Abstract
Yap functions as a transcriptional regulator by acting together with sequence-specific DNA binding factors and transcription cofactors to mediate cell proliferation in developing epithelial tissues and tumors. An upstream kinase cascade controls nuclear localization and function in response to partially identified exogenous signals, including cell-to-cell contact. Nevertheless, its role in CNS development is poorly understood. In order to investigate Yap function in developing CNS, we characterized the cellular outcomes after selective Yap gene ablation in developing ocular tissues. When Yap was lost, presumptive retinal pigment epithelium acquired anatomical and molecular characteristics resembling those of the retinal epithelium rather than of RPE, including loss of pigmentation, pseudostratified epithelial morphology and ectopic induction of markers for retinal progenitor cells, like Chx10, and neurons, like β-Tubulin III. In addition, developing retina showed signs of progressive degeneration, including laminar folding, thinning and cell loss, which resulted from multiple defects in cell proliferation and survival, and in junction integrity. Furthermore, Yap-deficient retinal progenitors displayed decreased S-phase cells and altered cell cycle progression. Altogether, our studies not only illustrate the canonical function of Yap in promoting the proliferation of progenitors, but also shed new light on its evolutionarily conserved, instructive role in regional specification, maintenance of junctional integrity and precise regulation of cell proliferation during neuroepithelial development.
Keywords: Yap, retina, RPE, transdifferentiation, cell cycle control, polarity, cell proliferation
INTRODUCTION
Hippo-Yap signaling pathway is emerging as a key player in cellular behaviors such as stem cell renewal, tissue regeneration, tumor homeostasis and organ size control. Core components of the pathway include two ser/thr kinase cascades, Mst [mammalian Ste 20-like] 1/2 kinases, Lats [large tumor suppressor] 1/2 kinases, nuclear targets, Yap [Yes-associated protein] and Taz (Transcriptional coactivator with PDZ-binding motif). When Hippo upstream kinases are activated by molecules that have not yet been identified, phosphorylated forms of Yap and/or Taz (pYap/pTaz) are inhibited from translocating into the nucleus, where they would bind with sequence-specific DNA binding factors like Tead family transcription factors (Tead1–4). Known transcription target genes of Yap/Taz-Tead include genes involved in the inhibition of apoptosis and genes essential for the control of cell proliferation (Bai et al., 2012; Camargo et al., 2007; Dong et al., 2007; Hsu et al., 2014; Huang et al., 2005; Zhang et al., 2011a; Zhang et al., 2008; Zhao et al., 2008). The mechanisms that initiate the upstream signal at the extracellular or plasma membrane level are not well understood, but cell to cell contact mediated by tight and adherens junctions has been proposed as one regulator of the pathway (Kim et al., 2011; Schlegelmilch et al., 2011; Varelas et al., 2010). Recent work has indicated multi-faceted cross-interactions with other signaling cascades mediated by Wnt, BMP, Notch and Akt (Alarcon et al., 2009; Barry et al., 2013; Ferrigno et al., 2002; Morgan et al., 2013). Although the numbers of transcription target genes and interacting regulatory proteins are accumulating, the mechanism by which Yap regulates cell cycle progression and re-entry (during organ development) remains elusive. Abnormal regulation of Hippo-Yap pathway has been implicated in various disease conditions. Most notably, either activation of Yap/Taz (nuclear accumulation) or mutations of MST1/2 and LATS1/2 occur in various tumors, including lung and liver (Lau et al., 2014; Xu et al., 2009). Importantly, mutations in TEAD and YAP have been implicated in ocular diseases. For example, a missense mutation in TEAD1 is linked to Sveinsson’s chorioretinal atrophy (SCRA), an autosomal dominant chorioretinal degenerative disease; mutated TEAD1 loses its ability to bind with Yap/Taz, but not with other cofactors, suggesting that inability to activate transcription of target genes may underlie the pathogenesis of SCRA (Kitagawa, 2007). Heterozygous Yap mutations also are linked to coloboma caused by abnormal eye development that leads to defective optic fissure closure (Williamson et al., 2014), highlighting the critical importance of Yap function in early ocular development.
The eye begins to develop around E8.5 from out-pouched optic vesicle (OV) of diencephalon. This then invaginates to form the two-layered optic cup (OC) upon close contact with the surface ectoderm where the lens placode is formed (Chow and Lang, 2001; Heavner and Pevny, 2012). The outer layer of the OC develops into a non-neural, pigmented sheet called retinal pigment epithelium (RPE), which surrounds the entire inner layer of the neural retina (NR). NR development within OC involves proliferation of multi-potent, lineage-limited retinal progenitors that can give rise to seven retinal cell types, orderly production of retinal cells and formation of pseudostratified epithelium comprising three nuclear and two plexiform layers(Cepko et al., 1996; Marquardt et al., 2001; Turner and Cepko, 1987; Young, 1985). Ocular progenitor cells in the OV are bi-potent progenitor cells which can adopt characteristics of either NR or RPE depending on their interaction with extraocular tissues (Fuhrmann et al., 2014): FGF signals from surface ectoderm promote NR fate by upregulating Chx10 in the inner layer of the OC; Wnt signaling dictates RPE fate. Genetic mutations or surgical manipulations disturbing the balance between these signals and their downstream activities during a restricted developmental window can facilitate adoption of the opposite fate, presumably due to their antagonistic relationship (Rowan et al., 2004; Zhao et al., 2001). The requirement for Yap activity during embryonic eye development was demonstrated in zebrafish, in which knock-down (KD) of Yap causes a smaller than normal eye (Jiang et al., 2009). Yap is expressed in late-stage progenitor cells in the mouse retina, and RNAi mediated functional analysis of Yap in postnatal retinas has identified Yap’s essential role in promoting the proliferation of retinal progenitors and inhibiting their cell cycle exit (Zhang et al., 2012). Yap is also essential in the lens; it maintains lens progenitor cells in lens epithelium, where it is specifically expressed, and promotes epithelial integrity by stabilizing apical polarity/adhesion complexes (Song et al., 2014). Although most previous reports define Hippo-Yap pathway as a general regulator of cell proliferation and survival, other evidence indicates its function in cell-, region- and tissue-fate determination. In developing Drosophila eye, for example, yorkie (yki) and its DNA binding partner scalloped (sd) are critical for the fate of peripodial epithelium (PE) (Zhang et al., 2011b). Mutation of either yki or sd transforms the PE layer into retina, whereas yki gain of function (GOF) suppresses eye formation. Recent studies using zebrafish mutants that result in the lack of RPE and/or coloboma have identified nuclear Yap, along with Taz, as a crucial regulator of RPE genesis (Miesfeld et al., 2015). Although these data suggest that the cellular outcomes determined by the Hippo-Yap pathway can be independently modulated in different cellular and molecular contexts, the specific requirement for Yap in each step of mammalian eye development has not been fully explored.
Here, our findings reveal tissue-dependent and stage-specific involvement of Yap during mouse eye development. First, Yap functions as a critical regulator of RPE regional specification at developing OV and early OC stages, a feature that is conserved between lower and higher vertebrates; when Yap is absent, prospective RPE transdifferentiates into NR, thus inducing retinal duplication. Second, Yap is indispensable for maintaining retinal epithelial polarity by stabilizing Crumbs polarity complex proteins. Third, Yap is essential for survival of retinal cells. Finally, Yap functions at the level of cell cycle progression to promote proliferation of retinal progenitors. Insufficient Yap activity partially inhibits G1 to S-phase transition and significantly lengthens the S- to M phase progression. Importantly, this study reveals the evolutionary conservation of Yap in vertebrates and yki in invertebrates for regulating regional fate choice in two adjacent primordial tissues during development.
