Abstract
Aim
Here, we assessed the expression of non-protein coding microRNAs (miRs), nerve growth factor and inflammatory molecules in the rat model of acetic acid induced bladder overactivity.
Main Methods
Under isoflurane anesthesia, adult female Sprague-Dawley rats were instilled for 30 min with either saline or NGF antisense oligonucleotides complexed with liposomes. 24h later, treated rats were exposed to either intravesical infusion of saline or saline containing 0.25% acetic acid at the rate of 0.04ml/min for 2h under urethane anesthesia (1g/kg; s.c). After CMG, bladder was harvested to study expression of NGF, cytokines and 8 specific miRNAs involved in bladder dysfunctions. The role of miR-132 in bladder overactivity was independently assessed through bladder wall transfection of plasmid encoding miR-132.
Key Findings
NGF overexpression in bladder overactivity was associated with ~2-fold upregulation and downregulation of miR-132 and miR-221, respectively. Pretreatment with NGF antisense restored the expression of miR-221 and miR-132 to control levels and also reduced the expression of cytokines (MCP-1 and sICAM-1). There was insignificant alteration in expression of miR-199a-5p, but expression of, miR-210, miR-212, miR-155, miR-134 and miR-206 remained similar across the experimental groups. Bladder wall transfection of miR-132 plasmid in absence of acetic acid exposure was able to independently induce bladder overactivity, bladder hypertrophy and upregulate the expression of NGF and other cytokines.
Significance
Overall, our work sheds light on the role of miR-132 in bladder overactivity, bladder hypertrophy, NGF signaling and expression of inflammatory mediators. Findings suggest that aberrant expression of miR-132 is involved in voiding dysfunctions.
Keywords: Neurotrophins, Bladder overactivity, MicroRNA, Antisense
1. Introduction
Previous studies have reported on the role of miR in the regulation of inflammatory responses, but the role of miR in bladder overactivity which is known to involve aberrant expression of neurotrophins such as nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), Glial derived neurotrophic factor (GDNF) and inflammatory signaling (Kashyap et al., 2013, Vizzard, 2000) is not completely known. Among neurotrophins, a causal relationship has been established for NGF and BDNF in lower urinary tract symptoms such as bladder overactivity.
Studies done on mouse brain reported intricate involvement of miR expression in the neuronal differentiation, synaptic plasticity (Kye and Goncalves Ido, 2014) and a reciprocal homeostatic relationship between miR expression and neuronal activity (Eacker et al., 2013, Karpova, 2014, Sim et al., 2014). However, studies in the peripheral organs have so far focused on the neurotrophin-induced expression of protein-encoding genes (Kashyap et al., 2013) and not on non-protein coding gene such as miRs. Since neurotrophin signaling is known to be regulated by miRs (Montalban et al., 2014) and the overexpression of neurotrophins is associated with most voiding dysfunctions, we surmised that miRs can open new avenues for understanding the voiding dysfunctions at molecular level and identify targets for genetic intervention in treatment of OAB. Here, we assessed the expression of miRs following NGF overexpression induced by acetic acid (Oddiah et al., 1998) and downregulation of NGF overexpression by intravesical NGF antisense. Various microRNAs (miRs) namely, miR-132, miR134, miR-221, miR-155, miR-199a-5p, miR-212, miR-210, and miR206 were selected based on the published literature on neuronal activation, inflammation and smooth muscle contractility (Tognini and Pizzorusso, 2012). Selection of miR-221, miR-199a-5p (Monastyrskaya et al., 2013) and miR-155 was guided by their reported role in the regulation of inflammation, which is relevant in cytokine expression linked to acetic acid induced bladder overactivity (Rao et al., 2015, Urbich et al., 2008). Selection of miR-206, miR-210 and miR-134 was predicated on their link to muscle function (Hu et al., 2010) and synaptic development (Schratt et al., 2006). Selection of miR-132 was supported by the upregulated expression of miR-132 in detrusor following bladder outlet obstruction (Sadegh et al., 2015) and a causal relationship between miR-132 transcription and neuronal activation (Nudelman et al., 2010). Here, we assessed the effect of NGF over expression and its genetic manipulation on the expression of miR-132 and other relevant miRs and cytokines in acetic acid induced bladder overactivity. Subsequently, the pathogenic role of miR-132 in bladder over activity was verified by the exogenous overexpression of miR-132 in rat bladder left unexposed to acetic acid.
2. Methods
2.1 Animals
Female adult Sprague-Dawley (SD) rats weighing 225–250 g were obtained from the Hilltop, PA. All rats were housed under pathogen free conditions and experiments were performed under protocols approved by the Institutional Animal Care and Use Committee (IACUC) protocol # 14053280.
2.2 Administration of NGF antisense
The study was conducted on three groups, with each group having 4 rats. Animals were anesthetized with 2% isoflurane and their bladders were temporarily catheterized for 30 min by 24-gauge angiocatheters (Becton Dickinson) through urethral opening to drain the urine and then instill 0.5mL of either saline or 18mer antisense phosphorothioateoligos (12µM) targeting nerve growth factor NGF complexed with liposomes. The dwell time period of instillation in bladder was 30 min, as described earlier (Kashyap et al., 2013), following which rats were returned back to regular housing for 24h. The NGF antisense sequence 5'GCCCGAGACGCCTCCCGA 3` was directed against unique sequence in the exon 3 of rat NGF mRNA, which were custom made by Integrated DNA technologies (San Diego, CA).
