolipids relates to their biophysical properties, which may govern their sites of action and consequently their downstream targets. For instance, sphingosine and dihydrosphingosine are sufficiently amphipathic to move freely between different membranes and to flip-flop across the bilayer. In contrast, ceramides are restricted to the compartments where they are formed; however, spontaneous transmembrane diffusion has been reported in human erythrocytes (6). Sphingomyelins are mainly found on the outer leaflet of the plasma membrane, and have very slow transmembrane diffusion as compared to other components of biological membranes such as cholesterol (7).
The multiple advances in mass spectrometry techniques have led to a better understanding of the complexity and interconnection of sphingolipid metabolism. However, little is known about the regulation of their synthesis and degradation. Therefore, better understanding of bioactive sphingolipids requires studying the mechanisms of their biosynthesis, their localization, as well as their downstream signaling pathways.
The underlying mechanisms that couple sphingolipids, and more specifically ceramide, to mitochondrial functions remain poorly understood. Early studies identified several ceramide-producing enzyme activities in mitochondria and mitochondria-associated membranes (MAMs), which includes CerS (8,9), CDases (10,11) and most recently a mitochondria-associated nSMase (MA-nSMase) (12). Furthermore, for the first time Birbes et al. demonstrated that a specific pool of ceramide generated in mitochondria (but not in other organelles) induced apoptosis in MCF-7 cells (13). Studies using pharamacological inhibitors to block ceramide generation or perfomed in genetically modified organisms and/or cells where ceramide generation is impaired have strongly supported a critical role of ceramide accumulation in the progression of mitochondrial apoptosis and mitophagy, defining ceramide as a bona fide transducer of mitochondrial function. In this review, we provide an overview of recent scientific literature, with a focus on the molecular mechanisms by which ceramide directly or inderectly regulates mitochondrial dysregulation and diseases.
2. Sphingolipid metabolism
2.1 Synthesis of sphingolipids
2.1.1 Serine palmitoyltransferase (SPT)
Sphingolipid metabolism consists of a complex network of interconnected metabolites with ceramide as a central hub (Figure 2). De novo sphingolipid biosynthesis starts with the condensation of serine and palmitoyl-CoA by the action of serine palmitoyltransferase (SPT), located on the ER membrane, to generate 3-ketodihydrosphingosine. SPT catalytic activity is pyridoxal 5′-phosphate (PLP) dependent, consistent with its homology to other PLP dependent decarboxylases. Active SPT is composed of a heterodimer complex of SPT1 and SPT2 subunits. A third subunit (SPT3) has been identified and shown to reconstitute the SPT activity in cells lacking the SPT2 subunit (14). Actually, SPT3 enables the heterodimer to utilize lauroyl- and myristoyl-CoA as a substrate (15). Mutations of SPT1 have been identified in patients with Hereditary Sensory and Autonomic Neuropathy Type I (HSANI), in which SPT1 can still associate with SPT2, but lacks enzymatic activity (16). In a recent study, mutation in SPT2 were also found in HSAN1 patients, leading to accumulation of neurotoxic 1-deoxysphingolipids in plasma from the patients (17).
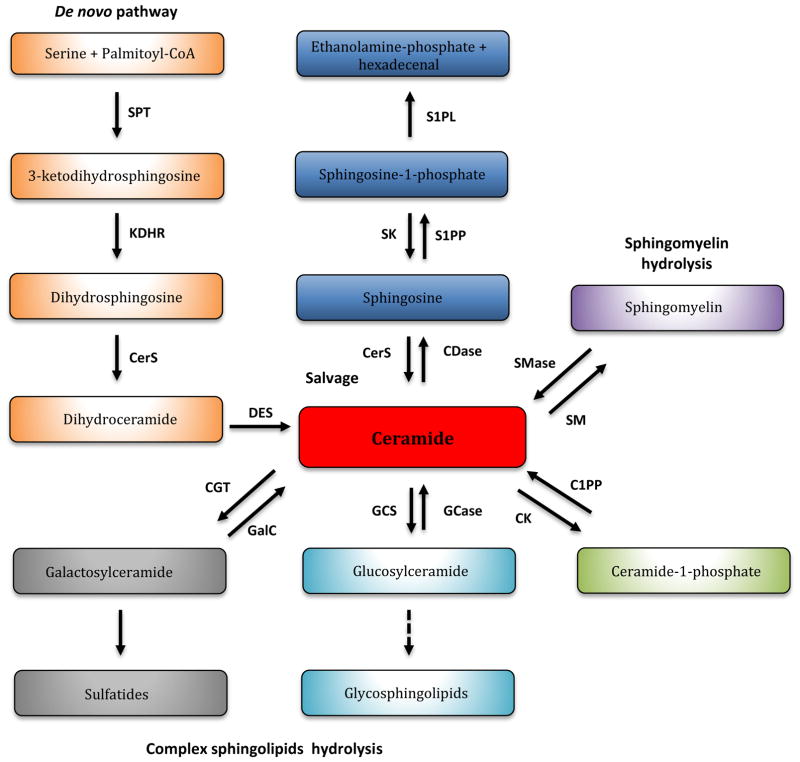
Figure 2. Sphingolipid metabolism.
Ceramide is the centerpiece of the sphingolipid metabolism and can be synthesized by multiple pathways. Ceramide can be generated by the de novo pathway (orange box) from the condensation of serine and palmitoyl-CoA to generate 3-ketodihydrosphingosine, which is then reduced to dihydrosphingosine (also known as sphinganine) and further acylated by ceramide synthases (CerS). Ceramide can be hydrolyzed to sphingosine and then reacylated back to ceramide in the salvage/ recycling pathway or phosphorylated to sphingosine-1-phosphate by sphingosine kinase (SK) and exit the sphingolipid metabolism by the action of the sphingosine-1-phosphate lyase (SPL) (blue box). Ceramide can also be formed by the hydrolysis of more complex sphingolipids such as sphingomyelin (purple box), glucosylceramide (turquoise box) and galactosylceramide (black box). Serine palmitoyltransferase (SPT); 3-ketodihydrosphingosine reductase (KDHR); (dihydro)ceramide synthase (CerS); dihydroceramide desaturase (DES); ceramidase (CDase); sphingosine-1-phosphate phosphatases (SPP); sphingomyelinases (SMase); sphingomyelin synthase (SMS); glucosylceramide synthase (GCS); glucosylceramidase (GCase); ceramide galactosyltransferase (CGT); galactosylceramidase (GALC); ceramide kinase (CERK); ceramide-1-phosphate phosphatase (C1PP).
2.1.2 3-ketodihydrosphingosine reductase (KDHR)
The second step in the de novo pathway is the conversion of 3-ketodihydrosphingosine to dihydrosphingosine by the enzyme 3-ketodihydrosphingosine reductase (KDHR). This reaction is NADPH dependent. The human KDHR was identified and cloned based on its yeast homologue (18). 3-ketodihydrosphingosine is a very short-lived metabolite of the sphingolipid metabolism, which suggests that KDHR catalyzes its conversion to dihydrosphingosine (dhSph) in a very efficient manner after the action of SPT (19).
2.1.3 (Dihydro)ceramide synthase (CerS)
The acylation of dihydrosphingosine and generation of dihydroceramide (dhCer) is carried out by (dihydro)ceramide synthase (CerS). In mammals, six different CerS have been identified (CerS1–6), each of which preferentially incorporate specific fatty acyl chains to dihydrosphingosine backbone and generate (dihydro)ceramide species with different chain lengths. While CerS1 and CerS4 are responsible for the generation of mainly C18-ceramide and to a lesser extent C20-ceramide, CerS5 and CerS6 have specificity towards C14 and C16-ceramide generation. CerS2 generates very long chain ceramide species such as C22- and C24-ceramide and CerS3 generates ultra-long chain ceramide species (C26-ceramide and longer) (8). In addition to being critical enzymes of the de novo pathway, CerS proteins also utilize sphingosine that is produced from the salvage (recycling) pathway to generate ceramide (20). CerSs have been found to localize to ER, nuclear envelope, mitochondria and MAMs (8,9).
2.1.4 Dihydroceramide desaturase (DES)
Desaturation of dihydroceramide to generate ceramide is carried out by the enzyme dihydroceramide desaturase (DES), which utilizes NADH or NADPH to produce a double bond in the C4–C5 position of the dihysphingosine backbone (21). The DES enzyme, encoded by the DES1 gene, has multiple transmembrane domains and is located in the ER membrane. In addition, its activation is regulated by myristoylation on its N-terminus (22). Mice lacking DES1 were described to have low ceramide levels and dramatically increased dihydroceramides as compared to wild-type. Moreover, Des1−/− null mice exhibited skin and hair disorders, tremors, hematological disorders, abnormal liver function, fail to gain weight and died 8–10 weeks after birth (23).
2.1.5 Sphingomyelin synthase (SMS)
Ceramide generated in the ER via the de novo pathway is then transferred to the Golgi complex for the generation of complex sphingolipids, such as, sphingomyelin, glucosylceramide (GlcCer) and ceramide-1-phosphate (C1P). Ceramide is transported from ER to Golgi by one of two mechanisms, namely vesicular transport and ceramide transfer protein (CERT). CERT is a cytosolic protein that can interact with the ER via its FAAT domain and extract ceramide. Once bound to ceramide on its START domain, CERT then interacts with the Golgi apparatus via its N-terminal PH domain that recognizes phosphatidylinositol 4-monophosphate on the Golgi. Although CERT was shown to transfer C22 and C24:1-fatty acid containing ceramides, it has preference for ceramides with shorter chain lengths as cargo (24).
Sphingomyelin synthesis is catalyzed by sphingomyelin synthases (SMS). SMS enzymes catalyze the transfer of phosphocholine from phosphatidylcholine (PC) to the C-1 position of ceramide. The two products of this reaction are sphingomyelin and diacylglycerol (DAG). SMS enzymes can regulate the levels of bioactive lipids DAG and ceramide, with opposing survival and antiproliferative roles, respectively (25). Sphingomyelin is the most abundant complex sphingolipid in human cells. In addition to being an important building block of membranes, sphingomyelin can regulate the levels of bioactive ceramide. Therefore, generation and hydrolysis of sphingomyelin is firmly regulated. There are two isoforms of SMS identified, SMS1 and SMS2. SMS1 is localized to trans-Golgi, whereas SMS2 is localized to the plasma membrane in addition to the trans-Golgi. Recently, a close family member of SMS1, SMS1-related (SMSr) has been identified to utilize ceramide to form ceramide phosphoethanolamine (CPE) in the ER lumen (26).
2.1.6 Ceramide galactosyltransferase (CGT) and Glucosylceramide synthase (GCS)
Another family of complex sphingolipids are glycosylceramides. Galactosylceramide and glucosylceramide can be generated by the actions of ceramide galactosyltransferase (CGT) and glucosylceramide synthase (GCS), respectively. The respective hexose substrates for CGT and GCS are UDP-galactose and UDP-glucose. While CGT is localized to the ER, GCS is shown to be on the cis-Golgi compartment. Galactosylceramides are further metabolized to sulfatides that are very important for Schwann cell and myelin functions. Glucosylceramide is the precursor for the majority of glycosphingolipids, which are major component of lamellar bodies in the skin and play essential role in neurite growth of primary hippocampal neurons (27). In addition, GCS has been widely implicated in multiple drug resistance of several cancer types (28). Moreover, defects in glycosphingolipid metabolizing enzymes and their contribution to multiple lysosomal storage diseases have been well characterized and reviewed (29).
