Abstract
Islets of Langerhans of all species harbor a small number of resident macrophages. These macrophages are found since birth, do not exchange with blood monocytes, and are maintained by a low level of replication. Under steady state conditions, the islet macrophages are in an activated state. Islet macrophages have an important homeostatic role in islet physiology. At the start of the autoimmune process in the NOD mouse, a small number of CD103+ dendritic cells (DC) are found at about the same time that CD4+ T cells also appear in islets. In the absence of the CD103+ DC in the Batf3 deficient mice, autoimmunity never develops. We discuss the interactions among the two phagocytes and beta cells that result in autoimmune diabetes in NOD mice.
The beta cells of the islets of Langerhans are the targets of diabetic autoimmunity. In human type 1 diabetes and in the diabetes of the NOD mouse, autoreactivity is directed to proteins produced by the beta cells, particularly insulin [1,2]. Examining the antigen presenting cells (APC) of the islets is important not only in the context of diabetic autoimmunity, but also for our general understanding of autoreactivity and host defense. Here we review recent studies on islet APCs in the context of autoimmunity. The islets of all species contain macrophages, and few, if any, DC. In the early stages of diabetic autoimmunity in the NOD mouse--by 3 to 4 weeks--a key DC appears in islets, that of the CD103+ DC lineage. This takes place concurrently with the arrival of CD4+ T cells and the development of a new gene expression program [3,4].
The resident myeloid cell in normal islets: the macrophage
Many of the early studies on islet APCs were made examining pancreata by histological procedures and few included islet isolation. In these early studies, including our own, the steady-state islet phagocytes were classified as dendritic cells based on their cell surface expression of CD11c. More critical marker studies, as well as gene expression analysis, identified them definitively as macrophages. Additionally, until inflammation begins, the macrophage is the only CD45+ cell readily detectable in the islets of Langerhans. See our review in 2014 that contains references to early reports [5].
Recent reports, most of them in the C57BL/6 (B6) mouse, have added new information on the development of tissue macrophages using lineage tracing procedures [6-10]. Most tissue resident macrophages develop during early embryogenesis. Under steady state conditions, the resident macrophages are maintained by a low level of replication and without the involvement of blood monocytes. Moreover, each tissue macrophage has unique features, an indication of their functional plasticity. In a recent study, we examined the islet macrophages, first focusing on islets from the non-diabetic (B6) mouse [11].
In the pancreas, macrophages are abundant in the inter-acinar stroma, while the islets contain a small number (~1-3% of total islet cellularity) [11-15 reviewed in ref 5]. Stromal and islet macrophages differ in their properties (Table 1). They are represented as a single set, positive for F4/80, CD11b, CD11c and, importantly, by high expression of class II-MHC molecules. These macrophages are found in the islets during embryogenesis and derive from definitive hematopoiesis. In contrast to the islets, two very different sets of macrophages are found in the inter-acinar stroma. These can be discriminated based on their expression of CD206, the mannose receptor, and CD301, CLEC10A. The CD206/301 positive macrophages cluster around ducts while the negative set shows no preference in localization and is found throughout the stroma. The CD206/CD301 set derives from yolk-sac hematopoiesis and has a slow turnover. The CD206/CD301-negative stromal set derives from blood monocytes and is constantly turning over. Islet macrophages do not exchange with blood monocytes, an issue that was examined by parabiosis distinguishing each of the parabionts by their differential expression of the two CD45 alleles, CD45.1 and CD45.2. After several weeks, at such times when there is blood interchange, the islet macrophages and stromal yolk-sac derived macrophages do not exchange between the two parabionts. The monocyte-derived stromal macrophages as well as those in the spleen exchange evenly between the two mice. Furthermore, using BrdU as a probe, we found a low level of replication in islet macrophages [11].
Table 1.
Features of pancreatic macrophages
| Islet macrophage | Stroma CD206+ macrophage | Stroma CD206− macrophage | |
|---|---|---|---|
| Properties | |||
| Derivation | Definitive* | Yolk-sac* | Monocytes |
| Exchange | No | No | With monocytes |
| Antigen presentation | + | Poor | + |
| Cell Surface Markers | |||
| F4/80 | + | + | + |
| CD11b | + | + | + |
| CD11c | + | + | + |
| MHC-II | + | +/− | + |
| CD64 | + | + | + |
| CD68 | + | + | + |
| LyzM | + | + | + |
| CX3CR1 | + | +/− | +/− |
| CD206 | − | + | − |
| CD301 | − | + | − |
| Gene Expression | |||
| II1b | + | − | − |
| Tnfa | + | − | − |
| Nos2 | − | − | − |
| Arg1 | − | +/− | +/− |
| II10 | − | + | − |
| Ym1 | − | − | + |
| Fizz1 | − | + | + |
This Table summarizes the reports in references 3 and 11. None of the three macrophages sets expressed genes typical of DC.