2.3 Induction of Bladder Overactivity
Transurethral open cystometry under urethane anesthesia (1.0 g/kg, s.c.) was performed in all groups by slowly filling the bladder with saline (0.04 mL/min) to elicit repetitive voiding for more than for 1 hour in both groups instilled with either saline or NGF antisense (12µM) twenty four hours earlier. A polyethylene catheter (PE-50) was inserted via urethra for recording the intravesical pressure and for infusing the solutions into the bladder. Inserted catheter was connected by a three-way stopcock to a pressure transducer and to a syringe pump. All the animal groups were first exposed to saline and then to either only saline or saline containing 0.25% acetic acid at the infusion rate of 0.04mL/min for 2h. The intravesical pressure was recorded with data-acquisition software (sampling rate 400 Hz; Char t) on a computer system equipped with an analog-to-digital converter. We evaluated different CMG parameters such as intercontractile intervals (ICI), bladder compliance and peak pressures. The body temperature of animals was maintained within physiologic range using a heating lamp. Rats were sacrificed at the end of cystometry and bladders were then harvested to extract total RNA for miR analysis.
2.4 miRNA Analysis- RNA Isolation
Total RNA from rat bladder of each group was extracted using Trizol reagent and converted to cDNA using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) according to manufacturer’s protocol. The following 8 TaqMan primers namely, hsa-miR-132; mmu-miR-212; hsa-miR-206; mmu-miR-134; mmu-miR-155; hsa-miR-199-5p; hsa-miR-210 and hsa-miR-221 were procured from Life Technologies for expressional study. Hsa prefix stands for human species and mmu stands for mouse species, and the commercially available primers are developed against sequences conserved from rodents to humans. The relative gene expression levels of miR were determined by quantitative real time PCR on a StepOnePlus™ System using TaqMan Universal PCR Master Mix (Applied Biosystems) according to manufacturer’s protocol. Relative expression levels were determined using the comparative CT method after normalization to U6 small nuclear RNA.
2.5 Analysis of NGF and Inflammatory Molecules
Whole bladder tissues were homogenized in RIPA lysis buffer system (Santa Cruz Biotechnology Inc., USA) containing 1× protein cocktail inhibitors. NGF content in homogenized tissue was determined using commercially available NGF Emax® ImmunoAssay System ELISA Kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Intercellular Adhesion Molecule-1 sICAM-1(Othumpangat et al., 2012a) and monocyte chemoattractant protein-1 (MCP-1)(Tyagi et al., 2016) were analyzed by Luminex xMAP kit obtained from EMD Millipore (Billerica, MA) as previously described and calculations were expressed as pg/mg of protein.
Bladder wall Transfection of miR-132 Plasmid
The plasmid vector pLV-[hsa-mir-132] was procured from Biosettia Inc, San Diego, CA (Catalog # mir-p098) for transfection in miR-132 plasmid group (n=5). Luciferase plasmid for transfection into control group was procured from Invitrogen. Procured plasmids were transformed in E. coli strain DH5α for propagation prior to transfection using liposomes. Plasmid (10µg) was complexed with protamine sulfate and cationic liposomes composed of DOTAP (N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N trimethylammonium methylsulfate) in a molar ratio of 1: 2: 3 (Kashyap et al., 2014) in nuclease free water. Bladder wall transfection involved, midline abdominal incision followed by injection of complexed plasmid (80 µl volume) at 4 separate sites (20 µL at each anterior, posterior and bilateral) with a 30-gauge needle. Abdomen was sutured back after surgery and after awakening, animals were returned to normal caging and monitored daily. 7 days later, transurethral CMG was performed under urethane anesthesia. At the end of cystometry, harvested bladder was weighed and then cryopreserved for histological analysis by hematoxylin and eosin (H&E) stain as previously described (Smaldone et al., 2009) and for quantitative real time PCR analysis with primers for NGF, MCP-1, sICAM-1 and connexin-43 (Cx-43).
3. Statistics
The results are presented as means ± the standard error of the mean (SEM). The differences in the expression of protein coding genes among the three groups were analyzed by ANOVA followed by Tukey’s multiple comparison test for statistical significance at p<0.05. Expression of miRs between acetic acid treated groups and transfected groups was analyzed by unpaired Student’s t test.
4. Results
4.1 Acetic acid exposure and Cystometry
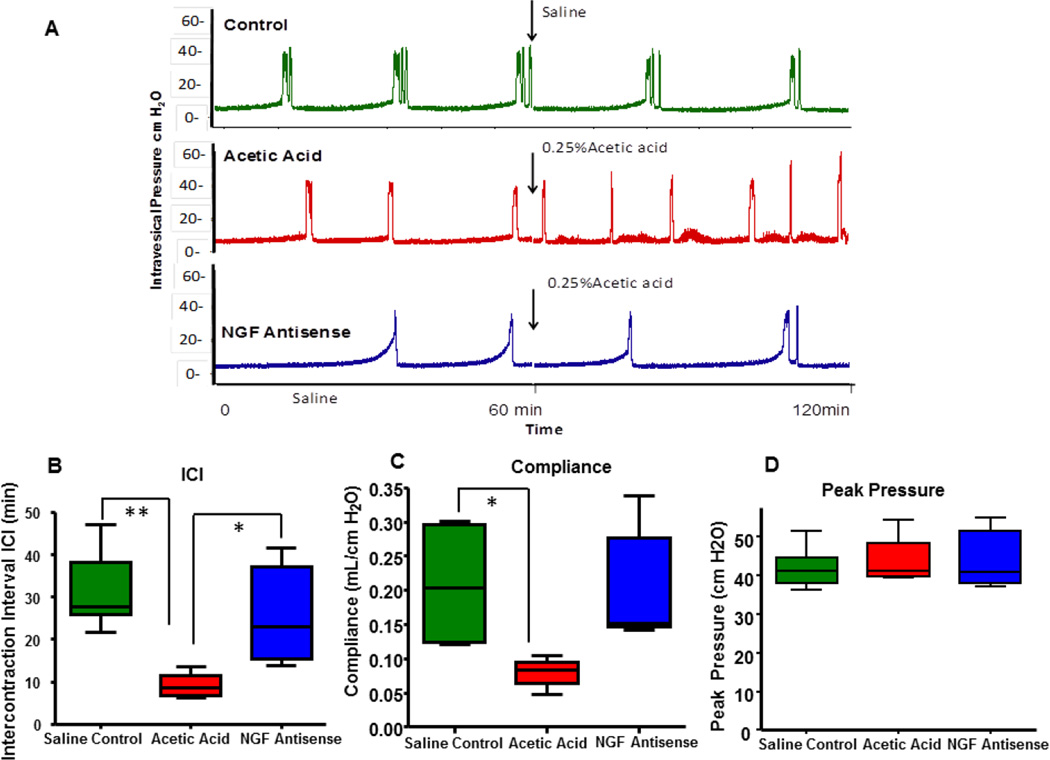
Baseline CMG under saline infusion was indistinct between the control and the NGF antisense treated groups. However, acetic acid infusion induced bladder overactivity in group instilled with saline previously, as evident from significantly reduced ICI from 29.64± 3.33 min to 9.18±1.59 min, (One way ANOVA followed by Tukey’s test #p<0.01 Fig. 1A–B). CMG after initiation of acetic acid appear very different from the CMG of the group continuously infused with saline showing significantly longer ICI (green tracing). Pretreatment with NGF antisense complexed with liposomes afforded protection against the acetic acid induced bladder overactivity (blue tracing) as is apparent from the longer ICI of 21.88±3.80 min in presence of acetic acid (Fig. 1B).