RESULTS
Yap is expressed in presumptive RPE, ciliary body/iris, lens and retina during early ocular development
For insight into Yap’s function in developing ocular tissues, we first carried out an immunofluorescence (IF) and immunohistochemical analysis of Yap in sections from embryonic day 10.5 (E10.5) (Fig. 1A – C). We used anti-Yap, which recognizes all forms of Yap. At the OV or invaginating OC stage, Yap was broadly distributed in tissues derived from neuroectoderm (RPE, NR, optic stalk and diencephalon), in surface ectoderm (SE) and periocular mesenchymal cells. Notably, the hinge region between NR and RPE, presumptive ciliary body (CB)/iris primordium and RPE showed moderately intense expression. Nuclear Yap was mainly detected in a portion of the SE and cells in the optic stalk, whereas a cytoplasmic distribution was clear in various regions. Localization at the apical surface of the neuroepithelium (diencephalon and RPE) was also evident. Some retinal and RPE cells expressing Yap also expressed Sox9, a gene essential for RPE and retina development (Masuda et al., 2014; Poche et al., 2008; Zhu et al., 2013). At OC stages, Yap was expressed in most developing ocular tissues of the OC, lens, retina, CB and iris primordia, RPE, cornea, extraocular muscles and periocular mesenchymal cells (Fig. 1D). Yap became selectively expressed in outer neuroblastic layer (ONBL) of the retina, where mainly retinal progenitors and migrating post-mitotic cells reside. In contrast, post-mitotic inner neuroblastic layer (INBL) cells expressed little or no Yap at any of the stages examined (Fig. 1E). When anti-pYap (recognizing Ser112 phosphorylated mouse Yap) was used, the distribution of pYap proteins in ONBL and INBL was not greatly different (Fig. 1F). In order to assess Yap and pYap localization in the retina at both tissue and cell levels, we carried out an IF assay using a confocal microscope. Anti-Yap and pYap staining were evident at the apical and basal surfaces and in the cytoplasm of the epithelial cells (Fig. 1G & H); that this distribution was commonly observed with Yap and pYap antibodies suggests that the Yap-stained pattern largely represents the pYap-stained pattern. However, staining with Yap, but not pYap, antibody also revealed a weak nuclear signal in subsets of retinal cells in ONBL, suggesting nuclear-specific localization of unphosphorylated Yap in retinal progenitors (Fig. 1G1 & H1). All three of these patterns of localization in retinal cells are likely to be genuine because they were partially or completely abolished when Yap gene was ablated by Rx-Cre (Fig. 3D & E compared to Fig. 1G & H). The pattern of embryonic Yap staining was similar to that of P0 in its concentration in the progenitor cells in ONBL (Zhang et al., 2012). However, in P0 retinas Yap staining was enriched in nuclei, whereas it was cytoplasm-dominant in embryonic retinas.
Fig. 1.
Yap is expressed in developing eye tissues. (A – C) Yap is slightly elevated in RPE, OS and border between RPE and retina (bracket), and co-stained with Sox9 in a subset of cells (arrowheads) at the invaginating OV stage (E10.5). (D – F) Yap and pYap antibody staining of WT eyes at both E13.5 (D) and E16.5 (E & F) shows Yap in the retina, RPE (red arrows, inset), extrinsic ocular muscles (arrows), RPE (arrowheads) and lens (Le). Note the absence of Yap in INBL (black line) compared to ONBL (white line). (G–H1) Staining with Yap and pYap antibodies of WT retinal sections on E15.5 shows Yap signal at the apical and basal surfaces (arrows) and in the processes (arrowheads). Magnified images within the blue boxes are shown in G1 and H1 (red line; ONBL, yellow line; INBL). * represents the subset of cells showing nuclear Yap staining. Di; diencephalon, LP; lens placode, NR; neural retina, OS; optic stalk, PM; periocular mesenchyme, RPE; retinal pigment epithelium, SE; surface ectoderm. Hoechst 33258 was used for nuclear counter staining (blue). Scale bars; 50 μm.
Fig. 3.
Phenotypic analysis of Yap-deficient eye tissues. (A & B) Compared to WT, Yap CKO mice show hypopigmented, microphthalmic eyes at P0. Note the protrusion of white epithelial tissues from the eyeballs (arrows). (C) Western blot analysis of E15.5 Yap heterozygotes (Het1 and 2) and CKOs shows the decrease of predicted Yap proteins at 65 kDa in CKO eyes (green, arrow). Numbers represent the relative percentage of the Yap proteins compared to the level in Het1. β-actin was used as a loading control (red, arrowhead). (D & E) An example of anti-Yap staining showing normal retina and Yap-deficient patch (dotted line) in Yap CKO at E16.5. Note that Yap expression is preserved in a small number of cells inside a Yap-deficient patch (*) and maintained in neighboring retina, which serves as an internal control for Yap staining. (F – M1) H&E staining of WT (F, H, J and L) and Yap CKO (G, I, K and M) eyes shows progressive deterioration of the retinal lamination at E12.5 ((F & G), E13.5 (H & I), E16.5 (J & K) and P0 (L & M). Arrows indicate pseudostratified epithelia derived from RPE. Areas of the ectopic pseudostratified epithelia contiguous with RPE are shown in the insets (G1 – M1). Le; Lens. Scale bars; 50 μm.
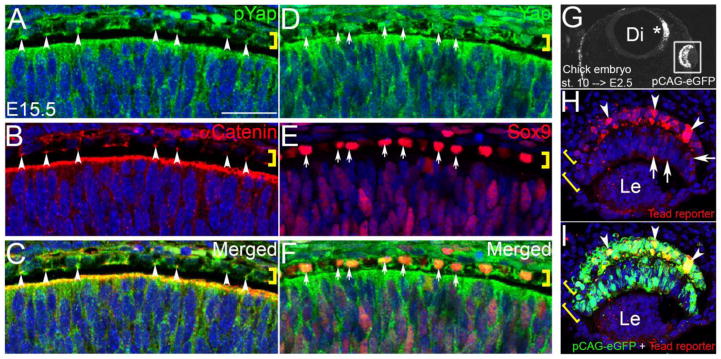
In order to confirm nuclear Yap staining, we next investigated the subcellular localization of Yap proteins in retinal and RPE cells. Consistent with the unique subcellular localization pattern shown in retinal cells by IF, cell fractionation followed by Western blot analysis revealed predominant localization of Yap proteins in non-nuclear, cytoplasmic, membrane and organelle –associated fractions (72%) and less in the nuclear fractions (28%) (Supp. Fig. 1A). pYap proteins were not detected in the nuclear-enriched fraction, which is also consistent with the results of IF staining (Fig. 1H & H1 and Supp. Fig. 1B). In developing RPE at E15.5, Yap proteins were localized in the apical junctions (colocalized with α-Catenin) and nucleus (colocalized with Sox9) (Fig. 2A – F). To determine whether Yap-Tead dependent transcriptional activation plays an important role in early primordial eye tissues, we performed a reporter assay. We co-electroporated the 8xGTIIC-luciferase (Dupont et al., 2011) reporter with pCAG:eGFP to the developing chicken OV at stage 10 and examined anti-luciferase staining at E2.5 (24 hours post-electroporation). Tead-mediated transcription was selectively elevated in RPE compared to the retinal counterpart (Fig. 2G – I), which is consistent with a previous finding in zebrafish (Miesfeld and Link, 2014). Weak reporter activity was also detected in the inner retinal layer. In summary, our studies of expression, cellular localization and electroporation suggest that Yap is involved in diverse cellular processes in the nucleus, cytoplasm and apical surface of the retinal epithelium and RPE, particularly during embryonic development.
Fig. 2.
IF photomicrographs showing that Yap proteins are localized in apical junctions and nucleus of RPE cells at E15.5. (A – C) pYap signal (green) partially overlaps with that of α-catenin (red). Arrowheads indicate the apical colocalization of pYap and α-catenin signals in RPE. (D – F) Nuclear Yap signal (green) overlaps with that of anti-Sox9 (red). Arrows indicate colocalization of Yap and Sox9 signals in the nucleus of RPE cells. Brackets represent RPE layers. (G) Cross section of chicken embryo showing GFP signal at E2.5 after in ovo electroporation of pCAG-eGFP (green) and 8xGIIC-luciferase reporter at stage 10. (H & I) Confocal and merged images of anti-luciferase (red) and eGFP signals (green). Arrowheads indicate RPE cells strongly positive for the Tead reporter assay. Upper and lower brackets represent outer RPE and inner retinal layers, respectively. Hoechst 33258 was used for nuclear counter staining (blue). Di, Diencephalon; Le, lens. Scale bars; 25 um.
Yap is expressed in retinal progenitor cells
In order to determine the identity of the cells expressing Yap, we performed double immunostaining with antibodies marking a subset or all retinal progenitor cells. We first studied whether Yap staining overlapped that of BrdU (5-Bromo-2′deoxyuridine), which identifies dividing cells in S-phase. We examined sections of retinas isolated from E15.5 wild type (WT) mice pretreated with BrdU for 30 min before assay. As shown in Supp. Fig. 2A–D, most BrdU (+) cells overlapped Yap (+) cells. This result is consistent with the interpretation that Yap is expressed in retinal progenitor cells and with a previous report that Yap and BrdU overlap in later-stage progenitor cells at P0 (Zhang et al., 2012). Retinal progenitors are lineage-restricted, multipotent progenitor cells that can give rise to six retinal neuronal types and one glial cell, Muller glia. Retinal progenitors at mid-embryonic stages selectively express genes such as Sox9 (Poche et al., 2008). Therefore, we next determined the extent of overlap with retinal progenitor gene Sox9. At E15.5, nearly all Sox9 (+) cells were Yap (+). For better cellular resolution and quantitation, we carried out antibody staining of dissociated WT E15.5 retinas (Supp. Fig. 2E – J). As with antibody staining of retinal sections, all BrdU (+) cells were Yap (+) (n=3) and nearly all (average 97.6%, n=3) Yap (+) cells were also Sox9 (+), which strengthens the notion that Yap is expressed in the Sox9 (+) progenitors. Together, these results suggest that within the retina, Yap is specifically expressed in most of the progenitor cells.
