Abstract
Background
Although used primarily in the pediatric population for decades, the use of intraosseous (IO) devices in the resuscitation of severely injured adult trauma patients has recently become more commonplace. The objective of this study was to determine the experience level, beliefs and attitudes of trauma practitioners in Canada, Australia and New Zealand regarding the use of IO devices in adult trauma patients.
Methods
We administered a web-based survey to all members of 4 national trauma and emergency medicine organizations in Canada, Australia and New Zealand. Survey responses were analyzed using descriptive statistics, univariate comparisons and a proportional odds model.
Results
Overall, 425 of 1771 members completed the survey, with 375 being trauma practitioners. IO devices were available to 97% (353 of 363), with EZ-IO being the most common. Nearly all physicians (98%, 357 of 366) had previous training with IO devices, and 85% (223 of 261) had previously used an IO device in adult trauma patients. Most respondents (79%, 285 of 361) were very comfortable placing an IO catheter in the proximal tibia. Most physicians would always or often use an IO catheter in a patient without intravenous access undergoing CPR for traumatic cardiac arrest (84%, 274 of 326) or in a hypotensive patient (without peripheral intravenous access) after 2 attempts or 90 s of trying to establish vascular access (81%, 264 of 326).
Conclusion
Intraosseous devices are readily available to trauma practitioners in Canada, Australia and New Zealand, and most physicians are trained in device placement. Most physicians surveyed felt comfortable using an IO device in resuscitation of adult trauma patients and would do so for indications broader than current guidelines.
Abstract
Contexte
Bien que le dispositif de perfusion intraosseuse soit depuis des décennies utilisé principalement chez les enfants, son utilisation lors de la réanimation d’adultes victimes de trauma grièvement blessés a récemment gagné en popularité. Notre étude vise à déterminer le niveau d’expérience, les croyances et les attitudes des spécialistes en traumatologie canadiens, australiens et néo-zélandais en ce qui concerne l’utilisation de ces dispositifs chez des patients adultes victimes de trauma.
Méthodes
Nous avons fait parvenir un sondage Web à tous les membres de 4 organisations nationales de traumatologie et d’urgentologie au Canada, en Australie et en Nouvelle-Zélande. Les réponses ont été analysées au moyen de statistiques descriptives, de comparaisons univariées et d’un modèle à cotes proportionnelles.
Résultats
Au total, parmi les 1771 personnes visées, 425 ont répondu au sondage, dont 375 spécialistes en traumatologie. De tous les répondants, 97 % avaient accès à un dispositif de perfusion intraosseuse, et le modèle EZ-IO était le plus répandu. Presque tous les médecins (98 %) avaient été formés pour utiliser cet appareil, et 85 % d’entre eux l’avaient déjà utilisé chez des adultes victimes de trauma. De plus, la plupart des répondants (79 %) étaient très à l’aise de poser un cathéter intraosseux dans la voie tibiale proximale. La plupart auraient toujours ou souvent recours à ces cathéters pour traiter un patient sans accès intraveineux subissant des manoeuvres de réanimation à la suite d’un arrêt cardiaque traumatique (84 %) ou un patient hypotendu (aucun accès veineux périphérique) sur lequel on a tenté à 2 reprises ou pendant 90 s d’établir un accès vasculaire (81 %).
Conclusion
Les spécialistes en traumatologie canadiens, australiens et néo-zélandais ont facilement accès à des dispositifs de perfusion intraosseuse, et la plupart d’entre eux ont été formés sur leur mise en place. La plupart des répondants au sondage se sont dits à l’aise d’utiliser le dispositif lors de la réanimation d’adultes victimes de trauma et prêts à s’en servir pour traiter des cas plus variés que ce que recommandent les lignes directrices actuelles.
The use of intraosseous (IO) devices for the purposes of achieving vascular access in adults has rapidly expanded in recent years1 and may still be underutilized. 2 While clinical use of IO devices over the last few decades has been largely confined to pediatric resuscitation, the use of IO devices in adults dates back to the early 20th century3 and was common during World War II.4,5 However, the advent of the over-the-needle plastic catheter in 1950 heralded the age of the peripheral intravenous (IV) cannula, and the use of IO infusions faded.6 The utility of IO access in pediatrics resurged in the 1980s and was introduced into the American Heart Association pediatric resuscitation guidelines in 1985.7 There were considerable limitations to the IO needles available at the time, until various manufacturers began producing new devices in the late 1990s. These decives included the FAST1 (Fast Access for Shock and Trauma, Pyng Medical Corporation) in 1997, the BIG (Bone Injection Gun, WaisMed) in 1998 and the EZ-IO (Vidacare Corporation) in 2004.
