Abstract
Major depressive disorder (MDD) is a common disorder with a high prevalence and significant social and economic impacts. Nevertheless, the treatment of MDD is far from satisfactory. Acupuncture treatment has emerged as a promising method for treating MDD. However, the neural mechanism by which acupuncture reduces depressive symptoms is not fully understood. Studies have shown that the corticostriatal reward circuitry is associated with the pathophysiology of MDD; thus, we investigated the corticostriatal resting-state functional connectivity (rsFC) before and after real and sham acupuncture treatments combined with the antidepressant fluoxetine. Forty-six female major depressive patients were assigned to either verum acupuncture plus fluoxetine (n = 22) or sham acupuncture plus fluoxetine (n = 24) treatment for 8 weeks, and resting state functional magnetic resonance imaging (fMRI) data were collected before the first and after the last treatment sessions. The results showed that compared with sham acupuncture, the verum acupuncture group showed: (1) significantly increased rsFC between inferior ventral striatum and medial prefrontal cortex, ventral rostral putamen and amygdala / parahippocampus, as well as dorsal caudate and middle temporal gyrus; (2) significantly decreased rsFC between right ventral rostral putamen and right dorsolateral prefrontal cortex, and right dorsal caudate and bilateral cerebellar tonsil. The increased rsFC between the inferior ventral striatum and medial prefrontal cortex, ventral rostral putamen and amygdala / parahippocampus were significantly positively associated with decreased clinical scores (Montgomery–Åsberg Depression Rating Scale and Self-Rating Depression Scale scores) at the end of the eight-week treatment. Our findings suggest that acupuncture may achieve treatment effects by modulating the corticostriatal reward / motivation circuitry in MDD patients.
Keywords: depression, acupuncture, striatum, resting state functional connectivity, reward, fluoxetine
Introduction
Major depressive disorder (MDD) has a high lifetime prevalence rate, and is the fourth leading cause of disability worldwide (Sackeim, 2001). Previous studies show that depression is more frequently observed in females than males (Abate, 2013; Soares & Zitek, 2008), with a female/male risk ratio roughly 2:1 (Kessler, 2003). This increased prevalence is speculated to be associated with female-specific reproductive events such as perimenstrual changes, pregnancy, the postpartum period and menopause (Cyranowski, Frank, Young, & Shear, 2000; Soares et al., 2008). Studies suggest that the fluctuations in sex hormones during female reproductive events could influence neurochemical pathways involved in the modulation of mood and behavior linked to depression (Bloch et al., 2000; Schmidt, Nieman, Danaceau, Adams, & Rubinow, 1998; Soares et al., 2008). Thus, it is reasonable to believe that the underlying pathophysiology between male and female MDD patients is not identical (Domes et al., 2008; Eisenberger, Inagaki, Rameson, Mashal, & Irwin, 2009; Moieni et al., 2015; Pitychoutis & Papadopoulou-Daifoti, 2010).
Although MDD affects a large proportion of the population by significantly impairing occupational, social and academic functioning (Johnson, Weissman, & Klerman, 1992; Lehtinen & Joukamaa, 1994), the antidepressant medications for MDD are not fully satisfactory due to undesirable side effects and a delay in the onset of therapeutic action (Arroll et al., 2005; Chan, Lo, Yang, Chen, & Lin, 2015). Acupuncture, however, has become a promising and effective alternative treatment for depression (Jorm, Christensen, Griffiths, & Rodgers, 2002; Kessler et al., 2001; Quah-Smith, Suo, Williams, & Sachdev, 2013; Quah-Smith, Wen, Chen, Williams, & Sachdev, 2012).
In addition, accumulating evidence has indicated that acupuncture combined with antidepressant medication is more effective than antidepressants alone, and is safe, well-tolerated, and has an early onset of action (Chan et al., 2015; Wang et al., 2016; Zhang, Yang, & Zhong, 2009), which embodies the potential of combining acupuncture and pharmacological treatments for depression.
A core characteristic of patients with depression is the loss of interest in pleasurable activities and limitations in multiple dimensions of well-being (Bogdan, Nikolova, & Pizzagalli, 2013; Hasler & Northoff, 2011; Hwang et al., 2016; Naranjo, Tremblay, & Busto, 2001; Russo & Nestler, 2013). The reward system (Bluhm et al., 2009; Naranjo et al., 2001; Pizzagalli et al., 2009) is involved in pleasure and motivation, which have been proven to play important roles in the pathophysiology of MDD (Bluhm et al., 2009; Soares et al., 2008). Reward processing is complex and consists of sensory, attention and valuation components (Schultz, 2015). A number of brain structures are involved in reward processing, including the orbitofrontal cortex, medial prefrontal cortex, dorsolateral prefrontal cortex, motor cortex, parietal association cortex, visual cortex, striatum, and amygdala (Carl et al., 2016; Schultz, 2015).
The striatum is a central region of the reward circuit (Alexander, DeLong, & Strick, 1986; Braunlich & Seger, 2013; Di Martino et al., 2008; Felger et al., 2015), which receives excitatory afferents from cortical areas modulating ventral striatal activity during encoding of reward prediction and the mediation of motivational state (Choi, Yeo, & Buckner, 2012; Di Martino et al., 2008). Previous studies have identified the dorsal and ventral divisions of the striatum (Alexander, Crutcher, & DeLong, 1990; Choi et al., 2012; Draganski et al., 2008; Leh, Ptito, Chakravarty, & Strafella, 2007). The dorsal striatal regions (including the dorsal caudate nucleus and dorsal putamen) receive inputs from the dorsolateral prefrontal cortex, which support motor and executive function, and the ventral striatal regions (including the nucleus accumbens, ventral putamen and ventromedial caudate) project to the orbitofrontal cortex, which is involved in affective division (Furman, Hamilton, & Gotlib, 2011; Selemon & Goldman-Rakic, 1985; Voorn, Vanderschuren, Groenewegen, Robbins, & Pennartz, 2004).
Reward circuit activity may be used as an effective predictor of treatment response in adolescent depression (Forbes et al., 2010; Phillips et al., 2015) with respect to neurotransmitter abnormalities in the striatum in depression (Ebert, Feistel, Loew, & Pirner, 1996; Martin, Martin, Rai, Richardson, & Royall, 2001). Interpersonal psychotherapy and venlafaxine hydrochloride can increase blood flow in the striatum (Martin et al., 2001). Deep brain stimulation to the ventral striatum, especially the nucleus accumbens, has also been applied to treat adults with severe depression (Schlaepfer et al., 2008).
Previous studies suggest that the autonomic nervous system (ANS), particularly the vagus nerve, may be an important mediator of acupuncture needle stimulation (Janig, 2006; Kavoussi & Ross, 2007). Lim and colleagues (Lim, Kim, Lee, & Namgung, 2016) found that acupuncture may achieve treatment effects through vagal nerve modulation of inflammatory responses in internal organs. A recent study also showed that direct stimulation of the peripheral branch of the vagus nerve can relieve symptoms in MDD patients (Rong et al., 2016). Taken together, these studies suggest that the vagus nerve may play an important role in acupuncture treatment of depression.
In addition, a number of neuroimaging studies indicate that acupuncture could modulate activity in multiple cortical and subcortical brain areas (Chae et al., 2013; Dougherty et al., 2008; Huang et al., 2012; Hui et al., 2000; Hui et al., 2005; Kong et al., 2007; Kong et al., 2002; Meng et al., 2014; Shan et al., 2014) involved in cognition, somatosensory processing, pain, and affective / emotional processing (Sun et al., 2011; Zhang, Wang, & McAlonan, 2012). Animal studies have shown that acupuncture stimulation facilitates the normalization of striatum activity and improves motor function in mouse models of Parkinson’s Disease (Kim et al., 2011). In a previous study, we found functional magnetic resonance imaging signal changes in the orbitofrontal cortex, which is a region involved in reward and endogenous opioid modulation during acupuncture analgesia (Dougherty et al., 2008).