Conditional ablation of Yap in optic primordia induces progressive defects in retinal and RPE organization
Study of Yap knock out mice (Yap−/−) indicated that Yap has pervasive functions during embryonic development; homozygous mutant embryos displayed developmental arrest at E8.5 and developmental perturbations (Morin-Kensicki et al., 2006). To determine whether Yap is necessary for normal visual system development, we used Rx-Cre to conditionally ablate Yap gene in developing optic progenitor cells starting in the OV stage. The Rx-Cre line initiates Cre expression as early as the invaginating OV stage (Swindell et al., 2006). Therefore, Yap gene can be removed early in primordial retinal tissues, including RPE and NR. Eyes from conditional knock out (CKO; Yapf/f; Rx-Cre, Yap CKO hereafter) mice displayed hypopigmentation and protrusion of retina-like epithelia at P0 (Fig. 3A and B, arrows). Hypopigmentation preferentially occurred in the central retina. Due to the known mosaic Cre expression of the Rx-Cre line (Cho et al., 2012), Yap CKO retinas showed decrease, but not complete absence, of Yap proteins at E16 (Fig. 3C; average 61% reduction in 4 CKO mice compared to WT or heterozygous littermates, p=0.0028). Anti-Yap staining of paraffin sections identified retinal areas devoid of Yap proteins (Fig. 3D & E). As expected from WB analysis and our previous observation of Cre protein mosaicism in the Rx-Cre line (Cho et al., 2012), Yap CKO retinas at E12.5, E16.5 and P0 contained retinal patches in which Yap proteins were deficient mixed with patches in which they were preserved (Supp. Fig. 3A – I). While the sizes varied, Yap-devoid retinal patches were more frequent in central than peripheral retina (Supp. Fig. 3J – L). IF assays were preferentially performed in the Yap-deficient retinal patches with horizontal lengths greater than 50 μm along the apical surface of the retina (Supp. Fig. 3). Histological analysis revealed that the retinas began to be disorganized with an extra epithelial layer at E12.5 (Fig. 3F – G1, n=6). At E13.5 (n=5), parts of the retina were folded and double-layered, and therefore formed thickened, irregular epithelia (Fig. 3H – I1). Retinal folding and thinning were observed primarily in the central retina and intensified as development proceeded (Fig. 3F – M1). The size of the vitreous body was severely reduced. The RPE layer, which is normally a single cell layer of cuboidal epithelium, was transformed into a multi-layered epithelium connected to the retina, which resembled the normal pseudostratified organization of retina (arrows, Fig. 3G, G1, I, I1, K and K1). At P0 (N=6), the overall morphology of the eyes was severely disrupted: the central to peripheral axis of the retina was indistinct, and the remnants of retinal epithelia were thin, folded and tightly associated with the lens (Fig. 3L – M1). Overall, the epithelial layers became progressively disorganized and thinned during embryonic and neonatal development. In summary, histological analysis of Yap CKO eyes revealed that Yap is essential for normal retinal integrity. Furthermore, loss of pigmentation and change of laminar organization from a single cell layer to pseudostratified epithelium suggest transdifferentiation of RPE to retina.
Loss of Yap in presumptive RPE induces transdifferentiation into retina
During early OV formation, regional fates are mainly specified by exogenous signaling cascades followed by activation of the transcription factors that initiate development of RPE, NR, CB and iris (Cho and Cepko, 2006; Fuhrmann et al., 2013). To determine whether the hypopigmented, multi-layered epithelium in Yap-deficient RPE resulted from the failure of regional fate specification, we used IF to study RPE- and retina-specific genes. All RPE cells in WT mice normally express Otx2 throughout development, and a salt-and-pepper distribution in ONBL shows the commitment of progenitor cells toward multiple retinal cell fates (Koike et al., 2007; Nishida et al., 2003; Rhee et al., 2012)(Fig. 4A). The abrupt loss of Otx2 was evident in the RPE of Yap CKO, where a multi-cell layered epithelium was forming. The Otx2 pattern within the pseudostratified epithelium was similar to that of normal retina, but apical concentration of Otx2 (+) cells was lost, suggesting that apicobasal polarity may be lost in the absence of Yap (Fig. 4B). We next tested whether the retina-like epithelium lost Ezrin, a marker for terminally differentiated RPE that is normally localized preferentially in the apical villi of RPE adjacent to outer limiting membrane (OLM), which is marked by Zo1 (Fig. 4C). In CKO, the retina-like epithelium also lost Ezrin staining while apical concentration in normal RPE was disrupted and it was broadly localized in the entire cytoplasm in the border cells seeming undergoing transdifferentiation (Fig. 4D). Ezrin staining was maintained in single-layered RPE, however, either because Yap gene was not deleted or because enough protein remained to elicit RPE phenotypes.
Fig. 4.
RPE transdifferentiates into retina in the absence of Yap. IF analysis of the RPE and retina from WT (A, C, E, G, J and L) and Yap CKO (B, D, F, H, I, K and M) at E16.5. (A) Otx2-positive RPE cells (arrows) show a regularly spaced, single-layered arrangement while retinal photoreceptor cells show an apically-enriched, scattered pattern (arrowheads). RPE and RPE derived tissues are marked by dashed lines. Note that the single-layered Otx2 positive pattern in WT RPE changes into that of retina in Yap-deficient eyes. (C & D) Ezrin (green), a marker for apical villi of RPE, is altered or absent in duplicated multilayered epithelium. Zo1 (red) marks the tight junction of the retinal and RPE epithelia. (E & F) Retinal progenitor gene, Chx10, is ectopically expressed in the multilayered tissue induced by Yap-deficiency. (G, H & I) Expression of RPE fate regulating genes, Sox9 and Mitf, is altered or absent in ectopic, multilayered epithelium. (J & K) Duplicated multilayer tissue expresses ectopic neuronal marker, β-Tubulin III, which is normally absent in WT RPE (green). Pax6 (+) cells (red) are ectopically located at the basal side of the duplicated epithelia. (L & M) Aberrant Pax2 (+) cells (red), an optic stalk marker, are scattered in the ectopic epithelium in Yap CKO (arrows). Hoechst 33258 was used for nuclear counter staining (blue). Le; lens, Scale bars; 50 μm.
We also looked for ectopic retinal gene expression in the multilayered epithelia. Chx10 is specific to progenitor cells during early retinal development and to bipolar cells at a late stage (Fig. 4E). Ectopic Chx10 expression was apparent in the epithelium derived from Yap-deleted RPE of Yap CKO (Fig. 4F). Within the region showing epithelial transformation, the RPE fate-regulating genes Mitf and Sox9 had also specifically disappeared from newly generated multilayers (Fig. 4G, H and I). Although these marker analyses clearly suggest transdifferentiation of RPE into tissues with retinal characteristics, we used β-Tubulin III antibody as an additional test of the neuronal identity of the multilayered epithelia derived from Yap-deficient RPE. At E16.5, INBL cells composed of ganglion and displaced amacrine cells, and post-mitotic migrating cells in ONBL specifically expressed β-Tubulin III (Fig. 4J). In Yap CKO, ectopic upregulation of β-Tubulin III was evident at the basal side of the newly formed, RPE-derived multilayered epithelium (Fig. 4K). To determine whether optic stalk was perturbed, we analyzed Pax2, a gene that is essential and specific for optic stalk (Schwarz et al., 2000). Interestingly, some RPE-derived retinal cells showed ectopic, abnormal induction of Pax2 (compare to normal, Fig. 4L and M). Together, these data indicate that Yap is necessary for RPE fate choice and that, when Yap is ablated, RPE transdifferentiates into retinal tissue.