The evolution of IO devices into an easy-to-use, rapidly placed and widely available method of achieving vascular access has made IO placement the emergent vascular access method of choice in the viewpoint of many.1,8,9 Furthermore, prospective studies have demonstrated superior speed of insertion with IO devices compared with the central venous catheter, which is generally considered the back-up technique for failed peripheral IV attempts.10,11 Although the use of IO devices is rapidly expanding for patients requiring fluid and cardiac resuscitation,1,9 some have expressed concern about their safety, especially in the setting of blood product transfusion.12 Despite these concerns, the European Resuscitation Council Guidelines,13 the American Heart Association Advanced Cardiac Life Support (ACLS)14 and the American College of Surgeons Advanced Trauma Life Support (ATLS)15 all support the use of IO devices for adult patients in extremis.
Intraosseous devices are currently being used in diverse settings, such as prehospital,16 the resuscitation bay10 and in-patient medical emergency settings.17 The purpose of this study was to investigate how physicians in Canada, Australia and New Zealand feel about using IO devices for resuscitation in adult trauma patients, how often they use these devices in their practices, and whether any physician characteristics are associated with choosing to use an IO device in various clinical scenarios. We hypothesized that IO devices are commonly being used for resuscitation in adult trauma patients.
Methods
Survey design and population
We conducted an electronic survey to assess the clinical experience and opinions of physicians in Canada, Australia and New Zealand regarding use of IO devices for resuscitation in trauma patients. After obtaining approval from the respective national organizations, the Trauma Association of Canada (TAC), the Canadian Association of Emergency Physicians (CAEP), the Australasian Trauma Society (ATS) and the Australia and New Zealand Association for the Surgery of Trauma (ANZAST), members were contacted via email and invited to participate in the survey via web link. The email explained how members were identified to participate in the survey, described the goals of the study, and assured them that the survey was confidential and that their participation would be anonymous. Owing to the voluntary nature of the study, we considered informed consent to be implied by completion of the survey. Because we sent participants a unique email link, they had the ability to complete the survey only once. This study was endorsed by the TAC Research Committee and received Health Research Ethics Board approval from the University of Alberta in Edmonton, Alta.
Survey administration and content
The survey was constructed by the research team using SelectSurvey (www.selectsurvey.net) and refined through pilot testing with multiple trauma researchers for content and response process validity. We are integrally involved in and provide content expertise in the field of trauma research. The survey was sent electronically to all members of TAC, CAEP, ATS and ANZAST. A reminder email was sent 2 weeks after the initial email. The survey was conducted over a 2-month period from April to June 2014.
We collected demographic information on practitioner roles and specialties, their practice and their level of training. Prior training with an IO device included ATLS 8th edition, ATLS 9th edition, Pediatric Advanced Life Support (PALS), accredited physician continuing medical education courses, departmental/hospital courses and training from a clinical nurse educator or colleague. We also collected data on clinical experience with IO devices and comfort levels using these devices. We evaluated the comfort level of physicians with placing an IO catheter in different body regions (proximal tibia, distal tibia, humerus, sternum) using a 4-point Likert scale ranging from “very comfortable” to “not at all comfortable.” Physicians were presented a variety of routes (IO, peripheral IV via gravity flow, peripheral IV via level 1/rapid infuser, central line, saphenous vein cutdown) and asked to indicate which were acceptable for administering blood products, crystalloids, medications or vasopressors by responding “yes,” “no,” or “I don’t know.” Using a 5-point Likert scale ranging from “always” to “never,” we assessed the attitudes of physicians regarding acceptable indications for using an IO catheter in adult/pediatric major trauma patients.
We also presented physicians with a clinical scenario and asked them to select the method they would use to establish access to the patient’s vascular system. We asked physicians to select the method they would use on their first attempt and, if unsuccessful, on their second, third, fourth, fifth and sixth attempts. The clinical scenario was as follows:
A 28-year-old male motorcyclist has been involved in a highway speed head-on collision with a truck. He is transported from the scene to your emergency department via ambulance, arriving 20 minutes after the crash. On exam, he has an unstable pelvic fracture, a large right hemothorax, and peritonitis with a distended abdomen. His vital signs are blood pressure 80/48, heart rate of 132, respiratory rate of 28, oxygen saturation of 96% on 15 L via mask, with a Glasgow Coma Score of 11. Emergency Medical Services have attempted to establish peripheral IVs but have been unsuccessful and he currently has no IV access.