In recent decades, rsFC has drawn the attention of investigators. The rsFC measures the temporal dependency of neuronal activation patterns between anatomically separated brain regions during rest (Biswal, Yetkin, Haughton, & Hyde, 1995). This approach helps to better elucidate the function of one brain region in terms of network and explore how brain regions sub-serve the common cognitive procedures (Bullmore & Sporns, 2009). For instance, Felger and colleagues (Felger et al., 2015) found that the corticostriatal reward circuitry rsFC decreased with increased symptoms of depression, and the rsFC between the ventral striatal and ventral medial prefrontal cortex was related to increased anhedonia, while the dorsal striatal rsFC was related to reduced motor function.
Recently, rsFC has been used to investigate the underlying mechanism of acupuncture in healthy subjects (Bai, Qin, Tian, Dai, & Yang, 2009; Dhond, Yeh, Park, Kettner, & Napadow, 2008; Hui et al., 2009; Liu et al., 2009; Qin et al., 2008; Zhong et al., 2012), and patient populations, such as patients with MDD (Deng et al., 2016; Yi et al., 2012), knee osteoarthritis (Chen et al., 2015; Egorova, Gollub, & Kong, 2015), migraines (Li et al., 2016) and Alzheimer’s disease (Wang et al., 2014). For instance, investigators found that peripheral nerve stimulation and acupuncture can significantly modulate the rsFC of the default mode network (Deng et al., 2016; Fang et al., 2016), amygdala (Liu et al., 2016), and anterior cingulate cortex (Yi et al., 2012) in MDD patients. Taken together, these results suggest that rsFC can be a useful tool to investigate the mechanism of acupuncture treatment of MDD.
In this study, we investigated the corticostriatal rsFC changes before and after verum and sham acupuncture treatment plus fluoxetine in females with depression. We only recruited female patients to increase the homogeneity of this study. Considering the different corticostriatal projections, we divided the striatum into ventral and dorsal striatal regions based on previous studies (Di Martino et al., 2011; Di Martino et al., 2008; Felger et al., 2015; Furman et al., 2011; Gabbay et al., 2013; Harrison et al., 2009; Kelly et al., 2009; Kwak et al., 2010). We hypothesized that compared with sham acupuncture plus fluoxetine, verum acupuncture plus fluoxetine treatment could effectively modulate the corticostriatal rsFC, and changes in rsFC before and after acupuncture treatment may be associated with corresponding changes in depressive symptoms.
Materials and Methods
In our previous study, we reported how acupuncture plus fluoxetine treatment modulates the rsFC of the amygdala related network (Wang et al., 2016). In this manuscript, we focus on how acupuncture modulates the rsFC of the extended corticostriatal network using a seed-to-whole-brain method, which has never been reported before.
Participants
The study protocol was approved by the Institutional Review Board of the 2nd Affiliated Hospital of Guangzhou University of Chinese Medicine. The study was enrolled online on the Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org.cn, ChiCTR-TRC-14005228). Each subject provided written informed consent before participation in the study. Patients with MDD were recruited for this study through community postings, and all eligible participants were required to meet the specific inclusion /exclusion criteria.
Inclusion criteria
1) Women meeting the criteria of depression in ICD-10; 2) aged 30–60 years old and able to provide voluntary informed consent; 3) standard scores of the Self-Rating Depression Scale (SDS) ≥ 53 or total scores of Montgomery-Åsberg Depression Rating Scale (MADRS) ≥ 14 (Ball et al., 2016; Daniel et al., 1999; Treasure & Treasure, 1987; van Noorden, van Fenema, van der Wee, Zitman, & Giltay, 2012); 4) exhibit normal cognitive functioning, and with no aphasia and intellectual disabilities; 5) have primary school education or higher; and 6) right-handed.
In this study, the liver function of all the participants was tested using alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and kidney function was tested using blood urea nitrogen (BUN) and creatinine tests (Cr). All tests were within normal ranges, and there were no significant differences between the two groups in the liver and kidney function tests. All patients received a physician screening and completed self-reports to determine comorbidities. The structural clinical interview was not applied.
Exclusion criteria
1) Pregnant or breastfeeding women; 2) patients with severe damage of liver, kidney function, or with neurological deficits, rheumatologic disorders, cardiac disease, diabetes, malignant tumors and any other significant systemic disorders that might affect the results; 3) presence of any somatic diseases (such as cerebral infarction, cerebral hemorrhage, Parkinson’s or cerebral tumor) and any other significant systemic disorders that might affect the results; 4) presence of severe psychoses (schizophrenia, mania, paranoid psychosis and depression with suicidal intent) or dementia; 5) use of antipsychotics or antidepressants within a month before the study; 6) consumption of alcohol or illicit substances; 7) women with metallic implants; and 8) refused to sign informed content.
Intervention
All participants were given fluoxetine (20 mg) by mouth once per day, and then they were randomly assigned to verum or sham acupuncture groups.
Verum and sham acupuncture administration
Verum acupuncture
Abdominal acupuncture was the school of acupuncture chosen for this study (Zhiyun, 2001), as several studies have shown that abdominal acupuncture treatment is effective for treatment of depression (Cheng & Tang, 2007; Wang, Li, Deng, & Bo, 2010; Wu, Yeung, Schnyer, Wang, & Mischoulon, 2012).
Abdominal acupuncture uses only acupoints in the abdomen. It only produces mild sensations, thus making it more acceptable to patients. The rationale of abdominal acupuncture is that CV 8 (umbilicus) plays a crucial role in propelling and regulating the flow of Qi (Zhiyun, 2001); thus, the acupoints around the CV 8 in the abdomen may regulate the flow of Qi more efficiently.
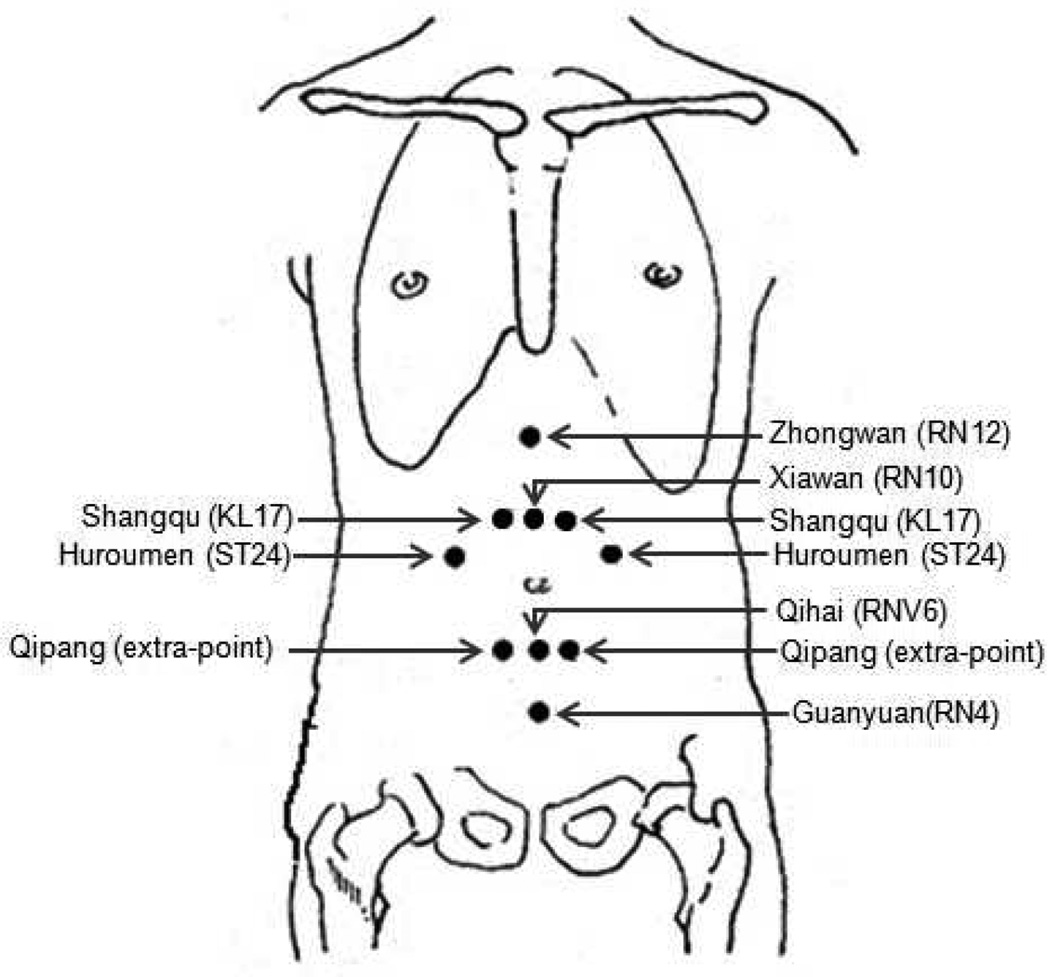
Based on a previous study from our group (Wang et al., 2008), the acupoints applied in this study were: Zhongwan (RN12), Xiawan (RN10), Qihai (RN6), Guanyuan (RN4), Shangqu (KL17), Huaroumen (ST24), and Qipang (extra-point) (Figure 1). These acupoints are close to important meridians and can easily communicate with five zang and six fu organs through channels like the Conceptional Vessel, Governor Vessel, Thoroughfare Meridian and Belt Meridian. This prescription could harmonize zang-fu five viscera, tonify qi, and replenish blood to relieve depression.