Most important are the findings of different gene expression patterns between the stromal and islet macrophages. The islet macrophages have an M1-like activation pattern, with expression of TNFα and IL-1β transcripts. Also of note is that they express high levels of MHC-II on their surface. The stromal macrophages have an M2-like pattern of transcripts, expressing Retnla, Arg1, Ym1, and IL-10, genes that are expressed in a typical M2-like macrophage [16]. Others have claimed, correctly, that the gene expression patterns of macrophages are diverse and complex when compared amongst macrophages from different tissues and under different conditions. Therefore, an M1-M2 classification is an oversimplification [17]. We agree, but still our point stands that the gene expression patterns of islets and stromal macrophages are distinct and compatible with the M1/M2-like patterns.
The pattern of gene expression in islet and stromal macrophages was maintained during adulthood. A definitive experiment in the study of Calderon et al. was to irradiate the B6 mouse and engraft it with bone marrow stem cells [11]. The reconstituted islet and stromal macrophages had the same features as the original inhabitants. In the islet, the replaced macrophage adopted the M1-like pattern, while in the stroma, it favored the M2-like. Thus, this led to the conclusion that the “pancreas anatomy conditions the origin and properties of the resident macrophages.” How the tissue microenvironment conditions the biology of macrophages is not known at present. Recent studies have discussed this issue [18-20]. An important report showed that macrophages isolated from yolk-sac, fetal liver, and fetal monocytes differentiated into alveolar macrophages when injected into lungs lacking alveolar macrophages. These macrophages expressed transcriptional profiles identical to the alveolar macrophage. As was pointedly stated, “tissue imprinting is a dominant factor in shaping the gene expression landscape of macrophages” [21].
The islet macrophage has an important homeostatic function, first reported when examining the osteopetrotic op/op mouse [22]. (Pollard has paid much attention to this homeostatic function of macrophages in many tissues; see his reviews in refs 23 and 24.) The op/op mouse has a spontaneous null mutation in the Csf1 gene leading to a reduction or absence in macrophages of many organs. The islets of the op/op mouse are highly deficient in macrophages, most having none at all, with a few islets having one or two. The islets are small, about half the size of control mice; about a third of the mice have an impaired glucose tolerance indicating a poor insulin response [11]. How the beta cell and the macrophages are interacting is not known. Beta cells express high levels of VEGF responsible for the development and maintenance of vascular structures. The Power laboratory just reported an experimental system in VEGF null mice [25]. Beta cell proliferation was related to the presence of recent monocyte migrants that differentiated into macrophages. They speculated that growth factors from the macrophages stimulated the growth of the beta cells [25, 26]. The intra-islet blood vessels could be another target of the macrophages. Islet macrophages are always located next to blood vessels. Live images show filopodia extending between beta cells with some of them protruding into the vessel lumen [27]. Macrophages also capture beta cell granules and can present antigens as we discuss below (Fig.1).
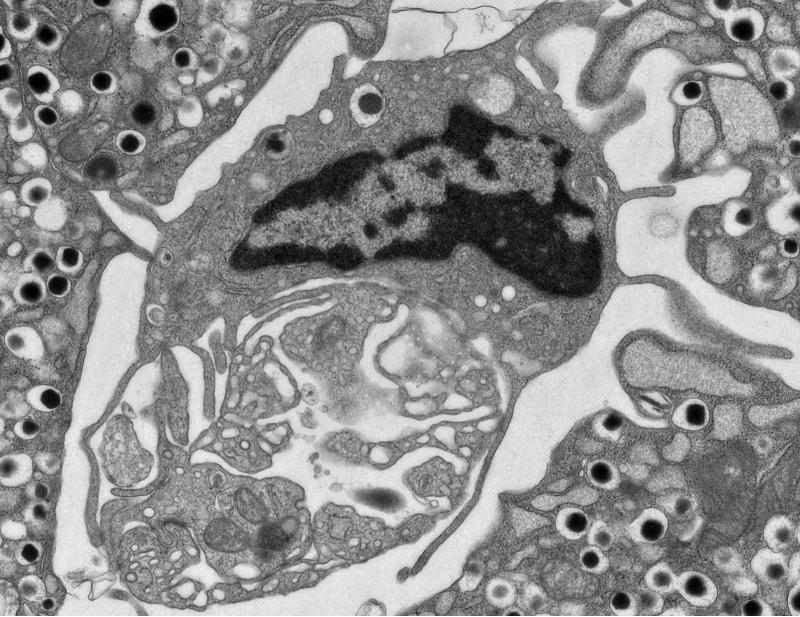
Figure 1.
