Abstract
Isoliquiritigenin is a botanical estrogen used as a dietary supplement. Previous studies show that other botanical estrogens affect ovarian estradiol synthesis, but isoliquiritigenin’s effects on the ovary are unknown. Thus, this study tested the hypothesis that isoliquiritigenin inhibits ovarian antral follicle growth and steroidogenesis. Antral follicles from CD-1 mice were cultured with vehicle control (dimethyl sulfoxide; DMSO) or isoliquiritigenin (0.6 μM, 6 μM, 36 μM, and 100 μM) for 48–96h. During culture, follicle diameters were measured daily to assess follicle growth. After culture, media were collected for hormone assays and follicles were collected for gene expression analysis of steroidogenic enzymes. Isoliquiritigenin inhibited antral follicle growth and altered estradiol, testosterone, and progesterone levels. Additionally, isoliquiritigenin altered the mRNA levels of cytochrome P450 steroid 17-α-hydroxylase 1, aromatase, 17β-hydroxysteroid dehydrogenase 1, and steroidogenic acute regulatory protein. These data indicate that exposure to isoliquiritigenin inhibits growth and disrupts steroid production in antral follicles.
Keywords: Isoliquiritigenin, Licorice root, Ovary, Antral follicle, Steroidogenesis
1. Introduction
Phytoestrogens are phenolic compounds found in plants that can interact with estrogen receptors and other targets in endogenous estrogen production and action [1]. Although potential benefits have been identified with the use of phytoestrogens [2], their adverse effects are less understood. Isoliquiritigenin is a flavonoid phytoestrogen extracted from the roots of Glycyrrhiza, a type of licorice. The U.S. Food and Drug Administration (FDA) classifies licorice and licorice extracts/derivatives as generally recognized as safe (GRAS) for use in foods (21 CFR 184.1408) and animal feeds (21 CFR 582.10; 582.20). They are also FDA-approved for use in certain over-the-counter drugs (21CFR 310.528; 310.544; 310.545) [3]. Licorice is used as a flavoring agent in candy, gum, tobacco, toothpaste, cough mixtures, herbal teas and other beverages. It is also frequently found in skin care products [4]. Licorice has been used extensively in traditional Asian and European medicine to treat conditions ranging from peptic ulcers, pharyngitis, and abdominal pain, to asthma, insomnia, malaria, and other infections [5]. Licorice may also be effective in weight loss and metabolic syndrome [5, 6]. Dietary supplements containing licorice are popular among women for relief from symptoms associated with premenopausal syndrome and menopause [5, 7].
Isoliquiritigenin, one of the bioactive components of licorice, is often used as an anti-inflammatory, antimicrobial, anti-diabetic, and anti-tussive agent [5, 8]. Moreover, isoliquiritigenin has been found to inhibit growth and aromatase activity of breast cancer cells and thus, has potential to be used as a chemotherapeutic agent in breast cancer [9–11]. It may also have potential utility as a therapy in other cancers, as it has been found to inhibit mouse colon cancer and proliferation of prostate cancer cells [12, 13]. Isoliquiritigenin reduces contraction in the mouse uterus, indicating that it may be useful for treating uterine pain due to excessive contraction [14, 15]. Thus, exposure to isoliquiritigenin commonly occurs through various licorice-containing foods and products, as well as through the clinical use of isoliquiritigenin and licorice supplements for a wide variety of medical conditions. Average daily human exposure to isoliquiritigenin through the diet and/or supplements is estimated to be 1–2 mg/kg [6]. Chronic exposure is possible with high intake of licorice-flavored tobacco and in individuals consuming licorice tablets or capsules as a heath product [3]. The female population may be more likely to be exposed to isoliquiritigenin, especially postmenopausal women seeking alternatives to traditional hormone replacement therapy [6, 7].
Unfortunately, to our knowledge, the effects of isoliquiritigenin on the ovary have not been published. Chemicals that target the ovary may disrupt ovarian function, resulting in decreased fertility, reduced estradiol synthesis, and premature ovarian failure [16]. The ovary contains numerous estrogen receptors and thus, it is an important target organ for phytoestrogens such as isoliquiritigenin, which can bind to estrogen receptors [7, 17, 18]. This, coupled with the fact that isoliquiritigenin has broad clinical and commercial applications, makes it imperative to understand how this botanical compound affects the ovary. Additionally, understanding the effects of isoliquiritigenin on the ovary may have applications in veterinary medicine for production animals that graze on Glycyrrhiza, and might be compromising their reproductive value.
Thus, the present study examined the effects of isoliquiritigenin on ovarian antral follicles, which are responsible for sex steroid production and further development into ovulatory follicles. Specifically, the present study tested the hypothesis that isoliquiritigenin inhibits antral follicle growth and sex steroid synthesis in the adult mouse ovary.
2. Materials and methods
2.1. Animals
Adult, cycling, female CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA) and acclimatized for 24–72 h in the College of Veterinary Medicine Animal Facility at the University of Illinois at Urbana-Champaign. The mice were housed in groups of four, in a controlled environment (22 ± 1 °C, 12 h light-dark cycles) and provided food and water ad libitum. All procedures involving animal care, euthanasia, and tissue collection were approved by the Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign.
2.2. Chemicals
Isoliquiritigenin (98% pure as ascertained by high-performance liquid chromatography) was supplied by the Botanical Estrogen Research Center, University of Illinois at Urbana-Champaign. Dimethylsulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) was used as the vehicle to dissolve the isoliquiritigenin and to prepare treatment concentrations of 0.6 μM, 6 μM, 36 μM, and 100 μM, which are approximately equivalent to in vivo doses of 0.1 mg/kg, 1.6mg/kg, 9.6 mg/kg, and 26.6 mg/kg respectively. To our knowledge, data are not available on the plasma isoliquiritigenin levels in women consuming licorice and licorice root supplements or on the potential toxicity of isoliquiritigenin on the ovary. Taking into account this paucity of information, a broad range of concentrations was chosen for the present study. The doses of 0.6 μM and 6 μM fall within the range of estimated human exposure to isoliquiritigenin (1–2 mg/kg) [6]. These doses are also representative of the plasma isoflavone levels that have been found in humans for other phytoestrogens such as daidzein and genistein [1, 19]. Previous studies have found genistein to be a toxicant for granulosa cells at concentrations of 50 μM, while daidzein, another phytoestrogen, was not toxic at dosages as high as 100 μM [20]. These varying results to different exposures of different phytoestrogens warrant testing isoliquiritigenin at concentrations ranging from 0.6 μM to 100 μM. The isoliquiritigenin concentrations tested in the present study are also similar to those used in previously published in vitro and in vivo studies investigating its effects on glioma cells, cervical cancer cells, and heart muscle [21–23].