Statistical analysis
Data collected through the survey instrument were entered into a Microsoft Excel spreadsheet. Data from partially completed surveys were included in the analysis. We grouped the responses in 2 ways: by physician type (emergency medicine physicians, surgeons, non–emergency medicine physicians [e.g., anesthesiologists, intensivists]) and by regional member organization (CAEP, TAC, ATS, ANZAST). We used simple descriptive statistics to report physician demographics and clinical experience with IO devices. The variable of interest was the attitudes of physicians toward using an IO device to establish vascular access in adult trauma patients. A proportional odds model was fitted using the following explanatory variables: previous IO device training, prior experience with IO devices for vascular access purposes, having access to an IO device, ATLS 8th edition certification, ATLS 9th edition certification and PALS certification. Associations were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). All analysis was performed using SPSS Statistics software version 21 (IBM).18
Results
Of the 1771 members who received the survey invitation, we received responses from 425 (24%) participants. Of these, 356 surveys were fully completed and 69 had 1 or more incomplete questions. The response rates among individual organizations were 26% (61 of 239) for TAC, 24% (322 of 1320) for CAEP and 20% (42 of 212) for ATS/ANZAST. For the purposes of our analysis, we included only surveys that were returned by staff or attending physician trauma practitioners, which left us with 375 responses. By physician type, most respondents were in the emergency medicine group (85%, 320 of 375), followed by the surgeon (8%, 30 of 375) and non–emergency medicine (7%, 25 of 375) groups. The majority of respondents practised in Canada (CAEP and TAC: 93%, 349 of 375; ATS and ANZAST: 7%, 26 of 375). Characteristics of study participants regarding their practices, previous IO training and having access to an IO device are summarized in Table 1.
Table 1.
Characteristics of survey participants, by physician type and by organization
| Characteristic | Physician group; no. (%)* | Organization; no. (%)* | |||
|---|---|---|---|---|---|
|
|
|
||||
| EM | Non-EM | Surgeons | ATS/ANZAST | CAEP/TAC | |
| Years in their role, no. of respondents | 316 | 25 | 30 | 25 | 346 |
|
| |||||
| > 15 | 118 (37) | 8 (32) | 9 (30) | 10 (40) | 125 (36) |
|
| |||||
| 11–15 | 62 (19) | 8 (32) | 3 (10) | 5 (20) | 68 (20) |
|
| |||||
| 6–10 | 64 (20) | 5 (20) | 9 (30) | 8 (32) | 70 (20) |
|
| |||||
| 1–5 | 65 (20) | 4 (16) | 9 (30) | 2 (8) | 76 (22) |
|
| |||||
| < 1 | 7 (2) | 0 (0) | 0 (0) | 0 (0) | 7 (2) |
|
| |||||
| Practice as a TTL, no. of respondents | 316 | 25 | 30 | 25 | 346 |
|
| |||||
| Yes | 142 (45) | 12 (48) | 25 (83) | 20 (80) | 159 (46) |
|
| |||||
| No | 174 (55) | 13 (52) | 5 (17) | 5 (20) | 187 (54) |
|
| |||||
| ATLS Certification, no. of respondents | 315 | 25 | 30 | 25 | 345 |
|
| |||||
| Yes | 215 (68) | 20 (80) | 29 (97) | 22 (88) | 242 (70) |
|
| |||||
| 8th edition | 116 (37) | 10 (40) | 16 (53) | 12 (48) | 130 (38) |
|
| |||||
| 9th edition | 106 (34) | 12 (48) | 28 (93) | 15 (60) | 131 (38) |
|
| |||||
| Other | 19 (6) | 4 (16) | 2 (7) | 5 (19) | 20 (6) |
|
| |||||
| Instructor | 17 (5) | 2 (8) | 2 (7) | 2 (8) | 19 (5) |
|
| |||||
| No | 100 (32) | 5 (20) | 1 (3) | 3 (12) | 103 (30) |
|
| |||||
| PALS Certification, no. of respondents | 313 | 25 | 30 | 25 | 343 |
|
| |||||
| Yes | 155 (50) | 11 (44) | 3 (10) | 8 (32) | 161 (47) |
|
| |||||
| No | 158 (50) | 14 (56) | 27 (90) | 17 (68) | 182 (53) |
|
| |||||
| Access to an IO device, no. of respondents | 308 | 25 | 30 | 25 | 338 |
|
| |||||
| Yes | 300 (97) | 23 (92) | 30 (100) | 24 (96) | 329 (97) |
|
| |||||
| No | 8 (3) | 2 (8) | 0 (0) | 1 (4) | 9 (3) |
|
| |||||
| Previous training with placing an IO device, no. of respondents | 311 | 25 | 30 | 25 | 341 |
|
| |||||
| Yes | 305 (98) | 24 (96) | 28 (93) | 24 (96) | 333 (98) |
|
| |||||
| No | 6 (2) | 1 (4) | 2 (7) | 1 (4) | 8 (2) |
|
| |||||
| Type of IO device available, no. of respondents† | 321 | 27 | 32 | 28 | 352 |
|
| |||||
| EZ-IO | 272 (85) | 20 (74) | 23 (72) | 23 (82) | 292 (83) |
|
| |||||
| Bone Injection Gun | 21 (7) | 2 (7) | 2 (6) | 2 (7) | 23 (7) |
|
| |||||
| FAST1 | 3 (1) | 0 (0) | 0 (0) | 0 (0) | 3 (1) |
|
| |||||
| Unknown | 18 (6) | 3 (11) | 6 (19) | 1 (4) | 26 (7) |
|
| |||||
| Other | 7 (2) | 2 (7) | 1 (3) | 2 (7) | 8 (2) |
|
| |||||
| Previously used IO device for vascular access in adult trauma patient, no. of respondents | 310 | 25 | 30 | 25 | 340 |
|
| |||||
| Yes | 223 (72) | 16 (64) | 24 (80) | 15 (60) | 248 (73) |
|
| |||||
| No | 87 (28) | 9 (36) | 6 (20) | 10 (40) | 92 (27) |
|
| |||||
| No. of times used IO device for vascular access in an adult trauma patient in past year, no. of respondents | 221 | 16 | 24 | 15 | 246 |
|
| |||||
| 0 | 33 (15) | 2 (13) | 3 (13) | 5 (33) | 33 (13) |
|
| |||||
| 1 | 65 (29) | 5 (31) | 10 (41) | 4 (27) | 76 (31) |
|
| |||||
| 2–5 | 109 (49) | 9 (56) | 8 (33) | 5 (33) | 121 (49) |
|
| |||||
| 6–10 | 12 (5) | 0 (0) | 3 (13) | 1 (7) | 14 (6) |
|
| |||||
| > 10 | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 2 (1) |
ANZAST = Australia and New Zealand Association for the Surgery of Trauma; ATLS = Advanced Trauma Life Support; ATS = Australasian Trauma Society; CAEP = Canadian Association of Emergency Physicians; EM = emergency medicine; FAST1 = Fast Access for Shock and Trauma; IO = intraosseous; PALS = Pediatric Advanced Life Support; TAC = Trauma Association of Canada; TTL = trauma team leader.