Yap is essential for the stability of apical polarity complex proteins in the retina
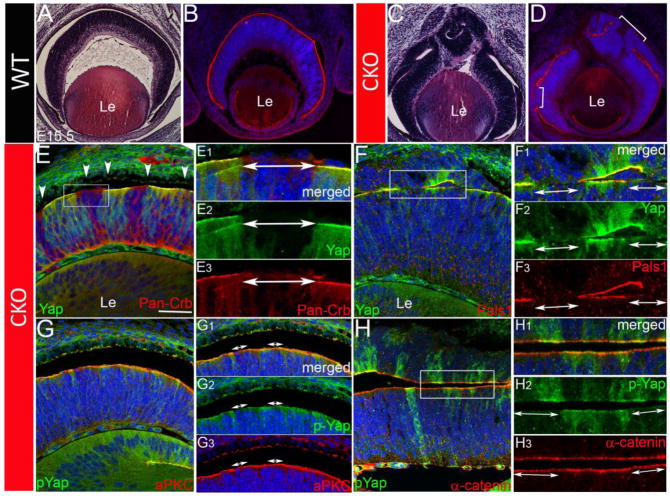
One of the most striking phenotypes of the Yap-deficient retina is progressive laminar disorganization, including folding, irregular thickness of the epithelium and fusion with ectopically formed epithelial tissue from the RPE. This type of disorganization was observed in retinas mutant for known polarity genes, such as Crb1, Crb2, Pals1 and aPKC (Alves et al., 2014; Alves et al., 2013; Koike et al., 2005). In order to test whether abnormal tissue polarity contributed to the disorganization of the retina, we examined apical polarity genes during eye development. Crumbs (Crb) 1 encodes a large transmembrane protein essential during and after development for maintenance of epithelial cell and tissue polarity and for adhesion between retinal cells (Pellissier et al., 2013; van de Pavert et al., 2007). Initial assessment using pan-Crb antibody (recognizing three different but related homologs, Crb1–3) revealed partial discontinuity of the presumptive OLM, which consists of a series of adherens junctions located in the apical surface of the developing retina and at the junctions between photoreceptor cells and Muller glial cells in adult retinas (Kantardzhieva et al., 2006) (Fig. 5A – D). The area showing a break in the presumptive OLM within the Yap CKO retinas correlated with a decrease in Yap staining, indicating that OLM disruption resulted from the absence or reduction of Yap activity (Fig. 5E - E3). Unaffected areas in Yap CKO provided an internal control. We next examined other apical complex proteins, such as Pals1, a Crumbs binding partner, and aPKC, a core component of PAR complex, which are essential for apical junctional stability of developing and mature OLM (Cho et al., 2012). As shown in Fig. 5F – F3, Yap-deficient regions of retinal epithelia concomitantly lost Pals1 proteins, whereas Yap-containing neighboring regions changed little or not at all. However, most, but not all, retinal areas without Yap showed partial disruption of aPKC and no alteration of α-catenin localization at the apical surface at E16.5 (Fig. 5G – H3), suggesting that the stability of the Crumbs polarity complex is especially vulnerable to the change in Yap gene function. Intriguingly, in the absence of both Yap and Crumbs polarity complex proteins, there was partial fusion when the duplicated retina abutted the original retina (Fig. 5F – F3). These results indicate that Yap is essential for the maintenance of protein complexes at the apical surface and thus for laminar integrity of the developing retina.
Fig. 5.
Yap-deficient retinal epithelia exhibit destabilized apical polarity complex proteins. (A – D) Antibody staining of Crb proteins at E15.5 illustrates the abrupt discontinuation of primitive OLM in Yap CKO retina (D, brackets) compared to WT (B). Adjacent H&E stained images are shown in A and C. Analysis of apical polarity/junction markers, Crumbs homologs (E – E3), Pals1 (F – F3), aPKC (G – G3) and α-Catenin (H – H3) from CKO at E15.5 (F & H) and E16.5 (E & G) using IF antibody staining. Insets correspond to the regions shown at higher magnification. Arrowheads in E mark normal pigmented RPE. E1, F1, G1 and H1 represent merged images. E2, F2, G2 and H2 show the absence of Yap or p-Yap. E3, F3, G3 and H3 show polarity proteins. Two-sided arrows indicate the patches of Yap deletion. Hoechst 33258 was used for nuclear counter staining (blue). Le; lens, Scale bars; 50 μm.
Yap is required for survival of retinal cells
Histological analysis of retinas affected by Yap loss suggested progressive hypocellularity in addition to laminar thinning and disruption (Fig. 3). Because Yap is an inhibitor of apoptosis, we analyzed cell death with activated cleaved caspase 3 (CC3) antibody. Comparison of retinal sections at E15.5 and E16.5 between Yap CKO and WT, revealed little or no increase in dying cells, although laminar disorganization had already begun (Fig. 6A – D). At P0 Yap-deficient retinas demonstrated a dramatic, widespread increase in dying cells (Fig. 6E – J). CC3 (+) cells were distributed in both INBL, where post-mitotic neurons reside, and ONBL, where most progenitors and migrating post-mitotic cells are located (Fig. 6H–J, brackets). Although highly variable, the increase in dying cells reached approximately 25-fold in Yap-deficient retinas (WT (n=4), CKO (n=6); Fig. 6K). We also attempted to determine the identity of dying cells using double antibody staining at P0. Antibodies included Pax6 (post-mitotic amacrine and ganglion cells in INBL), Chx10 (retinal progenitors and bipolar precursors), BrdU (S-phase progenitors), rhodopsin (photoreceptor), p27 (post-mitotic cells), and PCNA (progenitors). CC3 (+) cells rarely co-stained with these markers, suggesting that most were not dividing progenitors, post-mitotic amacrine or ganglion cells (Fig. 7). These results indicate that Yap is essential for the survival of retinal cells.
Fig. 6.
Yap-deficient epithelia show progressive increase of cleaved caspase 3 (+) cells. Anti-CC3 (red) staining of WT (A, C and E – G) and CKO (B, D and H – J). (A & B) At E 15.5, few CC3 (+) cells are detected in either retina or RPE-derived multilayered epithelium. Red signals surrounding the retinas originate from choroidal vasculature (arrows). (C & D) At E16.5, more CC3 (+) cells are detected in the epithelial areas where Yap (green) reduction is evident. (E – J) At P0, significant increase of CC3 (+) cells occurs preferentially in the Yap CKO epithelial area without Yap staining (green). (K) Comparison of CC3 (+) cells per section in CKO and WT retinas. Brackets in the insets indicate INBL (left) and ONBL (right). Hoechst 33258 was used for nuclear counter staining (blue). Le; lens, Scale bars; 50 um.
Fig. 7.
Representative images of WT (A, C, E, G, I and K) and Yap-deficient retinas (B, D, F, H, J and L) at P0 showing CC3 (+) cells (red) and additional markers (green): PCNA (A & B), Chx10 (C & D), p27 (E & F), Pax6 (G & H), rhodopsin (I & J) and BrdU (K & L). Arrows indicate rare double-positive cells. Hoechst 33258 was used for nuclear counter staining (blue). Scale bars; 50 um.
Yap regulates retinal progenitor cell proliferation
Yap’s function in maintaining progenitor pools by promoting cell proliferation has been well demonstrated in many tissues, including cardiac and lens (Heallen et al., 2011; Song et al., 2014). In addition, overexpression of Yap enhanced proliferation of progenitors in postnatal retinal explants, and RNA interference decreased proliferation (Zhang et al., 2012). More recently, Yap was shown to regulate cell-type specific S-phase entry by controlling the transcription of genes involved in replication origins (Shen and Stanger, 2015). In order to assay the in vivo proliferative activity of progenitor cells lacking Yap gene, we used BrdU pulse labeling followed by IF. Because of the mosaic pattern of Cre expression associated with Rx-Cre (Cho et al., 2012), we focused on the rare contiguous stretches of retina with clear Yap loss (Fig. 8A – H). Retinal patches lacking Yap showed an approximately 20% decrease in the fraction of S-phase cells [BrdU (+)] among total cells [Hoechst 33258 (+)] compared to WT littermates at E16.5 (Fig. 8I). Intriguingly, although their proliferation index was lower than in WT retina, Yap-deficient retinal progenitor cells retained progenitor markers like Sox9 and Chx10 (Fig. 8E – H and Supp. Fig. 4A – B), suggesting that Yap’s function might be specific to proliferation control, rather than to maintenance of progenitor status. To eliminate the possibility that Yap proteins remained at E16.5 due to the short interval after Yap gene deletion, we examined Chx10 and Sox9 expression in neonatal Yap-deficient retinas. Similar to the results at E16.5, progenitor gene expression such as Chx10 and Sox9 at P0 was relatively unaltered, supporting the idea that Yap is unlikely to regulate progenitor identity (Supp. Fig. 4C – F′). Therefore, our loss of function (LOF) study suggests that Yap activity is necessary for proliferation of retinal progenitors. To assess whether Yap is sufficient for activating the proliferation of mid-embryonic retinal progenitors, we used in vitro electroporation to express GFP-Yap fusion gene (pCAG:eGFP-Yap (Park et al., 2016)) ectopically in E15.5 retinal explant cultures and examined them 2 days later. We determined the fraction of BrdU (+) progenitor cells among all GFP (+) cells after dissociation following pulse labeling for 30 min (Matsuda and Cepko, 2004). We found that the retinas electroporated with GFP-Yap fusion construct produced an approximately 2-fold increase in the BrdU (+) dividing cell fraction compared to GFP electroporated counterparts (from 20% to 37%; Supp. Fig. 5). Therefore, both LOF and GOF studies confirm that Yap is necessary and sufficient to regulate the proliferation of retinal progenitors.
Fig. 8.