2.1.7 Ceramide kinase (CERK)
Phosphorylation of ceramide at its C-1position and formation of ceramide-1-phosphate (C1P) in the trans-Golgi is catalyzed by ceramide kinase (CERK), which was initially identified by its homology to sphingosine kinase. Although CERK is member of the DAG kinase family, it does not have activity towards DAG. CERK is shown to be involved in the generation of C1P from C16, C18 and C20-ceramides (30). In addition, CERK has substrate specificity towards ceramide, rather than dihydroceramide or phytoceramide. Development of cerk −/− mice and measurement of ceramide and C1P in their organs and blood identified important roles of CERK in regulating ceramide and C1P in cerebellar functions and immune responses (31,32). In addition, C1P was shown to control cell migration in different cell types, such as pancreatic cancer cells, macrophages, myoblasts and 3T3 pre-adipocytes (33). C1P can be dephosphorylated back to ceramide by the action of the ceramide-1-phosphate phosphatase (C1PP).
2.2 Sphingolipid catabolism
2.2.1 Sphingomyelinases (SMase)
Hydrolysis of sphingomyelin to generate free phosphocholine and ceramide is catalyzed by sphingomyelinases (SMase). There are three types of sphingomyelinases according to their pH optima: alkaline, acid and neutral sphingomyelinases. Alkaline sphingomyelinase is exclusively expressed in liver and intestine and shown to be important for the digestion of dietary sphingomyelin (34). Acid sphingomyelinase (aSMase), which is localized to the lysosomes due to its mannose-6 phosphorylation, metabolizes sphingomyelin in endosomal membranes. ASMase can also be secreted and control the levels of sphingomyelin in the plasma membrane (35), and can also have access to sphingomyelin in plasma lipoproteins (36). Amongst the neutral SMase (nSMase) family, nSMase2 has been the best characterized so far, which is shown to be localized to the plasma membrane with its catalytic site oriented to the inner leaflet (37). Furthermore, nSMase2 has been shown to be activated and localized to the plasma membrane upon stimulation with TNF-α and to be important for the regulation of ICAM and VCAM mediated biological responses (38). In addition to the plasma membrane, a mammalian nSMase that localizes to mitochondria (MA-nSMase) has been identified (12); however, its roles in mitochondrial function needs to be characterized further.
2.2.2 Ceramidases (CDases)
In addition to ceramide conversion into multiple complex sphingolipids, which is tightly regulated by multiple enzymes, catabolism of ceramide and its exit from metabolic pathways is also well ordered. The catabolism of ceramide starts with hydrolysis of the amide bond between the sphingosine backbone and the fatty acid moiety by the action of ceramidases (CDases). Three types of CDases have been identified according to their pH optima: acid ceramidase, neutral ceramidase, and alkaline ceramidase. Acid ceramidase (AC), encoded by ASAH1 gene, is localized to lysosomes and deacylates ceramide that is coming into the endosomal membrane system form the plasma membrane (39). Neutral ceramidase (NC) is localized to plasma membrane via its O-glycosylated mucin box domain and is an important regulator of sphingosine and S1P production and release across the plasma membrane (40). Moreover, NC has been shown to be important for dietary sphingolipid digestion in the intestine (41) and it may play a protective role against inflammation in an animal model of inflammatory bowel disease (42). There are three alkaline ceramidase (ACER) enzymes identified so far. ACER1, ACER2, and ACER3 that are encoded by separate genes and show distinct subcellular localizations. ACER1, which is localized to the ER, deacylates primarily C24 and C24:1-ceramide (43,44). The Golgi localized ACER2 metabolizes C16, C18 and C20-ceramide species in addition to C24 and C24:1-ceramide. ACER2 is also implicated in cell adhesion and integrin signaling (45). ACER3 was shown to catalyze the hydrolysis of ceramides carrying unsaturated long acyl chains and was implicated in cell proliferation and apoptosis (46).
2.2.3 Sphingosine kinases (SK)
Sphingosine that is generated via the hydrolysis of ceramide by ceramidases can be re-acylated to form ceramide by CerS enzymes in the ER via the salvage pathway. On the other hand, sphingosine can also be phosphorylated by one of two sphingosine kinases, SK1 or SK2, producing S1P. Once generated, S1P can act on five distinct G protein coupled receptors (S1PR1-5) on the plasma membrane to activate its downstream effector pathways. SK enzymes are at a critical junction in sphingolipid metabolism, such that their actions regulate cellular levels of pro-apoptotic and anti-proliferative sphingosine and ceramide in addition to controlling levels of angiogenic and pro-proliferative S1P. Sphingosine kinase 1 (SK1), which is primarily a cytosolic enzyme, can be stimulated by multiple growth factors and translocates to the plasma membrane, where it engages with sphingosine present on the membrane to generate S1P. SK1 can be activated by ERK-dependent phosphorylation and it is thought to interact with anionic phospholipids such as PS, PA, and Pl on the plasma membrane (47). Recently, the crystal structure of SK1 has been solved and key amino acids for ATP and sphingosine binding were identified (48). Sphingosine kinase 2 (SK2) is shown to localize to multiple cellular compartments, such as the nucleus, perinuclear regions and ER (49) and it was shown to be involved in the production of nuclear S1P (50).
2.2.4 Sphingosine-1-phosphate phosphatases (SPP)
The cellular S1P can be dephosphorylated back to sphingosine by the action of sphingosine-1-phosphate phosphatase 1 (SPP1) and sphingosine-1-phosphate phosphatase 2 (SPP2) (51–54). The actions of SPP1 and SPP2 were shown to be essential for the generation of ceramide from a pool of sphingosine originated from S1P in the ER (55). In addition, cooperative activities of SK2 and SPP1 were shown to generate sphingosine and subsequently ceramide (56).
2.2.5 Sphingosine-1-phosphate lyase (SPL)
Sphingosine-1-phosphate lyase (SPL), encoded by the SGPL1 gene, breaks down sphingosine-1-phosphate to phosphoethanolamine and hexadecenal. In addition, it regulates the levels of circulating S1P, this reaction is the only exit point of the sphingolipid pathway. SPL, which is localized to the ER, has wide tissue distribution with its highest expression in the intestine (57). Sgpl1−/− knockout mice exhibit metabolic and immunological alterations (58,59). Recently, SPL was recognized in colon carcinogenesis through the regulation of S1P levels (60).
3. Sphingolipids in mitochondria
3.1 Ceramide generation in the mitochondria
There is an increasing body of evidence suggesting that ceramide acts locally in mitochondria. Overexpression of bacterial SMase (bSMase) targeted to mitochondria increased ceramide levels and caused apoptosis in MCF-7 breast cancer cells, whereas targeted overexpression of bSMase to Golgi, ER, and plasma membrane increased ceramide levels without causing apoptosis (13). Furthermore, the critical role of mitochondrial ceramide in apoptosis has been demonstrated in a C. elegans germ cell line. The loss-of-function mutations of CerS in germ cells abolished apoptosis induced by ionizing radiation (IR), which was restored by microinjection of long chain ceramide (61).
Although several enzyme activities involved in ceramide generation and breakdown have been reported in mitochondria, the enzymes required for the synthesis of ceramide during mitochondrial apoptosis need to be clarified. Numerous studies have shown increased CerS activity induced by different cell stressors such as chemotherapeutics, TNFα or ionizing irradiation with subsequent ceramide accumulation within the cell and cell death (8,62,63). In purified rat liver mitochondria, CerS activity has been demonstrated in the outer and inner mitochondrial membranes (10). Moreover, several CerS isoforms (CerS1, 2 and 6) have been shown to localize to mouse brain mitochondria and CerS6 activity was induced by cerebral ischemia/reperfusion (I/R), which was associated with elevation in ceramide content and subsequent respiratory chain damage (64). Nevertheless, that association was not evident in HeLa cells suggesting that it could be dependent on tissue or cell type (65). Localization of CerS was recently described in isolated rat brain mitochondria by immunoprecipitation (66). It was shown that CerS6 is associated with adenine nucleotide translocase in the inner mitochondrial membrane, while CerS2 is associated with Tom20 in the mitochondrial outer membrane (MOM) (66).
MA-nSMases have been identified in zebrafish and mouse tissues (12,67). Moreover, inositol sphingolipid phospholipase C (Isc1p), the mammalian ortholog of nSMase in yeast, was shown to be associated with mitochondria and regulate sphingolipid metabolism in the post-diauxic phase of growth (68). Recent work from Rajagopalan et al. provided evidences that murine MA-nSMase localized to the MOM when expressed in MCF-7 cells (69).
Neutral ceramidase (NC) activity has been demonstrated in the mitochondria (10,11). A mechanism of ceramide generation from sphingosine and palmitate has been described in purified rat liver mitochondria that involves NC and thioesterase (70). It was shown that ceramide generation from sphingosine and palmitoyl-CoA or from sphingosine and palmitate was decreased in the mitochondria from NC-deficient mice compared to wild type mice. These findings suggest that mitochondrial reverse activity of NC in the liver participates in ceramide formation (70).
As mentioned above, many reports have highlighted the existence of a ceramide pool in the mitochondria generated by CerS, SMase and NC. In addition to the in situ synthesis of ceramide, another accepted hypothesis is that ceramide synthesized in the ER may reach the mitochondria through the interaction between these two cellular organelles. Indeed, some evidences showed that ER-generated long-chain ceramide via DES activity was transferred to mitochondria isolated from rat liver, which induced mitochondrial outer membrane permeabilization (MOMP) and cytochrome c release (71).
3.2 Mitochondrial ceramide and apoptosis
3.2.1 Apoptosis: brief overview
Programmed cell death, namely apoptosis, is a precise regulated form of cell death that is crucial for homeostasis and normal development of multicellular organisms all over the animal kingdom. It was originally recognized by changes in cell morphology in the form of shrinkage, chromatin condensation and nuclear fragmentation. During apoptosis, the cellular contents become enclosed in apoptotic bodies surrounded by the plasma membrane. All of these cellular changes are mainly caused by the activity of caspases that cleave several proteins leading to recognition and engulfment of apoptotic bodies by phagocytes.
Apoptosis in vertebrates classically progresses through one of two signaling pathways known as extrinsic, which implies the binding of a death ligand to its cell surface death receptors (e.g. TNF-α to TNFR1), and intrinsic pathways, initiated by intrinsic signals such as genotoxic stress. These two pathways merge at the level of MOMP and activation of effector caspases, such as caspase-3, 6 and 7 (Figure 3).