Fig. 1.
Representative tracings from cystometry under urethane anesthesia (1.0g/kg s.c.) from three groups, where saline control group represented by green tracing was not exposed to 0.25% acetic acidic. Groups represented by red and blue tracing were exposed to 0.25% acetic acid following the saline cystometry at baseline. Bladder overactivity in group pretreated with saline (red tracing) was clearly evident following the time point for infusion of acetic acid marked by arrow. Rat group pretreated with NGF antisense (blue tracing) was able to block the acetic acid induced overactivity, which is driven by overexpression of NGF and inflammatory mediators (Panel A). Different CMG parameters such as lower ICI and poor compliance were observed in rats exposed to acetic acid while NGF antisense was able to reverse these parameters (Panel B–D). We didn`t observe any change in the peak pressure amplitude in all these groups (Panel E) One way ANOVA followed by Tukey’s test #p<0.01; *p<0.05.
Acetic acid exposure in absence of NGF antisense reduced the compliance to 0.07±0.01 mL/cm H2O compared to 0.20 ± 0.03 mL/cm H2O in saline condition (Fig. 1C). NGF antisense treatment significantly reverse these parameters to normal as rats exposed to NGF antisense have significantly higher compliance (0.199 ± 0.37 mL/cm H2O) in presence of acetic acid. We did not observe observe significant changes in peak pressure amplitude among groups (Fig. 1D).
4.2 Expression of NGF and Inflammatory Mediators
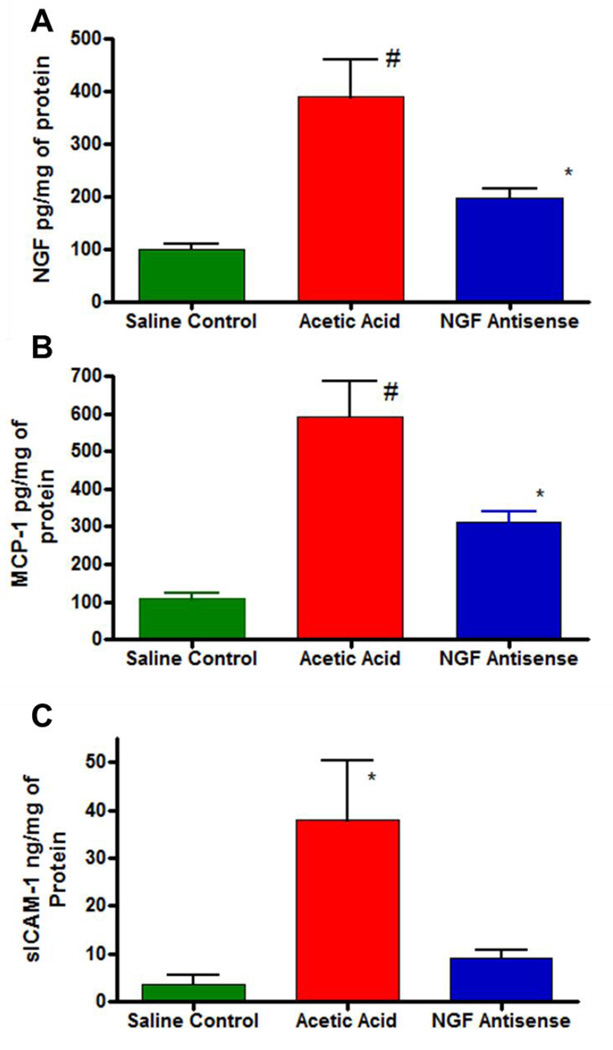
Acetic acid being a chemical irritant is known to cause massive release of chemokines and NGF in bladder. Exposure to acetic acid significantly increased the NGF expression to 363.3 ± 60.95pg/mg of protein compared to levels of 107.1± 10.15pg/mg of protein in saline group (One way ANOVA followed by Tukey’s test #p<0.01; n=4; Fig. 2A). Pretreatment with NGF antisense significantly reduced the NGF overexpression to 209.3 ± 20.18 pg/mg of protein (One way ANOVA followed by Tukey’s test; *p<0.05; Fig. 2A). Acetic acid also significantly increased the expression of inflammatory mediators, monocyte chemoattractant protein-1 (MCP-1) and soluble intracellular adhesion molecule-1 (sICAM-1). The expression of MCP-1 was 592.2± 94.2pg/mg in saline group after acetic acid exposure, which was significantly reduced to 312.9± 28.822pg/mg in the group pretreated with NGF antisense (Fig. 2B; *p<0.05). It is known that sICAM-1 is expressed in copious amounts in bladder and it is measured in ng range vs pg range for NGF and MCP-1. Exposure to acetic acid upregulated the expression of sICAM-1 in bladder to 37.83±12.63ng/mg of protein from 3.58±1.97 ng/mg of protein measured in the control group not exposed to acetic acid (Fig. 2C; *p<0.05). NGF antisense also reduced the acetic acid induced upregulation of sICAM-1 expression.
