Abstract
Objective
Evaluate the effects of aging on healthy Achilles tendon and aponeurosis shear wave speed (SWS), a quantitative metric which reflects tissue elasticity.
Methods
Shear wave elastography was used to measure spatial variations in Achilles tendon SWS in healthy young (n=15, 25±4 years), middle-aged (n=10, 49±4 years) and older (n=10, 68±5 years) adults. SWS was separately measured in the free Achilles tendon, soleus aponeurosis and gastrocnemius aponeurosis in resting (R), stretched (dorsiflexed 15 deg from R) and slack (plantarflexed 15 deg from R) postures.
Results
SWS significantly increased with stretch and varied with age in all tendon regions. Slack free tendon SWS was significantly higher in older adults than young adults (p=0.025). However, stretched soleus aponeurosis SWS was significantly lower in older adults than young adults (p=0.01). Stretched gastrocnemius aponeurosis SWS was significantly lower in both middle-aged (p=0.003) and older (p=0.001) adults, relative to younger adults.
Conclusions
These results suggest that aging alters spatial variations in Achilles tendon elasticity, which could alter deformations within the triceps surae muscle-tendon units, thus affecting injury potential. The observed location- and posture-dependent variations highlight the importance of controlling ankle posture and imaging location when using shear wave approaches clinically to evaluate tendon disorders.
Keywords: Ultrasound, Elastography, Tendon, Aging, Compliance
Introduction
Chronic tendon overuse injuries (i.e. tendinopathy) can severely reduce quality of life, with injury incidence related to age [1]. Age-related alterations in injury incidence may arise due to a variety of changes in tendon composition that have been identified in various models including an increase in stiffness of the interfascicular matrix (equine superficial digital flexor tendon [2]), an increase in collagen cross-linking (human patellar tendon [3]; rat tail tendon [4]), and a reduction in collagen fibril crimp angle (equine superficial and deep digital flexor tendons [5]). Despite evidence of these micro-scale alterations in tendon, it remains unclear how age-related changes in tendon composition affect whole tendon behaviour. For example, there remains considerable disagreement in the literature regarding whether aging leads to increased [6–8] or decreased [9, 10] tendon compliance. Tendon tissue heterogeneity may, in part, explain these discrepancies as recent studies have shown evidence that tendon material properties and strain may vary considerably within regions of the same tendon [11–15], and between different types of tendons [2, 16]. Nevertheless, to our knowledge, very few studies have measured region-specific effects of aging on tendon compliance, which may be key to understanding aging and injury in tendon.
In recent years, advanced ultrasound approaches have emerged for the evaluation of tendon elasticity. For example, sonoelastography techniques have shown potential for better sensitivity in diagnosing tendon injuries than traditional B-mode imaging [17–19]. Further, sonoelastography may be able to identify localized tissue softening and damage in pathological tendons [19, 20], with sonoelastography measures showing evidence of good agreement with histological observations [18]. However, traditional sonoelastography relies on manual tissue palpation, which can affect the reliability of results obtained, and limit the feasibility of inter-subject comparisons [21].
Shear Wave Elastography (SWE) is an ultrasound-based approach capable of noninvasively quantifying in vivo shear wave speeds in soft tissues with good repeatability [22] and high spatial resolution [23]. One advantage of SWE is that wave speeds are measured in response to a pulse from the ultrasound transducer, rather than via manual transducer palpation by the operator which is common in other techniques, thus limiting the operator dependence of SWE and increasing repeatability of results. In transversely isotropic materials, such as tendon, shear wave speed is dependent on the tissue shear elastic modulus [24, 25], and is thus an important mechanical characteristic. Although originally used to evaluate pathological compliance changes in tissues (e.g. liver [26]), SWE has gained increasing use as a technique for measuring tendon properties [27–30]. One advantage of this approach is that shear wave speeds are computed with high resolution, enabling the evaluation of shear wave speed heterogeneity over the length of the tendon. Using this approach, we have recently shown that Achilles tendon shear wave speeds vary significantly along the tendon’s length in healthy adults, and shear wave speeds show strain-stiffening effects, with increased stretch (i.e. ankle dorsiflexion) leading to increased shear wave speeds [12]. We also observed evidence of increased compliance in the proximal portion of the Achilles tendon (i.e. the gastrocnemius aponeurosis) in middle-aged adults when the ankle was dorsiflexed [11]. The purpose of this study was to build on this prior work and evaluate the effects of aging on Achilles tendon and aponeurosis shear wave speeds in older adults (aged > 60). We hypothesized that we would see evidence of increased compliance during Achilles tendon stretch in the proximal portion of the Achilles tendons of older adults compared with young adults, and that aging effects would be more pronounced in older adults than middle-aged adults.
Materials and Methods
Subject Population
Thirty-five healthy adults (aged 21–71 years, mean: 46 ± 19 years) with no history of Achilles tendon injury, surgery or systemic disease were recruited for this study (Table 1). A fellowship trained musculoskeletal radiologist with twelve years of experience reviewed B-mode images and found no evidence of subclinical tendinopathy changes in any subject. Data from young (n = 15, aged 21–40 years, mean: 25 ± 4 years) and middle-aged (n = 10, aged 41–60 years, mean: 49 ± 4 years) adults have been reported previously [11, 12] and were included in this study for further analysis. Institutional Review Board approval and informed consent were obtained. Subjects were asked to self-report their average weekly exercise on a questionnaire. Prior to imaging, each subject was asked to walk at a comfortable pace for six minutes to precondition their muscle-tendon structures [31].
Table 1.