An electron micrograph of an islet from an 8 week-old NOD mouse. The image shows a phagocyte wrapped around a blood vessel. Note a dense core granule inside a phagocytic vesicle. Islet phagocytes contain about 10 granules per cell. Unpublished micrograph.
In brief, there is a true symbiotic relationship between endocrine cells and the resident macrophages. Most likely the endocrine cells secrete molecules that make the macrophages convert to a trophic supportive role. Likewise, the macrophage exerts an influence on the endocrine cells as noted in the op/op mouse. We speculate that besides this homeostatic role, the islet macrophage may also have a protective role against pathogens in the circulation. This protective role has been suggested in infections with the beta cell trophic-encephalomyocarditis virus [28]. Finally, we are only considering here the resident macrophage, but we note that monocyte-derived macrophages participate as an important effector cell in the inflammatory stages of the autoimmune diabetic process.
The seminal role of the CD103+ DC
In the NOD mouse, and only in the NOD mouse, islets also contain the subset of DC characterized by expression of CD103 (αE integrin) [3]. The CD103+ DCs are found in the islets of NOD mice in very small numbers or not at all at the 3-4 week period of life, but increase shortly thereafter [3]. In NOD.Rag1−/− mice, their number is very low, about 1-2% of the islet myeloid cells. In B6 strains, there is no appreciably detectable number of CD103+ DCs.
The CD103+ DC are found in the islets at about the same time that a few CD4+ T cells enter, at the very initial stage of the autoimmune process. The early T cells, many of which recognize insulin epitopes, are found in tight contact with the islet APCs. With the presence of CD4 T cells and the CD103+ DC also appears an interferon-inducible gene expression signature [4]. This is the initial stage of diabetogenesis at ~3 weeks of age. Autoimmunity then progresses, with the pancreatic lymph node becoming an essential component where there is presentation of different MHC-I and MHC-II peptides, and with a seminal amplification of the response. How the islet communicates with the pancreatic node has not been ascertained. Islets lack detectable lymphatic vessels, but they are found in the stroma, sometimes next to the islets.
The CD103+ DC are characterized by their strong capacity to cross-present MHC-I epitopes to CD8 T cells [29]. Ken Murphy's group identified the Batf3 transcription factor as critical for the differentiation of the common DC precursor to the CD103+/CD8α+ lineage [30,31]. In his studies, the Batf3−/− mice were poor at cross-presenting antigens to CD8+ T cells. The mice were susceptible to viral infections and were poor at eliminating methylcholanthrene-induced sarcomas. We examined the role of the CD103+ DC in the Batf3 gene knockout mouse backcrossed to NOD, and in agreement, found that the NOD.Batf3−/− lacked the CD103/CD8α DC lineage of DCs in all tissues [3]. NOD.Batf3−/− mice never developed diabetes. Islets were never infiltrated by inflammatory leukocytes and did not show the gene expression signatures of inflammation that typically develops in the NOD by the 4th to 8th week of life. The gene expression analysis of NOD.Rag1−/− and the NOD.Batf3−/− were practically identical. Through a number of cell transfers, it became evident that the absence of diabetes in the Batf3-deficient mice was caused by the lack of the CD103+ DCs. The T cells in the Batf3-mice were fully competent and transferred diabetes into NOD.Rag1−/− mice. The Batf3−/− mice show no accumulation of the early CD4 T cells, and presentation of MHC-II epitopes was partially defective while presentation of MHC-I was completely absent.
In brief, several defects were noted in the absence of the CD103+DC lineage: (i) the early stage of diabetes was undetectable, (ii) there was no accumulation of the initial T cells into islets, (iii) the early inflammatory gene signature was absent, (iv) there was poor priming of diabetogenic CD4 and CD8 T cell responses, and (v) there was a lack of islet receptivity to T cell entrance. These results were found despite the presence of the islet-resident macrophage that expresses high levels of the insulin peptide-MHC-II complex. We hypothesize that that the CD103+ DC has a key role in the transport of immunogenic material to the pancreatic lymph node, a site that is required for the rapid amplification and progression of the autoreactive response. Still, there is a paucity of the intra-islet events in the absence of the CD103+DC. Thus, whether the macrophages are involved in the recruitment of DC and/or interacting with them are issues to be investigated. Indeed, the interactions among the three key cells, i.e., beta cells, macrophages and CD103+DC, needs to be explained.