Fig. 2.
Acetic acid exposure in bladder induced the expression of protein coding genes for NGF (panel A), MCP-1(panel B) and sICAM-1 (panel C) relative to saline control group. Normalized protein levels are expressed as pg/mg or ng/mg of protein and compared by ANOVA followed by Tukey’s test (*p<0.05; #p<0.01). Pretreatment with NGF antisense blunted the rapid rise of NGF, MCP-1 and sICAM-1 following acetic acid exposure.
4.3 Acetic acid exposure results in the dysregulation of several miR
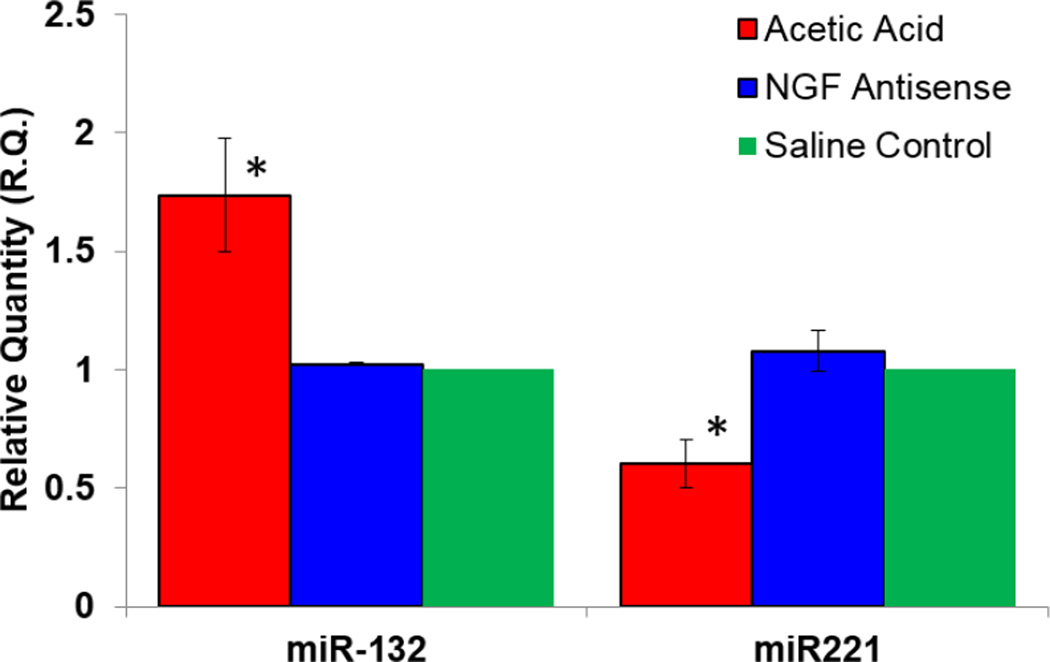
Upon investigating the effect of acetic acid exposure on the miR expression, we observed the aberrant expression of several miRs that could potentially contribute to acetic acid induced bladder overactivity. Eight specific miRs namely, miR-155, miR-132, miR 134, miR-199a-5p, miR-221, miR-210, miR-212 and miR-206 were predicted to either affect or be affected by neurotrophin upregulation consequent of bladder irritation. Pair wise comparison of miR-132 expression in acetic acid treated groups demonstrated a ~2 fold downregulation by NGF antisense (Fig. 3) compared to untreated group, suggesting that expression of miR-132 is linked to NGF overexpression (Fig. 2).
Fig. 3.
Acetic acid exposure caused a dysregulation in the expression of non-protein coding genes responsible for miR expression. Pair wise comparison of acetic acid treated experimental groups showed that the expression of miR-132 was downregulated ~2 fold by NGF antisense, while expression of miR-221 saw a ~ 2 fold increase. miR levels were calculated using the comparative CT method after normalization to U6 snRNA. *p <0.05; unpaired Student’s t test.
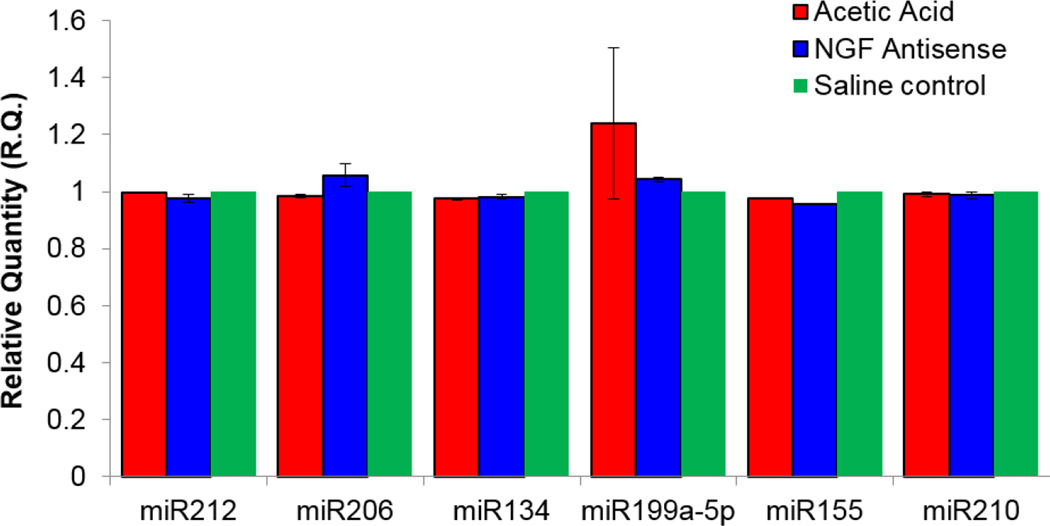
In contrast, pretreatment with NGF antisense caused a ~2-fold upregulation of miR-221 relative to group exposed to acetic acid in absence of NGF antisense (Fig. 3). Findings suggest that miR-132 expression is induced by NGF and the expression of miR-221 is suppressed by NGF overexpression. NGF antisense also altered the expression of miR-199a-5p, but without statistical significance, whereas expression levels of miR-212, miR-155, miR-210, miR-134 and miR-206 remained unchanged in all experimental groups (Fig. 4).