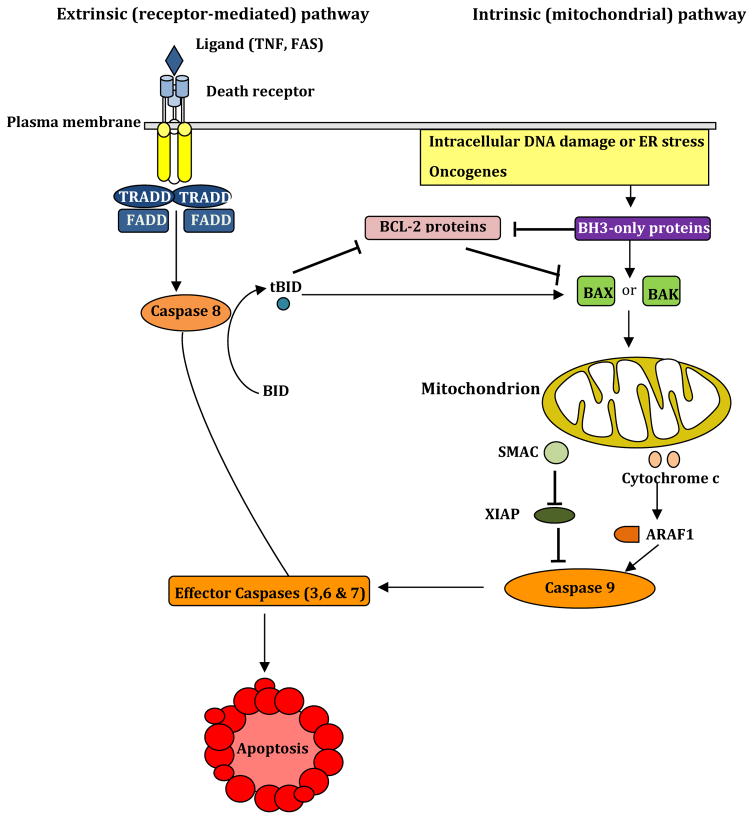
Figure 3. The extrinsic (receptor-mediated) and intrinsic (mitochondrial) pathways of apoptosis.
The receptor-mediated (extrinsic) pathway of apoptosis is initiated when ligands of TNF-α family such as FAS and TNF-α bind their plasma membrane-bound death receptors, this binding leads to activation of caspase-8 via TNFR-associated death domain (TRADD) and FAS-associated death domain (FADD) proteins. TRADD is required for induction of apoptosis in response to TNF-α a binding to TNFR. TRADD and FADD activate effector caspases (caspase-3, 6 and 7) causing apoptosis. Moreover, caspase-8 generates the active truncated form of BID (tBID) that inhibits pro-survival BCL-2-like proteins (BCL-2) and engages in the mitochondrial pathway. However, this engagement is only apparent in certain cells such as liver cells (type 2 cells), while it is absent in type 1 cells, such as thymocytes. Several stimuli such as DNA damaging agents, oncogenes, and ER stress stimulate the mitochondrial (intrinsic) pathway via activation of BH3-only family of proteins that inhibit pro-survival BCL-2 like proteins. Such inhibition leads to activation of pro-apoptotic BAX and BAK and subsequent disruption of the mitochondrial membrane and the release of cytochrome c and SMAC (second mitochondria-derived activator of caspases). Cytochrome c activates caspase-9 via APAF1 (apoptotic protease-activating factor 1) that activates effector caspases, whereas SMAC blocks the caspase inhibitor protein XIAP (X-linked inhibitor of apoptosis protein).
The mitochondrial (intrinsic) pathway of cell death involves the interactions between three subgroups of the BCL-2 protein family: the pro-survival BCL-2, BH3-only proteins, and pro-apoptotic proteins BAX and BAK on the MOM (Figure 3). BH3-only proteins are induced both at transcriptional and post-translational levels by a variety of cytotoxic stress signals. These proteins achieve their pro-apoptotic effect by neutralizing pro-survival BCL-2 family proteins and activating pro-apoptotic BAX and BAK. Upon stimulation of BH3-only proteins, oligomers are formed from BAK and BAX that lead to MOMP. Apoptogenic factors are then released into the cytosol, mainly cytochrome c that binds apoptotic protease activating factor 1 (APAF1) and stimulates the activation of caspase-9, which in turn cleaves and activates effector caspases-3, 6 and 7 causing apoptosis. MOMP is also associated with the release of a second mitochondria-derived activator of caspase (Smac/DIABLO) that impedes X-linked inhibitor of apoptosis protein (XIAP)-mediated inhibition of caspase. The effector caspases also cleave nuclear laminin that is involved in chromatin condensation, as well as the inhibitor of caspase-activated DNase (ICAD) that causes DNA fragmentation. Moreover, caspases cleave cytoskeletal proteins such as actin, gelsolin, and Rho kinase 1.
3.2.2 Role of ceramide in mitochondrial apoptosis
The essential role of ceramide during apoptosis has been extensively studied. For the first time, Obeid et al. demonstrated that exogenous ceramide treatment induces apoptosis in leukemic cells (72). Twenty years later, many other authors have corroborated these initial findings and demonstrated that levels of ceramide are increased in a variety of cell types in response to a myriad of pro-apoptotic insults. On the contrary, inhibition of sphingolipid enzymes involved in the synthesis of ceramide by pharmacological agents (e.g. fumonisin B1 or myriocin) or small interfering RNA (siRNA) has been shown to impair cell death (73).
Ceramide was shown to control several intracellular effectors that regulate apoptosis (Figure 4). Particularly, it activates serine/threonine protein phosphatases (PP1 and PP2A). Dephosphorylation of the product of the retinoblastoma susceptibility gene pRB by PP1 is involved in G1 phase cell cycle arrest in response to ceramide (74,75). Moreover, PP1 is also involved in dephosphorylation of SR proteins, which are known to generate pro-apoptotic splice variants of genes encoding BCL-XL and caspase-9. On the other hand, PP2A mediates dephosphorylation of BCL-2 that alters mitochondrial membrane potential and induces apoptosis (76). Furthermore, PP2A dephosphorylates the Ser184 residue of BAX causing its activation and translocation in response to exogenously added C2-ceramide (77). Ceramide is also implicated in cathepsin D activation, which activates the pro-apoptotic BID protein (74). PKC-ζ is also activated by ceramide, and its activation mediates AKT inhibition and JNK activation to induce apoptosis (78).
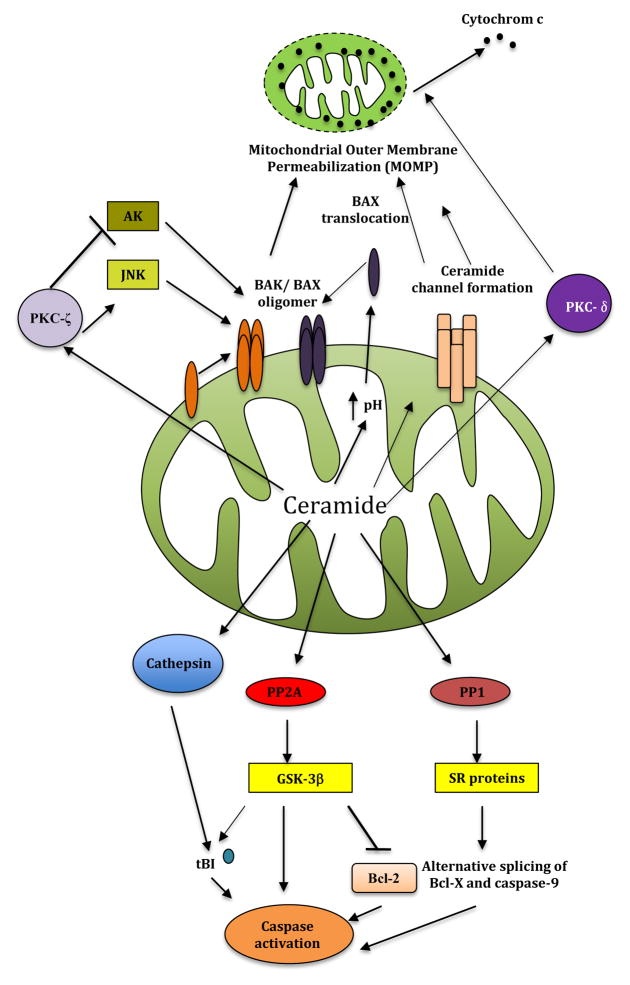
Figure 4. Regulation of mitochondrial apoptosis by ceramide.
Ceramide can regulate apoptosis via multiple mechanisms. Elevated ceramide levels controls MOMP, and subsequent cytochrome c release by BAX oligomerization and ceramide channel formation. In addition, ceramide-induced activation of PKC-ζ induces BAK oligomer formation and MOMP via AKT inhibition and JNK activation, whereas activation of PKC-δ leads to cytochrome c release from mitochondria to cytosol. On the other hand, dephosphorylation of SR protein or GSK-3β by ceramide-activated protein phosphatase PP1 and PP2A, respectively, results in activation of effectors caspases. Ceramide acts as activation of the protease cathepsin D, which in turn cleaves BID to its active form resulting in caspase activation.
Mitochondrial ceramide levels are increased in response to pro-apoptotic stimuli, such as IR, CD95/Fas and TNF-α, inducing key events in the apoptotic cascade, such as BAX translocation to the mitochondria (79) and MOMP (80). MOMP is crucial for apoptotic signaling, through the formation of ceramide channels (will be discussed in more details later). In human breast cancer cells, C6-ceramide was shown to induce MOMP (81). Some studies have reported that ceramide alone is not sufficient to induce MOMP; however, it was shown to act synergistically with BAX to induce apoptosis through MOMP (80). Interestingly, ceramide-enriched membrane platforms in the MOM was required for BAX oligomerization and pore formation, which in turn induced MOMP (82). In a histiocytic lymphoma cell line, ceramide was shown to transiently increase intracellular pH with a subsequent change in BAX conformation and apoptosis (83). In addition, ceramide was also able to induce BAX translocation to mitochondria through activation of p38 MAPK or downregulation of AKT (84,85). Ceramide has been reported to mediate MOMP by activating glycogen synthase kinase 3β (GSK3β) (86) through PP2A and through activation of cathepsin D (87,88), which then activates caspase-2, caspase-8 and BID cleavage to form tBID (89,90). In prostate cancer cells, ceramide was shown to induce activation and mitochondrial translocation of protein kinase C δ (PKCδ) with subsequent cytochrome c release and caspase-9 activation (91).
Furthermore, ceramide induced BAX-mediated apoptosis in various cancer types, including acute myeloid leukemia, glioblastoma, breast, colon, and prostate cancers (79,92,93). Thus, it is clear that the interplay between ceramide and mitochondria is an important feature in cancer biology. Moreover, ceramide could be an important therapeutic that could abrogate cancer cell resistance to mitochondrial apoptotic pathway.