2.3. Antral follicle culture
Adult, cycling, female CD-1 mice were euthanized on postnatal days (PND) 32–35, and based on relative follicle size (225–400 μm), antral follicles were manually isolated from the ovaries and cleaned of interstitial tissue using watchmaker forceps. The age selection of the mice in the current study is based on previous studies using the same follicle culture method [24, 25]. Follicles were pooled from four to five different mice for each experiment, with 20–40 follicles obtained from each mouse. The follicles were individually plated in wells of 96-well tissue culture plates, so that each treatment group contained 10–18 follicles. The different treatment groups (DMSO or vehicle control, isoliquiritigenin at 0.6 μM, 6 μM, 36 μM, and 100 μM) were prepared in supplemented α-MEM, containing 1% ITS (10ng/mL insulin, 5.5 ng/mL transferrin, 5.5 ng/mL selenium, Sigma-Aldrich, St. Louis, MO), 100 U/mL penicillin (Sigma-Aldrich, St. Louis, MO), 100 mg/mL streptomycin (Sigma-Aldrich, St. Louis, MO), 5 IU/mL human recombinant follicle-stimulating hormone (FSH; Dr. A. F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA), and 5% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA) as described previously [25]. Various stock concentrations of isoliquiritigenin (0.2, 2.05, 12.29, and 34.16 mg/mL) were prepared using DMSO, which allowed for an equal volume of each stock to be added to the culture wells to control for vehicle concentration (0.75 μL per mL of medium). This translated to final working concentrations of 0.15, 1.54, 9.22, and 25.62 μg of isoliquiritigenin per mL of culture medium, which is equivalent to 0.6 μM, 6 μM, 36 μM, and 100 μM, respectively (molecular weight of isoliquiritigenin: 256.25 g/mol). The cultures were carried out for total periods of 48, 72, and 96 h in an incubator supplying 5% CO2 at 37 °C. The experimental endpoints of follicle growth, sex steroid hormone levels, and mRNA levels of selected regulators of steroidogenesis were evaluated at these time-points. The cultures were performed for up to 96 h because previous studies indicate that non-treated follicles and vehicle control (DMSO) follicles continue to grow and remain viable up to 96 h without medium changes [25, 26].
2.4. Follicle growth analysis
The growth of antral follicles over time was assessed by measuring follicle diameters on perpendicular axes at every 24 h time point, with an inverted microscope equipped with a calibrated ocular micrometer. Follicles with diameters of 225–400 μm were considered to be antral follicles, which correlates with the histological appearance of these follicles [27]. The individual diameters were averaged within treatment group for each 24 h interval, and the average values were divided by the initial average measurement (0 h) of each of the respective treatment groups to calculate the percent change in follicle diameter over time. This percent change in antral follicle diameter was used for statistical analysis.
2.5. Hormone assays
After each 48, 72, and 96 h culture, medium from each well was collected and pooled according to treatment group, and then subjected to enzyme linked immunosorbent assays (ELISAs) for measuring the levels of progesterone, testosterone, and 17β-estradiol. These sex steroid hormones were chosen because they are essential for normal female reproductive function. The levels of these hormones were measured using ELISA kits purchased from Diagnostics Research Group (DRG, Springfield, NJ) according to manufacturer’s instructions. Samples were run in duplicate, and had inter-assay and intra-assay coefficients of variability of less than 15%. Samples were diluted as needed to match the dynamic range of each ELISA kit, and read with Multiskan Ascent software (Thermo Scientific). The mean values for each sample were used for statistical analysis. Cross reactivity of isoliquiritigenin was checked in all the assays, and when background was present, it was subtracted from sample means before analysis. Cross reactivity of isoliquiritigenin was negligible in the progesterone and testosterone assays, and less than 2% in the estradiol assay.
2.6. Quantitative real-time polymerase chain reaction
After every 48 and 96 h culture, antral follicles were pooled according to treatment group, and then subjected to quantitative real-time polymerase chain reaction (qPCR) analysis. Total RNA was extracted from the follicles using the RNeasy Micro Kit (Qiagen, Inc., Valencia, CA) and quantified with the Nanodrop spectrophotometer following manufacturer’s instructions. Subsequently, 100 ng total RNA per sample was reverse transcribed to complementary DNA (cDNA) using the iScript RT kit (Bio-Rad Laboratories, Inc., mHercules, CA) according to manufacturer’s protocol. qPCR was performed using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA) and accompanying CFX Manager Software as per manufacturer’s recommendations. The qPCR reactions were run in duplicate with 1 μL cDNA, 5 pmol gene specific primers (Integrated DNA Technologies, Inc., Coralville, IA; Table 1), and 5 μL SsoFast EvaGreen Supermix (Bio-Rad laboratories, Hercules, CA) for a final reaction volume of 10 μL. The primers and the qPCR program protocol were validated previously [28]. Standard curves obtained from serial dilutions of a combination of samples were used to calculate PCR efficiency. Beta-actin (Actb) was used as a reference gene because its mRNA level did not differ among the 5 treatment groups. The Pfaffl method for relative quantification was used to obtain expression data, and the calculated relative fold changes were analyzed. The mRNAs of the steroidogenic enzymes cytochrome P450 steroid 17-α-hydroxalase 1 (CYP17A1), cytochrome P450 aromatase (CYP19A1), and 17β-hydroxysteroid dehydrogenase 1 (HSD17B1), as well as of the transport protein steroidogenic acute regulatory protein (STAR) were tested because they are important regulators of steroid hormone production in the ovary [29]. A summary of ovarian steroidogenesis can be found below.
Table 1.