Age, gender, height and weekly exercise were self-reported. Resting ankle angle was measured manually with a goniometer with the subject lying prone. Tendon thickness was measured from B-mode images of the free Achilles tendon. No significant differences in weekly exercise or tendon thickness were observed between young, middle-aged or older groups. Presented are average ± standard deviation, where applicable.
| Young Adults (21–40) |
Middle-Aged Adults (41–60) |
Older Adults (61+) |
|
|---|---|---|---|
| Age: | 25 ± 4 years | 49 ± 4 years | 68 ± 5 years |
| Gender: | 8 M, 7 F | 5 M, 5 F | 5 M, 5 F |
| Height: | 69 ± 3 inches | 70 ± 5 inches | 67 ± 3 inches |
| Weekly exercise: | 11 ± 5 hours | 10 ± 5 hours | 10 ± 7 hours |
|
Resting ankle angle (deg. plantarflexion): |
25 ± 5 deg. | 25 ± 6 deg. | 23 ± 4 deg. |
| Tendon thickness: | 4.7 ± 0.3 mm | 4.9 ± 0.7 mm | 5.1 ± 0.8 mm |
Collection Protocol
Ultrasound images and shear wave speed measures were collected from the Achilles tendon in a manner described previously [11, 12]. Briefly, subjects were asked to lie fully relaxed in a prone position on an examination table. The foot was extended over the edge of the examination table and the resting ankle angle (R) of the right leg was measured with a handheld goniometer (Fig. 1). Subjects were assisted in reaching the stretched (dorsiflexed 15 deg from R) and slack (plantarflexed 15 deg from R) postures. To reach a stretched posture, the examination table was repositioned such that the sole made contact with a wall. For the slack posture, the subject was repositioned on the examination table such that the bridge of the foot rested on the table, and the foot was no longer freely extended off the table. A manual goniometer was used to assist with subject positioning to ensure that the final postures were ± 15 deg from the initial resting posture. Posture order was randomized for each subject. After the subject was positioned and asked to remain fully relaxed, ultrasound B-mode images and shear wave data (software version: 5; preset: superficial MSK persist: high; smoothing: 7) were collected along the length of the Achilles tendon (Fig. 2).
Figure 1.
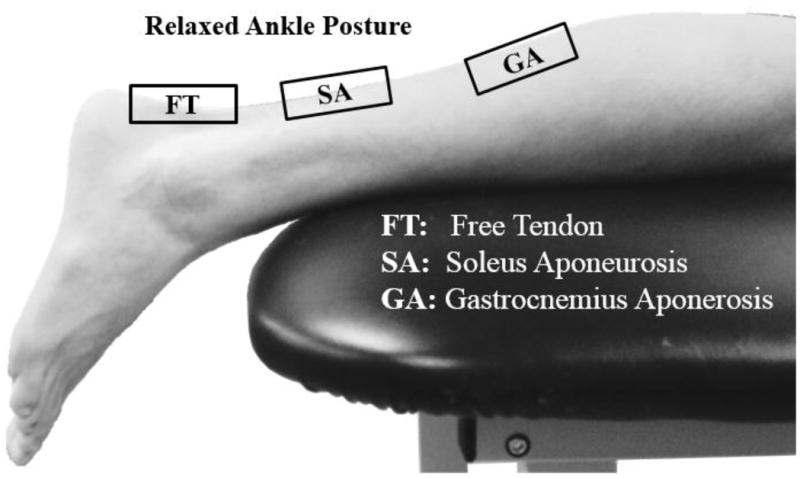
Subjects were asked to lie prone on an examination table. The resting ankle posture is shown here, in which the foot is extended off the edge of the table. Shear wave data were collected from the free tendon, soleus aponeurosis and medial gastrocnemius aponeurosis.
Figure 2.
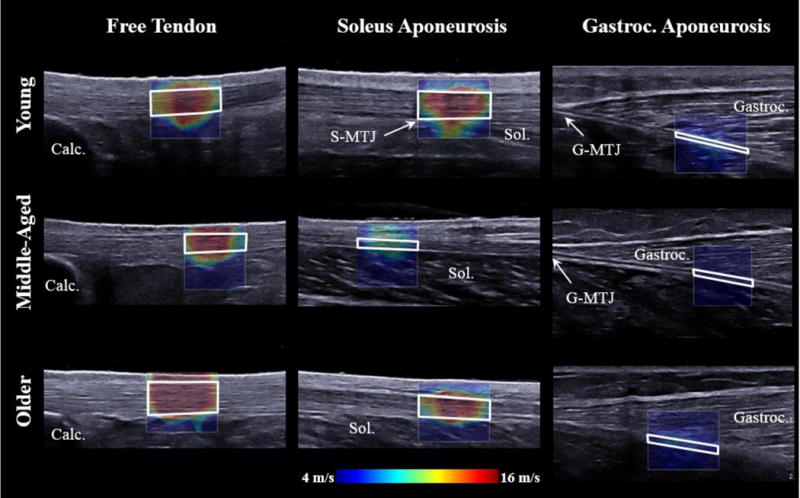
B-mode images with overlaying shear wave speed from representative subjects for each age group in the resting ankle posture. Tendon shear wave speeds were determined within regions of interest (ROIs; outlined in white) defined within the tendon boundaries. Shear wave speed in the gastrocnemius muscle overlying the gastrocnemius aponeurosis was also determined (outlined in grey). A standoff pad was used in the distal two most positions of the transducer. Please note that images have been cropped for space, but scaled accordingly. The slightly larger ROIs for the older adult subject group, and from one of the young adults is clear. Visible landmarks have been identified: Calc: Calcaneus, MTJ: Muscle-tendon junction, Sol: Soleus, Gastroc: Gastrocnemius.