Not all physicians answered every question.
Some physicians selected more than 1 of the choices.
Most physicians had at least 10 years of experience (56%, 208 of 371). Nearly half of respondents (48%, 179 of 371) practised as trauma team leaders (TTLs), with about 83% (25 of 30) of the surgeon group and 80% (20 of 25) of the ATS/ANZAST group doing so. ATLS certification was reported by 71% (264 of 370) of respondents, with 39% (146 of 370) having completed the 9th edition ATLS provider course. PALS certification was reported by 46% (169 of 368) of physicians, with the surgeon group having the lowest rate (10%, 3 of 30). Almost all respondents (98%, 357 of 366) had previous IO placement training, and only 3% (10 of 363) indicated they did not have access to an IO device. The most commonly available IO device was the EZ-IO, which was available to 83% (292 of 352) of Canadian respondents and 82% (23 of 28) of respondents from Australia and New Zealand. Overall, 72% (263 of 365) of respondents reported ever placing an IO device for the purpose of vascular access in a trauma patient, with 73% (248 of 340) of Canadians compared with 60% (15 of 25) of Australia/New Zealand respondents reporting so. Surgeons reported the highest rate of previous experience with IO devices for vascular access at 80% (24 of 30) compared with 72% (223 of 310) for emergency medicine physicians and 64% for non–emergency medicine physicians. Among Canadian respondents, 87% (213 of 246) reported having placed an IO device within the last year compared with 67% (10 of 15) of Australia/New Zealand respondents. Overall, 85% (223 of 261) of physicians had placed an IO device for the purpose of vascular access within the past year, with 55% (143 of 261) reporting they had done so at least twice during that period.
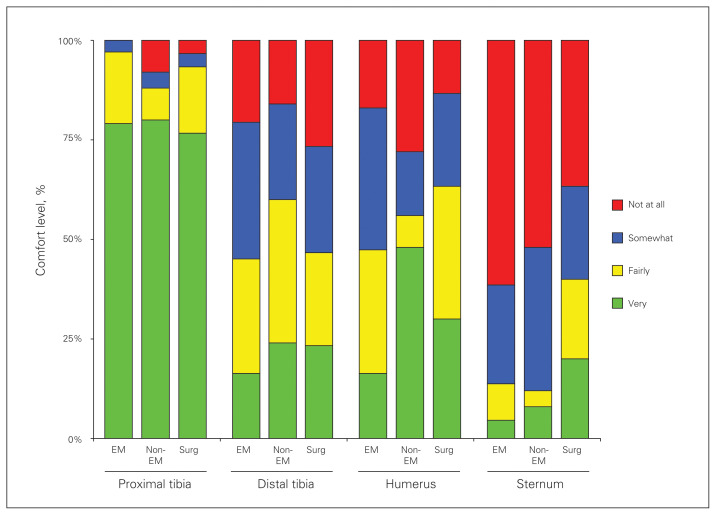
The comfort level of physicians with placing an IO device at various locations (proximal tibia, distal tibia, humeral head, sternum) is shown in Figure 1. Most physicians (79%, 285 of 361) were very comfortable placing an IO catheter in the proximal tibia, but most (51%, 183 of 361) were somewhat or not at all comfortable placing an IO catheter in the humeral head. The sternum was the location where physicians felt least comfortable placing an IO device, with 59% (212 of 361) responding they were not at all comfortable.
Fig. 1.
Comfort level of physicians with placing an intraosseous (IO) device at various locations (proximal tibia, distal tibia, humeral head, sternum). EM = emergency medicine.