It has been proposed that the formation of ceramide channels in the MOM is responsible for the ceramide-induced cytochrome c release (94). Siskind et al. showed that short-chain (C2-ceramide) and long-chain ceramides (C16-ceramide) form large and stable channels in membranes. On the other hand, the biologically inactive dihydroceramides do not form such channels (94). A structural model was proposed to describe the formation of channels by ceramide. This model is based on the formation of hydrogen bonds between ceramide molecules forming channels and the pore size is determined by the number of ceramide molecules making up these pores. Moreover, according to the model, the overall concentration of ceramide in the membrane is directly related to the membrane conductance and the individual conductance of the channels. Moreover, slight changes in ceramide concentration have dramatic effects on the size of the channels formed (94). The formation of ceramide channels and stability of existing ones are dependent on the steady-state concentration of ceramide in the membrane, determined by the rates of ceramide synthesis, breakdown, and transport. For instance, the interaction between ER membranes and the MOM allows ceramide exchange between the ER and the MOM (71). On the other hand, other sphingolipids, such as dihydroceramide and sphingosine interfere with channel formation (95,96). Interestingly, BCL-XL, an anti-apoptotic BCL-2 family protein, disassemble ceramide channels formed in phospholipid membranes (97). Moreover, cytochrome c release by ceramide is also inhibited by the addition of BCL-XL (97). It was also proposed that BAX and ceramide act in a synergistic manner to form channels in isolated mitochondria (80). However, the evidence for formation of ceramide channels in vivo is still lacking, due to the complicated landscape of cross-talk between sphingolipids and the BCL-2 family of proteins in the induction of MOMP. Recent data suggest a feed-forward mechanism by which the pro-apoptotic BAK regulates the activity of CerS in the context of cell death, leading to elevation in ceramides within the cell, inhibition of the anti-apoptotic BCL-2 proteins and subsequent ceramide channel formation (98).
3.3 Sphingosine and mitochondrial cell death
In addition to ceramide, sphingosine has been suggested to play a role in the control of apoptosis independently from ceramide in multiple cell lines (99). Igarashi and coworkers first reported that treatment of human neutrophils with TNF-α increased endogenous levels of sphingosine as well as ceramide, and induced apoptosis. Furthermore, treatment of neutrophils with exogenous sphingosine, but not ceramide, recapitulated the effects of TNF-α, suggesting that sphingosine generated by the deacylation of ceramide, and not ceramide itself, regulates apoptosis induced by TNF-α in human neutrophils (100). Since then, several studies have reported the involvement of sphingosine in the regulation of apoptosis induced by numerous stimuli in different cell lines (99). For instance, cell death induced by sphingosine in HL-60 cells was shown to be caspase-dependent and been triggered earlier in the apoptotic pathway than that induced by ceramide (101). Furthermore, pre-treatment with FB1 had no effect on sphingosine-mediated cell death in HL-60 and Jurkat T cells, which demonstrate that the induction of apoptosis by sphingosine in not due to its conversion to ceramide (101,102). In a cancer model, the treatment of MCF-7 cells with doxorubicin triggered the accumulation of sphingosine along with the generation of ceramide, which preceded cytochrome c release and caspase-7 activation. Exogenous sphingosine mimicked the effects of doxorubicin in those cells, and BCL-XL overexpression blocked both doxorubicin and sphingosine-induced apoptosis but did not affect endogenous sphingosine levels, suggesting that sphingosine effects might occur upstream of mitochondria (103). Interestingly, Cuvillier at al. observed that cell death induced by exogenous sphingosine was inhibited by the alkaline ceramidase inhibitor D-MAPP and by FB1, which may suggest that sphingosine needs to be acetylated to ceramide first and then deacylated to sphingosine later in order to trigger cell death in certain cell lines (103).
There are several mechanisms that underlie sphingosine-induced cell death including: inhibition of Akt/Protein kinase B, inhibition of ERK, BID cleavage, cytochrome c release, activation of effector caspase 3,7 and PARP cleavage. Moreover, sphingosine has been shown to decrease BCL-2 and BCL-XL expression and induce BAX cleavage and its association with BCL-2 (99). Sphingosine induced cell death can also be mediated through regulation of PKC. Sphingosine has initially been shown to inhibit PKC (104), an important prosurvival signal in the cell. Later on, a truncated form of proapoptotic PKCδ formed by caspase-3 mediated cleavage of full length PKCδ has been described as a sphingosine-dependent protein kinase (SDK1) (105). SDK1 has several substrates including the 14-3-3 proteins, which act as chaperones for members of the -2 family proteins, such as BAX. Nevertheless, in a cellular event where PKC is stimulated, sphingosine can still induce apoptosis suggesting the presence of PKC-independent pathway of cell death (106).
3.4 SK1/2, S1P and degradation products in mitochondrial cell death
There are multiple lines of evidence that link S1P to cell growth and cell survival (107). However, overexpression of SK2 (but not overexpression of SK1) and concomitant S1P elevation has been shown to sensitize cells to chemotherapeutics and caused apoptosis in multiple cell lines induced by different cell stressors, which was preceded by cytochrome c release from the mitochondria and caspase-3 activation. The pro-apoptotic effects of SK2 were demonstrated to be independent of the cell surface receptors of S1P, which suggested an intracellular role of SK2 in the regulation of apoptosis (108). Interestingly, the sequence analysis of human and mouse SK2 unveiled that N-terminal fragment of SK2, but not SK1, contains a BH3 domain that allows its binding to the pro-apoptotic BCL-XL and revokes its anti-apoptotic effect (108).
SK1 effect on apoptosis has also been examined in relation to BAX and BAK. SK1 knockdown by siRNA in breast cancer MCF-7 cells induced BAX oligomerization, caspase activation and cytochrome c release (109). Recently, in purified mitochondria, it was reported that C8-BID-induced cytochrome c release was dependent on two nSMases (SMPD3 and 4) (110). Consequent studies revealed that S1P induces BAK while hexadecenal induces BAX to trigger cytochrome c release (110). In MEFs, SK2, but not SK1, has been shown to be required for the sensitivity to TNFα and actinomycin D. Moreover, adding SK2 and minimal amount of C8-BID to WT heavy membrane fraction triggered cytochrome c release (110). These findings suggest that individual BCL-2 proteins could collaborate with different parts of the sphingolipid metabolic pathway to induce cell death.
3.5 Ceramide and mitophagy
3.5.1 Mitochondrial autophagy (Mitophagy)
Autophagy is a process by which many cytoplasmic elements, including ER, mitochondria, and peroxisomes are engulfed by autophagosomes. Recently, it was suggested that the autophagic process could be specific to a certain organelle that is initiated by specific organelle’s proteins. For example, Uth1p, is a MOM protein that initiates mitochondrial autophagy. The term mitophagy has been used to denote the autophagic degradation of mitochondria. Dysfunctional and aged mitochondria need to be removed from cells by autophagosomes as they can release pro-apoptotic proteins and generate reactive oxygen species (ROS). It has been reported that the mitochondria are engulfed by autophagosomes upon loss of their membrane potential or during permeability transition. It was suggested that accumulation of ROS in the damaged mitochondria act as a signal to LC3 positive autophagosomes (111).
Autophagy proceeds with formation of phagophores, cup-shaped structures that enlarge and engulf organelles and other cytosolic contents. Phagophores then mature and form autophagosome, which then fuse with lysosomes to form autophagolysosomes (112). The autophagy genes (Atg) were first discovered in yeast that facilitated our understanding of the mechanism underlying autophagy. Most of Atg genes are conserved in humans and have been shown to play roles in the autophagic process (113,114). LC3, the mammalian homologue of Atg8, plays an important role in the formation of mature autophagosome and has three forms LC3A, LC3B, and LC3C. LC3-I is the cytosolic form with a C-terminal glycine (Gly120) (115). LC3 is cleaved during autophagy by a protease, Atg4, and then activated by Atg7 to be transferred to E2-like ubiquitin ligase, Atg3. These events cause binding of C-terminal Gly120 residue to phosphatidylethanolamine (PE) to form LC3-II, which is then recruited to autophagosomal membranes leading to the formation of mature autophagosome (116).
Depending on the mechanism of mitochondrial sequestration into autophagosomes, three types of mitophagy have been proposed (117). The type 1 mitophagy occurs during deprivation of nutrients and involves mitochondrial fission. The process starts with phagophores formation. The phagophores then enlarge and surround the mitochondria to form what is called mitophagosomes, which once is acidified activates lysosomal enzymes. The type 2 mitophagy occurs during mitochondrial photo damage. In this type of mitophagy, the LC3 positive structures aggregate and surround depolarized mitochondria and degrade mitochondria into autophagosome. The type 3 mitophagy (micromitophagy) is associated with the formation of mitochondria-derived vesicles that move to the lysosomes. These vesicles are released following oxidative stress in the mitochondria. This type of mitophagy does not involve LC3, but it involves Pink 1 and Parkin proteins and is not associated with mitochondrial fission or depolarization. Micromitophagy allows selective removal of oxidized and damaged components without total mitochondrial degradation.
3.5.2 Role of ceramide in mitophagy
Depending on the context, mitophagy could play a role in either cell death or survival. Mitophagy could induce cell survival when it degrades mitochondria before the activation of caspase-dependent apoptosis. Conversely, excessive or sustained mitophagy could be associated with lysosomal enzymes release, such as cathepsins, that stimulate caspase-mediated apoptosis. Mitophagy could also function as an apoptosis-independent programmed cell death mechanism. Such a mechanism has been shown to be dependent on CerS1 and its product C18-ceramide. It was previously shown that CerS1/C18-ceramide induces lethal mitophagy in head and neck squamous cell carcinomas, which occurred independently of BAK, BAX, or caspases (118). The authors reported that either CerS1 overexpression or treatment with C18-pyridium-ceramide was associated with generation of LC3-II that interacts with ceramide on the mitochondrial membranes. Such binding triggers mitochondrial targeting by autophagosome. Moreover, the authors reported that CerS6-generated C16-ceramide did not induce mitophagy; however, mitophagy was induced by treatment with C16-pyridinium ceramide that accumulates in the mitochondria. From these findings it was suggested that the sub-cellular localization of ceramides, but not their acyl chain length, is the main determinant of their biological effect in mitophagy (118).
Ceramide has been shown to have higher affinity to LC3-II than LC3-I that was believed to involve LC3 central hydrophobic domain, which is structurally similar to ceramide transporter, CERT. The Ile35 and Phe52 residues within the hydrophobic domain are required for ceramide binding (118,119), suggesting that ceramide plays a novel role as a receptor at the mitochondrial membrane to bind LC3-II-containig autophagosomes.
3.6 Sphingolipids, altered apoptosis and disease
3.6.1 Sphingomyelin hydrolysis and ceramide generation in Alzheimer’s disease (AD)
Alzheimer’s disease (AD) is the most common chronic and progressive neurodegenerative disorder, which is characterized by extracellular deposits of amyloid β-peptides (Aβ) and intracellular deposits of hyperphosphorylated tau protein. Although the molecular mechanism underlying AD is poorly understood, an increasing body of literature has linked nSMase2-mediated SM hydrolysis to the pathogenesis of AD (120). Ayasolla et al. first reported that Aβ25–35 peptide increased the activation of nSMase activity and subsequent ceramide accumulation in rat primary astrocytes and neurons, which correlated with increased cell death (121). Furthermore, treatment of neuronal cells with Aβ1–42 or an elevated ratio of Aβ1–42/Aβ1–40 upregulated nSMase activity and elevated ceramide levels. Aβ1–42 was also shown to directly bind and activate nSMase in vitro (122).