The initial position of the transducer (L15-4, Aixplorer, Supersonic Imagine, Aix-en-Provence, France) was standardized across all subjects by placing the distal edge of the transducer approximately 12.5 mm distal (the width of one region of interest (ROI)) to the proximal edge of the calcaneus. The shear wave ROI (width: 12.5 mm) was then centered over the Achilles tendon and positioned in the distal portion of the image. Three repeat frames of shear wave data were collected for each shear wave scanning location. The scanning location was then progressively moved proximally in 12.5 mm increments until all four positions within the same 50 mm wide B-mode image were collected. The transducer was then translated proximally in 50 mm increments from the calcaneus to the medial gastrocnemius. Collections were obtained, on average, up until 120 mm proximal to the gastrocnemius muscle-tendon junction when the gastrocnemius aponeurosis was no longer visible in the ultrasound B-mode image, or if there were significant artifacts or missing shear wave speed data. Custom standoff pads (178 × 127 mm, 16 mm thick), that were created from commercial pads (Aquaflex, Parker Laboratories, Fairfield, NJ), were used in the two most distal transducer positions corresponding to the most superficial locations of the Achilles tendon.
Data Analysis
Shear wave data were analyzed post-hoc from exported DICOMs using a custom MATLAB (Mathworks, Inc., Natick, MA) script that enabled a single researcher to manually define ROIs within the tendon boundaries. During processing, the soleus muscle-tendon junction (S-MTJ) and the gastrocnemius muscle-tendon junction (G-MTJ) were identified within B-mode images for each trial. Shear wave speed measures were then divided into three regions: the free tendon (FT; from the calcaneus to S-MTJ), the soleus aponeurosis (SA; S-MTJ to G-MTJ) and the gastrocnemius aponeurosis (GA; G-MTJ to most proximal imaging location). Although other studies using this approach have reported Young’s Modulus, we chose to report shear wave speed, as the assumption of isotropy necessary for the Young’s Modulus computation is not valid in tendon [32, 33]. To evaluate any potential correlation between aponeurosis shear wave speed and the surrounding muscle tissue, shear wave speed was also computed in the adjacent gastrocnemius muscle that is visible superficial to the gastrocnemius aponeurosis. Measurement of wave speed in the soleus muscle was not performed because these measures are generally considered unreliable [30] due to the complex architecture and location of the muscle deep to the soleus aponeurosis. Saturation was evaluated as the percentage of pixels within each ROI that exhibited shear wave speed magnitudes equal to 16.3 m/s (the maximum shear wave speed capacity of the system). B-mode images were used to evaluate the anterior-posterior thickness of the tendon in the resting ankle posture. Achilles tendon thickness across the tendon mid-substance at approximately 10 mm proximal to the superior aspect of the calcaneus was measured using ImageJ (NIH, Bethesda, MD) by a musculoskeletal radiologist. To eliminate any potential bias in measurements, the radiologist was blinded to the subject age group and subject number, and thickness measurements were performed in a random order.
For each tendon region (free tendon, soleus aponeurosis, gastrocnemius aponeurosis), ANOVAs were used to evaluate any statistical relationship between age group (young, middle-aged, older) and ankle posture (slack, resting, stretched). Tukey post-hoc comparisons were used to investigate any interactions with significance set at p < 0.05. Regression analyses were also performed for each ankle region and posture to investigate the effects of age on shear wave speed. For postures and regions in which data saturation was high (>5%), regression analyses with age and saturation were also performed. A regression analysis between gastrocnemius muscle shear wave speed and aponeurosis shear wave speed was performed to test for a correlation between the two, with significance set at p < 0.05.
Potential differences between age groups in terms of tendon thickness and average weekly number of hours of exercise were evaluated using ANOVAs. Tukey post-hoc comparisons were used to investigate any interactions with significance set at p < 0.05.
Results
Shear wave speed varied with age in all tendon regions (Fig. 3), though significance was only found within certain ankle postures. In the slack free tendon, shear wave speed was significantly higher in older adults than young adults (p = 0.025). In the stretched soleus aponeurosis, shear wave speed was significantly lower in older adults than young adults (p = 0.01). In the stretched gastrocnemius aponeurosis, shear wave speed was significantly lower in middle-aged adults (p = 0.003) and older adults (p = 0.001) compared with young adults.
Figure 3.
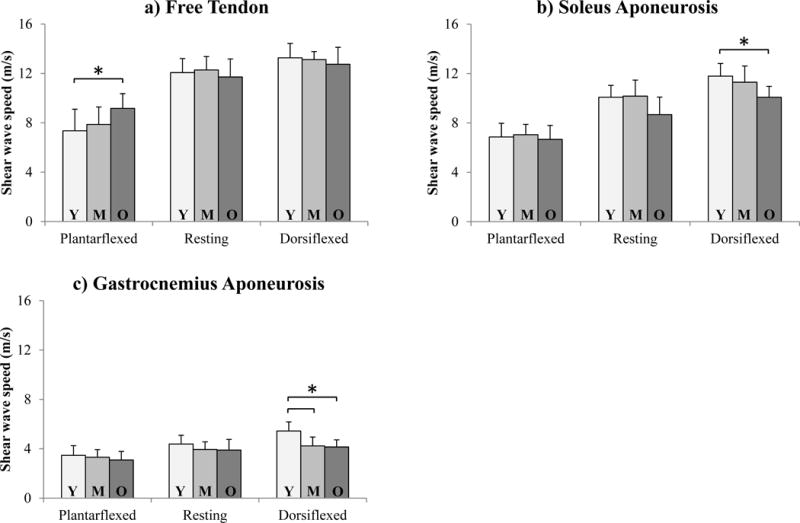
Average (±1 s.d.) regional shear wave speed for each age group (Y: Young, M: Middle-aged, O: Older) in the a) Achilles free tendon, b) soleus aponeurosis, and c) gastrocnemius aponeurosis. *p < 0.05.