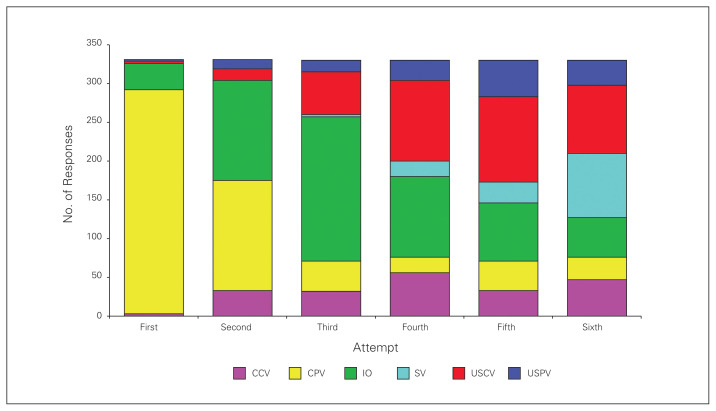
Physicians were presented with a clinical scenario of a severely injured motorcyclist and asked to rank their preferred methods for establishing IV access (Fig. 2). Most physicians (87%, 289 of 331) selected a peripheral IV as the first method they would attempt, followed by an IO device (10%, 34 of 331). If their first attempt was unsuccessful, using a peripheral IV remained the preferred choice for the second attempt (43%, 142 of 331), followed closely by using an IO device (39%, 129 of 331). By the third attempt, the majority of physicians (56%, 186 of 330) would use an IO device to establish vascular access, followed by ultrasound-guided central IV (17%, 55 of 330).
Fig. 2.
Physicians’ rankings of preferred methods for establishing intravenous (IV) access in a clinical scenario of a severely injured motorcyclist. CCV = conventional central vein; CPV = concentional peripheral vein; IO = intraosseous; SV = saphenous vein cutdown; USCV = ultrasound-guided central vein; USPV = ultrasound-guided peripheral vein.
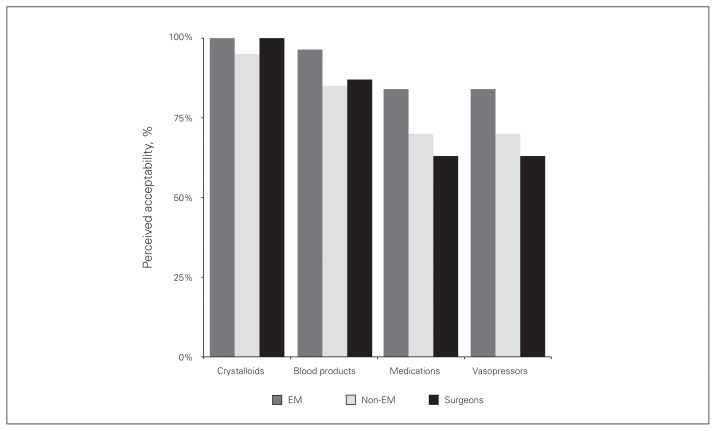
We asked physicians to consider the acceptability of administering blood products, crystalloids, medications, or vasopressors through various routes in a trauma patient. As shown in Figure 3, nearly all physicians believed that infusing crystalloids via an IO device was acceptable (emergency medicine group: 100%, 277 of 277; non–emergency medicine group: 95%, 19 of 20; surgeon group: 100%, 30 of 30). Most physicians also felt it was acceptable to give blood products using an IO device (emergency medicine group: 96%, 267 of 277; non–emergency medicine group: 85%, 17 of 20; surgeon group: 87%, 26 of 30). Physicians were least comfortable infusing either medications or vasopressors via an IO route (82%, 267 of 327), with 63% (19 of 30) of surgeons, 70% (14 of 20) of non–emergency medicine physicians, and 84% (234 of 277) of emergency medicine physicians believing this was acceptable. Owing to the small number of ATS/ANZAST responses to this question, we were unable to meaningfully compare the results according to organization.
Fig. 3.
Physicians’ perceived acceptability of administering blood products, crystalloids, medications, or vasopressors through various routes in a trauma patient. EM = emergency medicine.