Yap regulates retinal progenitor pool and cell cycle progression. (A – H) Yap-deficient retinal cells show reduced proliferation while sustaining expression of the retinal progenitor gene Sox9. In WT E16.5 retina (A – D), Yap (turquoise green) is expressed in ONBL where Sox9 (green) is expressed. (E – H) This progenitor specific pattern is maintained in a Yap-deficient retinal patch (dotted line), whereas BrdU signal is decreased. Arrows represent examples of Yap-deficient cells showing reduced BrdU stain. Brackets indicate the neighboring area with normal Yap (left) and partially decreased Yap due to mix of WT and Yap-deficient cells (right). Sox9 (+) RPE cells are indicated (arrowheads in C and G). (I) Plot showing percentage of BrdU (+) cells in WT and Yap CKO retinal sections. (J & K) Representative images of flow cytometry assay showing G0/G1 (red), G2/M (blue) and S (magenta) from E17.5 WT (J) and CKO (K) retinas. (L) Fractions of cells in G0/G1, G2/M and S-phase of the cell cycle are plotted and compared between WT (green) and CKO (orange). Hoechst 33258 was used for nuclear counter staining (blue). Scale bars; 50 μm.
Yap is essential for cell cycle progression
Yap is thought to influence cell cycle progression by upregulating multiple cell cycle regulators (Lu et al., 2010; Mizuno et al., 2012; Zhao et al., 2008). Consistent with this notion, several different types of cells with Yap knock-down in vitro showed partial arrest at the G1/S cell cycle check point (Mizuno et al., 2012; Muramatsu et al., 2011; Tsujiura et al., 2014). To address whether Yap controls progression of the progenitor cell cycle, we first determined the fraction of cells in each cell cycle phase by flow cytometry. To enhance the specific identification of S-phase cells, we introduced BrdU and sorted the cells with propidium iodide (Fig. 8J & K). Compared with age-matched WT tissues, retinas deficient in Yap at E17.5 (n=3 each) showed a significant decrease in the S-phase fraction (from 21.1% to 10.4%, p=0.0186) (Fig. 8J – L). Intriguingly, close examination of some patches containing Yap-deficient cells showed a lower level of BrdU signal than cells in WT retinas or in neighboring Yap-retaining regions (Fig. 8B & F), which is consistent with alteration of S-phase of the cell cycle in the absence of Yap. Although it is conceivable that suppressed G1 to S transition was the primary cause of the decreased BrdU (+) cell fraction in Yap CKO, altered cell cycle progression might affect other aspects of the cell cycle. We therefore investigated the cell cycle progression of S-phase cells by determining the percentage of M-phase cells that were in S-phase when EdU was pulse-labeled 2hrs before tissue harvest. We reasoned that EdU would label a decreased fraction of M-phase cells if Yap deficiency slowed down the progression of S- and/or G2 phases of the cell cycle. As predicted, 51.3% and 75.4% of the pH3 (+) cells at E16.5 and E17.5 were labeled with EdU in the center and periphery of Yap-deficient retinas, respectively (n=3), whereas 93.2% and 100% were labeled in the center and periphery of WT retinas (n=3) (p<0.006) (Supp. Fig. 6). Together these results suggest that Yap is required for retinal progenitor proliferation and cell cycle progression, but that it is not essential for the maintenance of retinal progenitor identity.
DISCUSSION
Our investigations identify features that establish Yap as a novel type of transcription cofactor in developing ocular tissues. First, Yap carries out essential stage- and region-specific functions in the eye. It is a critical RPE fate-determining factor in two neighboring regions of the developing OV and OC and is required primarily for proliferation and tissue polarity in subsequent retinal development. Therefore, Yap exerts qualitatively different effects on two adjacent tissues, and its functional outcomes depend on the cellular and tissue-level context. Second, Yap, a transcription cofactor, is essential for proliferation of retinal progenitors, but dispensable for maintenance of progenitor status. Yap therefore differs from other classical progenitor-specifying transcription factors like Sox2. In Sox2 mutant, developing retina loses competence to form NR and differentiates into non-neural ciliary epithelia (Matsushima et al., 2011). Expression and localization studies also suggest that Yap fulfills its diverse functions by localizing at three different subcellular sites: nucleus, cytoplasm and possibly also a cellular junction of retinal cells during embryonic development, a feature shared by β-catenin and ZONAB (Balda and Matter, 2009; Varelas et al., 2010).
Yap signaling serves pleiotropic functions in ocular development in diverse species, but its role and signal processing may be further modified in a species-specific manner. In contrast to Yap CKO mice, for example, in which RPE transdifferentiates into epithelium with retinal characteristics, zebrafish Yap (and Yap/Taz double) mutants do not display transdifferentiation while RPE genesis is severely affected (Miesfeld et al., 2015). This may be partly due to species difference because transdifferentiation does not occur in fish (Knight and Raymond, 1995). It occurs only at adult stages in amphibians and is limited to early developmental stages in chick (Coulombre and Coulombre, 1965; Zhao et al., 1997; Zhao et al., 1995). While selective upregulation of Yap in RPE is conserved between zebrafish and mice (Miesfeld and Link, 2014), the earliest of the gross abnormalities observed in Yap CKO eyes is the transdifferentiation of RPE to retina, which is followed by retinal defects, including apical junctional disruption and abnormal proliferation of retinal progenitor cells. Therefore, it is plausible that the retinal defects associated with Yap deletion are primarily caused by the non cell-autonomous effects of RPE transdifferentiation, rather than a primary outcome. However, previous reports that genetically modified mutant animals exhibit RPE to retinal transdifferentiation may not support this interpretation. For example, RPE-specific inactivation of β-catenin, Gas1 or Otx1/2, or activation of FGF2 signaling led to transdifferentiation of the RPE without obvious defects in retinal integrity (Azuma et al., 2005; Fujimura et al., 2009; Lee et al., 2001; Martinez-Morales et al., 2001; Nishihara et al., 2012; Sakaguchi et al., 1997). In contrast with our observations at similar stages like E13.5, in these mutants newly formed epithelia showing retinal characteristics did not fuse with normal underlying retina or induce rosette formation, and severe folding of transdifferentiated tissue was likely caused by a dramatic increase in cell number. Therefore, it is likely that RPE to retinal transdifferentiation by itself does not guarantee retinal laminar disorganization and disruption of the polarity complex proteins. Epithelial junctional defects in the transdifferentiated tissue mediated by Yap deletion, however, may contribute to localized fusion between two opposing epithelia, those of the original and transdifferentiated retinas. In our Yap CKO with Rx-Cre, which directs Cre expression in both tissues, junctional instability along with cell to cell adhesion defects further enhance fusion between original and duplicated retinas. Intriguingly, our results showed that Yap-deficiency-mediated disturbance of Crumbs polarity complex proteins, which are important for cell-to-cell adhesion in the retina, precedes the fusion between transdifferentiated and retina epithelia (Fig. 5E). Destabilized Crumbs polarity complex proteins and cell-cell junctions in Yap CKO are consistent with previous reports, including a comprehensive proteomics study showing significant overrepresentation of proteins associated with cell-cell contacts, among them apical polarity complex proteins, as interacting partners of Yap (Kohli et al., 2014; Varelas et al., 2010). Unstable cell-cell attachment and destabilization of apical polarity complex proteins were independently observed in developing ependymal cells and lens deficient in Yap (Park et al., 2016; Song et al., 2014). We also noted that Yap functions cell-autonomously within retinal epithelia (Fig. 8F), where BrdU staining, for example, was reduced in Yap-deficient patches but not in neighboring regions without Yap loss. Reduction in the staining intensity and number of cells labeled by BrdU closely correlated with Yap loss, suggesting cell-autonomous function of Yap in developing retina. This view is further supported by tight co-regulation of Yap and Crumbs polarity complex proteins in Yap-deficient retinal patches (Fig. 5E–F3).
Intriguingly, mutations of Yap and yki, the Drosophila homolog of Yap, induce transdifferentiation of RPE to retina or PM to retina (Zhang et al., 2011b), suggesting convergent evolution and conservation of the function of Yap and yki in ocular development. This observation also suggests that a mechanism of mutual antagonism between neural and non-neural tissues, in which Yap/yki plays a crucial role in determining non-neural tissue fate, is conserved between invertebrate and vertebrate (Supp. Fig. 7).
Recent findings suggest that Yap is critically important for cell cycle control. Yap expression in the mouse retina inversely correlates with neuronal differentiation (Zhang et al., 2012). Proneural bHLH transcription factors inhibit Yap by directly inhibiting Yap transcription and by activating upstream kinase Lats1/2. A recent study of frog CMZ stem cells illustrated Yap’s essential role in cell cycle regulation, especially at S phase (Cabochette et al., 2015). Total cell cycle length of Yap-deficient CMZ stem cells was increased while S-phase length was drastically shortened, presumably by skipping replication firing. In the present study we found that progenitor cells in Yap-deficient retina not only partially fail to maintain the pool of progenitors, but also experience two related inhibitions. First, Yap mutant retinas show reduction of the S-phase fraction. Dividing progenitors in Yap CKO retinas may be inhibited from passing the G1/S check point, resulting in an accumulation of G1 and/or G0 cells. In Yap mutant retinas, G0/G1 fraction was increased from 66.2% to 74.2% although statistical significance was not achieved (p=0.0717; Bonferroni’s multiple comparisons test). However, this view is consistent with previous findings suggesting Yap-regulated S-phase entry and showing decreased S-phase cells with Yap inactivation (Mizuno et al., 2012; Muramatsu et al., 2011; Tsujiura et al., 2014; Zhang et al., 2012). Second, Yap-deficient cells abnormally progress through cell cycle phases, as evidenced by delayed progression of EdU-labeled S-phase cells to mitosis. Therefore, Yap may be essential to ensure normal progression through the cell cycle and to inhibit cell cycle exit. It is plausible that Yap’s essential function in the G1/S involves timely upregulation of cyclin E, one of the known Yap-Tead transcription targets (Huang et al., 2005).