Regression analyses showed that shear wave speed also correlated with age at specific postures in all regions (Fig. 4, Table 2). In the free tendon, shear wave speed was positively correlated with age in a slack posture (p = 0.005). In the soleus aponeurosis, shear wave speed was negatively correlated with age in the stretched (p < 0.001) and resting (p = 0.008) postures. In the gastrocnemius aponeurosis, shear wave speed decreased with age in the stretched posture (p < 0.001) and was close to significance in the resting posture (p = 0.065). Shear wave speed in the gastrocnemius muscle and gastrocnemius aponeurosis showed a significant positive correlation (Fig. 5; p < 0.001, R2 = 0.32).
Figure 4.
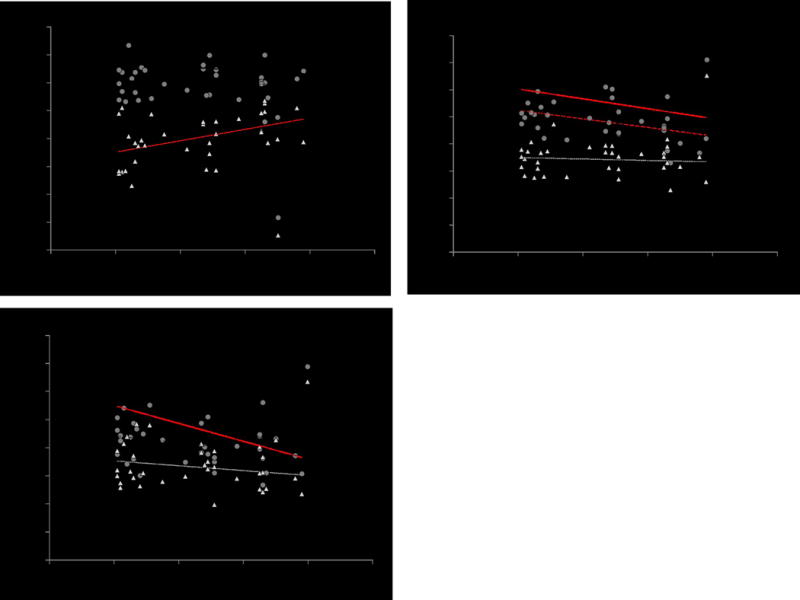
Regional shear wave speed vs. subject age in the a) Achilles free tendon, b) soleus aponeurosis, and c) gastrocnemius aponeurosis. Trend lines plotted in red exhibited statistical significance (p < 0.05).
Table 2.
Regression analyses identified significant relationships between age and shear wave speed for the plantarflexed Achilles free tendon, relaxed and dorsiflexed soleus aponeurosis, and dorsiflexed gastrocnemius aponeurosis.
| R2 | p-value | |
|---|---|---|
| a) Achilles Free Tendon | ||
|
| ||
| Plantarflexed posture | 0.21 | 0.005* |
|
| ||
| Relaxed posture | 0.01 | 0.57 |
|
| ||
| Dorsiflexed posture | 0.05 | 0.21 |
|
| ||
| b) Soleus Aponeurosis | ||
|
| ||
| Plantarflexed posture | 0.01 | 0.57 |
|
| ||
| Relaxed posture | 0.2 | 0.008* |
|
| ||
| Dorsiflexed posture | 0.3 | <0.001* |
|
| ||
| c) Gastrocnemius Aponeurosis | ||
|
| ||
| Plantarflexed posture | 0.053 | 0.183 |
|
| ||
| Relaxed posture | 0.1 | 0.065 |
|
| ||
| Dorsiflexed posture | 0.42 | <0.001* |
Figure 5.
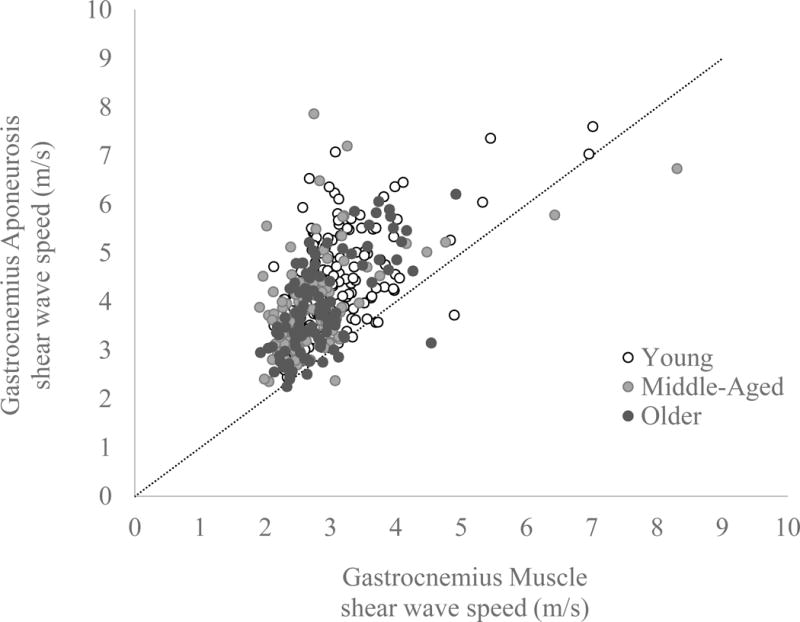
Resting gastrocnemius aponeurosis shear wave speed is plotted versus resting gastrocnemius muscle wave speed for all subjects, with a 1:1 line for reference. Multiple data points per subject are plotted from the different locations proximal to the gastrocnemius muscle-tendon junction, with different colours representing the data from the young, middle-aged and older adults. Most data points fall above the 1:1 line reflecting the fact that shear wave speeds in the gastrocnemius aponeurosis were generally higher than in the adjacent muscle.