Fig. 4.
Insignificant effect of acetic acid and NGF antisense on miR expression. Analysis found similar expression of miR212, miR-155, miR-210, miR134 and miR206 in control and acetic acid exposed groups with only a insignificant elevation of miR-199a-5p following acetic acid exposure.
4.4 Predicted targets of the dysregulated miR
When the miR expression data of experimental groups exposed to acetic acid was compared between saline and NGF antisense treated groups, we observed that the overexpression of miR-132 corresponded positively with the overexpression of inflammatory mediators. Amongst its target genes, we found that miR-132 was predicted to target transcription factors and genes involved in inflammation such as FOXO3 responsible for MCP-1 expression. Likewise, decreased miR-132 expression was associated with decreased expression of chemokines suggesting that reduced targeting of FOXO3 by miR-132 is probably responsible for the reduced production of chemokine expression (Rao et al., 2015). Reduced expression of miR-221 following acetic acid exposure was predicted by other reports (Othumpangat et al., 2012b), which indicate that silencing of miR-221 expression upregulates NGF expression. The expression of sICAM-1 is also regulated by miR-221 (Othumpangat et al., 2012a). These results suggest that the miR expression profile and its associated biological functions, as indicated in published studies for miR-132 and miR-221 correlate with observed bladder overactivity after acetic acid infusion.
4.5 Confirmation of the role of miR-132 in bladder overactivity
Bladder wall transfection of miR-132 plasmid (n=5) caused a >2 fold upregulation of miR-132 relative to control group transfected with Luciferase plasmid (Fig. 5). The upregulation of miR-132 in bladder following transfection is slightly higher than the fold change measured after acetic acid exposure. Physiological consequences from exogenous overexpression of miR-132 in bladder were the exhibition of bladder overactivity in absence of acetic acid ICI (Fig. 5A–B). The ICI of the rat group transfected with miR-132 was significantly reduced relative to the control group (Fig. 5B). As predicted by findings in Fig. 3, overexpression of miR-132 upregulates the expression of NGF and MCP-1 by 5 fold and sICAM-1 by 2 fold (Fig. 5C). Overexpression of miR-132 also upregulates the expression of Cx-43, which is a clinically relevant marker of bladder overactivity.
Fig. 5.
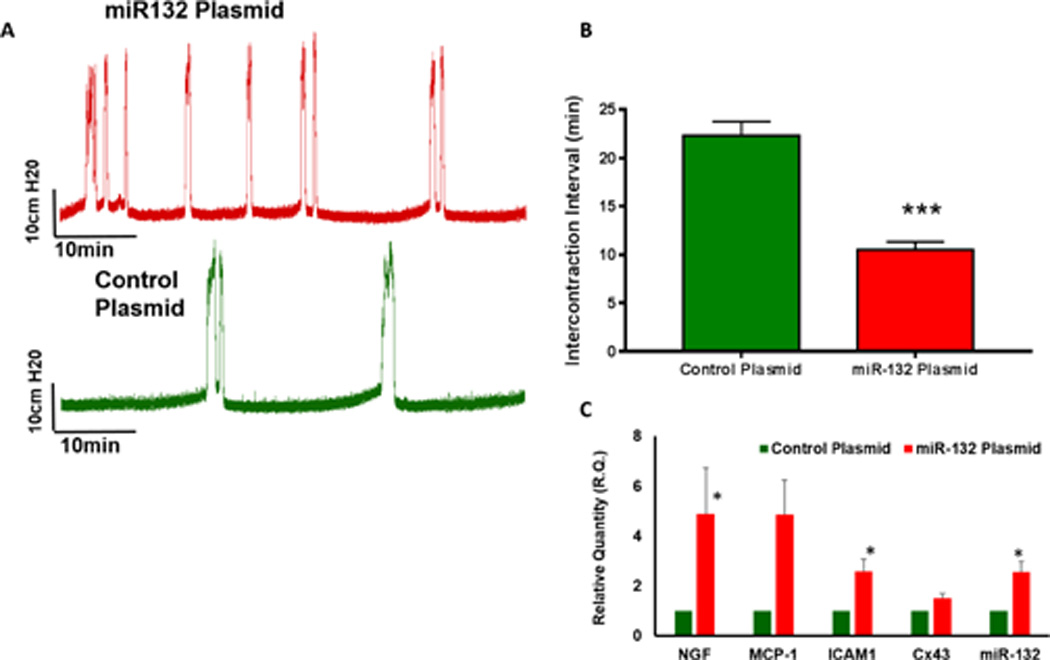
Representative tracings of saline cystometry performed 7 days after bladder wall transfection in luciferase (control) and miR-132 plasmid transfected group (n=5) under urethane anesthesia. Panel B- Unpaired analysis of ICI demonstrated that overexpression of miR-132 in bladder leads to bladder overactivity in absence of acetic acid exposure (Unpaired student’s t test; **p<0.001). Panel C: Bladder wall transfection of miR-132 plasmid vector drastically upregulates the expression of miR-132, which consequently causes 5 fold upregulation of NGF and MCP-1 and two fold upregulation of sICAM-1 and Cx-43 genes relative to the control group transfected with luciferase plasmid (Unpaired student’s t test; *p<0.01)
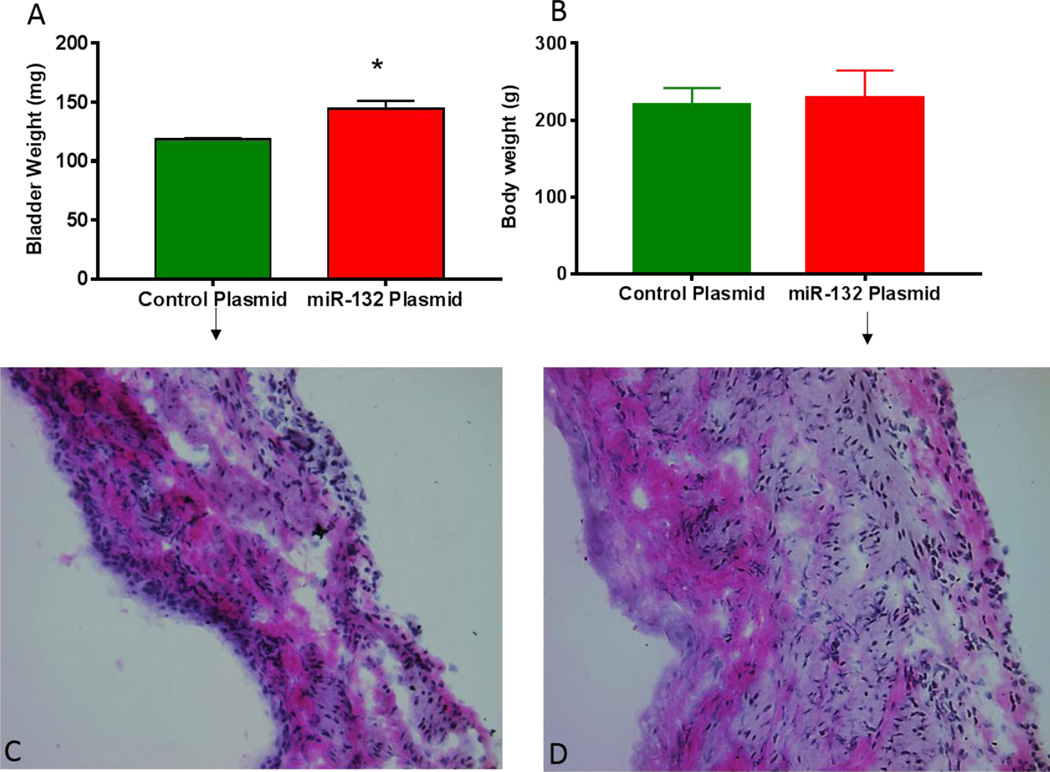