In summary, our study demonstrates that Yap’s multiple indispensable roles in ocular development impact regional fate choice, cell proliferation and survival and structural preservation and provides the molecular and cellular basis to understand human diseases caused by abnormal Yap or Yap binding partners. Understanding the evolutionarily conserved mechanisms of regional fate-determination further emphasizes the importance of Hippo-Yap signaling as a critical regulator in ocular development.
MATERIALS AND METHODS
Animals and genotyping
Mouse handling and housing were approved by the Temple University Institutional Animal Care and Use Committee. Rx-Cre line and Yapf/f allele were previously described (Swindell et al., 2006; Xin et al., 2011). PCR-based genotyping was performed using the primers previously described (Song et al., 2014). Yap CKO (Yap f/f; Rx-Cre), heterozygote (Yap+/−; Rx-Cre) and WT control littermates (mice not carrying Rx-Cre) used for the assays were generally obtained from the crosses of heterozygotes. Additional C57BL/6T and Swiss Webster (SW) mice (Taconic) were used as WT controls when needed. The genetic background of the ES cell lines used in constructing the Yap flox allele and Rx-Cre was not fully known, and rd1 and rd8 mutations were detected in the C57BL/6N mice and various ES cells(Mattapallil et al., 2012; Mehalow et al., 2003). Genomic DNA was therefore extracted from the paraffin sections used for the IF assay (QIAamp DNA FFPE Tissue Kit, Qiagen) according to the manufacturer’s instructions, and rd1 and rd8 genotyping performed with primers described previously (Gimenez and Montoliu, 2001; Mattapallil et al., 2012). We confirmed that all analyses of Yap CKO were performed on retinal samples negative for rd1 and rd8 mutations except for those used for flow cytometry.
Immunohistochemistry, histology and IF assay
Seven-micrometer thick paraffin sections were prepared from entire embryo heads or enucleated eyes after fixation with 4% paraformaldehyde, dehydration with ethanol and embedding in paraffin. Hematoxylin and eosin (H&E) staining and immunofluorescent antibody staining were done as described previously (Song et al., 2014). Images were obtained using an Axioplan 2 (Carl Zeiss Microimaging GmgH, Germany) or confocal microscope (TCS SP8, Leica Microsystems GmbH, Germany). The color of some images was changed from green to red or red to green to maintain consistency in the figures. DAB staining for Yap was done using the Diaminobenzidine (DAB) Histochemistry Kit (Molecular Probes) according to the manufacturer’s instructions. Prior to antibody staining of dissociated tissue, completely triturated E15.5 retinal cells were digested with DNase I at 37 °C for 5 min, plated on poly-D-lysine-treated slides, incubated at 37 °C for 30 min and fixed in 4% paraformaldehyde.
Antibodies
Primary antibodies used to stain tissue sections were aPKC (#610207; Becton Dickinson), BrdU (#347580; BD and ab6326; Abcam), α-Catenin (610193; BD), Chx10 (X1179P; Exalpha Biologicals), CC3 (#9661; Cell Signaling Technology (CST)), Pan-Crb (synthesized using VGARVPPTPNLKLPPEERLI), Ezrin (ab4069; Abcam), GFP (GFP-1020; Aves), Lamin B1 (#9087; CST), Luciferase (ab21176; Abcam), Mitf (#x2398M; Exalpha), Otx2 (ab9566; Millipore), p27 (#610241; BD), Pals1 (07-708; Millipore), Pax2 (PRB-276P; Covance), Pax6 (RBP-278P; Covance), PCNA (#2586; CST), pH3 (#06-570; Millipore), rhodopsin (#1840-RHO; Phosphosolutions), Sox9 (AB5809; Millipore), β-Tubulin III (MMS-435P; Covance), Yap (#4912; CST and ab56701; Abcam), pYap (#4911; CST) and Zo1 (#610966; BD). Secondary antibodies used were Alexa488 conjugated anti-mouse (Life Tech.), chicken (Life Tech), rabbit (Life Tech) and rat (Life Tech) antibodies, Cy3 conjugated anti-rabbit and mouse antibodies (Jackson Immuno Res.) and Cy5 conjugated anti-mouse and rabbit antibodies (Life Tech.).
Western blot analysis
Two whole retinas dissected from E15.5 Yap CKO and WT littermates were homogenized in a 1X sample lysis buffer with protease inhibitors (CST) following manufacturer’s directions. Protein concentration in the lysates was determined using a BCA Protein Assay Kit (Pierce) following manufacturer’s instructions. Fifty μg of the protein lysates were electrophoresed using 4–12% sodium dodecyl sulfate polyacrylamide gels and proteins were transferred to PVDF membranes. The membranes blocked with LiCor blocking buffer were simultaneously probed with two primary antibodies (Yap (rabbit, CST) and GAPDH (mouse, sc-166574; SCB)) overnight at 4°C, followed by secondary antibodies (goat anti-rabbit IRDYE800CW;LiCor or goat anti-mouse IRDYE680RD;LiCor) for 1 hr at room temp. The bands were detected using the Odyssey CLx Infrared Imaging System (LiCor). Signals corresponding to Yap and βactin were quantified using Image Studio software (ver. 3.1).
Cell proliferation assays
Timed pregnant females harboring E15.5 embryos were injected intraperitoneally (IP) with BrdU (50mg/kg body weight, B5002, Sigma-Aldrich) and tissues were harvested 30 min later for fixing and further processing. S-phase cell numbers were determined by staining the slides with BrdU antibodies, as described for immunohistochemistry analysis. Quantification was done on tissue sections from WT (n=3) and CKO (n=3) mice. Total cell number and the fraction of BrdU (+) cells were determined after staining the nuclei with Hoechst 33528. For the GOF study, pCAG:eGFP or pCAG:eGFP-Yap plasmids (1μg/μl in PBS) were electroporated into E15.5 WT retinal explants using an electroporation chamber (Platinum Block Petri dish Electrode (CUY520-P5)) with 5 square pulses (30 V) of 50 ms duration with 950 ms intervals (BTX ECM 830). Explants were cultured at 37 °C in neurobasal medium (Invitrogen) containing 1X B27 serum free supplement for 36 hrs, dissociated and subjected to BrdU antibody staining. To measure the progression of cells in S- and/or G2-phases, EdU (1ml of 10mM stock) was injected to label S-phase cells 2 hrs before tissue harvest at E16.5 or E17.5. EdU signal was detected using a Click-iT kit (Life Technologies) according to manufacturer’s instructions, which was followed by α-pH3 staining. Among pH3 (+) cells, the percentage of EdU (+) cells was independently determined from central and peripheral retinas of WT (n=3) and Yap CKO (n=3).
Flow cytometry analysis
Briefly, BrdU (500μl of 10mg/ml stock) was injected IP into pregnant mice at E16.5 30 min before tissue harvest. Two dissected retinas (3 replicates for WT, 4 replicates for CKO) per tube were trypsinized, fixed in ethanol and permeabilized with 2N HCl/0.5% Triton X-100 for 30 min. After blocking with 1% BSA in PBS, cells were stained with BrdU antibody overnight at 4°C. After 3 rounds of washing with PBS containing 1% BSA, cells were stained with Alexa488 conjugated goat anti-rat secondary antibody (Life Tech.). Antibody-labeled cells were treated with RNase (0.25μl of 20mg/ml) and propidium iodide (10μl of 1mg/ml) before undergoing flow cytometry analysis using Guava EasyCyte (Millipore). Cell cycle fraction analysis was done with GuavaExpressPro (Millipore). Age–matched C57BL/6 (Taconic) embryos were used as WT controls due to the difficulties in obtaining multiple WT or CKO from the same litter.
In ovo electroporation into chicken embryo
Fertilized eggs (Charles River) were incubated for approximately 36 hrs at 37 °C to Hamilton-Hamburger stage 9 or 10 (Hamburger V & Hamilton HL 1951). A plasmid mix containing pCAG:eGFP and 8xGIIC-luciferase (each in 1μg/μl PBS) was electroporated into the developing OV using a square electroporator (BTX, 17V, 50ms pulse 5-times followed by 950ms intervals) as described previously (Cho and Cepko, 2006).