Sequence of Primers used for qPCR.
| Gene Name | Gene symbol | Accession No. | Forward primer | Reverse primer |
|---|---|---|---|---|
| Beta-actin | Actb | NM_007393 | GGGCACAGTGTGGGTGAC | CTGGCACCACACCTTCTAC |
| Cytochrome P450 steroid 17-α-hydroxylase 1 | Cyp17a1 | NM_007809 | CCAGGACCCAAGTGTGTTCT | CCTGATACGAAGCACTTCTCG |
| Cytochrome P450 aromatase | Cyp19a1 | NM_007810 | CATGGTCCCGGAAACTGTGA | GTAGTAGTTGCAGGCACTTC |
| 17β-hydroxysteroid dehydrogenase 1 | Hsd17b1 | NM_010475 | ACTGTGCCAGCAAGTTTGCG | AAGCGGTTCGTGGAGAAGTAG |
| Steroidogenic acute regulatory protein | Star | NM_007810 | CAGGGAGAGGTGGCTATGCA | CCGTGTCTTTTCCAATCCTCTG |
2.7. Statistical analyses
Data were expressed as means ± standard error of the means (SEM), and analyzed using SPSS statistical software (SPSS, Inc., Chicago, IL). Comparisons between experimental groups were made using the Kruskal-Wallis test, followed by the Mann-Whitney test. Statistical significance was assigned at p ≤ 0.05.
3. Results
3.1. Effect of isoliquiritigenin on antral follicle growth
Exposure of follicles to 36 and 100μM of isoliquiritigenin inhibited follicle growth from 24 h through 96 h of culture when compared to the vehicle control group (Fig. 1; n = 4–11, p ≤ 0.05). Additionally, exposure to 0.6 μM and 6 μM of isoliquiritigenin inhibited follicle growth at 24 and 72 h, respectively (Fig. 1; n = 4–11, p ≤ 0.05). However, the two lower concentrations of isoliquiritigenin (0.6 μM and 6 μM) did not affect follicle growth at any other time-point (Fig. 1).
Fig. 1.
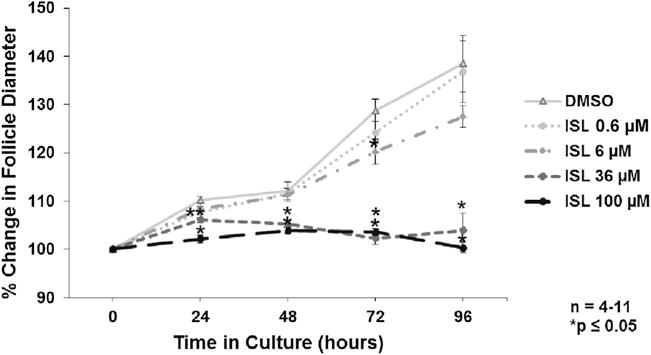
Effect of isoliquiritigenin on follicle growth overtime. Antral follicles were isolated from adult CD-1 mice and cultured with vehicle (DMSO) or isoliquiritigenin (ISL) for total culture periods of 48, 72, and 96 h. Follicle diameters were measured every 24 h along perpendicular axes and percent change in growth was determined over each total culture period. The graph represents the means ± SEM of percent change in follicle growth from 4 to 11 separate experiments. Asterisks (*) represent a significant difference between vehicle control and isoliquiritigenin (p ≤ 0.05).
3.2. Effect of isoliquiritigenin on sex steroid hormone levels
Follicles cultured with 100 μM of isoliquiritigenin showed decreased production of estradiol and progesterone at 48, 72, and 96 h when compared to the vehicle control group (Figs. 2 and 4; n = 3–4, p ≤ 0.05). Follicles cultured with 100 μM of isoliquiritigenin also showed decreased production of testosterone at 48 and 96h when compared to vehicle control (Fig. 3; n = 3–4, p ≤ 0.05). Additionally, while isoliquiritigenin at 100 μM suppressed follicle testosterone levels at 72 h, this decrease was not statistically significant (Fig. 3; n = 3–4, p = 0.083). Follicles exposed to 36 μM of isoliquiritigenin showed decreased estradiol levels at 48, 72, and 96 h (Fig. 2; n = 3–4, p ≤ 0.05), but increased progesterone levels at 72 h (Fig 4; n = 3–4, p ≤ 0.05). Further, exposure to 6 μM of isoliquiritigenin caused an increase in progesterone production in the follicles at 72 h when compared to control (Fig. 4; n = 3–4, p ≤ 0.05). Isoliquiritigenin at 0.6 μM did not significantly affect hormone levels when compared to DMSO (Figs. 2–4).
Fig. 2.
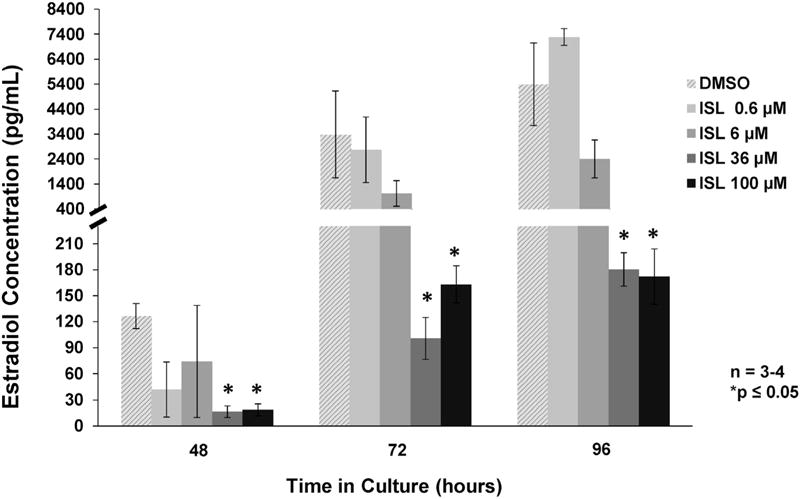
Effect of isoliquiritigenin on follicle estradiol production. Antral follicles were isolated from adult CD-1 mice and cultured with vehicle (DMSO) or isoliquiritigenin (ISL) for 48, 72, and 96 h. Subsequently, media were pooled within experiment by treatment group and subjected to enzyme-linked immunosorbent assays for estradiol. The graph represents the means ± SEM from 3 to 4 separate experiments. Asterisks (*) represent a significant difference between vehicle control and isoliquiritigenin (p ≤ 0.05).
Fig. 4.