We checked whether overexpression of miR-132 by comparing the gross weights of harvested bladder at the end of cystometry and the body weights of animals in two groups. Gross weight comparison of harvested bladder demonstrated a significant increase in group transfected with miR-132 plasmid (Fig. 6A; unpaired student’s t test; p<0.01). Significant increase in bladder weight was noted without any significant change in the body weight of miR-132 plasmid group relative to the control group (Fig. 6B). Increased bladder weight suggested that bladder overactivity observed following transfection of miR-132 is linked to bladder hypertrophy. Histological analysis of cryosections obtained from two groups indicated thickening of the bladder wall following transfection of miR-132 plasmid (Fig. 6D) compared to rat bladder transfected with control plasmid (Fig. 6C). Therefore, overexpression of miR-132 has a pathogenic role in the bladder overactivity and bladder hypertrophy.
Fig. 6.
Panel A: Exogenous overexpression of miR-132 significantly increased the gross weight of bladder harvested 7 days after transfection of luciferase (control) and miR-132 plasmids (Unpaired student’s t test; * p<0.01). Significant increase in bladder weight of group transfected with miR-132 plasmid was noted without any significant change in body weight relative to control group (Panel B). Histological analysis indicated signs of bladder hypertrophy following overexpression of miR-132 as apparent from thickened bladder wall (Panel D) relative to control plasmid (Panel C).
5. Discussion
MicroRNA (miR) is an emerging class of highly conserved, non-coding small RNA, whose discovery has advanced the understanding of gene regulation. These non-coding RNA molecules can be located in the introns, exons or the intragenic regions of genes to facilitate mRNA degradation and/or translational repression (Gheinani et al., 2013). Earlier reports indicated that chemically induced bladder overactivity involves upregulation of neurotrophins, genes involved in neuronal development, and increased maturation of neural progenitor cells (Saban et al., 2006, Saban et al., 2002). Here we extended our earlier work (Kashyap et al., 2013) to demonstrate that changes in the transcriptional repertoire of inflammatory molecules in rat bladder following acetic acid exposure are also accompanied with perturbations in miR expression.
The exhibition of bladder overactivity following transfection of miR-132 in absence of acetic acid confirms the critical role played by miR-132 in mediating bladder overactivity. Bladder wall transfection of miR-132 upregulated the expression of miR-132 in bladder leading to bladder hypertrophy and bladder overactivity. Observed bladder hypertrophy after transfection of miR-132 is concordant with the results of miR-132 upregulation following bladder outlet obstruction (Sadegh et al., 2015). Bladder hypertrophy may be a consequence of smooth cell proliferation and angiogenesis from increased Ras activity following upregulation of miR-132 (Anand et al., 2010). Overexpression of miR-132 also raised the expression of Cx-43, which is a clinically relevant marker for bladder overactivity (Heinrich et al., 2011) and may explain the observed bladder overactivity observed. Overexpression of Cx-43 in miR-132 plasmid transfected group is consistent with the literature reports indicating decreased Cx43 expression due to disruption in miR-132 expression (Xu et al., 2015).