3.6.2 Role of ceramide in Ischemia/Reperfusion (IR)
Accumulation of ceramide has been reported to mediate apoptosis in the injured tissue in several in vivo IR models, such as rat brain, mouse kidney and liver, and rat heart (123). Several lines of evidence have linked the production of cytokines in the IR tissue (e.g. TNF-α and IL-1β) to ceramide accumulation and tissue apoptosis (123). For instance, in a rat left coronary artery occlusion IR model, ceramide levels increased up to 50% in reperfused myocardium which was associated with decreased ceramidase activity (124). On the other hand, increased ceramide levels that was attributed to SM hydrolysis has been observed in brain (for a review see (120)).
Altered ceramide metabolism within the mitochondria has been demonstrated to regulate mitochondrial dysfunction, which plays an essential role in tissue damage after brain IR (125). In mild IR ceramide accumulation is mainly caused by increased de novo biosynthesis. The elevated ceramide levels were observed in mitochondria (for C18, C18:1, and C16 ceramides) and ER (for all of ceramide species) suggesting the activation of different CerS in these two compartments. Among different CerS, CerS6 activity has been shown to be increased in the mitochondria in response to IR (64). Increased de novo ceramide biosynthesis has been shown in response to hypoxia, glucose deprivation, or TNF-α in cultured brain cells. Similarly, in neuronal precursor NT-2 cells, a massive increase in C14 and C16-ceramide and a small increase in C18, C18:1, and C20-ceramide were induced by exposure to hypoxia/reoxygenation that were primarily mediated by aSMase and CerS5 (for more details see (125).
3.6.3 Ceramide in diabetes mellitus
Diabetes mellitus is one of the top causes of death in the world. Multiple studies have suggested that excessive apoptosis of pancreatic β-cells contributes to the development of type 1 and type 2 diabetes (126). Over the past twenty years, several lines of evidence have implicated ceramide in cytokine-induced apoptosis of β-cells (127). For instance, in vitro models showed that the treatment of insulin-producing MIN6 cells and RINm5F cells with TNF-α and IL-1β, respectively resulted in accumulation of endogenous ceramide and subsequent apoptosis. Moreover, exogenous ceramide recapitulated the effects of the cytokines (128,129). Results from an in vivo model demonstrated that caspase-8 is necessary for cytokine-induced diabetes Interestingly, β-cell–specific caspase-8 knockout mice, RIPcre+Casp8fl/fl, were protected from apoptosis triggered by IL-1β/ FasL as well as ceramide treatment, whereas wild-type mice, RIPcre+Casp8+/+, were not (130).
Similarly, ceramide has also been suggested to mediate free fatty acid (FFA)-induced cell death in β-cells. In the diabetic rat model, palmitate has been reported to induce β-cell apoptosis, and a similar effect has also been reported in healthy rats and human β-cells. In the Zucker diabetic fatty (ZDF) rat model, de novo synthesized ceramide has been shown to mediate β-cell apoptosis induced by FFA (126,131). FFA-induced β-cell apoptosis involves the intrinsic apoptotic pathway, as increased BAX and decreased BCL-2 expressions have been reported in FFA-treated β-cells (132).
3.6.4 Sphingolipids in cancer
Sphingolipids, and more particularly ceramide and S1P, have been extensively implicated in multiple aspects of carcinogenesis, cancer progression, and drug resistance. Due to its essential role in the regulation of physiological as well as pharmacologically-induced apoptosis, ceramide has been considered a tumor-suppressor lipid. In contrary, S1P is considered a tumor-promoting lipid. As a result, multiple strategies for cancer treatment target sphingolipid metabolism (133).
The generation of ceramide via aSMase was shown to be crucial in the modulation of cancer progression and its inhibition was linked to resistance to a variety of anti-cancer drugs (134). Likewise, upregulated GCS in MCF-7 breast cancer cells induced resistance to pro-apoptotic stimuli such as TNF-α and doxorubicin (135). Similarly, CerS expression has been demonstrated to regulate the sensitivity to cancer chemotherapeutic agents and radiation. Overexpression of CerS1in HEK-293 cells was demonstrated to sensitize those cells to several anti-cancer drugs such as cisplatin, carboplatin, doxorubicin and vincristine, whereas CerS5 increased sensitivy to doxorubicin and vincristine (136). CerS6 expression was found to be decreased in the TRAIL-resistant SW620 colon cancer cells as compared with the TRAIL-sensitive SW480 cells. CerS6 expression was demonstrated to affect the translocation of caspase-3 to the nucleus and subsequent nuclear permeability (137).
Ceramide synthases have been also related to the pathobiology of cancer (8,138). Decreased CerS1 expression, and its product C18-ceramide, was found in head and neck squamous cell carcinomas (HNSCC) as compared to normal tissue, which correlated with lymphovascular invasion and nodal metastasis (139). Subsequent work demonstrated that overexpression of CerS1 and elevated C18-ceramide generation decreased cell growth in HNSCC via inhibition of the activity of telomerase and increased sensitivity to gemcitabine/doxorubicin treatment by upregulating caspase-3 and -9 activation (9). On the other hand, silencing of CerS6 and decreased C16-ceramide triggered ER stress through activation of the ATF6-CHOP branch of the unfolded protein response (140).
The role of SK and its product S1P have been widely demonstrated to promote tumor growth and progression (for a review (107,138,141,142)). Increased levels of SK/S1P were found in a multitude of cancer and tumor tissues such as colon, lung, brain, stomach, kidney and breast cancer (143). SK1 has been shown to play an essential role in the malignant transformation and tumor formation of immortalized 3T3 fibroblasts (138,144) and have been demonstrated to correlate with drug resistance and poor prognosis (107). For instance, over-expression of SK1 in MCF-7 cells increased drug resistance and formed more and larger tumors when injected into mice as compared to vector control (145). Similarly, elevated SK1 conferred resistance to UV-induced and adriamycin-induced glioma cells apoptosis, whereas knockdown of SK1 decreased resistance to apoptotic insults. SK1 inhibitor (SK1-I) mimicked the siRNA SK1 results (146). Treatment with SK inhibitors has been observed to sensitize pancreatic, prostate and pancreatic cells to chemotherapeutic drugs (143), opening a new avenue for cancer therapy by targeting the SK1/S1P axis. SK2 inhibitor ABC294640 showed to increase death in cancer cells. However, these results have to be carefully taken due to the lack of specificity.
The role of S1P in angiogenesis has been extensevely studied. Multiple growth factors as well as cytokines increase SK1 activity by inducing its phosphorylation, followed by the elevation of S1P levels, which binds S1P receptors (S1PR1-S1PR5) leading to activation of G protein-coupled receptors (GPCRs) and subsequent angiogenesis (4). A recent study by Salama et al. showed that SK1 mediates angiogenesis and invasion of VHL deficient clear cell renal cell carcinoma cells (147). On the other hand, recent work have linked SK2-S1P, and not SK1, in Ezrin-Radixin-Moesin (ERM) phosphorylation and cell invasion induced by EGF in HeLa cells, defining a new mechanism of cell invasion and novel role of SK2 (148).
4. Summary and future directions
In this review, we have attempted to summarize the current knowledge about sphingolipids and their role in mitochondrial functions. Ceramide has been linked to the control of important cellular functions, such as apoptosis, that is tightly regulated by cellular components (e.g. cytochrome c release), which reside within mitochondria. Understanding the mechanism by which ceramides control mitochondrial permeability has become of crucial importance as altered regulation of cell death results in human diseases such as cancer or diabetes. Although enormous advances have been made in the last decades, there are many questions that remain unanswered with regard to ceramide biosynthetic pathways. One of them is to pinpoint the specific subcompartments where ceramide is elevated and its specific mechanism of action. On the other hand, the relationship between ceramide metabolism, ceramide channel formation and BCL-2 family members is not completely understood. As stated above, the pro-apoptotic protein BAK has been indentified to regulate CerS activity during apoptosis; however, further studies will be necessary in order to elucidate which CerS is regulated by BAK and what is the mechanism involved.
Ceramide has been reported to regulate mitophagy by acting as a mitochondrial receptor for LC3-II-containing autophagosomes and recruiting autophagolysosomes to damaged mitochondria. The role of ceramide in mitophagy is of great significance as the binding of ceramide to LC3-II has been linked to HNSCC cell death and tumor suppression and unveiled the direct binding of ceramide to proteins. Furthermore, ceramide-mediated mitophagy has been shown to be downstream of DRP1 (DNLM1)- mediated mitochondrial fission (118). Mitochondrial fission induced by DRP1 (DNML1) has been described to have a protective role. Thus, future studies may shed light on the the activation mechanism of DNML1 in ceramide-mediated lethal mitophagy.
The fact that enzymatic activity involved in ceramide generation has been found within mitochondria has demonstrated the existence of a specific pool of ceramide that may regulate processes such as mitochondrial apoptosis and/or mitophagy. Furthermore, the transport of ER-generated ceramide to mitochondria has been reported in an in vitro model, suggesting that exchange of ceramide between these two compartments may also contribute to increased ceramide in mitochondria during apoptosis. The isolation and characterization of MAMs further supports the later mechanism. Although there is no supporting data so far, another possibility is that some unknown protein transporters (similar to CERT) may deliver ER-generated ceramide to mitochondria.
As mention above, ceramide has the capacity to promote cell death by inducing MOMP (by the formation of ceramide channels and the interaction of ceramide with the BCL-2 family proteins) or by activating proteins involved in MOMP. Thus, the manipulation of sphingolipid metabolism by targeting the enzymes involved in the synthesis and degradation of mitochondrial ceramide (and therefore its metabolites) by using specific inhibitors, alone or in combination with other drugs, offers a new and promising therapeutic approach that needs further investigation.
Acknowledgments
This work was supported by Veterans Affairs Merit Award, NIH Grants GM097741 and PO1CA097132 (LMO).