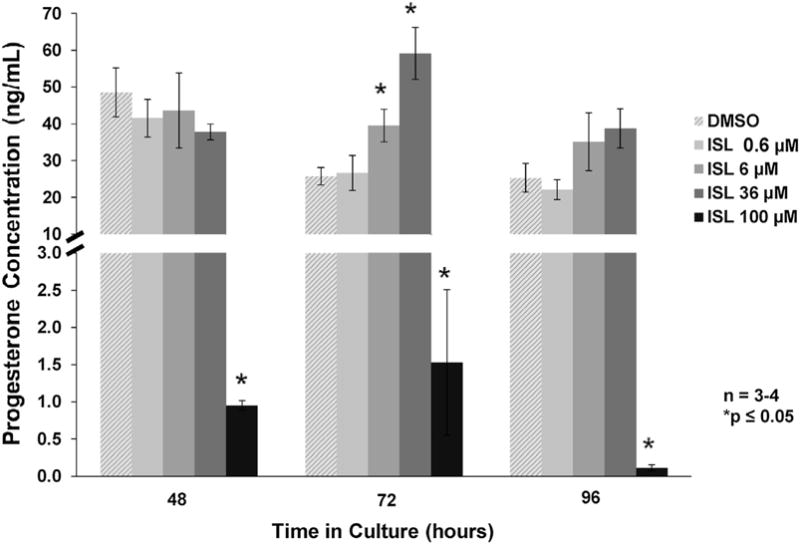
Effect of isoliquiritigenin on follicle progesterone production. Antral follicles were isolated from adult CD-1 mice and cultured with vehicle (DMSO) or isoliquiritigenin (ISL) for 48, 72, and 96 h. Subsequently, media were pooled within experiment by treatment group and subjected to enzyme-linked immunosorbent assays for progesterone. The graph represents the means ± SEM from 3 to 4 separate experiments. Asterisks (*) represent a significant difference between vehicle control and isoliquiritigenin (p ≤ 0.05).
Fig. 3.
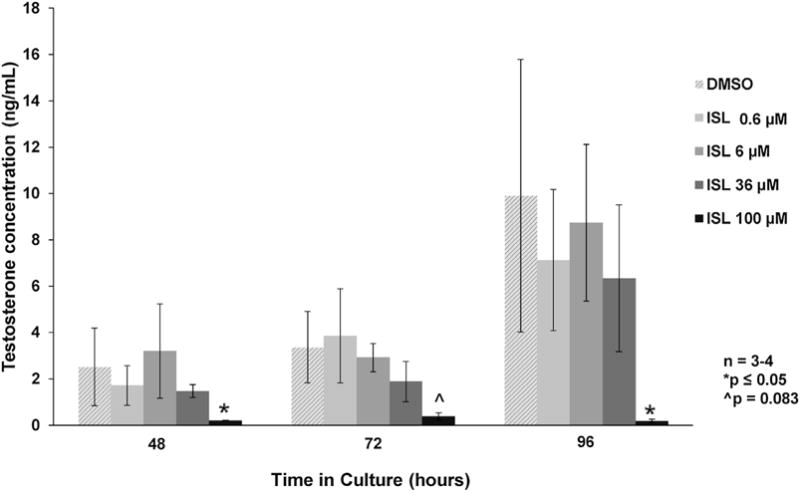
Effect of isoliquiritigenin on follicle testosterone production. Antral follicles were isolated from adult CD-1 mice and cultured with vehicle (DMSO) or isoliquiritigenin (ISL) for 48, 72, and 96 h. Subsequently, media were pooled within experiment by treatment group and subjected to enzyme-linked immunosorbent assays for testosterone. The graph represents the means ± SEM from 3 to 4 separate experiments. Asterisks (*) represent a significant difference between vehicle control and isoliquiritigenin (p ≤ 0.05).
3.3. Effect of isoliquiritigenin on steroidogenic enzyme and transport protein mRNA levels
Exposure of follicles to 100 μM of isoliquiritigenin caused a decrease in the mRNA level of Cyp17a1 at 96 h, and of Cyp19a1 and Hsd17b1 at both 48 and 96 h of culture (Fig. 5A–C; n = 3–4, p ≤ 0.05). It also caused a significant increase in the mRNA level of Star at 96 h (Fig. 5D; n = 3–4, p ≤ 0.05). Isoliquiritigenin at 36 μM significantly decreased Cyp19a1 mRNA level at 96 h (Fig. 5B; n = 3–4, p ≤ 0.05). Further, exposure of follicles to 0.6 μM and 6 μM of isoliquiritigenin did not affect the mRNA levels of Cyp17a1, Cyp19a1, Hsd17b1, or Star when compared to control (Fig. 5A–D).
Fig. 5.
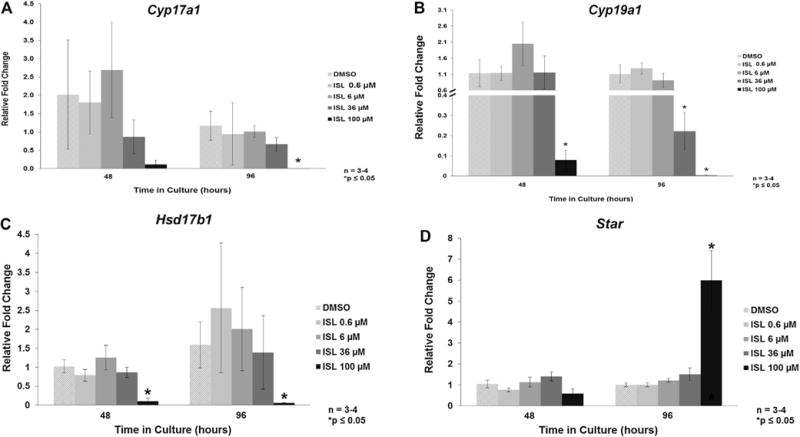
Effect of isoliquiritigenin on follicle mRNA level of regulators of steroidogenesis. Antral follicles were isolated from adult CD-1 mice and cultured with vehicle (DMSO) orisoliquiritigenin (ISL) for 48 and 96 h. Subsequently, follicles were pooled within experiment by treatment group and subjected to quantitative polymerase chain reaction for Cyp17a1 (panel A), Cyp19a1 (panel B), Hsd17b1 (panel C), and Star (panel D). Values were normalized to Actb. The graph represents the means ± SEM from 3 to 4 separate experiments. Asterisk (*) represents a significant difference between vehicle control and isoliquiritigenin (p ≤ 0.05).
4. Discussion
We investigated the effects of isoliquiritigenin on growth and steroidogenesis of antral follicles, which are crucial for normal female reproductive function. We observed that exposure of mouse antral follicles to isoliquiritigenin inhibited their growth over 96 h of culture and altered their production of the sex steroid hormones estradiol, testosterone, and progesterone. Decreased mRNA levels of Cyp17a1, Cyp19a1, and Hsd17b1, along with increased mRNA levels of Star in isoliquiritigenin-treated follicles suggest disruption of upstream regulators of steroidogenesis as a factor in the altered sex steroid synthesis of these follicles. These are novel findings on the actions of isoliquiritigenin on the ovary, and warrant further investigation of the potential reproductive toxicity of this widely-used phytoestrogen.