Data saturation was highest in the free tendon when in stretched (19.6 ± 14.2 %) and resting (9.5 ± 8.0 %) postures. The soleus aponeurosis also showed notable data saturation when in a stretched posture (9.6 ± 8.5 %). In all other regions and postures, data saturation was low (< 2%). Age was not found to correlate with data saturation in the free tendon (p > 0.2), but data saturation did correlate negatively with age in the soleus aponeurosis when stretched (R2 = 0.23, p = 0.004).
No significant differences between average weekly hours of exercise (p = 0.89) or tendon thickness (p = 0.205) were observed between groups. Likewise, no significant effects of gender or weekly exercise were observed on tendon shear wave speeds.
Discussion
We observed complex interactions of age, ankle posture and region on in vivo tendon shear wave speeds. Notably, aging was associated with an increase in shear wave speed in the slack Achilles tendon, and a decrease in shear wave speed in the stretched soleus and gastrocnemius aponeuroses. Prior theoretical studies have shown that wave speed is dependent on the shear elastic modulus [24], while empirical studies have also found that shear wave speed is correlated with the along-fiber tendon elastic modulus [33]. Our observations would thus suggest that aging alters the inherent spatial variation in Achilles tendon elasticity. Consistent with our hypothesis, we observed evidence to support an increase in compliance in the stretched aponeuroses with aging. Further, although we did not observe group-wise differences in compliance between middle-aged and older adults, low p-values from regression analyses suggest that such aging effects are progressive. However, we also observed the unexpected result that the slack Achilles tendon appears less compliant in older adults. These varied aging effects could influence spatial tissue deformations within the triceps surae muscle-tendon units, and thus affect both muscle performance and injury potential.
It is well recognized that age-related changes in tendon composition and architecture can alter the inherent mechanical properties of tissue. Notably, both animal and human studies have shown evidence that aged tendon exhibits increased stiffness of the inter-fascicular matrix [2], an increase in collagen cross-linking [3, 4], and a reduction in collagen fibril crimp angle [34]. Although tendon elasticity measures in humans have produced conflicting evidence regarding an age-related decrease in tendon compliance [6–10, 35], the ambiguity may arise in part from methodological factors or from spatial definitions of the tendon, which may span regions of free tendon and aponeurosis. For example, one traditional ultrasound approach for measuring tendon elasticity involves tracking anatomical landmarks (e.g. the gastrocnemius muscle-tendon junction) while the muscle-tendon unit is passively or actively loaded [6–8, 36]. Tendon modulus is then computed from estimates of the tendon force and strain, thereby providing an average elasticity measure over a large portion of the tendon. In contrast, the SWE imaging [23] used in this study provides much more regional assessments of tissue elasticity than anatomical landmark tracking, and in fact reveals complex spatial variations in aging tendon elasticity that would not be apparent using traditional B-mode imaging.
It is important to recognize that we measured shear wave speeds at low tendon loads, where the tendon exhibits strain-stiffening behaviour. Indeed, the monotonic increase in shear wave speed from the slack to stretched postures would likely reflect the uncrimping of tendon fibres with passive stretch (Figs 2 and 3). It is interesting that age-related changes in shear wave speed in the free Achilles tendon were only evident in the most plantarflexed posture (mean: 39 ± 5 deg. plantarflexion). This posture is comparable to the average ankle angle at which the Achilles tendon reaches its slack length (43 ± 3 deg plantarflexion [30]). Thus, the age-related increase in free tendon shear wave speed would seem to reflect the material properties of the unloaded tissue, which could arise from increased stiffness of the interfascicular matrix [2]. Likewise, a recent study using a sonoelastography technique observed that older adult Achilles tendons exhibit less deformation with manual palpation [35], possibly reflecting greater transverse stiffness.
In contrast, the reduction in older adult aponeurosis shear wave speed occurred in a taut muscle-tendon unit, such that the change in compliance could arise from material property effects and/or altered loading. Loading effects are further complicated by the aponeurosis being both in series and parallel with muscle fibres, making it challenging to infer the relative loading transmitted through the two tissues [37]. It is thus conceivable that the lower shear wave speed results from reduced loading of the aponeurosis with muscle-tendon stretch, and hence a reduced strain-stiffening response. Alternatively, the aging Achilles tendon may undergo localized changes in material properties. Further study is needed to identify the underlying factors giving rise to altered aponeurosis behaviour.