Figure 1.
Locations of acupoints applied in this study.
An acupuncturist with at least 3 years of experience administered all acupuncture treatments. Before abdominal acupuncture administration, subjects were instructed to lie in a supine position and place a mask over their eyes. Next, the subject’s abdomen was exposed and the skin was disinfected at the acupuncture points. Then, an acupuncture specialist inserted fine needles (0.22 mm × 40 mm) through short plastic tubes or sheaths. The needles were inserted intramuscularly to a depth of 15–20 mm and were left in situ for 20 minutes. The patient’s abdomen was covered with a basket underneath a sheet during the treatment period.
To accumulate the effect of acupuncture quickly and enhance the motivation and compliance of patients at the beginning of the study, patients received abdominal acupuncture once a day for the first three days and subsequently once every three days for the reminder of the 8-week trial.
Sham acupuncture operation
The acupoints were the same as in the treatment group. Before abdominal acupuncture administration, subjects were instructed to lie in a supine position and put a mask over the eyes. Then, the subject’s abdomen was exposed and the skin was disinfected at the acupoints. Next, short plastic needle sheaths not containing any needles were tapped against the skin at the patient’s acupoints, but no needles were inserted into the skin through the sheaths. The patient’s abdomen was covered with a basket underneath a sheet during the ‘treatment’ period. The time and frequency of the sham abdominal acupuncture treatments were the same as in the acupuncture group.
Clinical outcomes
The evaluations used to assess clinical outcomes of this study were MADRS and SDS, evaluated before the first treatment and after the last treatment.
MRI data acquisition
The fMRI brain imaging acquisition was conducted on a 1.5 Tesla Siemens Avanto scanner. High-resolution brain structural images were acquired with a T1-weighted three-dimensional multi-echo magnetization-prepared rapid gradient-echo (MP-RAGE) sequence (repetition time: 1,900 ms, echo time: 2.3 ms, data matrix: 256×256, field of view: 256 mm ×256 mm, slice thickness 1 mm, flip angle: 15°, and 176 sagittal slices covering the whole brain). T2*-weighted functional images encompassing the whole brain were acquired with the gradient-echo EPI sequence (echo time: 30 ms, repetition time: 2,000 ms, data matrix: 64×64, field of view: 240 mm, flip angle: 90°, slice thickness: 4 mm, interslice gap: 0.88 mm, 31 slices paralleled by anterior commissure-posterior commissure line, and 180 time points). Two 6-minute resting state fMRI scans were applied while the subjects were required to keep their eyes closed.
Statistical analysis
Clinical data analysis
Statistical analysis was performed using SPSS 19.0 Software (SPSS Inc., Chicago, IL, USA). Two sample t-tests were applied to compare the baseline characteristics of the subjects between groups. Analysis of covariance ANCOVA was applied to compare primary and secondary outcomes. Age was included in the model to adjust the effects.
Resting-state fMRI data analysis
Functional BOLD data were preprocessed using SPM8 (Statistical Parametric Mapping. Welcome Department of Cognitive Neurology, London, UK; implemented by MATLAB R3012b, Math Works, Inc., Natick, MA, USA). During the preprocessing, images were realigned, segmented and co-registered to each subject’s high-resolution T1 scan, which was used to normalize to the standard Montreal Neurological Institute (MNI) template. Images were also smoothed using an 8 mm full-width at half-maximum (FWHM) Gaussian kernel, filtered with a frequency window of 0.008–0.09 Hz. In addition to these steps, we employed segmentation of gray matter, white matter, and cerebrospinal fluid (CSF) areas for the removal of temporal confounding factors (white matter and CSF) (Whitfield-Gabrieli & Nieto-Castanon, 2012). Finally, data were then submitted to motion correction using the artifact detection toolbox (http://www.nitrc.org/projects/artifact_detect/). Time points in subjects’ scans were marked as outliers if the global signal exceeded three standard deviations from the mean or if scan to scan motion exceeded 0.5 mm deviation (Redcay et al., 2013).
Resting-state functional connectivity analysis was conducted using the CONN toolbox v15.g (Whitfield-Gabrieli et al., 2012) (http://www.nitrc.org/projects/conn). We used a priori ventral striatum seeds centered on ventral and dorsal striatal regions, (Figure. 1a) (Supplementary Table S1) (Felger et al., 2015). The ventral striatal areas are inferior ventral striatum (iVS) (including nucleus accumbens) and ventral rostral putamen (vrP), and the dorsal striatal subdivisions are dorsal caudal putamen (dcP) and dorsal caudate (dC). The ventral and dorsal regions were assessed separately. Functional connectivity measures were computed between each of these seeds and every other voxel in the brain. First-level correlation maps were produced by extracting the residual BOLD time course from each ventral striatal seed and by computing Pearson’s correlation coefficients between that time course and the time courses of all other voxels in the brain. Correlation coefficients were transformed into Fisher’s z scores, which increases normality and allows for improved second-level General Linear Model analyses.
The treatment effect (post-treatment minus pre-treatment) on striatum seed-to-voxel rsFC between group analyses (verum vs sham) was performed using two sample t-tests. A threshold of a voxel-wise p < 0.005 (uncorrected) and cluster-level p < 0.05 (FDR correction) were applied for data analyses.
We also conduct an exploratory regression analysis to investigate the association between corticostriatal rsFC modulated by acupuncture treatment effect and the clinical outcome changes (Cullen et al., 2014; Esterman, Tamber-Rosenau, Chiu, & Yantis, 2010). To do this, we extracted the peak z value from the significant cluster resulting from group analysis from each participant’s corticostriatal rsFC z score map. Within the verum and sham acupuncture group, regression analyses were computed on theses z scores with changes of clinical outcomes, with ages included as covariates.