Cell fractionation assay
Dissociated retinal cells were analyzed by cell fractionation assay performed following the manufacturer’s instructions (CST). Briefly, 12 dissected retinas from E15.5 SW embryos were pooled and dissociated in 25μl cytoplasm isolation buffer by trituration. The cytoplasmic fraction was collected after 10 min incubation on ice following centrifugation at 500 × g. Pellets were resuspended in 25μl of the membrane isolation buffer, vortexed, incubated for 10 min on ice and centrifuged at 500 × g to collect the membrane and organelle fraction. The remaining pellets were resuspended in 50μl of cytoskeleton/nucleus isolation buffer and sonicated for 5 sec at 20% power 3 times. Five μl of the cytoplasmic and membrane fractions and 10μl of the nuclear fraction were loaded for Western blot analysis with Yap and pYap antibodies. Lamin B1 (#9087; CST) and GAPDH (#10494-1-AP; Proteintech) antibodies were used to detect nuclear and cytoplasmic fractions, respectively.
Statistical analysis
Data are shown as average +/− standard error. Statistical analyses used throughout the study (BrdU, CC3, and GFP-Yap counts, and evaluation of EdU-pH3-mediated cell cycle progression) were two-tailed, paired Student’s t-test to identify differences between WT and CKO samples. Differences were only considered significant when P was <0.05. N represents the number of eyes analyzed in each assay except for the EdU-pH3 assay, where central and peripheral retinas from 3 WT and 3 CKO mice were counted separately. For flow cytometry analysis, we employed the more stringent two-way ANOVA followed by Bonferroni post-hoc test using Prism 6 (GraphPad Prism software).
Supplementary Material
Highlights.
Yap is essential for regional fate determination of the RPE during early eye development. RPE transdifferentiates into neural retina when Yap is deleted.
Yap is indispensable for maintenance of the apical polarity complex essential for cell to cell adhesion.
Yap is essential for the proliferation of retinal progenitor cells. The progenitor pool decreases and cell cycle progression is delayed when Yap is ablated.
Acknowledgments
We thank Dr. Eric Olson for his generous gift of Yapf/f mouse. This work was supported by National Institute of Health grant EY020578 (S.-H.C.) and NS073112 (S.K.), and by research grants from Shriners Hospitals for Children (S.-H.C. and S.K.).
Footnotes
CONFLICT OF INTEREST STATEMENT
None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcon C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, Sapkota G, Pan D, Massague J. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves CH, Pellissier LP, Wijnholds J. The CRB1 and adherens junction complex proteins in retinal development and maintenance. Prog Retin Eye Res. 2014;40:35–52. doi: 10.1016/j.preteyeres.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Alves CH, Sanz AS, Park B, Pellissier LP, Tanimoto N, Beck SC, Huber G, Murtaza M, Richard F, Sridevi Gurubaran I, Garcia Garrido M, Levelt CN, Rashbass P, Le Bivic A, Seeliger MW, Wijnholds J. Loss of CRB2 in the mouse retina mimics human retinitis pigmentosa due to mutations in the CRB1 gene. Human molecular genetics. 2013;22:35–50. doi: 10.1093/hmg/dds398. [DOI] [PubMed] [Google Scholar]
- Azuma N, Tadokoro K, Asaka A, Yamada M, Yamaguchi Y, Handa H, Matsushima S, Watanabe T, Kida Y, Ogura T, Torii M, Shimamura K, Nakafuku M. Transdifferentiation of the retinal pigment epithelia to the neural retina by transfer of the Pax6 transcriptional factor. Human molecular genetics. 2005;14:1059–1068. doi: 10.1093/hmg/ddi098. [DOI] [PubMed] [Google Scholar]
- Bai H, Gayyed MF, Lam-Himlin DM, Klein AP, Nayar SK, Xu Y, Khan M, Argani P, Pan D, Anders RA. Expression of Yes-associated protein modulates Survivin expression in primary liver malignancies. Human pathology. 2012;43:1376–1385. doi: 10.1016/j.humpath.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochimica et biophysica acta. 2009;1788:761–767. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, Kuo CJ, Camargo FD. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabochette P, Vega-Lopez G, Bitard J, Parain K, Chemouny R, Masson C, Borday C, Hedderich M, Henningfeld KA, Locker M, Bronchain O, Perron M. YAP controls retinal stem cell DNA replication timing and genomic stability. eLife. 2015:4. doi: 10.7554/eLife.08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Current biology: CB. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Cepko CL. Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133:3167–3177. doi: 10.1242/dev.02474. [DOI] [PubMed] [Google Scholar]
- Cho SH, Kim JY, Simons DL, Song JY, Le JH, Swindell EC, Jamrich M, Wu SM, Kim S. Genetic ablation of Pals1 in retinal progenitor cells models the retinal pathology of Leber congenital amaurosis. Human molecular genetics. 2012;21:2663–2676. doi: 10.1093/hmg/dds091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annual review of cell and developmental biology. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Developmental biology. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Ferrigno O, Lallemand F, Verrecchia F, L’Hoste S, Camonis J, Atfi A, Mauviel A. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene. 2002;21:4879–4884. doi: 10.1038/sj.onc.1205623. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Zou C, Levine EM. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Experimental eye research. 2013 doi: 10.1016/j.exer.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S, Zou C, Levine EM. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Experimental eye research. 2014;123:141–150. doi: 10.1016/j.exer.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura N, Taketo MM, Mori M, Korinek V, Kozmik Z. Spatial and temporal regulation of Wnt/beta-catenin signaling is essential for development of the retinal pigment epithelium. Developmental biology. 2009;334:31–45. doi: 10.1016/j.ydbio.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Gimenez E, Montoliu L. A simple polymerase chain reaction assay for genotyping the retinal degeneration mutation (Pdeb(rd1)) in FVB/N-derived transgenic mice. Laboratory animals. 2001;35:153–156. doi: 10.1258/0023677011911525. [DOI] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heavner W, Pevny L. Eye development and retinogenesis. Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YL, Hung JY, Chou SH, Huang MS, Tsai MJ, Lin YS, Chiang SY, Ho YW, Wu CY, Kuo PL. Angiomotin decreases lung cancer progression by sequestering oncogenic YAP/TAZ and decreasing Cyr61 expression. Oncogene. 2014 doi: 10.1038/onc.2014.333. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Liu D, Gong Y, Wang Y, Sun S, Gui Y, Song H. yap is required for the development of brain, eyes, and neural crest in zebrafish. Biochemical and biophysical research communications. 2009;384:114–119. doi: 10.1016/j.bbrc.2009.04.070. [DOI] [PubMed] [Google Scholar]
- Kantardzhieva A, Alexeeva S, Versteeg I, Wijnholds J. MPP3 is recruited to the MPP5 protein scaffold at the retinal outer limiting membrane. The FEBS journal. 2006;273:1152–1165. doi: 10.1111/j.1742-4658.2006.05140.x. [DOI] [PubMed] [Google Scholar]
- Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M. A Sveinsson’s chorioretinal atrophy-associated missense mutation in mouse Tead1 affects its interaction with the co-factors YAP and TAZ. Biochemical and biophysical research communications. 2007;361:1022–1026. doi: 10.1016/j.bbrc.2007.07.129. [DOI] [PubMed] [Google Scholar]
- Knight JK, Raymond PA. Retinal pigmented epithelium does not transdifferentiate in adult goldfish. Journal of neurobiology. 1995;27:447–456. doi: 10.1002/neu.480270402. [DOI] [PubMed] [Google Scholar]
- Kohli P, Bartram MP, Habbig S, Pahmeyer C, Lamkemeyer T, Benzing T, Schermer B, Rinschen MM. Label-free quantitative proteomic analysis of the YAP/TAZ interactome. American journal of physiology. Cell physiology. 2014;306:C805–818. doi: 10.1152/ajpcell.00339.2013. [DOI] [PubMed] [Google Scholar]
- Koike C, Nishida A, Akimoto K, Nakaya MA, Noda T, Ohno S, Furukawa T. Function of atypical protein kinase C lambda in differentiating photoreceptors is required for proper lamination of mouse retina. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:10290–10298. doi: 10.1523/JNEUROSCI.3657-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Nishida A, Ueno S, Saito H, Sanuki R, Sato S, Furukawa A, Aizawa S, Matsuo I, Suzuki N, Kondo M, Furukawa T. Functional roles of Otx2 transcription factor in postnatal mouse retinal development. Molecular and cellular biology. 2007;27:8318–8329. doi: 10.1128/MCB.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AN, Curtis SJ, Fillmore CM, Rowbotham SP, Mohseni M, Wagner DE, Beede AM, Montoro DT, Sinkevicius KW, Walton ZE, Barrios J, Weiss DJ, Camargo FD, Wong KK, Kim CF. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. The EMBO journal. 2014;33:468–481. doi: 10.1002/embj.201386082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, May NR, Fan CM. Transdifferentiation of the ventral retinal pigmented epithelium to neural retina in the growth arrest specific gene 1 mutant. Developmental biology. 2001;236:17–29. doi: 10.1006/dbio.2001.0280. [DOI] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, Johnson RL. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Signore M, Acampora D, Simeone A, Bovolenta P. Otx genes are required for tissue specification in the developing eye. Development. 2001;128:2019–2030. doi: 10.1242/dev.128.11.2019. [DOI] [PubMed] [Google Scholar]