The abbreviations used are
- CerS
ceramide synthase
- (dh)Cer
(dihydro)ceramide
- SMase
sphingomyelinase
- SM
sphingomyelin
- (dh)Sph
(dihydro)sphingosine
- S1P
sphingosine-1-phosphate
- C1P
ceramide-1-phosphate
- MOMP
mitochondrial outer membrane permeabilization
- MAM
mitochondrial-associated membrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goni FM, Alonso A. Biophysics of sphingolipids I. Membrane properties of sphingosine, ceramides and other simple sphingolipids. Biochim Biophys Acta. 2006;1758:1902–1921. doi: 10.1016/j.bbamem.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 3.Ando J, Kinoshita M, Cui J, Yamakoshi H, Dodo K, Fujita K, Murata M, Sodeoka M. Sphingomyelin distribution in lipid rafts of artificial monolayer membranes visualized by Raman microscopy. Proc Natl Acad Sci U S A. 2015;112:4558–4563. doi: 10.1073/pnas.1418088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 5.Canals D, Jenkins RW, Roddy P, Hernandez-Corbacho MJ, Obeid LM, Hannun YA. Differential effects of ceramide and sphingosine 1-phosphate on ERM phosphorylation: probing sphingolipid signaling at the outer plasma membrane. J Biol Chem. 2010;285:32476–32485. doi: 10.1074/jbc.M110.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Montero I, Rodriguez N, Cribier S, Pohl A, Velez M, Devaux PF. Rapid transbilayer movement of ceramides in phospholipid vesicles and in human erythrocytes. J Biol Chem. 2005;280:25811–25819. doi: 10.1074/jbc.M412052200. [DOI] [PubMed] [Google Scholar]
- 7.Frickenhaus S, Heinrich R. Kinetic and thermodynamic aspects of lipid translocation in biological membranes. Biophys J. 1999;76:1293–1309. doi: 10.1016/S0006-3495(99)77292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J. 2012;441:789–802. doi: 10.1042/BJ20111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senkal CE, Ponnusamy S, Rossi MJ, Bialewski J, Sinha D, Jiang JC, Jazwinski SM, Hannun YA, Ogretmen B. Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol Cancer Ther. 2007;6:712–722. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- 10.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. Molecular cloning and characterization of a human mitochondrial ceramidase. J Biol Chem. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 12.Wu BX, Rajagopalan V, Roddy PL, Clarke CJ, Hannun YA. Identification and characterization of murine mitochondria-associated neutral sphingomyelinase (MA-nSMase), the mammalian sphingomyelin phosphodiesterase 5. J Biol Chem. 2010;285:17993–18002. doi: 10.1074/jbc.M110.102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birbes H, El Bawab S, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- 14.Hornemann T, Richard S, Rutti MF, Wei Y, von Eckardstein A. Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J Biol Chem. 2006;281:37275–37281. doi: 10.1074/jbc.M608066200. [DOI] [PubMed] [Google Scholar]
- 15.Hornemann T, Penno A, Rutti MF, Ernst D, Kivrak-Pfiffner F, Rohrer L, von Eckardstein A. The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J Biol Chem. 2009;284:26322–26330. doi: 10.1074/jbc.M109.023192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat Genet. 2001;27:309–312. doi: 10.1038/85879. [DOI] [PubMed] [Google Scholar]
- 17.Murphy SM, Ernst D, Wei Y, Laura M, Liu YT, Polke J, Blake J, Winer J, Houlden H, Hornemann T, Reilly MM. Hereditary sensory and autonomic neuropathy type 1 (HSANI) caused by a novel mutation in SPTLC2. Neurology. 2013;80:2106–2111. doi: 10.1212/WNL.0b013e318295d789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beeler T, Bacikova D, Gable K, Hopkins L, Johnson C, Slife H, Dunn T. The Saccharomyces cerevisiae TSC10/YBR265w gene encoding 3-ketosphinganine reductase is identified in a screen for temperature-sensitive suppressors of the Ca2+-sensitive csg2Delta mutant. J Biol Chem. 1998;273:30688–30694. doi: 10.1074/jbc.273.46.30688. [DOI] [PubMed] [Google Scholar]
- 19.Kihara A, Igarashi Y. FVT-1 is a mammalian 3-ketodihydrosphingosine reductase with an active site that faces the cytosolic side of the endoplasmic reticulum membrane. J Biol Chem. 2004;279:49243–49250. doi: 10.1074/jbc.M405915200. [DOI] [PubMed] [Google Scholar]
- 20.Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Usta J, Szulc Z, Bielawska A, Obeid LM, Hannun YA. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J Biol Chem. 2002;277:12960–12969. doi: 10.1074/jbc.M110699200. [DOI] [PubMed] [Google Scholar]
- 21.Savile CK, Fabrias G, Buist PH. Dihydroceramide delta(4) desaturase initiates substrate oxidation at C-4. J Am Chem Soc. 2001;123:4382–4385. doi: 10.1021/ja010088w. [DOI] [PubMed] [Google Scholar]
- 22.Beauchamp E, Goenaga D, Le Bloc’h J, Catheline D, Legrand P, Rioux V. Myristic acid increases the activity of dihydroceramide Delta4-desaturase 1 through its N-terminal myristoylation. Biochimie. 2007;89:1553–1561. doi: 10.1016/j.biochi.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Kudo N, Kumagai K, Tomishige N, Yamaji T, Wakatsuki S, Nishijima M, Hanada K, Kato R. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc Natl Acad Sci U S A. 2008;105:488–493. doi: 10.1073/pnas.0709191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villani M, Subathra M, Im YB, Choi Y, Signorelli P, Del Poeta M, Luberto C. Sphingomyelin synthases regulate production of diacylglycerol at the Golgi. Biochem J. 2008;414:31–41. doi: 10.1042/BJ20071240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vacaru AM, Tafesse FG, Ternes P, Kondylis V, Hermansson M, Brouwers JF, Somerharju P, Rabouille C, Holthuis JC. Sphingomyelin synthase-related protein SMSr controls ceramide homeostasis in the ER. J Cell Biol. 2009;185:1013–1027. doi: 10.1083/jcb.200903152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jennemann R, Sandhoff R, Langbein L, Kaden S, Rothermel U, Gallala H, Sandhoff K, Wiegandt H, Grone HJ. Integrity and barrier function of the epidermis critically depend on glucosylceramide synthesis. J Biol Chem. 2007;282:3083–3094. doi: 10.1074/jbc.M610304200. [DOI] [PubMed] [Google Scholar]
- 28.Liu YY, Hill RA, Li YT. Ceramide glycosylation catalyzed by glucosylceramide synthase and cancer drug resistance. Adv Cancer Res. 2013;117:59–89. doi: 10.1016/B978-0-12-394274-6.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabourdy F, Kedjouar B, Sorli SC, Colie S, Milhas D, Salma Y, Levade T. Functions of sphingolipid metabolism in mammals--lessons from genetic defects. Biochim Biophys Acta. 2008;1781:145–183. doi: 10.1016/j.bbalip.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Lamour NF, Stahelin RV, Wijesinghe DS, Maceyka M, Wang E, Allegood JC, Merrill AH, Jr, Cho W, Chalfant CE. Ceramide kinase uses ceramide provided by ceramide transport protein: localization to organelles of eicosanoid synthesis. J Lipid Res. 2007;48:1293–1304. doi: 10.1194/jlr.M700083-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Mitsutake S, Yokose U, Kato M, Matsuoka I, Yoo JM, Kim TJ, Yoo HS, Fujimoto K, Ando Y, Sugiura M, Kohama T, Igarashi Y. The generation and behavioral analysis of ceramide kinase-null mice, indicating a function in cerebellar Purkinje cells. Biochem Biophys Res Commun. 2007;363:519–524. doi: 10.1016/j.bbrc.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Graf C, Zemann B, Rovina P, Urtz N, Schanzer A, Reuschel R, Mechtcheriakova D, Muller M, Fischer E, Reichel C, Huber S, Dawson J, Meingassner JG, Billich A, Niwa S, Badegruber R, Van Veldhoven PP, Kinzel B, Baumruker T, Bornancin F. Neutropenia with impaired immune response to Streptococcus pneumoniae in ceramide kinase-deficient mice. J Immunol. 2008;180:3457–3466. doi: 10.4049/jimmunol.180.5.3457. [DOI] [PubMed] [Google Scholar]
- 33.Presa N, Gomez-Larrauri A, Rivera IG, Ordonez M, Trueba M, Gomez-Munoz A. Regulation of cell migration and inflammation by ceramide 1-phosphate. Biochim Biophys Acta. 2016;1861:402–409. doi: 10.1016/j.bbalip.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Cheng Y, Hansen GH, Niels-Christiansen LL, Koentgen F, Ohlsson L, Nilsson A, Duan RD. Crucial role of alkaline sphingomyelinase in sphingomyelin digestion: a study on enzyme knockout mice. J Lipid Res. 2011;52:771–781. doi: 10.1194/jlr.M012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhein C, Reichel M, Muhle C, Rotter A, Schwab SG, Kornhuber J. Secretion of Acid Sphingomyelinase is Affected by its Polymorphic Signal Peptide. Cell Physiol Biochem. 2014;34:1385–1401. doi: 10.1159/000366345. [DOI] [PubMed] [Google Scholar]
- 36.Jenkins RW, Canals D, Hannun YA. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal. 2009;21:836–846. doi: 10.1016/j.cellsig.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tani M, Hannun YA. Analysis of membrane topology of neutral sphingomyelinase 2. FEBS Lett. 2007;581:1323–1328. doi: 10.1016/j.febslet.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke CJ, Truong TG, Hannun YA. Role for neutral sphingomyelinase-2 in tumor necrosis factor alpha-stimulated expression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecule-1 (ICAM) in lung epithelial cells: p38 MAPK is an upstream regulator of nSMase2. J Biol Chem. 2007;282:1384–1396. doi: 10.1074/jbc.M609216200. [DOI] [PubMed] [Google Scholar]
- 39.Park JH, Schuchman EH. Acid ceramidase and human disease. Biochim Biophys Acta. 2006;1758:2133–2138. doi: 10.1016/j.bbamem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 40.Ito M, Okino N, Tani M. New insight into the structure, reaction mechanism, and biological functions of neutral ceramidase. Biochim Biophys Acta. 2014;1841:682–691. doi: 10.1016/j.bbalip.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Ohlsson L, Palmberg C, Duan RD, Olsson M, Bergman T, Nilsson A. Purification and characterization of human intestinal neutral ceramidase. Biochimie. 2007;89:950–960. doi: 10.1016/j.biochi.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Snider AJ, Wu BX, Jenkins RW, Sticca JA, Kawamori T, Hannun YA, Obeid LM. Loss of neutral ceramidase increases inflammation in a mouse model of inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 2012;99:124–130. doi: 10.1016/j.prostaglandins.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun W, Xu R, Hu W, Jin J, Crellin HA, Bielawski J, Szulc ZM, Thiers BH, Obeid LM, Mao C. Upregulation of the human alkaline ceramidase 1 and acid ceramidase mediates calcium-induced differentiation of epidermal keratinocytes. J Invest Dermatol. 2008;128:389–397. doi: 10.1038/sj.jid.5701025. [DOI] [PubMed] [Google Scholar]
- 44.Mao C, Xu R, Szulc ZM, Bielawski J, Becker KP, Bielawska A, Galadari SH, Hu W, Obeid LM. Cloning and characterization of a mouse endoplasmic reticulum alkaline ceramidase: an enzyme that preferentially regulates metabolism of very long chain ceramides. J Biol Chem. 2003;278:31184–31191. doi: 10.1074/jbc.M303875200. [DOI] [PubMed] [Google Scholar]