The in vitro follicle culture system that we utilized for the present study enabled us to track the growth of individual antral follicles over time, and thus easily observe the changes brought about by exposure to isoliquiritigenin. This allowed us to study endpoints that would be difficult to assess in vivo, such as follicle growth progression in response to treatment. It also enabled us to determine whether isoliquiritigenin was directly toxic to antral follicles. Further, using this in vitro system more closely mimicked in vivo conditions than using isolated ovarian cells, as it evaluated the effects on intact antral follicles as a whole unit, rather than isolated oocytes, granulosa cells, or theca cells. Although our in vitro model precludes the effects of absorption, distribution, and metabolism, it would complement future in vivo studies necessary for full understanding of isoliquiritigenin toxicity.
Our data indicate that exposure of antral follicles to 0.6 μM of isoliquiritigenin initially suppressed their growth at 24 h, but this effect did not persist beyond that time-point in culture. We also found that isoliquiritigenin at 6 μM decreased follicle growth at a single time-point in culture (i.e., 72 h). However, exposure to isoliquiritigenin at 36 and 100 μM inhibited the growth of antral follicles beginning at 24 h, and persisted throughout 96 h of culture. Normal development of antral follicles is necessary for adequate sex steroid synthesis and the process of ovulation [30]. Although the effects of isoliquiritigenin on ovarian follicle growth have not been reported, previous studies have shown the antiproliferative and cytotoxic effects of licorice root and isoliquiritigenin on human breast cancer cells [11, 31–33]. One study found that isoliquiritigenin inhibited cancer cell proliferation and induced apoptosis by deactivating the PI3K/Akt pathway involved in cell cycle regulation [33]. Another study found that licorice root extract caused apoptosis of MCF-7 breast cancer cells partly through induction of the pro-apoptotic factor Bax, inhibition of Cdk 2 and cyclin E, and induction of G1 cell cycle arrest [32]. Previous studies with genistein, a commonly used phytoestrogen found in soy, showed that it could induce atresia of rat follicles [34] and inhibit growth of mouse antral follicles via Cdkn1a upregulation [35]. Equol, a metabolite of the soy phytoestrogen daidzein, was found to inhibit mouse antral follicle growth and increase follicle atresia [36]. The isoliquiritigenin-induced inhibition of follicle growth in the present study may thus potentially be due to disruption of cell cycle regulators causing cell cycle arrest, increased apoptosis causing follicle atresia, or a combination of both. Although the goal of the current study was to evaluate whether isoliquiritigenin affects antral follicle growth, future studies investigating these plausible mechanisms in more detail will help determine the exact cause of the observed follicle growth inhibition due to isoliquiritigenin exposure.
Ovarian antral follicles are a major source of sex steroid hormones, and we measured the levels of estradiol and two of its main precursors, testosterone and progesterone. Normal levels of estradiol, testosterone, and progesterone are required for proper functioning of the reproductive system [37, 38]. Our data indicate that isoliquiritigenin at 36 and 100 μM reduced the capacity of antral follicles to synthesize estradiol at 48, 72, and 96 h. Treatment with 100 μM of isoliquiritigenin also reduced the production of testosterone, which is the immediate metabolic precursor to estradiol. Further, while isoliquiritigenin at 100 μM decreased follicle production of progesterone from 48 to 96 h, we found that isoliquiritigenin at 6 and 36 μM increased progesterone levels at 72 h of culture. We would like to note here that the high degree of variability in the testosterone levels may have made it more difficult to be conclusive about low dose effects on this hormone.
The alterations in hormone levels in our study could partly be attributed to the lack of normal follicle growth in response to isoliquiritigenin exposure. Adequate growth of antral follicles with sufficient numbers of healthy theca and granulosa cells is necessary for estradiol production [30, 39, 40]. Further, since estradiol is produced from precursor hormones in the theca and granulosa cells of the follicles, an adequate level of the precursor hormones is another prerequisite for normal estradiol synthesis [39]. Inhibition of growth as early as 24 h, with continued inhibition at 48, 72, and 96 h of culture in follicles treated with 100 μM isoliquiritigenin could have resulted in decreased estradiol levels in this group. A decrease in the levels of the precursor steroids, testosterone and progesterone from 48 through 96 h of culture may further have contributed to the low estradiol levels in the 100 μM treatment group. Similarly, follicle growth inhibition from 24 through 96 h in the 36 uM treatment group could explain the decreased estradiol levels observed at 48, 72, and 96 h in this group. The increased progesterone levels at 72 h in follicles treated with 36 uM isoliquiritigenin may be a compensatory effect to maintain downstream hormones levels. Although this compensatory increase in progesterone appears to help maintain normal levels of testosterone, which is downstream of progesterone in the sex steroid synthesis pathway (refer to Fig. 6), it does not appear sufficient to prevent low estradiol levels in the 36 uM treatment group. Similarly, the increase in progesterone at 72 h in the 6uM group could be a compensatory response to growth inhibition at 72 h in this treatment group. This response appears to be able to maintain normal levels of both testosterone and estradiol at this lower dose.
Fig. 6.
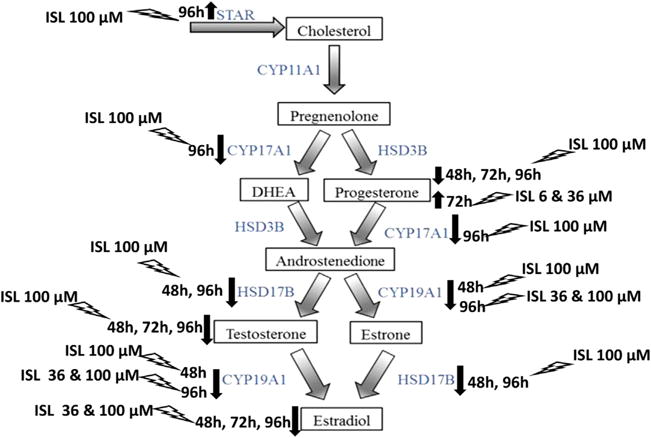
Effect of isoliquiritigenin on follicle steroidogenesis. This schematic of the estradiol biosynthesis pathway shows how estradiol is produced from cholesterol through the action of steroidogenic enzymes in the ovarian antral follicle. The effects of different concentrations of isoliquiritigenin (ISL) on the levels of progesterone, testosterone, and estradiol, as well as the transport protein STAR and the enzymes CYP17A1, CYP19A1, and HSD17B1 are summarized.