- Masuda T, Wahlin K, Wan J, Hu J, Maruotti J, Yang X, Iacovelli J, Wolkow N, Kist R, Dunaief JL, Qian J, Zack DJ, Esumi N. Transcription factor SOX9 plays a key role in the regulation of visual cycle gene expression in the retinal pigment epithelium. The Journal of biological chemistry. 2014;289:12908–12921. doi: 10.1074/jbc.M114.556738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima D, Heavner W, Pevny LH. Combinatorial regulation of optic cup progenitor cell fate by SOX2 and PAX6. Development. 2011;138:443–454. doi: 10.1242/dev.055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, Caspi RR. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Investigative ophthalmology & visual science. 2012;53:2921–2927. doi: 10.1167/iovs.12-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehalow AK, Kameya S, Smith RS, Hawes NL, Denegre JM, Young JA, Bechtold L, Haider NB, Tepass U, Heckenlively JR, Chang B, Naggert JK, Nishina PM. CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Human molecular genetics. 2003;12:2179–2189. doi: 10.1093/hmg/ddg232. [DOI] [PubMed] [Google Scholar]
- Miesfeld JB, Gestri G, Clark BS, Flinn MA, Poole RJ, Bader JR, Besharse JC, Wilson SW, Link BA. Yap and Taz regulate retinal pigment epithelial cell fate. Development. 2015;142:3021–3032. doi: 10.1242/dev.119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld JB, Link BA. Establishment of transgenic lines to monitor and manipulate Yap/Taz-Tead activity in zebrafish reveals both evolutionarily conserved and divergent functions of the Hippo pathway. Mechanisms of development. 2014;133:177–188. doi: 10.1016/j.mod.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Murakami H, Fujii M, Ishiguro F, Tanaka I, Kondo Y, Akatsuka S, Toyokuni S, Yokoi K, Osada H, Sekido Y. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012;31:5117–5122. doi: 10.1038/onc.2012.5. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Murphy CJ, Russell P. What do mechanotransduction, Hippo, Wnt, and TGFbeta have in common? YAP and TAZ as key orchestrating molecules in ocular health and disease. Experimental eye research. 2013;115:1–12. doi: 10.1016/j.exer.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O’Neal W, Milgram SL. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Molecular and cellular biology. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T, Imoto I, Matsui T, Kozaki K, Haruki S, Sudol M, Shimada Y, Tsuda H, Kawano T, Inazawa J. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011;32:389–398. doi: 10.1093/carcin/bgq254. [DOI] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nature neuroscience. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- Nishihara D, Yajima I, Tabata H, Nakai M, Tsukiji N, Katahira T, Takeda K, Shibahara S, Nakamura H, Yamamoto H. Otx2 is involved in the regional specification of the developing retinal pigment epithelium by preventing the expression of sox2 and fgf8, factors that induce neural retina differentiation. PloS one. 2012;7:e48879. doi: 10.1371/journal.pone.0048879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park R, Moon UY, Park JY, Hughes LJ, Johnson RL, Cho SH, Kim S. Yap is required for ependymal integrity and is suppressed in LPA-induced hydrocephalus. Nature communications. 2016;7:10329. doi: 10.1038/ncomms10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier LP, Alves CH, Quinn PM, Vos RM, Tanimoto N, Lundvig DM, Dudok JJ, Hooibrink B, Richard F, Beck SC, Huber G, Sothilingam V, Garcia Garrido M, Le Bivic A, Seeliger MW, Wijnholds J. Targeted ablation of CRB1 and CRB2 in retinal progenitor cells mimics Leber congenital amaurosis. PLoS genetics. 2013;9:e1003976. doi: 10.1371/journal.pgen.1003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poche RA, Furuta Y, Chaboissier MC, Schedl A, Behringer RR. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Muller glial cell development. The Journal of comparative neurology. 2008;510:237–250. doi: 10.1002/cne.21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee KD, Yu J, Zhao CY, Fan G, Yang XJ. Dnmt1-dependent DNA methylation is essential for photoreceptor terminal differentiation and retinal neuron survival. Cell Death Dis. 2012;3:e427. doi: 10.1038/cddis.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan S, Chen CM, Young TL, Fisher DE, Cepko CL. Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development. 2004;131:5139–5152. doi: 10.1242/dev.01300. [DOI] [PubMed] [Google Scholar]
- Sakaguchi DS, Janick LM, Reh TA. Basic fibroblast growth factor (FGF-2) induced transdifferentiation of retinal pigment epithelium: generation of retinal neurons and glia. Developmental dynamics: an official publication of the American Association of Anatomists. 1997;209:387–398. doi: 10.1002/(SICI)1097-0177(199708)209:4<387::AID-AJA6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M, Cecconi F, Bernier G, Andrejewski N, Kammandel B, Wagner M, Gruss P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127:4325–4334. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- Shen Z, Stanger BZ. YAP regulates S-phase entry in endothelial cells. PloS one. 2015;10:e0117522. doi: 10.1371/journal.pone.0117522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Park R, Kim JY, Hughes L, Lu L, Kim S, Johnson RL, Cho SH. Dual function of Yap in the regulation of lens progenitor cells and cellular polarity. Developmental biology. 2014;386:281–290. doi: 10.1016/j.ydbio.2013.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell EC, Bailey TJ, Loosli F, Liu C, Amaya-Manzanares F, Mahon KA, Wittbrodt J, Jamrich M. Rx-Cre, a tool for inactivation of gene expression in the developing retina. Genesis. 2006;44:361–363. doi: 10.1002/dvg.20225. [DOI] [PubMed] [Google Scholar]
- Tsujiura M, Mazack V, Sudol M, Kaspar HG, Nash J, Carey DJ, Gogoi R. Yes-associated protein (YAP) modulates oncogenic features and radiation sensitivity in endometrial cancer. PloS one. 2014;9:e100974. doi: 10.1371/journal.pone.0100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- van de Pavert SA, Sanz AS, Aartsen WM, Vos RM, Versteeg I, Beck SC, Klooster J, Seeliger MW, Wijnholds J. Crb1 is a determinant of retinal apical Muller glia cell features. Glia. 2007;55:1486–1497. doi: 10.1002/glia.20561. [DOI] [PubMed] [Google Scholar]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Developmental cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Williamson KA, Rainger J, Floyd JA, Ansari M, Meynert A, Aldridge KV, Rainger JK, Anderson CA, Moore AT, Hurles ME, Clarke A, van Heyningen V, Verloes A, Taylor MS, Wilkie AO, Fitzpatrick DR Consortium UK. Heterozygous loss-of-function mutations in YAP1 cause both isolated and syndromic optic fissure closure defects. American journal of human genetics. 2014;94:295–302. doi: 10.1016/j.ajhg.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Science signaling. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. The Anatomical record. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Zhang H, Deo M, Thompson RC, Uhler MD, Turner DL. Negative regulation of Yap during neuronal differentiation. Developmental biology. 2012;361:103–115. doi: 10.1016/j.ydbio.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proceedings of the National Academy of Sciences of the United States of America. 2011a;108:2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Smolen GA, Haber DA. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer research. 2008;68:2789–2794. doi: 10.1158/0008-5472.CAN-07-6205. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhou Q, Pignoni F. Yki/YAP, Sd/TEAD and Hth/MEIS control tissue specification in the Drosophila eye disc epithelium. PloS one. 2011b;6:e22278. doi: 10.1371/journal.pone.0022278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes & development. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Hung FC, Colvin JS, White A, Dai W, Lovicu FJ, Ornitz DM, Overbeek PA. Patterning the optic neuroepithelium by FGF signaling and Ras activation. Development. 2001;128:5051–5060. doi: 10.1242/dev.128.24.5051. [DOI] [PubMed] [Google Scholar]
- Zhao S, Rizzolo LJ, Barnstable CJ. Differentiation and transdifferentiation of the retinal pigment epithelium. International review of cytology. 1997;171:225–266. doi: 10.1016/s0074-7696(08)62589-9. [DOI] [PubMed] [Google Scholar]
- Zhao S, Thornquist SC, Barnstable CJ. In vitro transdifferentiation of embryonic rat retinal pigment epithelium to neural retina. Brain Res. 1995;677:300–310. doi: 10.1016/0006-8993(95)00163-k. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Gasperowicz M, Chow RL. The expression of NOTCH2, HES1 and SOX9 during mouse retinal development. Gene Expr Patterns. 2013;13:78–83. doi: 10.1016/j.gep.2012.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.