When interpreting aponeurosis data, it is important to consider the possibility that shear wave speeds are influenced by surrounding tissue. Indeed, we did find a significant correlation between shear wave speeds measured in the gastrocnemius muscle and aponeurosis. Biomechanically, however, this is to be expected. It is intuitive that Achilles tendon shear wave speeds would be highest close to its insertion at the stiff calcaneus bone, and lowest proximally near the compliant gastrocnemius muscle. Likewise, proximal to the gastrocnemius muscle-tendon junction, it is predictable that muscle and aponeurosis tissue would show similar patterns of wave speed variations due to the anatomical and mechanical link between the tissues. However, it is also possible that the computation of wave speed in the thin aponeurosis is influenced by the wave propagation in the surrounding muscle tissue. Without a readily available alternate technique for analysis, we are limited to presenting the data as collected and acknowledging this relationship between gastrocnemius muscle and aponeurosis shear wave speed.
This study highlights the importance of carefully controlling tendon region and stretch (i.e. ankle posture) when measuring tendon shear wave speed. Shear wave speed began reaching the maximum measurable value (16.3 m/s) in the relaxed and stretched posture. Ex vivo studies have also reported saturation with moderate (1–2%) tendon strain [38, 39], which reflects relatively low tendon loading. Our free tendon results that show no aging effects in a resting or stretched posture are consistent with prior data collected from similar ankle postures [27, 29]. Our stretched observations (angle range: 28–50 deg plantarflexion) however, contrast with Aubry et al., who found no aging effects at maximum plantarflexion, and reduced shear wave speeds with age at an angle of 45 deg plantarflexion [29]. The results from the current study, however, demonstrate that even small changes in ankle posture (15 deg) can reveal characteristics of aging that may not be present in other ankle postures. Thus it will be critical that future studies include a wide range of ankle angles to better elucidate the complex relationship between aging, ankle posture and shear wave speed.
Age-related changes in tendon behaviour may be relevant to consider in assessing the causes of altered injury and disease incidence with aging [40]. Tendon disorders, such as Achilles tendinopathy/rupture and calf muscle-tendon injuries, tend to occur in consistent locations, most notably, 3–6 cm from the calcaneus insertion [41], and near the medial gastrocnemius muscle-tendon junction [42–44], respectively. Interestingly, we did observe specific aging effects in these regions of the tendon. The subjects did not self-report prior Achilles injuries, and no evidence of damage was visible within B-mode images, but it is possible that subjects could have had sub-clinical tendon damage. For example, sonoelastography has revealed evidence of potential damage in asymptomatic patients [20].
Recent studies have found evidence that SWE may be capable of discriminating between healthy and injured tendons [28, 45] indicating the translational potential of this new technique. However, although we observed age-related changes in shear wave speed that had very high significance, these low p-values (range: <0.001 – 0.01) were often paired with relatively low R2 values (range: 0.42 – 0.19). Therefore, although our results have implications for interpreting population-level age-related results, the reality of interpreting data for an individual patient as a measure of tendon health remains unclear.
There are a few limitations in this study. We observed high data saturation in the Achilles free tendon and the soleus aponeurosis when stretched. We do not anticipate that saturation affected the trends we observed in this study; the only significant variation with age that also showed data saturation (stretched soleus aponeurosis) showed a decrease with age, which would presumably only emphasize the observation of reduced shear wave speeds with aging. However, it is important to recognize that the most stretched posture used in this study (mean: 9 deg plantarflexion) is still far from fully dorsiflexed and yet high data saturation occurred, emphasizing the need for careful protocol development when using SWE in tendon studies. It is also relevant to note that the research protocol for older adult subjects and five of the young adult subjects was slightly altered from the earlier protocols used for young [12] and middle-aged [11] subjects in that we increased the width of our shear wave speed box (+2.5 mm) to reduce collection time and require only four (rather than five) positions of the shear wave speed box for each transducer position. Shear wave speeds may be affected by depth, as acoustic waves are known to exhibit depth-dependent attenuation [46], and spatial smoothing is inherent in sonoelastography methodologies [47]. In our previous study, we investigated the relationship between wave speed and depth, and did find a weak correlation [12]. Unfortunately, as tendon thickness and spatial variations in material properties are also varying, it is not possible to discriminate the relative influence of these factors. It is also possible, though unlikely, that shear wave measures are affected by the use of gel pads, which could affect regional comparisons as gel pads were only used in distal locations. To minimize the effects of any of these parameters on age-group comparisons, we maintained all machine collection settings, including smoothing, for all research subjects. Finally, the sample size in this study is relatively small (35 subjects), with only ten subjects in each of our middle-aged and older adult groups. We hypothesize that larger sample sizes will further reinforce the results from this study.
Achilles tendon shear wave speeds show age-related changes that vary based on location, with evidence of reduced compliance in the free Achilles tendon and increased compliance in the gastrocnemius aponeurosis. Tendon shear wave speeds were highly dependent on ankle posture, emphasizing the clinical relevance of controlling ankle posture when using shear wave approaches clinically to evaluate tendon. Future work will be critical to understanding how these age-related changes may contribute to the increased incidence of injury that is observed in middle- aged and older adults.
Key Points.
Shear wave elastography shows promise as a clinical quantitative ultrasound-based technique.
Aging induces location-dependent changes in Achilles tendon shear wave speed.
Spatial and postural dependence necessitates careful integration of this approach clinically.
Acknowledgments
The scientific guarantor of this publication is Dr. Kenneth Lee. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding from the Radiological Society of North America (Scholar Grant), the University of Wisconsin-Madison Radiology Department Research and Development Fund (#1204-001), and the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Some study subjects or cohorts have been previously reported in Physiological Measurement (10) and the Journal of Biomechanics (11). The study was prospective, cross-sectional and performed at one institution.