Antigen presentation in islets
Both the islet macrophages and the CD103+ DC can process and present insulin epitopes to autoreactive T cells [5]. This has been shown by direct isolation of each of the islet phagocytes and then testing whether they can elicit IL-2 production by antigen-specific T cell hybridomas. At the same time, antigen presentation of islet products also takes place in the draining pancreatic lymph node. This can be visualized by transferring beta-cell antigen specific primary T cell clones into mice and observing their division using fluorescent dyes. In non-diabetogenic strains of mice, islets only have macrophages, and these are still effective antigen-presenting cells when isolated and tested.
One important concern is how the beta cell passes its antigens to the local APCs. Our laboratory just reported on a series of studies examining the interactions between beta cells and APCs combining in vivo and ex vivo experiments [32]. Beta cells were isolated from normal mice and incubated with various APC. After a period of time, presentation to CD4 T cells reactive against insulin was then examined. Presentation to CD4 T cells reactive to different insulin epitopes was strong, indicating that the APC had acquired immunogenic material from the beta cells. We examined T cells that respond to two different registers of the insulin 9-23 segment [33,34]. One group of T cells responds to both the insulin protein and the 9-23 peptide, whereas the second group responds the 9-23 peptide, not to insulin protein. The presentation of insulin epitopes to T cells required close contact between the APC and beta cells; separating the beta cells from the APC resulted in an absence of transfer. It also required live beta cells, indicating an active transport process. The transfer was increased when the level of glucose in the media was high in a process that depended on mobilization of intracellular Ca++. Beta cells from NOD.Rag1−/− mice, the non-diabetic C57BL/6 mouse, and human beta cells all transferred granules to NOD mouse APCs [32]. (The transfer of beta-cell protein to APCs has also been observed, albeit in less detail, in the non-diabetic B10.BR mouse.)
We concluded that inflammation or beta cell death was not a requirement for the initial presentation of islet antigens to take place. The granules that were transferred included the typical dense core granule that has a high content of insulin, as well as granules that mainly contain insulin peptides. These are the crinophagic granules identified by cell biologists in endocrine organs that contain degradative products of the secretory granule [35, 36]. Our data indicate that these granules contain the peptides that activate the peptide specific T cells.
Ultrastructural examination of islets from various strains of mice showed phagocytes next to blood vessels. Of interest, all phagocytes had taken up the dense core granules that contain insulin. Surprisingly the granules were transferred intact; the phagocyte's endocytic vesicle contained the granule within its own membrane. This is a process very different from how the insulin-containing granule is released after a glucose challenge in which its membrane is incorporated into the plasma membrane of the beta cell. We concluded that beta cells interact closely with the APC forming a beta cell:APC synapse. In this synapse, the dense core granules are concentrated and are transferred intact to the APC. This process is taking place constitutively in all islets and in all strains of mice. It becomes relevant in the autoimmune situation in which the genetic program favors autoreactivity.
Conclusions
Under steady state conditions, the pancreas contains macrophages both in the inter-acinar stroma and in the islets of Langerhans. These are very different in their origin and properties. We discussed the resident macrophage of the islets having a state of activation and an important homeostatic role in islet development. The start of the autoimmune process in the NOD mouse is characterized by the appearance in islets of a small number of CD103+ DC together with insulin-reactive CD4 T cells and a new gene inflammatory program. Gene ablation of the CD103+ DC lineage results in the absence of diabetes. We discussed the interactions among beta cells, macrophages, and CD103+DC in the autoimmune process.