- 45.Sun W, Hu W, Xu R, Jin J, Szulc ZM, Zhang G, Galadari SH, Obeid LM, Mao C. Alkaline ceramidase 2 regulates beta1 integrin maturation and cell adhesion. FASEB J. 2009;23:656–666. doi: 10.1096/fj.08-115634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu W, Xu R, Sun W, Szulc ZM, Bielawski J, Obeid LM, Mao C. Alkaline ceramidase 3 (ACER3) hydrolyzes unsaturated long-chain ceramides, and its down-regulation inhibits both cell proliferation and apoptosis. J Biol Chem. 2010;285:7964–7976. doi: 10.1074/jbc.M109.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stahelin RV, Hwang JH, Kim JH, Park ZY, Johnson KR, Obeid LM, Cho W. The mechanism of membrane targeting of human sphingosine kinase 1. J Biol Chem. 2005;280:43030–43038. doi: 10.1074/jbc.M507574200. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Min X, Xiao SH, Johnstone S, Romanow W, Meininger D, Xu H, Liu J, Dai J, An S, Thibault S, Walker N. Molecular basis of sphingosine kinase 1 substrate recognition and catalysis. Structure. 2013;21:798–809. doi: 10.1016/j.str.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 49.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH, Jr, Milstien S, Spiegel S. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 50.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandala SM, Thornton R, Galve-Roperh I, Poulton S, Peterson C, Olivera A, Bergstrom J, Kurtz MB, Spiegel S. Molecular cloning and characterization of a lipid phosphohydrolase that degrades sphingosine-1-phosphate and induces cell death. Proc Natl Acad Sci U S A. 2000;97:7859–7864. doi: 10.1073/pnas.120146897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogawa C, Kihara A, Gokoh M, Igarashi Y. Identification and characterization of a novel human sphingosine-1-phosphate phosphohydrolase, hSPP2. J Biol Chem. 2003;278:1268–1272. doi: 10.1074/jbc.M209514200. [DOI] [PubMed] [Google Scholar]
- 53.Mao C, Obeid LM. Yeast sphingosine-1-phosphate phosphatases: assay, expression, deletion, purification, and cellular localization by GFP tagging. Methods Enzymol. 2000;311:223–232. doi: 10.1016/s0076-6879(00)11085-7. [DOI] [PubMed] [Google Scholar]
- 54.Johnson KR, Johnson KY, Becker KP, Bielawski J, Mao C, Obeid LM. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. J Biol Chem. 2003;278:34541–34547. doi: 10.1074/jbc.M301741200. [DOI] [PubMed] [Google Scholar]
- 55.Long J, Darroch P, Wan KF, Kong KC, Ktistakis N, Pyne NJ, Pyne S. Regulation of cell survival by lipid phosphate phosphatases involves the modulation of intracellular phosphatidic acid and sphingosine 1-phosphate pools. Biochem J. 2005;391:25–32. doi: 10.1042/BJ20050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Stunff H, Giussani P, Maceyka M, Lepine S, Milstien S, Spiegel S. Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J Biol Chem. 2007;282:34372–34380. doi: 10.1074/jbc.M703329200. [DOI] [PubMed] [Google Scholar]
- 57.Fyrst H, Saba JD. Sphingosine-1-phosphate lyase in development and disease: sphingolipid metabolism takes flight. Biochim Biophys Acta. 2008;1781:448–458. doi: 10.1016/j.bbalip.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aguilar A, Saba JD. Truth and consequences of sphingosine-1-phosphate lyase. Adv Biol Regul. 2012;52:17–30. doi: 10.1016/j.advenzreg.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allende ML, Bektas M, Lee BG, Bonifacino E, Kang J, Tuymetova G, Chen W, Saba JD, Proia RL. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem. 2011;286:7348–7358. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Degagne E, Pandurangan A, Bandhuvula P, Kumar A, Eltanawy A, Zhang M, Yoshinaga Y, Nefedov M, de Jong PJ, Fong LG, Young SG, Bittman R, Ahmedi Y, Saba JD. Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J Clin Invest. 2014;124:5368–5384. doi: 10.1172/JCI74188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, Fuks Z, Shaham S, Kolesnick R. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322:110–115. doi: 10.1126/science.1158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park JW, Park WJ, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta. 2014;1841:671–681. doi: 10.1016/j.bbalip.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 63.Hernandez-Corbacho MJ, Canals D, Adada MM, Liu M, Senkal CE, Yi JK, Mao C, Luberto C, Hannun YA, Obeid LM. Tumor Necrosis Factor-alpha (TNFalpha)-induced Ceramide Generation via Ceramide Synthases Regulates Loss of Focal Adhesion Kinase (FAK) and Programmed Cell Death. J Biol Chem. 2015;290:25356–25373. doi: 10.1074/jbc.M115.658658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu J, Novgorodov SA, Chudakova D, Zhu H, Bielawska A, Bielawski J, Obeid LM, Kindy MS, Gudz TI. JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J Biol Chem. 2007;282:25940–25949. doi: 10.1074/jbc.M701812200. [DOI] [PubMed] [Google Scholar]
- 65.Mesicek J, Lee H, Feldman T, Jiang X, Skobeleva A, Berdyshev EV, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010;22:1300–1307. doi: 10.1016/j.cellsig.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Novgorodov SA, Chudakova DA, Wheeler BW, Bielawski J, Kindy MS, Obeid LM, Gudz TI. Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+ loading capacity and promotes apoptosis. J Biol Chem. 2011;286:4644–4658. doi: 10.1074/jbc.M110.164392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yabu T, Shimuzu A, Yamashita M. A novel mitochondrial sphingomyelinase in zebrafish cells. J Biol Chem. 2009;284:20349–20363. doi: 10.1074/jbc.M109.004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kitagaki H, Cowart LA, Matmati N, Vaena de Avalos S, Novgorodov SA, Zeidan YH, Bielawski J, Obeid LM, Hannun YA. Isc1 regulates sphingolipid metabolism in yeast mitochondria. Biochim Biophys Acta. 2007;1768:2849–2861. doi: 10.1016/j.bbamem.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rajagopalan V, Canals D, Luberto C, Snider J, Voelkel-Johnson C, Obeid LM, Hannun YA. Critical determinants of mitochondria-associated neutral sphingomyelinase (MA-nSMase) for mitochondrial localization. Biochim Biophys Acta. 2015;1850:628–639. doi: 10.1016/j.bbagen.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Novgorodov SA, Wu BX, Gudz TI, Bielawski J, Ovchinnikova TV, Hannun YA, Obeid LM. Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J Biol Chem. 2011;286:25352–25362. doi: 10.1074/jbc.M110.214866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stiban J, Caputo L, Colombini M. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. J Lipid Res. 2008;49:625–634. doi: 10.1194/jlr.M700480-JLR200. [DOI] [PubMed] [Google Scholar]
- 72.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 73.Mullen TD, Obeid LM. Ceramide and apoptosis: exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med Chem. 2012;12:340–363. doi: 10.2174/187152012800228661. [DOI] [PubMed] [Google Scholar]
- 74.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 75.Dbaibo GS, Pushkareva MY, Jayadev S, Schwarz JK, Horowitz JM, Obeid LM, Hannun YA. Retinoblastoma gene product as a downstream target for a ceramide-dependent pathway of growth arrest. Proc Natl Acad Sci U S A. 1995;92:1347–1351. doi: 10.1073/pnas.92.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruvolo PP, Deng X, Ito T, Carr BK, May WS. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J Biol Chem. 1999;274:20296–20300. doi: 10.1074/jbc.274.29.20296. [DOI] [PubMed] [Google Scholar]
- 77.Xin M, Deng X. Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. J Biol Chem. 2006;281:18859–18867. doi: 10.1074/jbc.M512543200. [DOI] [PubMed] [Google Scholar]
- 78.Young MM, Kester M, Wang HG. Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res. 2013;54:5–19. doi: 10.1194/jlr.R031278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ganesan V, Perera MN, Colombini D, Datskovskiy D, Chadha K, Colombini M. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis. 2010;15:553–562. doi: 10.1007/s10495-009-0449-0. [DOI] [PubMed] [Google Scholar]
- 81.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee H, Rotolo JA, Mesicek J, Penate-Medina T, Rimner A, Liao WC, Yin X, Ragupathi G, Ehleiter D, Gulbins E, Zhai D, Reed JC, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS One. 2011;6:e19783. doi: 10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Belaud-Rotureau MA, Leducq N, Macouillard Poulletier de Gannes F, Diolez P, Lacoste L, Lacombe F, Bernard P, Belloc F. Early transitory rise in intracellular pH leads to Bax conformation change during ceramide-induced apoptosis. Apoptosis. 2000;5:551–560. doi: 10.1023/a:1009693630664. [DOI] [PubMed] [Google Scholar]
- 84.Kong JY, Klassen SS, Rabkin SW. Ceramide activates a mitochondrial p38 mitogen-activated protein kinase: a potential mechanism for loss of mitochondrial transmembrane potential and apoptosis. Mol Cell Biochem. 2005;278:39–51. doi: 10.1007/s11010-005-1979-6. [DOI] [PubMed] [Google Scholar]
- 85.Kim HJ, Oh JE, Kim SW, Chun YJ, Kim MY. Ceramide induces p38 MAPK-dependent apoptosis and Bax translocation via inhibition of Akt in HL-60 cells. Cancer Lett. 2008;260:88–95. doi: 10.1016/j.canlet.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 86.Lin CF, Chen CL, Chiang CW, Jan MS, Huang WC, Lin YS. GSK-3beta acts downstream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J Cell Sci. 2007;120:2935–2943. doi: 10.1242/jcs.03473. [DOI] [PubMed] [Google Scholar]
- 87.De Stefanis D, Reffo P, Bonelli G, Baccino FM, Sala G, Ghidoni R, Codogno P, Isidoro C. Increase in ceramide level alters the lysosomal targeting of cathepsin D prior to onset of apoptosis in HT-29 colon cancer cells. Biol Chem. 2002;383:989–999. doi: 10.1515/BC.2002.106. [DOI] [PubMed] [Google Scholar]
- 88.Heinrich M, Neumeyer J, Jakob M, Hallas C, Tchikov V, Winoto-Morbach S, Wickel M, Schneider-Brachert W, Trauzold A, Hethke A, Schutze S. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11:550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 89.Darios F, Lambeng N, Troadec JD, Michel PP, Ruberg M. Ceramide increases mitochondrial free calcium levels via caspase 8 and Bid: role in initiation of cell death. J Neurochem. 2003;84:643–654. doi: 10.1046/j.1471-4159.2003.01590.x. [DOI] [PubMed] [Google Scholar]
- 90.Yuan H, Williams SD, Adachi S, Oltersdorf T, Gottlieb RA. Cytochrome c dissociation and release from mitochondria by truncated Bid and ceramide. Mitochondrion. 2003;2:237–244. doi: 10.1016/S1567-7249(02)00106-X. [DOI] [PubMed] [Google Scholar]
- 91.Sumitomo M, Ohba M, Asakuma J, Asano T, Kuroki T, Asano T, Hayakawa M. Protein kinase Cdelta amplifies ceramide formation via mitochondrial signaling in prostate cancer cells. J Clin Invest. 2002;109:827–836. doi: 10.1172/JCI14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sawada M, Nakashima S, Banno Y, Yamakawa H, Takenaka K, Shinoda J, Nishimura Y, Sakai N, Nozawa Y. Influence of Bax or Bcl-2 overexpression on the ceramide-dependent apoptotic pathway in glioma cells. Oncogene. 2000;19:3508–3520. doi: 10.1038/sj.onc.1203699. [DOI] [PubMed] [Google Scholar]
- 93.von Haefen C, Wieder T, Gillissen B, Starck L, Graupner V, Dorken B, Daniel PT. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 2002;21:4009–4019. doi: 10.1038/sj.onc.1205497. [DOI] [PubMed] [Google Scholar]