References
- 1.Alfredson H. Chronic midportion Achilles tendinopathy: an update on research and treatment. Clin Sports Med. 2003;22:727–741. doi: 10.1016/s0278-5919(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 2.Thorpe CT, Udeze CP, Birch HL, et al. Capacity for Sliding between Tendon Fascicles Decreases with Ageing in Injury Prone Equine Tendons: A Possible Mechanism for Age-Related Tendinopathy? Eur Cell Mater. 2013;25:48–60. doi: 10.22203/ecm.v025a04. [DOI] [PubMed] [Google Scholar]
- 3.Couppe C, Hansen P, Kongsgaard M, et al. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol. 2009;107:880–886. doi: 10.1152/japplphysiol.00291.2009. [DOI] [PubMed] [Google Scholar]
- 4.Viidik A. Connective Tissues – Possible Implications of the Temporal Changes for the Aging Process. Mech Ageing Dev. 1979;9:267–285. doi: 10.1016/0047-6374(79)90104-0. [DOI] [PubMed] [Google Scholar]
- 5.Patterson-Kane JC, Parry DA, Birch HL, et al. An age-related study of morphology and cross-link composition of collagen fibrils in the digital flexor tendons of young thoroughbred horses. Connect Tissue Res. 1997;36:253–260. doi: 10.3109/03008209709160225. [DOI] [PubMed] [Google Scholar]
- 6.Csapo R, Malis V, Hodgson J, Sinha S. Age-related greater Achilles tendon compliance is not associated with larger plantar flexor muscle fascicle strains in senior women. J Appl Physiol. 2014;116:961–969. doi: 10.1152/japplphysiol.01337.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol. 2006;100:2048–2056. doi: 10.1152/japplphysiol.01442.2005. [DOI] [PubMed] [Google Scholar]
- 8.Stenroth L, Peltonen J, Cronin NJ, et al. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol. 2012;113:1537–1544. doi: 10.1152/japplphysiol.00782.2012. [DOI] [PubMed] [Google Scholar]
- 9.Kubo K, Morimoto M, Komuro T, et al. Age-related differences in the properties of the plantar flexor muscles and tendons. Med Sci Sports Exerc. 2007;39:541–547. doi: 10.1249/01.mss.0000247006.24965.74. [DOI] [PubMed] [Google Scholar]
- 10.Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol. 2011;111:999–1006. doi: 10.1152/japplphysiol.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reference removed to comply with double-blind review.
- 12.Reference removed to comply with double-blind review.
- 13.Arruda EM, Calve S, Dennis RG, et al. Regional variation of tibialis anterior tendon mechanics is lost following denervation. J Appl Physiol. 2006;101:1113–1117. doi: 10.1152/japplphysiol.00612.2005. [DOI] [PubMed] [Google Scholar]
- 14.Pearson SJ, Ritchings T, Mohamed ASA. Regional strain variations in the human patellar tendon. Med Sci Sports Exerc. 2014;46:1343–51. doi: 10.1249/MSS.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 15.De Zordo T, Fink C, Feuchtner GM, et al. Real-time sonoelastography findings in healthy Achilles tendons. AJR Am J Roentgenol. 2009;193:W134–8. doi: 10.2214/AJR.08.1843. [DOI] [PubMed] [Google Scholar]
- 16.Thorpe CT, Udeze CP, Birch HL, et al. Specialization of tendon mechanical properties results from interfascicular differences. J R Soc Interface. 2012;9:3108–3117. doi: 10.1098/rsif.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ooi CC, Richards PJ, Maffulli N, et al. A soft patellar tendon on ultrasound elastography is associated with pain and functional deficit in volleyball players. J Sci Med Sport. 2015 doi: 10.1016/j.jsams.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Klauser AS, Miyamoto H, Tamegger M, et al. Achilles tendon assessed with sonoelastography: histologic agreement. Radiology. 2013;267:837–42. doi: 10.1148/radiol.13121936. [DOI] [PubMed] [Google Scholar]
- 19.De Zordo T, Lill SR, Fink C, et al. Real-time sonoelastography of lateral epicondylitis: comparison of findings between patients and healthy volunteers. Am J Roentgenol. 2009;193:180–185. doi: 10.2214/AJR.08.2020. [DOI] [PubMed] [Google Scholar]
- 20.De Zordo T, Chhem R, Smekal V, et al. Real-time sonoelastography: findings in patients with symptomatic achilles tendons and comparison to healthy volunteers. Ultraschall der Medizin. 2010;31:394–400. doi: 10.1055/s-0028-1109809. [DOI] [PubMed] [Google Scholar]
- 21.Ooi CC, Malliaras P, Schneider ME, Connell DA. “Soft, hard, or just right?” Applications and limitations of axial-strain sonoelastography and shear-wave elastography in the assessment of tendon injuries. Skeletal Radiol. 2014;43:1–12. doi: 10.1007/s00256-013-1695-3. [DOI] [PubMed] [Google Scholar]
- 22.Lacourpaille L, Hug F, Bouillard K, et al. Supersonic shear imaging provides a reliable measurement of resting muscle shear elastic modulus. Physiol Meas. 2012;33:N19–28. doi: 10.1088/0967-3334/33/3/N19. [DOI] [PubMed] [Google Scholar]
- 23.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- 24.Brum J, Bernal M, Gennisson JL, Tanter M. In vivo evaluation of the elastic anisotropy of the human Achilles tendon using shear wave dispersion analysis. Phys Med Biol. 2014;59:505–523. doi: 10.1088/0031-9155/59/3/505. [DOI] [PubMed] [Google Scholar]