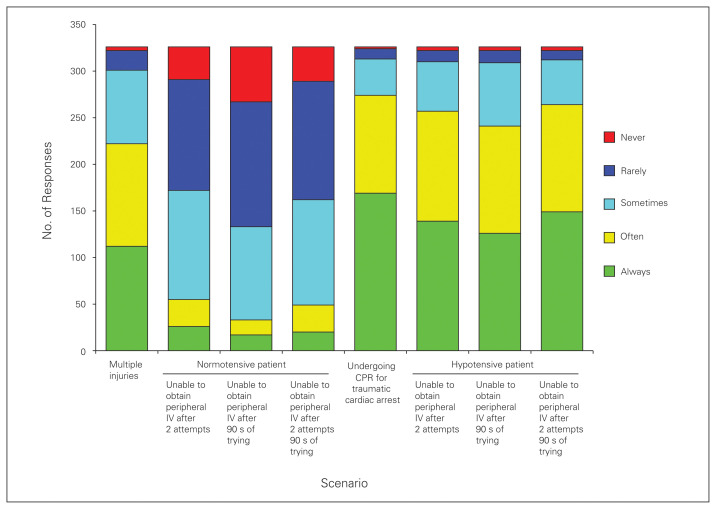
Figure 4 summarizes overall physician impressions of when to use an IO device in the setting of an adult trauma patient. More than 75% of respondents believed that an IO device would “always” or “often” be indicated in the following scenarios: in patients undergoing CPR for traumatic cardiac arrest (84%, 274 of 326); in patients who are hypotensive, without peripheral IVs, after 2 attempts (79%, 257 of 326); and in patients who are hypotensive, without peripheral IVs, after 90 s of trying to establish vascular access (74%, 241 of 326). Furthermore, 81% (264 of 326) of respondents replied that an IO device is indicated “always/often” in a hypotensive patient without peripheral IVs and after 90 s of trying to establish vascular access, and 68% (222 of 326) said an IO device is indicated in the setting of a patient with multiple injuries.
Fig. 4.
Overall physician impressions of when to use an intraosseous (IO) device in the setting of an adult trauma patient. CPR = cardiopulmonary resuscitation; IV = intravenous.
We used a proportional odds model to identify physician characteristics associated with an increased likelihood of an IO device being used. As shown in Table 2, physicians who had previous IO device training (OR 4.02, 95% CI 1.06–15.34, p = 0.040), prior experience with IO devices for vascular access purposes (OR 1.80, 95% CI 1.09–2.99, p = 0.020) and PALS certification (OR 2.07, 95% CI 1.32–3.26, p = 0.001) were were more likely to use an IO device. Interestingly, having ATLS certification (8th or 9th edition) was not significantly associated with increased likelihood of using an IO device.
Table 2.
Ordinal regression of factors associated with increased likelihood of physicians using an IO device in an adult trauma patient with no IV access
| Variable | OR (95% CI) | p value |
|---|---|---|
| ATLS 8th edition | 0.85 (0.47–1.53) | 0.60 |
| ATLS 9th edition | 0.66 (0.38–1.11) | 0.12 |
| PALS | 2.07 (1.32–3.26) | 0.001 |
| Previous IO device training | 4.02 (1.06–15.34) | 0.040 |
| Experience with IO devices for vascular access purposes | 1.80 (1.09–2.99) | 0.020 |
| Access to an IO device | 2.50 (0.61–10.26) | 0.20 |
ATLS = Advanced Trauma Life Support; CI = confidence interval; IO = Intraosseous; OR = odds ratio; PALS = Pediatric Advanced Life Support.
Discussion
Our study demonstrates that IO devices for the purpose of vascular access are widely available and are being actively used to resuscitate trauma patients in Canada, Australia and New Zealand. While the literature is replete with studies examining the use of IO devices in various settings (prehospital,16 resuscitation,1 in-patient,17 military19), it isn’t clear exactly how widely implemented this technique is in each setting. For instance, in Canada, the availability of IO devices in the prehospital setting varies among provinces. However, our responses indicate that IO devices are commonly available and used in the emergency department setting.
Our respondents were most comfortable placing the IO device in the proximal tibia. The benefit of this location is quicker time to insertion,20 although time to peak medication concentration is slower than via the sternal route.21 Flow rates via the tibia are also slower than via the humerus.22 Tibial placement is also a subdiaphragmatic point of vascular access, which is not the preferred location23 as blood return to the heart from this infusion point can be thwarted by other potential injuries (e.g., lower-extremity injuries, pelvic fractures, major intra-abdominal injuries). For these reasons, just as the subclavian vein is the preferred insertion site for a central venous catheter in trauma, the humerus may be the preferred IO route in trauma. Additional training focusing on the humerus as an insertion site could be considered.
Current resuscitation guidelines from the American Heart Association and the European Resuscitation Council state that the IO route is an effective method for administering drugs in adults and that it may be used if peripheral IV access cannot be readily established.13,14 The most recent ATLS manual states that IO access can be used in all age groups and suggests that in children 2 attempts at peripheral IV access can be made before resorting to IO devices; however, the manual doesn’t make any such statements regarding the adult population. The respondents in our survey clearly felt comfortable expanding the indications for IO placement, and one-third responded that they consider it to be the vascular access modality of first choice in trauma patients with multiple injuries. In comparison, in a 2009 survey of American emergency medicine residency program directors regarding unstable patients requiring emergent vascular access, 62% chose central line insertion as their second-line choice (after peripheral IV), and IO access became the dominant choice only if a fourth attempt was required.24
The vast majority of our respondents felt it acceptable to transfuse blood products and crystalloids via IO access, with slightly fewer respondents in favour of administering medications or vasopressors using this method. Despite a recent article making a theoretical argument to the contrary, 12 there exists a preponderance of historical5 and recent combat data19 supporting the transfusion of blood products via the IO route. Lewis and Wright19 recently reported on more than 1000 IO devices used in the combat setting of Afghanistan and the overall transfusion of 1881 units of packed red blood cells, 1497 units of fresh frozen plasma, 619 units of platelets and 410 units of cryoprecipitate with no major complications. The translation of lessons learned from military combat into civilian practice is often delayed; however, evidence supporting the administration of medications via the IO route is mounting. 1 The responses to our international survey provide evidence that IO devices are readily available and being used for resuscitation purposes and suggest that there is an opportunity to educate IO practitioners and train them to make sound and competent judgments regarding the use of these devices in adult trauma patients.