Overexpression of miR-132 in bladder caused parallel overexpression of NGF and MCP-1 following acetic acid exposure or transfection. The parallel increase of miR-132 and inflammatory chemokine MCP-1 is consistent with the known role of miR-132 in mediating inflammation via the transcription factor FOXO3 (Rao et al., 2015). FOXO3 is also known to be involved in cardiac hypertrophy (Ucar et al., 2012) and may also explain the miR-132 mediated bladder hypertrophy. The effect of NGF antisense on the expression of miR-132 and miR-221 provides a mechanistic link for the reduced NGF expression leading to reduced expression of inflammatory cytokines and chemokines (Kashyap et al., 2013) through the action of FOXO3.
NGF overexpression following exogenous overexpression of miR-132 is in agreement with the literature reports on miR mediated regulation of neurotrophin expression (Gao et al., 2010, Giannotti et al., 2014). Deep sequencing studies of cultured primary cortical mouse neurons identified miR-132 as BDNF-induced miR (Remenyi et al., 2010), which taken together with reduced expression of BDNF in mouse urothelium following transgenic overexpression of NGF (Girard et al., 2011) led us to postulate a mediatory role for BDNF in the observed changes in miR-132 expression. Slight elevation of miR-199a-5p following acetic acid induced bladder irritation is consistent with its reported role in impairment of tight junctions and bladder permeability (Monastyrskaya et al., 2013). Unchanged expression of miR-134, miR-155, miR-206 and miR-210 (Fasanaro et al., 2009) together with parallel overexpression of NGF and miR-132 in bladder suggests intricate miR interactions with genes encoding neurotrophins.
Differences in expression of MCP-1 and sICAM-1 following transfection of miR-132 indicates that different signaling pathways and miRs are involved in production of MCP-1 and sICAM-1. The overexpression of sICAM-1 and NGF accompanying reduced expression of miR-221 following acetic acid exposure (Othumpangat et al., 2012b) can be explained by the complementarity of miR-221 to the 3'-UTR of sICAM-1 mRNA (Hu et al., 2010). Treatment with NGF antisense was able to reduce both NGF and ICAM-1 expression and also the silencing of miR-221 expression by acetic acid. The miR-221 is known to target the 3'-UTR of stem cell factor receptor c-kit (Koelz et al., 2011) and indirectly regulate endothelial nitric oxide synthase expression (Urbich et al., 2008).
NGF antisense restored the miR-221 expression to levels seen in saline treated animals. Future studies will need a method to independently reduce the miR-221 expression (Hamada et al., 2012) to demonstrate per se the effect of miR-221 on bladder overactivity. These findings provide evidence that observed bladder overactivity can be explained, at least in part, to the intricate miR alterations and miR interactions with genes encoding neurotrophins or inflammatory genes. Overall, expression of miR-132 is involved in bladder overactivity and hypertrophy through changes in the expression neurotrophins, chemokines and gap junction communication.
6. Conclusions
Overall, present work sheds light on the involvement of miR-132 in the NGF signaling as well as in the therapeutic effect of NGF antisense in the bladder. These findings suggest that aberrant expression of miR-132 is involved in overactive bladder.
Acknowledgments
This work was partly supported by funding from NIH (DK088836)
List of Abbreviations Used
- miR
MicroRNA
- NGF
Nerve Growth factor
- BDNF
Brain derived neurotrophic factor
- MCP-1
Monocyte chemoattractant protein-1
- ICAM-1
Intracellular adhesion molecule 1
- CMG
Cystometrogram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: None of the authors have any interest to disclose
References
- Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nature medicine. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacker SM, Dawson TM, Dawson VL. The interplay of microRNA and neuronal activity in health and disease. Frontiers in cellular neuroscience. 2013;7:136. doi: 10.3389/fncel.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanaro P, Greco S, Lorenzi M, Pescatori M, Brioschi M, Kulshreshtha R, et al. An integrated approach for experimental target identification of hypoxia-induced miR-210. J Biol Chem. 2009;284:35134–35143. doi: 10.1074/jbc.M109.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheinani AH, Burkhard FC, Monastyrskaya K. Deciphering microRNA code in pain and inflammation: lessons from bladder pain syndrome. Cellular and molecular life sciences : CMLS. 2013;70:3773–3789. doi: 10.1007/s00018-013-1275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti G, Caffino L, Calabrese F, Racagni G, Riva MA, Fumagalli F. Prolonged abstinence from developmental cocaine exposure dysregulates BDNF and its signaling network in the medial prefrontal cortex of adult rats. Int J Neuropsychopharmacol. 2014;17:625–634. doi: 10.1017/S1461145713001454. [DOI] [PubMed] [Google Scholar]