Our results on hormone levels align to some extent with the observation that endocrine disrupting chemicals often have different effects at different doses [41]. This is the first time, to our knowledge, that the effects of isoliquiritigenin on ovarian sex steroid hormone levels have been investigated. However, our findings are consistent with a few studies examining the effects of other phytoestrogens on the levels of steroid hormones [35, 42–44]. For instance, genistein was found to reduce estradiol and increase progesterone synthesis of cultured mouse antral follicles [35]. It was also found to decrease estradiol levels in porcine granulosa cells [44]. Equol was found to inhibit the production of estradiol, testosterone, and progesterone by mouse antral follicles in vitro [36]. Daily consumption of soy products containing genistein and daidzein for one month reduced circulating plasma levels of estradiol and progesterone in healthy, premenopausal women [42].
The production of sex steroid hormones in the theca and granulosa cells of the antral follicles is brought about by several enzymes that convert cholesterol to estradiol. The transfer of cholesterol into the mitochondria of these cells for the purpose of steroid production is a rate-limiting step in the process. The present study examined the mRNA levels of cytochrome P450 steroid 17-α-hydroxylase 1 (CYP17A1), cytochrome P450 aromatase (CYP19A1), and 17β-hydroxysteroid dehydrogenase 1 (HSD17B1), three of the steroidogenic enzymes in this estradiol biosynthesis pathway. CYP17A1 converts progesterone to androstenedione, as well as pregnenolone to dehydroepiandrosterone (DHEA). CYP19A1 converts testosterone to estradiol, as well as androstenedione to estrone. HSD17B1 converts androstenedione to testosterone, as well as estrone to estradiol [29]. The study also measured the mRNA level of steroidogenic acute regulatory protein (STAR), which regulates the transport of cholesterol from the outer to the inner mitochondrial membrane, where it can be further converted to the various sex steroid hormones in the pathway [45]. Our data indicate that isoliquiritigenin at 100 μM decreased follicle mRNA level of Cyp17a1 at 96h of culture, when compared to the vehicle control. It should be noted that lower dose effects on Cyp17a1 mRNA may not be apparent in the present study due to the variability observed in the levels of this enzyme. Further, the significance of decreased Cyp17a1 mRNA due to the high dose is not immediately clear, and future studies are warranted to determine whether androstenedione or DHEA levels are affected by the 100 μM dose of isoliquiritigenin. We found a decrease in the mRNA level of Cyp19a1 at both 48 and 96 h in the 100 μM treatment group. Additionally, isoliquiritigenin at 36 μM decreased Cyp19a1 mRNA at 96 hours, with no changes seen at 48h. The downregulation of Cyp19a1 is a likely factor in the reduced estradiol levels observed in the 36 and 100 μM treatment groups, by hampering the conversion of testosterone to estradiol. Our data on the downregulation of this enzyme are consistent with a previous study which found that isoliquiritigenin could suppress the mRNA expression and activity of CYP19A1 in MCF-7 breast cancer cells as well as in an animal model of breast cancer [10]. The downregulation of Hsd17b1 at 48 and 96 h by exposure to 100 μM isoliquiritigenin in the present study is another probable cause of decreased estradiol and testosterone levels observed at this concentration. Reduced levels of this enzyme could have resulted in inadequate conversion of estrone to estradiol and of androstenedione to testosterone. Interestingly, we found that isoliquiritigenin at 100 μM upregulated the mRNA of Star at 96 h, when compared to control. This could be an attempt to compensate for the depleted hormone levels observed over 96 hours of culture in this treatment group. Future studies which examine the protein levels and activity of the steroidogenic enzymes, as well as measure the levels of other steroid hormones in the pathway will help to better understand the changes observed due to isoliquiritigenin. Another possible mechanism to explain the reduced estradiol levels in our study is that isoliquiritigenin increases estradiol metabolism in the antral follicles by enhancing the expression of cytochrome P4501A1 (CYP1A1) and cytochrome P450 1B1 (CYP1B1). This is a likely alternative mechanism that should be investigated because a previous study found that isoliquiritigenin stimulated the expression and activity of CYP1B1 in mammary epithelial cells [46].
Based on the data from the present study, we can conclude that inhibition of follicle growth and perturbed expression of key regulators of steroidogenesis are both likely contributing factors in the altered hormone levels of follicles exposed to 36 and 100 μM of isoliquiritigenin. However, additional studies are required to evaluate whether cytotoxicity and atresia of follicles at these doses could also be playing a role. Moreover, since ovarian steroid synthesis and follicular growth are interdependent processes [30, 37], detailed time-course studies would clarify if isoliquiritigenin causes follicle growth inhibition and/or follicle death before disrupting steroidogenesis.
In conclusion, our results indicate that isoliquiritigenin inhibits the growth of mouse antral follicles in vitro, and disrupts their normal production of estradiol and its precursor hormones, testosterone and progesterone. Our data also indicate that a decrease in mRNA level of steroidogenic enzymes is a likely factor in altered hormone production in isoliquiritigenin-treated follicles. These findings are important because inhibition of follicle growth and altered steroidogenesis due to isoliquiritigenin could pose a threat to reproductive health and fertility. The lower doses of isoliquiritigenin used in the study (0.6 and 6 μM), which fall within the estimated general range of daily human exposure, affect follicle growth and hormone production to a lesser extent than the higher doses (36 and 100 μM). However, the cumulative exposure to isoliquiritigenin may be greater in certain populations, for example, those regularly consuming excessive amounts of candy, licorice-flavored tobacco products, and isoliquiritigenin health supplements. Isoliquiritigenin is one of the active components of licorice, and the use of licorice in the tobacco, food, and pharmaceutical industries is significant. Additionally, isoliquiritigenin is widely consumed as a dietary supplement for a variety of ailments, and research on its potential utility in cancer therapy is on the rise. Thus, it is important to further evaluate the low-dose and high-dose effects of isoliquiritigenin on the ovary, and how this impacts reproductive function in vivo.