Results
Patient characteristics
Forty-six subjects (22 in acupuncture group, 24 in sham group) were recruited into the study, and only thirty-six subjects (18 in acupuncture group, 18 in sham group) were scanned twice (week 0 and week 8). Four subjects from the verum acupuncture group dropped out (3 due to only scan once and 1 due to a scheduling conflict), six subjects from the sham acupuncture group dropped out (2 uncomfortable with treatment, 1 due to a scheduling conflict, and 3 due to only scan once). All analyses were based on these 36 subjects who completed the study.
There were no significant differences between the two groups with respect to age, scale of MADRS, and SDS at baseline (Table 1). After treatment, ANCOVA analysis (dependent variable is the change (post- minus pre-treatment) of MADRS/SDS score, the independent is the type of treatments (verum or sham), and covariate variables are age of participants) showed a significant difference between the verum and sham acupuncture groups on both MADRS and SDS scores and verum acupuncture showed significantly greater clinical improvement (Table 1).
Table 1.
Demographic and clinical characteristics of participants in this study.
| Characteristic | Conditions | Acupuncture Mean (SD) |
Sham Mean (SD) |
Statistic |
p value |
|---|---|---|---|---|---|
| N | 18 | 18 | |||
| Age (years) | 44.5 (10.47) | 43.78 (9.10) | t(34) = 0.22 | 0.83 | |
| BMI | 21.45 (2.51) | 20.82 (2.36) | t(34) = 0.77 | 0.45 | |
| MADRS | pre-treatment | 22.94 (7.36) | 22.83 (9.17) | F(1,33) = 0.001 | 0.97 |
| post-treatment | 5.44 (5.37) | 14.06 (4.39) | F(1,33) = 26.86 | < 0.01 | |
| post-pre | −17.5 (7.89) | −8.78 (7.94) | F(1,33) = 10.86 | < 0.01 | |
| SDS | pre-treatment | 47.833 (6.46) | 47.44 (9.23) | F(1,33) = 0.01 | 0.94 |
| post-treatment | 26.83 (6.46) | 34.94 (5.40) | F(1,33) = 16.29 | < 0.01 | |
| post-pre | −21.00 (9.11) | −12.50 (8.65) | F(1,33) = 8.21 | < 0.01 |
Abbreviations: BMI, Body Mass Index; MADRS, Montgomery-Asberg Depression Rating Scale; SDS, Self-rating Depression Scale; ‘post-pre’ means treatment effect between pre- and post-treatment.
Given the important role of the reward network in anhedonia, we also compared the anhedonia changes between the two treatment groups using the item “Inability to feel” from MADRS. ANCOVA analysis showed that there was no significant anhedonia difference between the verum acupuncture plus fluoxetine group and sham group at baseline (F (1, 33) = 0.001, p = 0.972), but there was a significant difference after treatment between the two groups (F (1, 33)= 7.353, p = 0.011). The verum acupuncture plus fluoxetine treatment produced greater treatment effects (post-treatment minus pre-treatment) (F (1, 33) = 4.551, p = 0.040) in the anhedonia subscale (Table 1).
Resting state functional connectivity results
After 8 weeks of treatment, compared with sham acupuncture plus fluoxetine, the verum acupuncture plus fluoxetine group showed significantly increased rsFC in both the ventral and dorsal striatal areas with cortical cortices. In particular, there were greater rsFC increases in the rsFC of right iVS-left rostral medial prefrontal cortex (rMPFC), left vrP – right amygdala (AMY) / parahippocampus (PHC) and right dC – left middle temporal gyrus (MTG). Verum acupuncture plus fluoxetine induced greater decreased rsFC between right vrP-right dorsolateral prefrontal cortex (DLPFC), left dC-bilateral cerebellar tonsil compared with sham acupuncture treatment. We also found increased rsFC between striatum seeds and occipital regions in verum acupuncture plus fluoxetine group than sham group, i.e. rsFC increased between right iVS-bilateral lingual gyrus, right iVS-right fusiform gyrus, and right iVS-left cuneus gyrus; right dcP – bilateral fusiform gyrus, right dcP- right cuneus gyrus and left dC – bilateral cuneus (Table 2).
Table 2.
Regions showed significantly increased connectivity of corticostriatum to other brain regions after acupuncture treatment and sham treatment, controlling for age as a covariate (voxel-wise, p < 0.005, uncorrected; cluster–wise, p < 0.05, FDR corrected).
| Cluster centroid (MNI) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Conditions | Seeds | Brain regions | Brodmann area |
mm3 | x | y | z | z- value |
| Verum acupuncture > sham acupuncture |
iVS | |||||||
| Right | Left rMPFC | BA10 | 246 | −6 | 60 | 24 | 3.54 | |
| Right occipital gyrus | BA18 | 1,028 | 42 | −94 | −4 | 4.19 | ||
| Left inferior occipital gyrus |
BA19 | 314 | −40 | −74 | −4 | 4.1 | ||
| Left | No region survive the threshold | |||||||
| vrP | ||||||||
| Left | Right amygdala/ parahippocampus |
398 | 22 | −10 | −10 | 4.72 | ||
| Right | No region survive the threshold | |||||||
| dcP | ||||||||
| Right | Right lingual/fusiform gyrus |
BA18 | 621 | 20 | −72 | −4 | 4.27 | |
| Left lingual/fusiform gyrus |
BA19 | 364 | −34 | −72 | 0 | 4.14 | ||
| Right cuneus gyrus | BA17 | 214 | 18 | −88 | 8 | 3.6 | ||
| Left | No region survive the threshold | |||||||
| dC | ||||||||
| Left | Bilateral cuneus gyrus | BA18 | 2,003 | −16 | −82 | 22 | 4.82 | |
| Right | Left middle temporal Gyrus |
BA39 | 344 | −26 | −52 | 34 | 4.63 | |
| Verum acupuncture < sham acupuncture | ||||||||
| vrP | ||||||||
| Right | Right DLPFC | BA9 | 276 | 36 | 42 | 38 | 3.81 | |
| dC | ||||||||
| Left | Right cerebellar tonsil | 235 | 16 | −38 | −40 | 4.09 | ||
| Left cerebellar tonsil | 270 | −20 | −44 | −44 | 4.07 | |||
Abbreviations: iVS, inferior ventral striatal; vrP, ventral rostral putamen; dcP, dorsal caudal putamen; dC, dorsal caudate; rMPFC, rostral medial prefrontal cortex; DLPFC, dorsolateral prefrontal cortex.
To further explore the association between the rsFC changes and the corresponding clinical outcome changes, we extracted the increased rsFC z scores (peak with 3mm radius) of left rMPFC, right AMY/PHC and MTG. We only focused on the rsFC changes from rMPFC, AMY/PHC and MTG because previous studies suggested these regions may play important roles in the neuropathology of MDD (Drevets, 2001; Ma et al., 2012). Then we applied a multiple regression analysis to investigate the association between the rsFC changes with the corresponding improvement of clinical outcomes, including age as a covariate across all participants. The results showed that the standardized reduction of depressive symptoms of MADRS negatively correlated (FDR corrected) with rsFC of iVS-rMPFC (p = 0.009), vrP-AMY/PHC (p = 0.048), and dC-MTG (p = 0.009). In addition, the standardized reduction of depressive symptoms of SDS was significantly negatively correlated with rsFC of vrP-AMY/PHC (p = 0.048) and dC-MTG (p = 0.016).
Discussion
In this study, we investigated the corticostriatal rsFC changes before and after 8 weeks of acupuncture plus fluoxetine treatment as compared with sham acupuncture plus fluoxetine in females with depression. The results showed a significant remission of depression symptoms in the real acupuncture plus fluoxetine group compared with the sham group. In addition, we also found verum acupuncture plus fluoxetine can significantly increase the rsFC of the corticostriatal reward circuits and decrease the rsFC of the striatal-cerebellar regions. The rsFC changes in the corticostriatum are also significantly associated with the symptom severity changes as indicated by MADRS and SDS scores, implying that acupuncture plus fluoxetine may achieve the treatment effect by modulating the rsFC of corticostriatal reward circuits.