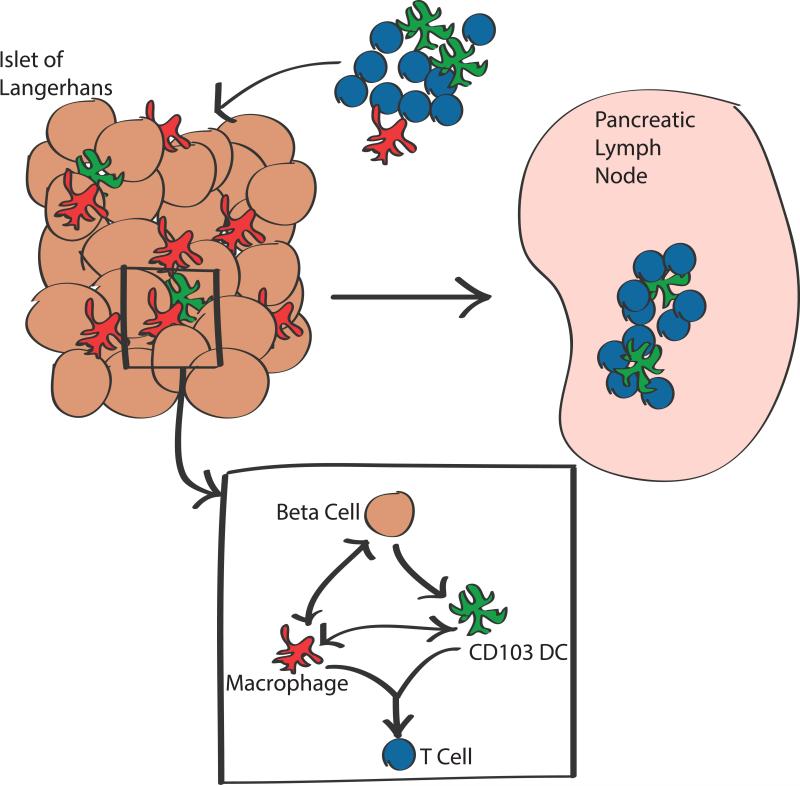
Figure 2.
The graph summarizes the main points made in the review. Islets of all species contain macrophages that are in true symbiosis with beta cells. In NOD mice at the start of the autoimmune process, the CD103+ DC join the macrophages in the islets. Both capture dense core granules and present to insulin-reactive CD4 T cells. However, it is the CD103+DC that is essential to move the autoimmune process forward. At the same time CD4 T cells to insulin appear in islets together with a new gene signature. The process readily extends to the pancreatic node where the autoimmune process is amplified.
Highlights.
Pancreatic islets contain resident macrophages that self-replicate
Islet macrophages capture secretory vesicles and present beta cell antigens
Macrophages have an M1-pattern of gene expression
A CD103+ dendritic cell enters islets during autoimmune diabetes
Autoimmune diabetes does not develop in mice lacking CD103+ dendritic cells
Acknowledgments
Our work cited in the text is supported by grants from the NIH and the Juvenile Diabetes Research Foundation. We thank all the members of the laboratory who have participated in the experiments mentioned here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brezar V, Carel JC, Boitard C, Mallone R. Beyond the hormone: insulin as an autoimmune target in type 1 diabetes. Endocr Rev. 2011;32:623–669. doi: 10.1210/er.2011-0010. [DOI] [PubMed] [Google Scholar]
- 2*.Unanue ER. Antigen presentation in the autoimmune diabetes of the NOD mouse. Annu Rev Immunol. 2014;32:579–608. doi: 10.1146/annurev-immunol-032712-095941. [Both these reviews consider the clinical and experimental evidence for insulin as a major autoantigen driving diabetic autoimmunity.] [DOI] [PubMed] [Google Scholar]
- 3**.Ferris ST, Carrero JA, Mohan JF, Calderon B, Murphy KM, Unanue ER. A minor subset of Batf3-dependent antigen presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity. 2014;41:657–669. doi: 10.1016/j.immuni.2014.09.012. [This report shows that the absence of the cD103+DC in the NOD. Batf3−/− results in a complete absence of diabetes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER. Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS One. 2013;8:e59701. doi: 10.1371/journal.pone.0059701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderon B, Carrero JA, Unanue ER. The central role of antigen presentation in islets of Langerhans in autoimmune diabetes. Curr Opin Immunol. 2014;26C:32–40. doi: 10.1016/j.coi.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Ann Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 7.Boyer SW, Schroeder AV, Smith-Berdan S, Forsberg ED. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell. 2011;9:64–73. doi: 10.1016/j.stem.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas S. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17:34–40. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- 9.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2016;17:2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [References 6 to 10 are important contributions on the development of tissue resident macrophages.] [DOI] [PubMed] [Google Scholar]
- 11**.Calderon B, Carrero JA, Ferris ST, Sojka DK, Moore L, Epelman S, Murphy KM, Yokoyama WM, Randolph GJ, Unanue ER. The pancreas anatomy the origin and properties of resident macrophages. J Exp Med. 2015;212:1497–1512. doi: 10.1084/jem.20150496. [This report examined the origin and biology of pancreatic macrophages and underscored the differences between those residing in the interacinar stroma and those of the islets.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geutskens SB, Otonkoski T, Pulkkinen MA, Drexhage HA, Leenen PJ. Macrophages in the murine pancreas and their involvement in fetal endocrine development in vitro. J Leukoc Biol. 2005;78:845–852. doi: 10.1189/jlb.1004624. [DOI] [PubMed] [Google Scholar]