- Girard BM, Malley SE, Vizzard MA. Neurotrophin/receptor expression in urinary bladder of mice with overexpression of NGF in urothelium. Am J Physiol Renal Physiol. 2011;300:F345–F355. doi: 10.1152/ajprenal.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada N, Fujita Y, Kojima T, Kitamoto A, Akao Y, Nozawa Y, et al. MicroRNA expression profiling of NGF-treated PC12 cells revealed a critical role for miR-221 in neuronal differentiation. Neurochemistry international. 2012;60:743–750. doi: 10.1016/j.neuint.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Oberbach A, Schlichting N, Stolzenburg JU, Neuhaus J. Cytokine effects on gap junction communication and connexion expression in human bladder smooth muscle cells and suburothelial myofibroblasts. PLoS One. 2011;6:e20792. doi: 10.1371/journal.pone.0020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Gong AY, Liu J, Zhou R, Deng C, Chen XM. miR-221 suppresses ICAM-1 translation and regulates interferon-gamma-induced ICAM-1 expression in human cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2010;298:G542–G550. doi: 10.1152/ajpgi.00490.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova NN. Role of BDNF epigenetics in activity-dependent neuronal plasticity. Neuropharmacology. 2014;76(Pt C):709–718. doi: 10.1016/j.neuropharm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Kashyap M, Kawamorita N, Tyagi V, Sugino Y, Chancellor M, Yoshimura N, et al. Down-regulation of nerve growth factor expression in the bladder by antisense oligonucleotides as new treatment for overactive bladder. J Urol. 2013;190:757–764. doi: 10.1016/j.juro.2013.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap M, Yoshimura N, de Groat WC, Tyagi P. Exogenous Overexpression Of Brain Derived Neurotrophic Factor (BDNF) In Rat Bladder Evokes Bladder Overactivity. J Urol. 2014;191:4. [Google Scholar]
- Koelz M, Lense J, Wrba F, Scheffler M, Dienes HP, Odenthal M. Down-regulation of miR-221 and miR-222 correlates with pronounced Kit expression in gastrointestinal stromal tumors. International journal of oncology. 2011;38:503–511. doi: 10.3892/ijo.2010.857. [DOI] [PubMed] [Google Scholar]
- Kye MJ, Goncalves Ido C. The role of miRNA in motor neuron disease. Frontiers in cellular neuroscience. 2014;8:15. doi: 10.3389/fncel.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastyrskaya K, Sanchez-Freire V, Hashemi Gheinani A, Klumpp DJ, Babiychuk EB, Draeger A, et al. miR-199a-5p regulates urothelial permeability and may play a role in bladder pain syndrome. Am J Pathol. 2013;182:431–448. doi: 10.1016/j.ajpath.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Montalban E, Mattugini N, Ciarapica R, Provenzano C, Savino M, Scagnoli F, et al. MiR-21 is an Ngf-modulated microRNA that supports Ngf signaling and regulates neuronal degeneration in PC12 cells. Neuromolecular medicine. 2014;16:415–430. doi: 10.1007/s12017-014-8292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, et al. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus. 2010;20:492–498. doi: 10.1002/hipo.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddiah D, Anand P, McMahon SB, Rattray M. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport. 1998;9:1455–1458. doi: 10.1097/00001756-199805110-00038. [DOI] [PubMed] [Google Scholar]
- Othumpangat S, Regier M, Piedimonte G. Nerve growth factor modulates human rhinovirus infection in airway epithelial cells by controlling ICAM-1 expression. American journal of physiology Lung cellular and molecular physiology. 2012a;302:L1057–L166. doi: 10.1152/ajplung.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othumpangat S, Walton C, Piedimonte G. MicroRNA-221 modulates RSV replication in human bronchial epithelium by targeting NGF expression. PLoS One. 2012b;7:e30030. doi: 10.1371/journal.pone.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, Nagarkatti P, Nagarkatti M. Role of miRNA in the Regulation of Inflammatory Genes in Staphylococcal enterotoxin B-Induced Acute Inflammatory Lung Injury and Mortality. Toxicological sciences : an official journal of the Society of Toxicology. 2015 doi: 10.1093/toxsci/kfu315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenyi J, Hunter CJ, Cole C, Ando H, Impey S, Monk CE, et al. Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. The Biochemical journal. 2010;428:281–291. doi: 10.1042/BJ20100024. [DOI] [PubMed] [Google Scholar]
- Saban MR, Hellmich HL, Turner M, Nguyen NB, Vadigepalli R, Dyer DW, et al. The inflammatory and normal transcriptome of mouse bladder detrusor and mucosa. BMC Physiol. 2006;6:1. doi: 10.1186/1472-6793-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban MR, Nguyen NB, Hammond TG, Saban R. Gene expression profiling of mouse bladder inflammatory responses to LPS, substance P, antigen-stimulation. The American journal of pathology. 2002;160:2095–2110. doi: 10.1016/S0002-9440(10)61159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadegh MK, Ekman M, Krawczyk K, Svensson D, Goransson O, Dahan D, et al. Detrusor induction of miR-132/212 following bladder outlet obstruction: association with MeCP2 repression and cell viability. PLoS One. 2015;10:e0116784. doi: 10.1371/journal.pone.0116784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Sim SE, Bakes J, Kaang BK. Neuronal Activity-Dependent Regulation of MicroRNAs. Molecules and cells. 2014 doi: 10.14348/molcells.2014.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaldone MC, Vodovotz Y, Tyagi V, Barclay D, Philips BJ, Yoshimura N, et al. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis. Urology. 2009;73:421–426. doi: 10.1016/j.urology.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognini P, Pizzorusso T. MicroRNA212/132 family: molecular transducer of neuronal function and plasticity. The international journal of biochemistry & cell biology. 2012;44:6–10. doi: 10.1016/j.biocel.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Tyagi P, Tyagi V, Qu X, Chuang YC, Kuo HC, Chancellor M. Elevated CXC chemokines in urine noninvasively discriminate OAB from UTI. Am J Physiol Renal Physiol. 2016;311:F548–F554. doi: 10.1152/ajprenal.00213.2016. [DOI] [PubMed] [Google Scholar]
- Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nature communications. 2012;3:1078. doi: 10.1038/ncomms2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Xu B, Chen M, Ji X, Yao M, Mao Z, Zhou K, et al. Metabolomic profiles reveal key metabolic changes in heat stress-treated mouse Sertoli cells. Toxicol In Vitro. 2015;29:1745–1752. doi: 10.1016/j.tiv.2015.07.009. [DOI] [PubMed] [Google Scholar]