Consistent with previous studies (Chan et al., 2015; Duan, Tu, & Chen, 2008; Liu, Zhang, Jin, & Liu, 2008; Roschke et al., 2000; Zhang et al., 2009), we found that the acupuncture stimulation plus fluoxetine could significantly relieve symptoms of depression, including anhedonia, compared with sham acupuncture plus fluoxetine treatment. In addition, we found that the symptoms in the sham acupuncture plus fluoxetine group showed a significant decrease after treatment (MADRS: p < 0.001; SDS: p < 0.001). We speculate that this may be attributed to the treatment effect of fluoxetine (Lam et al., 2016). The additional effect of verum acupuncture demonstrated that acupuncture can be combined with pharmacological treatment to achieve greater therapeutic effects in MDD patients.
Our results showed that the ventral corticostriatum (iVS and vrP) was more affected by verum acupuncture plus fluoxetine treatment compared with sham acupuncture plus fluoxetine treatment. Especially, we found that the rsFC was significantly increased between the right iVS-left rMPFC and the left vrP-right AMY/PHC. This result is consistent with previous studies showing the ventral striatum plays an important role in acupuncture treatment (Lee et al., 2015; Li et al., 2016; Pariente, White, Frackowiak, & Lewith, 2005). The rMPFC is an important region dedicated to representing the hedonic properties of reward, focusing on learning appropriate action-reward contingencies, and selecting those actions that potentially lead to reward (Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004). Studies found that the MPFC showed significant rsFC with subdivisions of striatal areas, especially with the ventral striatum (Felger et al., 2015; Meng et al., 2014). A previous study in MDD patients (Furman et al., 2011) showed attenuated rsFC between VS-MPFC in MDD compared with healthy controls. A previous study from our group also found verum acupuncture can significantly increase the rsFC between the MPFC and ventral striatum in knee osteoarthritis patients (Chen, Spaeth, Retzepi, Ott, & Kong, 2014).
Previous studies found that the rate of reduction of anxiety symptoms was associated with the reactivity of the ventral striatum and MPFC (Forbes et al., 2010; Fu, Steiner, & Costafreda, 2013; Phillips et al., 2015). Felger et al. (Felger et al., 2015) observed a negative correlation between decreased rsFC of left iVS-vmPFC and increased anhedonia subscale of the Inventory of Depressive Symptomatology-Self-Report in MDD. Consistent with the above studies, we also found the increased rsFC of iVS-mPFC was negatively correlated with standardized symptom reduction as measured by MADRS and SDS scores.
At a less conservative subthreshold (voxel wise p < 0.01, cluster wise p < 0.05, FDR correction), we found that verum acupuncture increased the rsFC of right vrP- rMPFC (p = 0.02; peak Z value: 3.66; peak: 12, 72, 14 and voxel number: 503). The rsFC between vrP- MPFC is also found in healthy controls (Di Martino et al., 2008). Felger and colleagues also found (2015) decreased right vrP-MPFC rsFC was negatively correlated with increased anhedonia. We also observed verum acupuncture plus fluoxetine treatment enhanced the rsFC between left vrP-AMY/PHC, which is negatively associated with the rate of symptom reduction. The vrP-AMY/PHC circuit is important to explain the increased memory sensitivity for negative stimuli in depressed subjects compared with healthy controls (Hamilton & Gotlib, 2008). The verum acupuncture plus fluoxetine treatment decreased the right vrP-right DLPFC more than sham acupuncture plus fluoxetine treatment. The DLPFC is one important region involved in emotion regulation (Erk et al., 2010; Goldin, McRae, Ramel, & Gross, 2008; Hwang et al., 2015; Staudinger, Erk, & Walter, 2011), and the DLPFC could modulate the putamen activity during reappraisal of reward anticipation (Staudinger et al., 2011).
In our study, the verum acupuncture plus fluoxetine treatment significantly increased rsFC changes between right dC-left MTG and right dC-bilateral cuneus compared with the sham acupuncture plus fluoxetine treatment. Recent studies found that brains of patients with MDD showed reduced activity in the temporal lobe and caudate during reward / decision-making processing as compared to healthy controls (Segarra et al., 2016; Yang et al., 2016). Resting-state fMRI studies also found that depressive disorders were accompanied by increased amplitude of low-frequency fluctuations in the temporal gyrus (Liu et al., 2011) and lower effective connectivity from the temporal lobe to caudate (Gao, Zou, He, Sun, & Chen, 2016). As the temporal lobe is involved in motor-sensory control and perception (Kilintari, Raos, & Savaki, 2014; Xu et al., 2015), we speculated that the dorsal caudate’s influence on the temporal cortex and visual areas may have an impact on the somatization effects of MDD, including sleep disturbance, and pain conditions such as tension headaches and musculo-tendinous pain. More studies are needed to investigate the association between the striatum and temporal lobe.
Another finding of our present study is that we found increased rsFC changes between subdivisions of striatal regions with occipital regions and decreased rsFC changes with the cerebellum (Table 2). Despite its roles in motor coordination and motor behavior (Flourens, 1842), the cerebellum is also involved in cognition and emotional processing through interaction with other brain areas (D’Angelo & Casali, 2013; Xi-Jian et al., 2013), such as the prefrontal cortex, temporal lobe, amygdala and striatum. Many previous studies have demonstrated the abnormal activity of the cerebellum and occipital areas (Guo et al., 2011; Liu, Wang, Liu, Liu, & Zhou, 2010; Peng et al., 2011; Wang, Hermens, Hickie, & Lagopoulos, 2012) in MDD. Thus, it is important to consider whether the posterior brain is home to the longer-term consolidated effects of acupuncture (Huang et al., 2012; Hui et al., 2005; Li et al., 2008; Yoo, Teh, Blinder, & Jolesz, 2004; Zhang et al., 2015). In this case, acupuncture treatment could benefit the depressed patient by increasing his or her resilience to a recurrent depressive episode.
There are several limitations in our study. First, we only recruited females with depression to increase the homogeneity of the study, and thus our external validity may only be applied to interpret the acupuncture mechanism on female patients. Secondly, the exploratory association analyses may induce variance inflation between the rsFC changes and the corresponding clinical outcome changes (Esterman et al., 2010), and thus other approaches are needed to explore the relationship between the acupuncture treatment effect and clinical outcome changes. Finally, we only focus on the effect of acupuncture treatment plus fluoxetine in this study; there is no placebo fluoxetine, which prevents us from exploring the effect and mechanism of fluoxetine as well as the interaction between fluoxetine and acupuncture.
In conclusion, we found that verum acupuncture modulates the rsFC of the ventral and dorsal portions of the striatum differently; the ventral portion of the striatum showed increased rsFC with the medial prefrontal cortex following acupuncture treatment, but the dorsal portion increased at temporal cortex and visual areas. The observed rsFC change of the corticostriatum was associated with clinical improvement in MDD patients, and the modulation process is believed to be underlying acupuncture treatment of MDD.
Supplementary Material
Figure 2.
Top row indicates locations of striatum seeds. Bottom row indicates the corresponding significant rsFC changes (post minus pre) evoked by verum acupuncture as compared with sham treatment. iVS, inferior ventral striatal; vrP, ventral rostral putamen; Dc, dorsal caudate; dcP, dorsal caudal putamen; rMPFC, ventral medial prefrontal cortex; PHC, parahippocampus; AMY, amygdala; MTG, middle temporal gyrus. Images were presented in neurological orientation.