Acknowledgments
The authors thank the members of Dr. Flaws’ laboratory for their assistance. This work was supported by R03ES023972 (JAF) from the National Institute of Environmental Health (NIEHS), T35OD011145 (JAF and Dr. Lois Hoyer) from the Office of the Director, National Institutes of Health, and P50AT006268 (WGH) from the National Center for Complementary and Alternative Medicine (NCCAM), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NCCAM, ODS, or NCI.
Footnotes
Conflict of interest
None.
Contributor Information
Sharada Mahalingam, Email: mahalin2@illinois.edu.
Liying Gao, Email: lgao@illinois.edu.
Jacqueline Eisner, Email: jeisner2@illinois.edu.
William Helferich, Email: helferic@illinois.edu.
References
- 1.Whitten PL, Patisaul HB. Cross-species and interassay comparisons of phytoestrogen action. Environ Health Perspect. 2001;109(Suppl 1):5–20. doi: 10.1289/ehp.01109s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham DM, Gardner CD, Haskell WL. Clinical review 97: potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab. 1998;83(7):2223–2235. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- 3.Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.) its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol: RTP. 2006;46(3):167–192. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res: PTR. 2008;22(6):709–724. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pizzorno JE, Murray Michae T. Chapter 96 glycyrrhiza glabra (Licorice) In: Pizzorno JE, Murray Michae T, editors. Textbook of Natural Medicine. fourth. Churchill Livingstone, an imprint of Elsevier Inc.; St Louis, Missouri: 2013. p. 63043. [Google Scholar]
- 6.Madak-Erdogan Z, Gong P, Zhao YC, Xu L, Wrobel KU, Hartman JA, Wang M, Cam A, Iwaniec UT, Turner RT, Twaddle NC, Doerge DR, Khan IA, Katzenellenbogen JA, Katzenellenbogen BS, Helferich WG. Dietary licorice root supplementation reduces diet-induced weight gain, lipid deposition, and hepatic steatosis in ovariectomized mice without stimulating reproductive tissues and mammary gland. Mol Nutr Food Res. 2016;60(2):369–380. doi: 10.1002/mnfr.201500445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajirahimkhan A, Simmler C, Yuan Y, Anderson JR, Chen SN, Nikolic D, Dietz BM, Pauli GF, van Breemen RB, Bolton JL. Evaluation of estrogenic activity of licorice species in comparison with hops used in botanicals for menopausal symptoms. PLoS One. 2013;8(7):e67947. doi: 10.1371/journal.pone.0067947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng F, Du Q, Peng C, Wang N, Tang H, Xie X, Shen J, Chen J. A review: the pharmacology of isoliquiritigenin. Phytother Res: PTR. 2015;29(7):969–977. doi: 10.1002/ptr.5348. [DOI] [PubMed] [Google Scholar]
- 9.Lorusso V, Marech I. Novel plant-derived target drugs: a step forward from licorice? Expert Opin Ther Targets. 2013;17(4):333–335. doi: 10.1517/14728222.2013.773312. [DOI] [PubMed] [Google Scholar]
- 10.Ye L, Gho WM, Chan FL, Chen S, Leung LK. Dietary administration of the licorice flavonoid isoliquiritigenin deters the growth of MCF-7 cells overexpressing aromatase. Int J Cancer. 2009;124(5):1028–1036. doi: 10.1002/ijc.24046. [DOI] [PubMed] [Google Scholar]
- 11.Maggiolini M, Statti G, Vivacqua A, Gabriele S, Rago V, Loizzo M, Menichini F, Amdo S. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J Steroid Biochem Mol Biol. 2002;82(4–5):315–322. doi: 10.1016/s0960-0760(02)00230-3. [DOI] [PubMed] [Google Scholar]
- 12.Kanazawa M, Satomi Y, Mizutani Y, Ukimura O, Kawauchi A, Sakai T, Baba M, Okuyama T, Nishino H, Miki T. Isoliquiritigenin inhibits the growth of prostate cancer. Eur Urol. 2003;43(5):580–586. doi: 10.1016/s0302-2838(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi T, Takasuka N, Iigo M, Baba M, Nishino H, Tsuda H, Okuyama T. Isoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci development. Cancer Sci. 2004;95(5):448–453. doi: 10.1111/j.1349-7006.2004.tb03230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia J, Li Y, Lei Z, Hao Y, Wu Y, Zhao Q, Wang H, Ma L, Liu J, Zhao C, Jiang Y, Wang Y, Tan H, Dai X, Zhang W, Sun T, Yu J. Relaxative effect of core licorice aqueous extract on mouse isolated uterine horns. Pharm Biol. 2013;51(6):744–748. doi: 10.3109/13880209.2013.764536. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Wu D, Sun Z, Yang J, Chai H, Tang L, Guo Y. Analgesic and uterine relaxant effects of isoliquiritigenin, a flavone from Glycyrrhiza glabra. Phytother Res: PTR. 2012;26(9):1410–1417. doi: 10.1002/ptr.3715. [DOI] [PubMed] [Google Scholar]
- 16.Patel S, Zhou C, Rattan S, Flaws JA. Effects of endocrine-disrupting chemicals on the ovary. Biol Reprod. 2015;93(1):20. doi: 10.1095/biolreprod.115.130336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boonmuen N, Gong P, Ali Z, Chittiboyina AG, Khan I, Doerge DR, Helferich WG, Carlson KE, Martin T, Piyachaturawat P, Katzenellenbogen JA, Katzenellenbogen BS. Licorice root components in dietary supplements are selective estrogen receptor modulators with a spectrum of estrogenic and antiestrogenic activities. Steroids. 2016;105:42–49. doi: 10.1016/j.steroids.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamir S, Eizenberg M, Somjen D, Izrael S, Vaya J. Estrogen-like activity of glabrene and other constituents isolated from licorice root. J Steroid Biochem Mol Biol. 2001;78(3):291–298. doi: 10.1016/s0960-0760(01)00093-0. [DOI] [PubMed] [Google Scholar]