- 13.Ehses JA, Perren A, Eppler E, Ribaux P, Pspisilik JA, Maor-Cahn R, Bueripel X, Ellingsgaard H, Schneider MK, Biollaz G, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 14.Criscimanna A, Coudriet GM, Gittes GK, Piganelli JD, Esni F. Activated macrophages create lineage-specific microenvironments for pancreatic acinar- and β-cell regeneration in mice. Gastroenterology. 2014;147:1106–1118. doi: 10.1053/j.gastro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Westwell-Roper CY, Ehses JA, Verchere CB. Resident macrophages mediate islet amyloid polypeptide-induced islet IL1β production and β-cell dysfunction. Diabetes. 2014;63:1698–1711. doi: 10.2337/db13-0863. [DOI] [PubMed] [Google Scholar]
- 16.Gordon S. Alternative activation of macrophages. Nature Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 17*.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [A cogent analysis and discussion of the gene expression patterns of macrophages under different conditions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2016;17:18–25. doi: 10.1038/ni.3325. [DOI] [PubMed] [Google Scholar]
- 21*.van de Laar L, Saelens W, De Prijck S, Martens L, Scott CL, Isterdael GV, Hoffmann E, Beyaert R, Saeys Y, Lambrecht BN, et al. Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue-resident macrophages. Immunity. 2016;44:755–768. doi: 10.1016/j.immuni.2016.02.017. [Macrophages from different embryological origens or monocytes can develop into alveolar macrophages indicating that the tissue environment is a major factor in their final differentiation.] [DOI] [PubMed] [Google Scholar]
- 22.Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, Polak M. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol. 2004;76:359–367. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- 23.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brissova M, Aarnodt K, Brahmachary P, Prasad N, Hong JY, Dai C, Mellati M, Shostak A, Poffenberger G, Aramandia R, et al. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration. Cell Metab. 2014;19:498–511. doi: 10.1016/j.cmet.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao X, Gittes GK. Concise Review: New insights into the role of macrophages in β-cell proliferation. Stem Cells Transl Me. 2015;4:655–8.7. doi: 10.5966/sctm.2014-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calderon B, Carrero JA, Miller MJ, Unanue ER. Cellular and molecular events in the localization of diabetogenic T cells to islets of Langerhans. Proc Natl Acad Sci USA. 2011;108:1567–1572. doi: 10.1073/pnas.1018973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCartney SA, Vermi W, Lonardi S, Rossini C, Otero K, Calderon B, Gilfillan S, Diamond MS, Unanue ER, Colonna M. RNA sensor-induced type I IFN prevents diabetes caused by a β cell-tropic virus in mice. J Clin Invest. 2011;121:1497–1507. PMC3069767. doi: 10.1172/JCI44005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 30**.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [An important paper showing the role of the Batf3 transcription factor in the differentiation of the DC lineage. The gene ablated mice lack the CD8 alpha/CD103+ DC.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edelson BT, KC W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vomund AN, Zinselmeyer BH, Hughes J, Calderon B, Valderrama C, Ferris ST, Wan X, Kanekura K, Carrero JA, Urano F, Unanue ER. Beta cell transfer vesicles containing insulin to phagocytes for presentation to T cells. Proc Natl Acad Sci USA. 2015;112:5496–5502. doi: 10.1073/pnas.1515954112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohan JF, Calderon B, Anderson MS, Unanue ER. Pathogenic CD4+ T cells recognizing an unstable peptide of insulin are directly recruited into islets bypassing local lymph nodes. J Exp Med. 2013;210:2403–2414. doi: 10.1084/jem.20130582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arvan P, Halban PA. Sorting ourselves out: Seeking consensus on trafficking in the beta-cell. Traffic. 2004;5:53–61. doi: 10.1111/j.1600-0854.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 36.Orci L, Ravazzola M, Amherdt M, Yanaihara C, Yanaihara N, Halban P, Renold AE, Perrelet A. Insulin, not C-peptide (proinsulin), is present in crinophagic bodies of the pancreatic B-cell. J Cell Biol. 1984;98:222–228. doi: 10.1083/jcb.98.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]