- 25.Eby SF, Song P, Chen S, et al. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013;46:2381–2387. doi: 10.1016/j.jbiomech.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bavu E, Gennisson JL, Couade M, et al. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 2011;37:1361–1373. doi: 10.1016/j.ultrasmedbio.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Arda K, Ciledag N, Aktas E, et al. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am J Roentgenol. 2011;197:532–536. doi: 10.2214/AJR.10.5449. [DOI] [PubMed] [Google Scholar]
- 28.Aubry S, Nueffer JP, Tanter M, et al. Viscoelasticity in Achilles Tendonopathy: Quantitative Assessment by Using Real-time Shear-Wave Elastography. Radiology. 2014;140434 doi: 10.1148/radiol.14140434. [DOI] [PubMed] [Google Scholar]
- 29.Aubry S, Risson JR, Kastler A, et al. Biomechanical properties of the calcaneal tendon in vivo assessed by transient shear wave elastography. Skeletal Radiol. 2013;42:1143–1150. doi: 10.1007/s00256-013-1649-9. [DOI] [PubMed] [Google Scholar]
- 30.Hug F, Lacourpaille L, Maisetti O, Nordez A. Slack length of gastrocnemius medialis and Achilles tendon occurs at different ankle angles. J Biomech. 2013;46:2534–2538. doi: 10.1016/j.jbiomech.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Hawkins D, Lum C, Gaydos D, Dunning R. Dynamic creep and pre-conditioning of the Achilles tendon in-vivo. J Biomech. 2009;42:2813–2817. doi: 10.1016/j.jbiomech.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Kuo P-L, Li P-C, Li M-L. Elastic properties of tendon measured by two different approaches. Ultrasound Med Biol. 2001;27:1275–1284. doi: 10.1016/s0301-5629(01)00442-2. [DOI] [PubMed] [Google Scholar]
- 33.Royer D, Gennisson JL, Deffieux T, Tanter M. On the elasticity of transverse isotropic soft tissues. J Acoust Soc Am. 2011;129:2757–2760. doi: 10.1121/1.3559681. [DOI] [PubMed] [Google Scholar]
- 34.Patterson-Kane JC, Firth EC, Goodship AE, Parry DA. Age-related differences in collagen crimp patterns in the superficial digital flexor tendon core region of untrained horses. Aust Vet J. 1997;75:39–44. doi: 10.1111/j.1751-0813.1997.tb13829.x. [DOI] [PubMed] [Google Scholar]
- 35.Turan A, Teber MA, Yakut ZI, et al. Sonoelastographıc assessment of the age-related changes of the Achilles tendon. Med Ultrason. 2015;17:58–61. doi: 10.11152/mu.2013.2066.171.ayt. [DOI] [PubMed] [Google Scholar]
- 36.Karamanidis K, Arampatzis A. Mechanical and morphological properties of different muscle-tendon units in the lower extremity and running mechanics: effect of aging and physical activity. J Exp Biol. 2005;208:3907–3923. doi: 10.1242/jeb.01830. [DOI] [PubMed] [Google Scholar]
- 37.Epstein M, Wong M, Herzog W. Should tendon and aponeurosis be considered in series? J Biomech. 2006;39:2020–2025. doi: 10.1016/j.jbiomech.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Blevins FT, Hecker AT, Bigler GT, et al. The effects of donor age and strain rate on the biomechanical properties of bone-patellar tendon-bone allografts. Am J Sports Med. 1994;22:328–333. doi: 10.1177/036354659402200306. [DOI] [PubMed] [Google Scholar]
- 39.Vogel HG. Influence of maturation and aging on mechanical and biochemical properties of connective tissue in rats. Mech Ageing Dev. 1980;14:283–292. doi: 10.1016/0047-6374(80)90002-0. [DOI] [PubMed] [Google Scholar]
- 40.McKean KA, Manson NA, Stanish WD. Musculoskeletal injury in the masters runners. Clin J Sport Med. 2006;16:149–154. doi: 10.1097/00042752-200603000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Astrom M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop Relat Res. 1995;316:151–164. [PubMed] [Google Scholar]
- 42.Garrett WE. Muscle Strain Injuries – Clinical and Basic Aspects. Med Sci Sports Exerc. 1990;22:436–443. [PubMed] [Google Scholar]
- 43.Kirkendall DT, Garrett WE. Clinical perspectives regarding eccentric muscle injury. Clin Orthop Relat Res. 2002:S81–S89. doi: 10.1097/00003086-200210001-00010. [DOI] [PubMed] [Google Scholar]
- 44.Speer KP, Lohnes J, Garrett WE., Jr Radiographic imaging of muscle strain injury. Am J Sports Med. 1993;21:89–95. doi: 10.1177/036354659302100116. discussion 96. [DOI] [PubMed] [Google Scholar]
- 45.DeWall RJ, Jiang J, Wilson J, Lee KS. Visualizing tendon elasticity in an ex vivo partial tear model. Ultrasound Med Biol. 2013 doi: 10.1016/j.ultrasmedbio.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 46.Barbosa DC, Bamber JC, Cosgrove DO, Nassiri D. Ultrasound Attenuation Measurements to Assess the Progress of Malignant-Tumors. Br J Radiol. 1986;59:742. [Google Scholar]
- 47.Havre RF, Elde E, Gilja OH, et al. Freehand real-time elastography: impact of scanning parameters on image quality and in vitro intra- and interobserver validations. Ultrasound Med Biol. 2008;34:1638–50. doi: 10.1016/j.ultrasmedbio.2008.03.009. [DOI] [PubMed] [Google Scholar]