- 19.Cederroth CR, Zimmermann C, Nef S. Soy, phytoestrogens and their impact on reproductive health. Mol Cell Endocrinol. 2012;355(2):192–200. doi: 10.1016/j.mce.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 20.Tiemann U, Schneider F, Vanselow J, Tomek W. In vitro exposure of porcine granulosa cells to the phytoestrogens genistein and daidzein: effects on the biosynthesis of reproductive steroid hormones. Reprod Toxicol (Elmsford, NY) 2007;24(3–4):317–325. doi: 10.1016/j.reprotox.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Wegener JW, Nawrath H. Cardiac effects of isoliquiritigenin. Eur J Pharmacol. 1997;326(1):37–44. doi: 10.1016/s0014-2999(97)00134-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, Yuan X, Li D, Chen H, Jiang J, Wang Z, Sun X, Zheng Q. Isoliquiritigen enhances the antitumour activity and decreases the genotoxic effect of cyclophosphamide. Mol (Basel Switzerland) 2013;18(8):8786–8798. doi: 10.3390/molecules18088786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao S, Chang H, Ma P, Gao G, Jin C, Zhao X, Zhou W, Jin B. Inhibitory effect of DNA topoisomerase inhibitor isoliquiritigenin on the growth of glioma cells. Int J Clin Exp Pathol. 2015;8(10):12577–12582. [PMC free article] [PubMed] [Google Scholar]
- 24.Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93(1):180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- 25.Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci. 2005;88(1):213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- 26.Nayudu PL, Osborn SM. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil. 1992;95(2):349–362. doi: 10.1530/jrf.0.0950349. [DOI] [PubMed] [Google Scholar]
- 27.Cortvrindt RG, Smitz JE. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update. 2002;8(3):243–254. doi: 10.1093/humupd/8.3.243. [DOI] [PubMed] [Google Scholar]
- 28.Hannon PR, Brannick KE, Wang W, Gupta RK, Flaws JA. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol Appl Pharmacol. 2015;284(1):42–53. doi: 10.1016/j.taap.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43(8):779–804. doi: 10.1016/0960-0760(92)90307-5. [DOI] [PubMed] [Google Scholar]
- 30.Lunenfeld B, Kraiem Z, Eshkol A. The function of the growing follicle. J Reprod Fertil. 1975;45(3):567–574. doi: 10.1530/jrf.0.0450567. [DOI] [PubMed] [Google Scholar]
- 31.Hu C, Liu H, Du J, Mo B, Qi H, Wang X, Ye S, Li Z. Estrogenic activities of extracts of Chinese licorice (Glycyrrhiza uralensis) root in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2009;113(3–5):209–216. doi: 10.1016/j.jsbmb.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Jo EH, Kim SH, Ra JC, Kim SR, Cho SD, Jung JW, Yang SR, Park JS, Hwang JW, Aruoma OI, Kim TY, Lee YS, Kang KS. Chemopreventive properties of the ethanol extract of chinese licorice (Glycyrrhiza uralensis) root: induction of apoptosis and G1 cell cycle arrest in MCF-7 human breast cancer cells. Cancer Lett. 2005;230(2):239–247. doi: 10.1016/j.canlet.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Zhao H, Wang Y, Zheng H, Yu W, Chai H, Zhang J, Falck JR, Guo AM, Yue J, Peng R, Yang J. Isoliquiritigenin induces growth inhibition and apoptosis through downregulating arachidonic acid metabolic network and the deactivation of PI3K/Akt in human breast cancer. Toxicol Appl Pharmacol. 2013;272(1):37–48. doi: 10.1016/j.taap.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Medigovic I, Ristic N, Trifunovic S, Manojlovic-Stojanoski M, Milosevic V, Zikic D, Nestorovic N. Genistein affects ovarian folliculogenesis: a stereological study. Microsc Res Tech. 2012;75(12):1691–1699. doi: 10.1002/jemt.22117. [DOI] [PubMed] [Google Scholar]
- 35.Patel S, Peretz J, Pan YX, Helferich WG, Flaws JA. Genistein exposure inhibits growth and alters steroidogenesis in adult mouse antral follicles. Toxicol Appl Pharmacol. 2016;293:53–62. doi: 10.1016/j.taap.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahalingam S, Gao L, Gonnering M, Helferich W, Flaws JA. Equol inhibits growth, induces atresia, and inhibits steroidogenesis of mouse antral follicles in vitro. Toxicol Appl Pharmacol. 2016;295:47–55. doi: 10.1016/j.taap.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drummond AE. The role of steroids in follicular growth. Reprod Biol Endocrinol: RB&E. 2006;4:16. doi: 10.1186/1477-7827-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Findlay JK, Britt K, Kerr JB, O’Donnell L, Jones ME, Drummond AE, Simpson ER. The road to ovulation: the role of oestrogens. Reprod Fertil Dev. 2001;13(7–8):543–547. doi: 10.1071/rd01071. [DOI] [PubMed] [Google Scholar]
- 39.Falck B. Site of production of oestrogen in the ovary of the rat. Nature. 1959;184(Suppl. 14):1082. doi: 10.1038/1841082a0. [DOI] [PubMed] [Google Scholar]
- 40.Armstrong DT. Gonadotropins, ovarian metabolism, and steroid biosynthesis. Recent Prog Horm Res. 1968;24:255–319. doi: 10.1016/b978-1-4831-9827-9.50012-5. [DOI] [PubMed] [Google Scholar]
- 41.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu LJ, Anderson KE, Grady JJ, Nagamani M. Effects of soya consumption for one month on steroid hormones in premenopausal women: implications for breast cancer risk reduction. Cancer Epidemiol Biomarkers Prev: Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 1996;5(1):63–70. [PubMed] [Google Scholar]
- 43.Lu LJ, Anderson KE, Grady JJ, Nagamani M. Effects of an isoflavone-free soy diet on ovarian hormones in premenopausal women. J Clin Endocrinol Metab. 2001;86(7):3045–3052. doi: 10.1210/jcem.86.7.7684. [DOI] [PubMed] [Google Scholar]
- 44.Basini G, Bussolati S, Santini SE, Grasselli F. The impact of the phyto-oestrogen genistein on swine granulosa cell function. J Anim Physiol Anim Nutr. 2010;94(6):e374–82. doi: 10.1111/j.1439-0396.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 45.Miller WL, Strauss JF., 3rd Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol. 1999;69(1–6):131–141. doi: 10.1016/s0960-0760(98)00153-8. [DOI] [PubMed] [Google Scholar]
- 46.Dunlap TL, Wang S, Simmler C, Chen SN, Pauli GF, Dietz BM, Bolton JL. Differential effects of glycyrrhiza species on genotoxic estrogen metabolism: licochalcone a downregulates P450 1B1, where as isoliquiritigenin stimulates it. Chem Res Toxicol. 2015;28(8):1584–1594. doi: 10.1021/acs.chemrestox.5b00157. [DOI] [PMC free article] [PubMed] [Google Scholar]