Our search of the contemporary literature revealed only 3 published surveys examining IO use. Hallas and colleagues25 surveyed Scandinavian emergency physicians, anesthesiologists and pediatricians to assess users’ experiences of complications with IO placement. In 68% of cases the user reported a complication or difficulty using the IO device, raising the concern that perceived difficulties with IO insertion could affect the willingness of medical staff to use IO devices.25 This finding suggests there is a need for improved device insertion education. In 1999, Lavis and colleagues26 surveyed members of the British Association for Accident and Emergency Physicians on their familiarity with and use of IO devices. While 74% responded they were aware that IO devices could be used in adult resuscitation, only 7% reported using the technique.26 James Cheung and colleagues27 performed an electronic survey of residents and attending physicians from a variety of specialties at a Canadian hospital in an effort to uncover barriers and facilitators to IO placement in adult resuscitations when peripheral IVs could not be achieved. They concluded that in order to increase IO use, future educational interventions should address physicians’ attitudinal, normative and control beliefs.27 Our results indicate widespread dissemination of the IO technique and significant attitudinal buy-in among physicians on the usefulness of IO devices in the setting of adult trauma resuscitation.
Limitations
There are limitations to our study. With its self-reporting survey design, validity is inherently limited by the response rate. Our response rate was 24%, hence there is the possibility that nonresponder bias may threaten the validity of our findings. Portions of our survey relied on self-reporting of volume and experience; thus, it is subject to recall bias. Given the smaller number of responses from the ATS/ANZAST group compared with the CAEP/TAC component, we were limited in our ability to compare these groups. Despite these limitations, our study has a number of strengths. The survey was designed by experts in the fields of trauma and emergency medicine using a rigorous methodology. The analysis was focused on active staff/attending-level trauma care practitioners, approximately 50% of whom were working as active TTLs. Although the response rate was 24%, this survey represents the opinions of 375 physicians and, to our knowledge, is the largest such survey conducted to date on this topic.
Conclusion
IO devices for the purposes of rapid vascular access are readily available to trauma practitioners in Canada, Australia and New Zealand, and these devices are in fact being used by trained practitioners in caring for trauma patients. Furthermore, the trauma physicians surveyed believed that indications for IO device placement could be expanded beyond current guidelines, and in particular could include the hypotensive adult trauma patient without peripheral IV access after 2 attempts or 90 s trying to obtain access in the setting of traumatic cardiac arrest or in patients with multiple injuries. Future efforts to optimize the use of IO devices in trauma may include local quality care initiatives aimed at encouraging IO device use for these expanded indications with corresponding outcome tracking and assessment.
Footnotes
This manuscript has been presented in oral form at the Trauma Association of Canada Annual Scientific Meeting (Apr. 11, 2015, Calgary, Alta., Canada) and as a poster at the Canadian Association of Emergency Physicians Annual Meeting, May 30, 2015, Edmonton, Alta.
Competing interests: None declared.
Contributors: P. Engels, S. Widder and R. Green designed the study. P. Engels, M. Erdogan and K. Martin acquired the data, which P. Engels. M. Erdogan, S. Widder, M. Butler, N. Kureshi and R. Green analyzed. P. Engels. M. Erdogan, S. Widder, M. Butler, N. Kureshi and R. Green wrote the article, which all authors reviewed and approved for publication.