- 94.Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem. 2000;275:38640–38644. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stiban J, Fistere D, Colombini M. Dihydroceramide hinders ceramide channel formation: Implications on apoptosis. Apoptosis. 2006;11:773–780. doi: 10.1007/s10495-006-5882-8. [DOI] [PubMed] [Google Scholar]
- 96.Elrick MJ, Fluss S, Colombini M. Sphingosine, a product of ceramide hydrolysis, influences the formation of ceramide channels. Biophys J. 2006;91:1749–1756. doi: 10.1529/biophysj.106.088443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siskind LJ, Feinstein L, Yu T, Davis JS, Jones D, Choi J, Zuckerman JE, Tan W, Hill RB, Hardwick JM, Colombini M. Anti-apoptotic Bcl-2 Family Proteins Disassemble Ceramide Channels. J Biol Chem. 2008;283:6622–6630. doi: 10.1074/jbc.M706115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beverly LJ, Howell LA, Hernandez-Corbacho M, Casson L, Chipuk JE, Siskind LJ. BAK activation is necessary and sufficient to drive ceramide synthase-dependent ceramide accumulation following inhibition of BCL2-like proteins. Biochem J. 2013;452:111–119. doi: 10.1042/BJ20130147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cuvillier O. Sphingosine in apoptosis signaling. Biochim Biophys Acta. 2002;1585:153–162. doi: 10.1016/s1388-1981(02)00336-0. [DOI] [PubMed] [Google Scholar]
- 100.Ohta H, Yatomi Y, Sweeney EA, Hakomori S, Igarashi Y. A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-alpha in human neutrophils. FEBS Lett. 1994;355:267–270. doi: 10.1016/0014-5793(94)01218-0. [DOI] [PubMed] [Google Scholar]
- 101.Sweeney EA, Inokuchi J, Igarashi Y. Inhibition of sphingolipid induced apoptosis by caspase inhibitors indicates that sphingosine acts in an earlier part of the apoptotic pathway than ceramide. FEBS Lett. 1998;425:61–65. doi: 10.1016/s0014-5793(98)00198-7. [DOI] [PubMed] [Google Scholar]
- 102.Cuvillier O, Edsall L, Spiegel S. Involvement of sphingosine in mitochondria-dependent Fas-induced apoptosis of type II Jurkat T cells. J Biol Chem. 2000;275:15691–15700. doi: 10.1074/jbc.M000280200. [DOI] [PubMed] [Google Scholar]
- 103.Cuvillier O, Nava VE, Murthy SK, Edsall LC, Levade T, Milstien S, Spiegel S. Sphingosine generation, cytochrome c release, and activation of caspase-7 in doxorubicin-induced apoptosis of MCF7 breast adenocarcinoma cells. Cell Death Differ. 2001;8:162–171. doi: 10.1038/sj.cdd.4400793. [DOI] [PubMed] [Google Scholar]
- 104.Hannun YA, Bell RM. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987;235:670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- 105.Hamaguchi A, Suzuki E, Murayama K, Fujimura T, Hikita T, Iwabuchi K, Handa K, Withers DA, Masters SC, Fu H, Hakomori S. Sphingosine-dependent protein kinase-1, directed to 14-3-3, is identified as the kinase domain of protein kinase C delta. J Biol Chem. 2003;278:41557–41565. doi: 10.1074/jbc.M305294200. [DOI] [PubMed] [Google Scholar]
- 106.Hung WC, Chang HC, Chuang LY. Activation of caspase-3-like proteases in apoptosis induced by sphingosine and other long-chain bases in Hep3B hepatoma cells. Biochem J. 1999;338( Pt 1):161–166. [PMC free article] [PubMed] [Google Scholar]
- 107.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278:40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- 109.Taha TA, Kitatani K, El-Alwani M, Bielawski J, Hannun YA, Obeid LM. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. FASEB J. 2006;20:482–484. doi: 10.1096/fj.05-4412fje. [DOI] [PubMed] [Google Scholar]
- 110.Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 112.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thumm M. Structure and function of the yeast vacuole and its role in autophagy. Microsc Res Tech. 2000;51:563–572. doi: 10.1002/1097-0029(20001215)51:6<563::AID-JEMT6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 114.Hemelaar J, Lelyveld VS, Kessler BM, Ploegh HL. A single protease, Apg4B, is specific for the autophagy-related ubiquitin-like proteins GATE-16, MAP1-LC3, GABARAP, and Apg8L. J Biol Chem. 2003;278:51841–51850. doi: 10.1074/jbc.M308762200. [DOI] [PubMed] [Google Scholar]
- 115.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo j. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tanida I, Ueno T, Kominami E. Human light chain 3/MAP1LC3B is cleaved at its carboxyl-terminal Met121 to expose Gly120 for lipidation and targeting to autophagosomal membranes. J Biol Chem. 2004;279:47704–47710. doi: 10.1074/jbc.M407016200. [DOI] [PubMed] [Google Scholar]
- 117.Lemasters JJ. Variants of mitochondrial autophagy: Types 1 and 2 mitophagy and micromitophagy (Type 3) Redox Biol. 2014;2:749–754. doi: 10.1016/j.redox.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, Ramshesh VK, Peterson YK, Lemasters JJ, Szulc ZM, Bielawski J, Ogretmen B. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8:831–838. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang W, Ogretmen B. Ceramide stress in survival versus lethal autophagy paradox: ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Autophagy. 2013;9:258–259. doi: 10.4161/auto.22739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bienias K, Fiedorowicz A, Sadowska A, Prokopiuk S, Car H. Regulation of sphingomyelin metabolism. Pharmacol Rep. 2016;68:570–581. doi: 10.1016/j.pharep.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 121.Ayasolla K, Khan M, Singh AK, Singh I. Inflammatory mediator and beta-amyloid (25–35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radic Biol Med. 2004;37:325–338. doi: 10.1016/j.freeradbiomed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 122.Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, Tschape JA, De Strooper B, Muller U, Shen J, Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- 123.Beresewicz A, Dobrzyn A, Gorski J. Accumulation of specific ceramides in ischemic/reperfused rat heart; effect of ischemic preconditioning. J Physiol Pharmacol. 2002;53:371–382. [PubMed] [Google Scholar]
- 124.Zhang DX, Fryer RM, Hsu AK, Zou AP, Gross GJ, Campbell WB, Li PL. Production and metabolism of ceramide in normal and ischemic-reperfused myocardium of rats. Basic Res Cardiol. 2001;96:267–274. doi: 10.1007/s003950170057. [DOI] [PubMed] [Google Scholar]
- 125.Novgorodov SA, Gudz TI. Ceramide and mitochondria in ischemic brain injury. Int J Biochem Mol Biol. 2011;2:347–361. [PMC free article] [PubMed] [Google Scholar]
- 126.Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis. 2013;12:98. doi: 10.1186/1476-511X-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lang F, Ullrich S, Gulbins E. Ceramide formation as a target in beta-cell survival and function. Expert Opin Ther Targets. 2011;15:1061–1071. doi: 10.1517/14728222.2011.588209. [DOI] [PubMed] [Google Scholar]
- 128.Welsh N. Interleukin-1 beta-induced ceramide and diacylglycerol generation may lead to activation of the c-Jun NH2-terminal kinase and the transcription factor ATF2 in the insulin-producing cell line RINm5F. J Biol Chem. 1996;271:8307–8312. doi: 10.1074/jbc.271.14.8307. [DOI] [PubMed] [Google Scholar]
- 129.Ishizuka H, Watanabe M, Kubota T, Matsuzaki SW, Otani Y, Kitajima M. Antitumor activity of murine monoclonal antibody NCC-ST-421 on human cancer cells by inducing apoptosis. Anticancer Res. 1998;18:2513–2518. [PubMed] [Google Scholar]
- 130.Liadis N, Salmena L, Kwan E, Tajmir P, Schroer SA, Radziszewska A, Li X, Sheu L, Eweida M, Xu S, Gaisano HY, Hakem R, Woo M. Distinct in vivo roles of caspase-8 in beta-cells in physiological and diabetes models. Diabetes. 2007;56:2302–2311. doi: 10.2337/db06-1771. [DOI] [PubMed] [Google Scholar]
- 131.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51:1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 133.Oskouian B, Saba JD. Cancer treatment strategies targeting sphingolipid metabolism. Adv Exp Med Biol. 2010;688:185–205. doi: 10.1007/978-1-4419-6741-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Adada M, Luberto C, Canals D. Inhibitors of the sphingomyelin cycle: Sphingomyelin synthases and sphingomyelinases. Chem Phys Lipids. 2016;197:45–59. doi: 10.1016/j.chemphyslip.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 135.Liu YY, Yu JY, Yin D, Patwardhan GA, Gupta V, Hirabayashi Y, Holleran WM, Giuliano AE, Jazwinski SM, Gouaze-Andersson V, Consoli DP, Cabot MC. A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB J. 2008;22:2541–2551. doi: 10.1096/fj.07-092981. [DOI] [PubMed] [Google Scholar]
- 136.Min J, Mesika A, Sivaguru M, Van Veldhoven PP, Alexander H, Futerman AH, Alexander S. (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol Cancer Res. 2007;5:801–812. doi: 10.1158/1541-7786.MCR-07-0100. [DOI] [PubMed] [Google Scholar]
- 137.White-Gilbertson S, Mullen T, Senkal C, Lu P, Ogretmen B, Obeid L, Voelkel-Johnson C. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene. 2009;28:1132–1141. doi: 10.1038/onc.2008.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ponnusamy S, Meyers-Needham M, Senkal CE, Saddoughi SA, Sentelle D, Selvam SP, Salas A, Ogretmen B. Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010;6:1603–1624. doi: 10.2217/fon.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koybasi S, Senkal CE, Sundararaj K, Spassieva S, Bielawski J, Osta W, Day TA, Jiang JC, Jazwinski SM, Hannun YA, Obeid LM, Ogretmen B. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem. 2004;279:44311–44319. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- 140.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24:296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heffernan-Stroud LA, Obeid LM. Sphingosine kinase 1 in cancer. Adv Cancer Res. 2013;117:201–235. doi: 10.1016/B978-0-12-394274-6.00007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12:688–702. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 144.Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, Hannun YA, Obeid LM. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res. 2002;281:115–127. doi: 10.1006/excr.2002.5658. [DOI] [PubMed] [Google Scholar]
- 146.Guan H, Song L, Cai J, Huang Y, Wu J, Yuan J, Li J, Li M. Sphingosine kinase 1 regulates the Akt/FOXO3a/Bim pathway and contributes to apoptosis resistance in glioma cells. PLoS One. 2011;6:e19946. doi: 10.1371/journal.pone.0019946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Salama MF, Carroll B, Adada M, Pulkoski-Gross M, Hannun YA, Obeid LM. A novel role of sphingosine kinase-1 in the invasion and angiogenesis of VHL mutant clear cell renal cell carcinoma. FASEB J. 2015;29:2803–2813. doi: 10.1096/fj.15-270413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Adada MM, Canals D, Jeong N, Kelkar AD, Hernandez-Corbacho M, Pulkoski-Gross MJ, Donaldson JC, Hannun YA, Obeid LM. Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion. FASEB J. 2015;29:4654–4669. doi: 10.1096/fj.15-274340. [DOI] [PMC free article] [PubMed] [Google Scholar]