Acknowledgments
The study was funded by 2013 project of South Korean Health Ministry. Jian Kong is supported by R01AT006364, R01 R01AT008563, R21AT008707, and P01 AT006663 from NIH / NCCIH. Ming Liu is supported by the Natural Science Foundation of China (No. 31371049).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Experimental design: XYW, BL, GNN, KCS
Data collection: JL, JC, XL, BL, GNN, KCS
Data analysis: ZJW, JK
Manuscript preparation: ZJW, JK, XYW, JL, KJ, RWH, ML, BL
Reference
- Abate KH. Gender disparity in prevalence of depression among patient population: a systematic review. Ethiop J Health Sci. 2013;23:283–288. doi: 10.4314/ejhs.v23i3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Arroll B, Macgillivray S, Ogston S, Reid I, Sullivan F, Williams B, Crombie I. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: a meta-analysis. Ann Fam Med. 2005;3:449–456. doi: 10.1370/afm.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Qin W, Tian J, Dai J, Yang W. Detection of dynamic brain networks modulated by acupuncture using a graph theory model. Progress in Natural Science. 2009;19:827–835. [Google Scholar]
- Ball SG, Ferguson MB, Martinez JM, Pangallo BA, Nery ES, Dellva MA, Sparks J, Zhang Q, Liu P, Bangs M, Goldberger C. Efficacy outcomes from 3 clinical trials of edivoxetine as adjunctive treatment for patients with major depressive disorder who are partial responders to selective serotonin reuptake inhibitor treatment. J Clin Psychiatry. 2016;77:635–642. doi: 10.4088/JCP.14m09619. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Theberge J, Densmore M, Bartha R, Neufeld R, Osuch E. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci. 2009;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Nikolova YS, Pizzagalli DA. Neurogenetics of depression: a focus on reward processing and stress sensitivity. Neurobiol Dis. 2013;52:12–23. doi: 10.1016/j.nbd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunlich K, Seger C. The basal ganglia. Wiley Interdiscip Rev Cogn Sci. 2013;4:135–148. doi: 10.1002/wcs.1217. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, Gibbs D, Petty C, Bizzell J, Dichter GS, Smoski MJ. Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. J Affect Disord. 2016;203:204–212. doi: 10.1016/j.jad.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae Y, Chang DS, Lee SH, Jung WM, Lee IS, Jackson S, Kong J, Lee H, Park HJ, Lee H, Wallraven C. Inserting needles into the body: a meta-analysis of brain activity associated with acupuncture needle stimulation. J Pain. 2013;14:215–222. doi: 10.1016/j.jpain.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Chan YY, Lo WY, Yang SN, Chen YH, Lin JG. The benefit of combined acupuncture and antidepressant medication for depression: A systematic review and meta-analysis. J Affect Disord. 2015;176:106–117. doi: 10.1016/j.jad.2015.01.048. [DOI] [PubMed] [Google Scholar]
- Chen X, Spaeth RB, Freeman SG, Scarborough DM, Hashmi JA, Wey HY, Egorova N, Vangel M, Mao J, Wasan AD, Edwards RR, Gollub RL, Kong J. The modulation effect of longitudinal acupuncture on resting state functional connectivity in knee osteoarthritis patients. Mol Pain. 2015;11:67. doi: 10.1186/s12990-015-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Spaeth RB, Retzepi K, Ott D, Kong J. Acupuncture modulates cortical thickness and functional connectivity in knee osteoarthritis patients. Sci Rep. 2014;4:6482. doi: 10.1038/srep06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Tang QS. Abdominal acupuncture in treating liver-qi stagnation and spleen deficiency in the elderly with post-stroke depression: A randomized and controlled observation. Journal of Clinical Rehabilitative Tissue Engineering Research. 2007:39. [Google Scholar]
- Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, Lim KO. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 2014;71:1138–1147. doi: 10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- D’Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits. 2013;6:116. doi: 10.3389/fncir.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel DG, Zimbroff DL, Potkin SG, Reeves KR, Harrigan EP, Lakshminarayanan M. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology. 1999;20:491–505. doi: 10.1016/S0893-133X(98)00090-6. [DOI] [PubMed] [Google Scholar]
- Deng D, Liao H, Duan G, Liu Y, He Q, Liu H, Tang L, Pang Y, Tao J. Modulation of the Default Mode Network in First-Episode, Drug-Naive Major Depressive Disorder via Acupuncture at Baihui (GV20) Acupoint. Front Hum Neurosci. 2016;10:230. doi: 10.3389/fnhum.2016.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136:407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, Lord C, Castellanos FX, Milham MP. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Domes G, Schulze L, Bottger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum Brain Mapp. 2008;31:758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Kong J, Webb M, Bonab AA, Fischman AJ, Gollub RL. A combined [11C]diprenorphine PET study and fMRI study of acupuncture analgesia. Behav Brain Res. 2008;193:63–68. doi: 10.1016/j.bbr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Duan DM, Tu Y, Chen LP. Assessment of effectiveness of electroacupuncture and fluoxetine for treatment of depression with physical symptoms. Zhongguo Zhen Jiu. 2008;28:167–170. [PubMed] [Google Scholar]
- Ebert D, Feistel H, Loew T, Pirner A. Dopamine and depression--striatal dopamine D2 receptor SPECT before and after antidepressant therapy. Psychopharmacology (Berl) 1996;126:91–94. doi: 10.1007/BF02246416. [DOI] [PubMed] [Google Scholar]
- Egorova N, Gollub RL, Kong J. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. NeuroImage: Clinical. 2015;9:430–435. doi: 10.1016/j.nicl.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30:15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Tamber-Rosenau BJ, Chiu YC, Yantis S. Avoiding non-independence in fMRI data analysis: leave one subject out. Neuroimage. 2010;50:572–576. doi: 10.1016/j.neuroimage.2009.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, Zhang G, Chen X, Shi S, Wang L, Liu R, Hwang J, Li Z, Tao J, Wang Y, Zhu B, Kong J. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol Psychiatry. 2016;79:266–273. doi: 10.1016/j.biopsych.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flourens P. Recherches experimentales sur les animaux vertebres. 1842 [Google Scholar]
- Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, Dahl RE. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Neurosci. 2010;10:107–118. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Furman DJ, Hamilton JP, Gotlib IH. Frontostriatal functional connectivity in major depressive disorder. Biol Mood Anxiety Disord. 2011;1:11. doi: 10.1186/2045-5380-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52:628–41. doi: 10.1016/j.jaac.2013.04.003. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Zou K, He Z, Sun X, Chen H. Causal connectivity alterations of cortical-subcortical circuit anchored on reduced hemodynamic response brain regions in first-episode drug-naive major depressive disorder. Sci Rep. 2016;6:21861. doi: 10.1038/srep21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WB, Sun XL, Liu L, Xu Q, Wu RR, Liu ZN, Tan CL, Chen HF, Zhao JP. Disrupted regional homogeneity in treatment-resistant depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1297–1302. doi: 10.1016/j.pnpbp.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, Deus J, Alonso P, Yucel M, Pantelis C, Menchon JM, Cardoner N. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol Psychiatry. 2011;16:604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- Huang W, Pach D, Napadow V, Park K, Long X, Neumann J, Maeda Y, Nierhaus T, Liang F, Witt CM. Characterizing acupuncture stimuli using brain imaging with FMRI--a systematic review and meta-analysis of the literature. PLoS One. 2012;7:e32960. doi: 10.1371/journal.pone.0032960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Liu J, Makris N, Gollub RL, Chen AJ, Moore CI, Kennedy DN, Rosen BR, Kwong KK. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum Brain Mapp. 2000;9:13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage. 2005;27:479–496. doi: 10.1016/j.neuroimage.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Hui KK, Marina O, Claunch JD, Nixon EE, Fang J, Liu J, Li M, Napadow V, Vangel M, Makris N, Chan ST, Kwong KK, Rosen BR. Acupuncture mobilizes the brain’s default mode and its anti-correlated network in healthy subjects. Brain Res. 2009;1287:84–103. doi: 10.1016/j.brainres.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JW, Egorova N, Yang XQ, Zhang WY, Chen J, Yang XY, Hu LJ, Sun S, Tu Y, Kong J. Subthreshold depression is associated with impaired resting-state functional connectivity of the cognitive control network. Transl Psychiatry. 2015;5:e683. doi: 10.1038/tp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JW, Xin SC, Ou YM, Zhang WY, Liang YL, Chen J, Yang XQ, Chen XY, Guo TW, Yang XJ, Ma WH, Li J, Zhao BC, Tu Y, Kong J. Enhanced default mode network connectivity with ventral striatum in subthreshold depression individuals. J Psychiatr Res. 2016;76:111–120. doi: 10.1016/j.jpsychires.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janig W. The intergative action of the autonomic nervous system. Cambridge University Press; 2006. [Google Scholar]
- Johnson J, Weissman MM, Klerman GL. Service utilization and social morbidity associated with depressive symptoms in the community. Jama. 1992;267:1478–1483. [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Griffiths KM, Rodgers B. Effectiveness of complementary and self-help treatments for depression. Med J Aust. 2002;176:S84–S96. doi: 10.5694/j.1326-5377.2002.tb04508.x. [DOI] [PubMed] [Google Scholar]
- Kavoussi B, Ross BE. The neuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther. 2007;6:251–257. doi: 10.1177/1534735407305892. [DOI] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Soukup J, Davis RB, Foster DF, Wilkey SA, Van Rompay MI, Eisenberg DM. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158:289–294. doi: 10.1176/appi.ajp.158.2.289. [DOI] [PubMed] [Google Scholar]
- Kilintari M, Raos V, Savaki HE. Involvement of the superior temporal cortex in action execution and action observation. J Neurosci. 2014;34:8999–9011. doi: 10.1523/JNEUROSCI.0736-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SN, Doo AR, Park JY, Bae H, Chae Y, Shim I, Lee H, Moon W, Lee H, Park HJ. Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson’s disease. PLoS One. 2011;6:e27566. doi: 10.1371/journal.pone.0027566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Webb JM, Kong JT, Vangel MG, Kwong K. Test-retest study of fMRI signal change evoked by electroacupuncture stimulation. Neuroimage. 2007;34:1171–1181. doi: 10.1016/j.neuroimage.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Ma L, Gollub RL, Wei J, Yang X, Li D, Weng X, Jia F, Wang C, Li F, Li R, Zhuang D. A pilot study of functional magnetic resonance imaging of the brain during manual and electroacupuncture stimulation of acupuncture point (LI-4 Hegu) in normal subjects reveals differential brain activation between methods. J Altern Complement Med. 2002;8:411–419. doi: 10.1089/107555302760253603. [DOI] [PubMed] [Google Scholar]
- Kwak Y, Peltier S, Bohnen NI, Muller ML, Dayalu P, Seidler RD. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson’s disease. Front Syst Neurosci. 2010;4:143. doi: 10.3389/fnsys.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RW, Levitt AJ, Levitan RD, Michalak EE, Cheung AH, Morehouse R, Ramasubbu R, Yatham LN, Tam EM. Efficacy of Bright Light Treatment, Fluoxetine, and the Combination in Patients With Nonseasonal Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2016;73:56–63. doi: 10.1001/jamapsychiatry.2015.2235. [DOI] [PubMed] [Google Scholar]
- Lee I-S, Wallraven C, Kong J, Chang D-S, Lee H, Park H-J, Chae Y. When pain is not only pain: Inserting needles into the body evokes distinct reward-related brain responses in the context of a treatment. Physiology & behavior. 2015;140:148–155. doi: 10.1016/j.physbeh.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Leh SE, Ptito A, Chakravarty MM, Strafella AP. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen V, Joukamaa M. Epidemiology of depression: prevalence, risk factors and treatment situation. Acta Psychiatr Scand Suppl. 1994;377:7–10. doi: 10.1111/j.1600-0447.1994.tb05794.x. [DOI] [PubMed] [Google Scholar]
- Li L, Liu H, Li YZ, Xu JY, Shan BC, Gong D, Li KC, Tang XW. The human brain response to acupuncture on same-meridian acupoints: evidence from an fMRI study. J Altern Complement Med. 2008;14:673–678. doi: 10.1089/acm.2008.0036. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu M, Lan L, Zeng F, Makris N, Liang Y, Guo T, Wu F, Gao Y, Dong M, Yang J, Li Y, Gong Q, Liang F, Kong J. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep. 2016;6:20298. doi: 10.1038/srep20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HD, Kim MH, Lee CY, Namgung U. Anti-Inflammatory Effects of Acupuncture Stimulation via the Vagus Nerve. PLoS One. 2016;11:e0151882. doi: 10.1371/journal.pone.0151882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Li F, Li SF, Wang YJ, Tie CL, Wu HY, Zhou Z, Zhang D, Dong J, Yang Z, Wang CY. Abnormal baseline brain activity in bipolar depression: a resting state functional magnetic resonance imaging study. Psychiatry Res. 2011;203:175–179. doi: 10.1016/j.pscychresns.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Liu J, Fang J, Wang Z, Rong P, Hong Y, Fan Y, Wang X, Park J, Jin Y, Liu C, Zhu B, Kong J. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J Affect Disord. 2016;205:319–326. doi: 10.1016/j.jad.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Liu P, Zhang Y, Zhou G, Yuan K, Qin W, Zhuo L, Liang J, Chen P, Dai J, Liu Y, Tian J. Partial correlation investigation on the default mode network involved in acupuncture: an fMRI study. Neurosci Lett. 2009;462:183–187. doi: 10.1016/j.neulet.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Y, Liu H, Liu Z, Zhou W. Diffusion tensor imaging and resting state functional magnetic resonance imaging on young patients with major depressive disorder. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:25–31. doi: 10.3969/j.issn.1672-7347.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang YH, Jin M, Liu WJ. Study on clinical effect enhancement of acupuncture for depression with chronic pain treated with SSRI antidepressants. Zhongguo Zhen Jiu. 2008;33:689–691. [PubMed] [Google Scholar]
- Ma C, Ding J, Li J, Guo W, Long Z, Liu F, Gao Q, Zeng L, Zhao J, Chen H. Resting-state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in major depression. PLoS One. 2012;7:e45263. doi: 10.1371/journal.pone.0045263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SD, Martin E, Rai SS, Richardson MA, Royall R. Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride: preliminary findings. Arch Gen Psychiatry. 2001;58:641–648. doi: 10.1001/archpsyc.58.7.641. [DOI] [PubMed] [Google Scholar]
- Meng C, Brandl F, Tahmasian M, Shao J, Manoliu A, Scherr M, Schwerthoffer D, Bauml J, Forstl H, Zimmer C, Wohlschlager AM, Riedl V, Sorg C. Aberrant topology of striatum’s connectivity is associated with the number of episodes in depression. Brain. 2014;137:598–609. doi: 10.1093/brain/awt290. [DOI] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:781–823. doi: 10.1016/s0278-5846(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Pariente J, White P, Frackowiak RS, Lewith G. Expectancy and belief modulate the neuronal substrates of pain treated by acupuncture. Neuroimage. 2005;25:1161–1167. doi: 10.1016/j.neuroimage.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Peng DH, Jiang KD, Fang YR, Xu YF, Shen T, Long XY, Liu J, Zang YF. Decreased regional homogeneity in major depression as revealed by resting-state functional magnetic resonance imaging. Chin Med J (Engl) 2011;124:369–373. [PubMed] [Google Scholar]
- Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JR, Deckersbach T, Trivedi MH. Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am J Psychiatry. 2015;172:124–138. doi: 10.1176/appi.ajp.2014.14010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitychoutis PM, Papadopoulou-Daifoti Z. Of depression and immunity: does sex matter? International Journal of Neuropsychopharmacology. 2010;13:675–689. doi: 10.1017/S1461145710000465. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Tian J, Bai L, Pan X, Yang L, Chen P, Dai J, Ai L, Zhao B, Gong Q, Wang W, von Deneen KM, Liu Y. FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network. Mol Pain. 2008;4:55. doi: 10.1186/1744-8069-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah-Smith I, Suo C, Williams MA, Sachdev PS. The Antidepressant Effect of Laser Acupuncture: A Comparison of the Resting Brain’s Default Mode Network in Healthy and Depressed Subjects During Functional Magnetic Resonance Imaging. Med Acupunct. 2013;25:124–133. doi: 10.1089/acu.2012.0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah-Smith I, Wen W, Chen X, Williams MA, Sachdev PS. The brain effects of laser acupuncture in depressed individuals: an fMRI investigation. Medical Acupuncture. 2012;24:161–171. doi: 10.1371/journal.pone.0012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Moran JM, Mavros PL, Tager-Flusberg H, Gabrieli JD, Whitfield-Gabrieli S. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front Hum Neurosci. 2013;7:573. doi: 10.3389/fnhum.2013.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rong P, Liu J, Wang L, Liu R, Fang J, Zhao J, Zhao Y, Wang H, Vangel M, Sun S, Ben H, Park J, Li S, Meng H, Zhu B, Kong J. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J Affect Disord. 2016;195:172–179. doi: 10.1016/j.jad.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschke J, Wolf C, Muller MJ, Wagner P, Mann K, Grozinger M, Bech S. The benefit from whole body acupuncture in major depression. J Affect Disord. 2000;57:73–81. doi: 10.1016/s0165-0327(99)00061-0. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neuronal Reward and Decision Signals: From Theories to Data. Physiol Rev. 2015;95:853–951. doi: 10.1152/physrev.00023.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, Dudas RB, Arrondo G, Robbins TW, Clark L, Fletcher PC, Murray GK. Abnormal Frontostriatal Activity During Unexpected Reward Receipt in Depression and Schizophrenia: Relationship to Anhedonia. Neuropsychopharmacology. 2016;41:2001–2010. doi: 10.1038/npp.2015.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y, Wang ZQ, Zhao ZL, Zhang M, Hao SL, Xu JY, Shan BC, Lu J, Li KC. An FMRI study of neuronal specificity in acupuncture: the multiacupoint siguan and its sham point. Evid Based Complement Alternat Med. 2014;2014:103491. doi: 10.1155/2014/103491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? Journal of psychiatry & neuroscience: JPN. 2008;33:331. [PMC free article] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Walter H. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb Cortex. 2011;21:2578–2588. doi: 10.1093/cercor/bhr041. [DOI] [PubMed] [Google Scholar]
- Sun R, Yang Y, Li Z, Li Y, Cheng S, Zeng F. Connectomics: a new direction in research to understand the mechanism of acupuncture. Evid Based Complement Alternat Med. 2011;2014:568429. doi: 10.1155/2014/568429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treasure J, Treasure T. Depression and outcome in acute myocardial infarction. Br Med J (Clin Res Ed) 1987;294:645–646. doi: 10.1136/bmj.294.6572.645-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noorden MS, van Fenema EM, van der Wee NJA, Zitman FG, Giltay EJ. Predicting outcome of depression using the depressive symptom profile: the Leiden Routine Outcome Monitoring Study. Depression and anxiety. 2012;29:523–530. doi: 10.1002/da.21958. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang L, Hermens DF, Hickie IB, Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord. 2012;142:6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang Z, Liu J, Chen J, Liu X, Nie G, Byun J-S, Liang Y, Park J, Huang R. Repeated acupuncture treatments modulate amygdala resting state functional connectivity of depressive patients. NeuroImage: Clinical. 2016 doi: 10.1016/j.nicl.2016.07.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Li XY, Deng AJ, Bo ZY. Comparative study on abdominal acupuncture and western medicine for treatment of menopause depressive disorder. Zhongguo zhen jiu= Chinese acupuncture & moxibustion. 2010;30:913–917. [PubMed] [Google Scholar]
- Wang XY, Yuan SH, Yang HY, Sun YM, Cheng FP, Zhang CL, Huang XC. Abdominal acupuncture for insomnia in women: a randomized controlled clinical trial. Acupunct Electrother Res. 2008;33:33–41. doi: 10.3727/036012908803861203. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liang P, Zhao Z, Han Y, Song H, Xu J, Lu J, Li K. Acupuncture modulates resting state hippocampal functional connectivity in Alzheimer disease. PLoS One. 2014;9:e91160. doi: 10.1371/journal.pone.0091160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wu J, Yeung AS, Schnyer R, Wang Y, Mischoulon D. Acupuncture for depression: a review of clinical applications. Canadian Journal of Psychiatry. 2012;57:397. doi: 10.1177/070674371205700702. [DOI] [PubMed] [Google Scholar]
- Xi-Jian D, Bi-Xia L, You-Jiang M, Jian J, Xian-Jun Z, Hong-Han G. Different Cerebellar Responding to Acupuncture at SP6 under Different Sleep States: an fMRI Study. Sleep Disorders: Treatment and Care. 2013 [Google Scholar]
- Xu J, Wang J, Fan L, Li H, Zhang W, Hu Q, Jiang T. Tractography-based Parcellation of the Human Middle Temporal Gyrus. Sci Rep. 2015;5:18883. doi: 10.1038/srep18883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XH, Huang J, Lan Y, Zhu CY, Liu XQ, Wang YF, Cheung EF, Xie GR, Chan RC. Diminished caudate and superior temporal gyrus responses to effort-based decision making in patients with first-episode major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:52–59. doi: 10.1016/j.pnpbp.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Yi Y, Xu F-m, Xie P, Lv F-j, Lin Y, Wu Y, Fucha J-s. Acupuncturing Taichong point for regulating the brain function of depression patients: restingstate fMRI study. China Journal of Traditional Chinese Medicine and Pharmacy. 2012;27:369–373. [Google Scholar]
- Yoo SS, Teh EK, Blinder RA, Jolesz FA. Modulation of cerebellar activities by acupuncture stimulation: evidence from fMRI study. Neuroimage. 2004;22:932–940. doi: 10.1016/j.neuroimage.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Zhang SQ, Wang YJ, Zhang JP, Chen JQ, Wu CX, Li ZP, Chen JR, Ouyang HL, Huang Y, Tang CZ. Brain activation and inhibition after acupuncture at Taichong and Taixi: resting-state functional magnetic resonance imaging. Neural Regen Res. 2015;10:292–297. doi: 10.4103/1673-5374.152385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WJ, Yang XB, Zhong BL. Combination of acupuncture and fluoxetine for depression: a randomized, double-blind, sham-controlled trial. J Altern Complement Med. 2009;15:837–844. doi: 10.1089/acm.2008.0607. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Wang XM, McAlonan GM. Neural acupuncture unit: a new concept for interpreting effects and mechanisms of acupuncture. Evid Based Complement Alternat Med. 2012;2012:429412. doi: 10.1155/2012/429412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiyun B. On abdominal acupuncture therapy. Chinese Acuponcture & Moxibustion. 2001;8:012. [Google Scholar]
- Zhong C, Bai L, Dai R, Xue T, Wang H, Feng Y, Liu Z, You Y, Chen S, Tian J. Modulatory effects of acupuncture on resting-state networks: a functional MRI study combining independent component analysis and multivariate Granger causality analysis. J Magn Reson Imaging. 2012;35:572–581. doi: 10.1002/jmri.22887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.