References
- 1.Anson JA. Vascular access in resuscitation: Is there a role for the intraosseous route? Anesthesiology. 2014;120:1015–31. doi: 10.1097/ALN.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 2.Voigt J, Waltzman M, Lottenberg L. Intraosseous vascular access for in-hospital emergency use: a systematic clinical review of the literature and analysis. Pediatr Emerg Care. 2012;28:185–99. doi: 10.1097/PEC.0b013e3182449edc. [DOI] [PubMed] [Google Scholar]
- 3.Paxton JH. Intraosseous vascular access: a review. Trauma. 2012;14:195–232. [Google Scholar]
- 4.Doud EA, Tysell JE. Massive intramedullary infusions. JAMA. 1942;120:1212–3. [Google Scholar]
- 5.Dubick MA, Hocomb JB. A review of intraosseous vascular access: current status and military application. Mil Med. 2000;165:552–9. [PubMed] [Google Scholar]
- 6.Millam D. The history of intravenous therapy. J Intraven Nurs. 1996;19:5–14. [PubMed] [Google Scholar]
- 7.American Heart Association. National conference on standard and guidelines for cardiopulmonary resuscitation and emergency cardiac care. Standard and guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiac are (ECC). Part VI: pediatric advanced life support. JAMA. 1986;255:2961–4. [PubMed] [Google Scholar]
- 8.Paxton JH, Knuth TE, Klausne HA. Proximal humerus intraosseous infusion: a preferred emergency venous access. J Trauma. 2009;67:606–11. doi: 10.1097/TA.0b013e3181b16f42. [DOI] [PubMed] [Google Scholar]
- 9.Davis DP. The use of intraosseous devices during cardiopulmonary resuscitation: Is this the answer for which we have been searching? Resuscitation. 2012;83:7–8. doi: 10.1016/j.resuscitation.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Leidel BA, Kirchhoff C, Braunstein V, et al. Comparison of intraosseous versus central venous vascular access in adults under resuscitation in the emergency department with inaccessible peripheral veins. Resuscitation. 2012;83:40–5. doi: 10.1016/j.resuscitation.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Leidel BA, Kirchhoff C, Bogner V, et al. Is the intraosseous access route fast and efficacious compared to the conventional central venous catheterization in adult patients under resuscitation in the emergency department? A prospective observational pilot study. Patient Saf Surg. 2009;3:24. doi: 10.1186/1754-9493-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris M, Balog R, Devries R. What is the evidence of utility for intraosseous blood transfusion in damage-control resuscitation? J Trauma Acute Care Surg. 2013;75:904–6. doi: 10.1097/TA.0b013e3182a85f71. [DOI] [PubMed] [Google Scholar]
- 13.Nolan JP, Soar J, Zideman DA, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation. 2010;81:1219–76. doi: 10.1016/j.resuscitation.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Neumar RW, Ott CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S729–67. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 15.American College of Surgeons. Advanced Trauma Life Support Student Manual, 9th edition. Chicago, IL: American College of Surgeons; 2012. [Google Scholar]
- 16.Santos D, Carron PN, Yersin B, et al. EZ-IO® intraosseous device implementation in a pre-hospital emergency service: a prospective study and review of the literature. Resuscitation. 2013;84:440–5. doi: 10.1016/j.resuscitation.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Lee PMJ, Lee C, Rattern P, et al. Intraosseous versus central venous catheter utilization and performance during inpatient medical emergencies. Crit Care Med. 2015;43:1233–8. doi: 10.1097/CCM.0000000000000942. [DOI] [PubMed] [Google Scholar]
- 18.Corp IBM. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp; [Google Scholar]
- 19.Lewis P, Wright C. Saving the critically injured trauma patient: a retrospective analysis of 1000 uses of intraosseous access. Emerg Med J. 2015;32:463–7. doi: 10.1136/emermed-2014-203588. [DOI] [PubMed] [Google Scholar]
- 20.Reades R, Studnek JR, Vandeventer S, et al. Intraosseous versus intravenous vascular access during out-of-hospital cardiac arrest: a randomized controlled trial. Ann Emerg Med. 2011;58:509–16. doi: 10.1016/j.annemergmed.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Hoskins SL, do Nascimento P, Jr, Lima RM, et al. Pharmacokinetics of intraosseous and central venous drug delivery during cardiopulmonary resuscitation. Resuscitation. 2012;83:107–12. doi: 10.1016/j.resuscitation.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 22.Lairet J, Bebarta V, Lairet K, et al. A comparison of proximal tibia, distal femur, and proximal humerus infusion rates using the EZ-IO intraosseous device on the adult swine model. Prehosp Emerg Care. 2013;17:280–4. doi: 10.3109/10903127.2012.755582. [DOI] [PubMed] [Google Scholar]
- 23.American College of Surgeons. Advanced Trauma Life Support Student Manual, 9th edition. Chicago, IL: American College of Surgeons; 2012. Chapter 1: Initial assessment and management; p. 11. [Google Scholar]
- 24.Bloch SA, Bloch AJ, Silva P. Adult intraosseous use in academic EDs and simulated comparison of emergent vascular access techniques. Am J Emerg Med. 2013;31:622–4. doi: 10.1016/j.ajem.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Hallas P, Brabrand M, Folkestad L. Complication with intraosseous access: scandinavian users’ experience. West J Emerg Med. 2013;14:440–3. doi: 10.5811/westjem.2013.1.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavis M, Vaghela A, Tozer C. Adult intraosseous infusion in accident and emergency departments in the UK. J Accid Emerg Med. 2000;17:29–32. doi: 10.1136/emj.17.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James Cheung W, Rosenberg H, Vaillancourt C. Barriers and facilitators to intraosseous access in adult resuscitations when peripheral intravenous access is not achievable. Acad Emerg Med. 2014;21:250–6. doi: 10.1111/acem.12329. [DOI] [PubMed] [Google Scholar]