Abstract
Background
In February 2013, Alberta Health Services established an Enhanced Recovery After Surgery (ERAS) implementation program for adopting the ERAS Society colorectal guidelines into 6 sites (initial phase) that perform more than 75% of all colorectal surgeries in the province. We conducted an economic evaluation of this initiative to not only determine its cost-effectiveness, but also to inform strategy for the spread and scale of ERAS to other surgical protocols and sites.
Methods
We assessed the impact of ERAS on patients’ health services utilization (HSU; length of stay [LOS], readmissions, emergency department visits, general practitioner and specialist visits) within 30 days of discharge by comparing pre- and post-ERAS groups using multilevel negative binomial regressions. We estimated the net health care costs/savings and the return on investment (ROI) associated with those impacts for post-ERAS patients using a decision analytic modelling technique.
Results
We included 331 pre- and 1295 post-ERAS patients in our analyses. ERAS was associated with a reduction in all HSU outcomes except visits to specialists. However, only the reduction in primary LOS was significant. The net health system savings were estimated at $2 290 000 (range $1 191 000–$3 391 000), or $1768 (range $920–$2619) per patient. The probability for the program to be cost-saving was 73%–83%. In terms of ROI, every $1 invested in ERAS would bring $3.8 (range $2.4–$5.1) in return.
Conclusion
The initial phase of ERAS implementation for colorectal surgery in Alberta is cost-saving. The total savings has the potential to be more substantial when ERAS is spread for other surgical protocols and across additional sites.
Abstract
Contexte
En février 2013, les Services de santé de l’Alberta ont mis en place le programme ERAS (Enhanced Recovery After Surgery — récupération postchirurgicale améliorée) dans le but de faire adopter les lignes directrices en matière d’interventions colorectales de la ERAS Society à 6 établissements (première phase) où sont prati-quées plus de 75 % des interventions chirurgicales colorectales de la province. Nous avons réalisé une évaluation économique du programme, non seulement pour en mesurer la rentabilité, mais aussi pour élaborer une stratégie visant à étendre le programme ERAS à d’autres protocoles chirurgicaux et services de chirurgie.
Méthodes
Nous avons mesuré les effets du programme ERAS sur l’utilisation des services de santé (durée de séjour, réadmissions, visites au service des urgences, visites d’un omnipraticien ou d’un spécialiste) dans les 30 jours suivant le congé en comparant les groupes pré- et post-ERAS à l’aide de régressions binomiales négatives multiniveaux. Nous avons évalué le coût net des soins de santé, les économies réalisées et le rendement sur investissement (RSI) associés aux mesures ci-dessus chez les patients post-ERAS à l’aide d’une technique de modélisation analytique décisionnelle.
Résultats
Nos analyses ont porté sur 331 patients pré-ERAS et 1295 patients post-ERAS. Nous avons observé une réduction de toutes les mesures de l’utilisation des services de santé étudiées, sauf les visites d’un spécialiste. Toutefois, seule la réduction de la durée du premier séjour était significative. Les économies nettes pour le système de santé ont été estimées à 2 290 000 $ (de 1 191 000 $ à 3 391 000 $), soit 1768 $ (de 920 $ à 2619 $) par patient. La probabilité que le programme soit économique était de 73 % à 83 %. En ce qui concerne le RSI, nous avons établi que chaque dollar investi dans le programme ERAS rapporterait 3,8 $ (de 2,4 $ à 5,1 $).
Conclusion
La première phase de la mise en oeuvre du programme ERAS en Alberta, appliqué à la chirurgie colorectale, a été économique. Les économies pour le système de santé pourraient être plus importantes si l’on étendait le programme à d’autres protocoles chirurgicaux et services de chirurgie.
Enhanced Recovery After Surgery (ERAS) is an evidence-based multimodal care pathway that aims to attenuate surgical stress and has proven earlier recovery for patients undergoing major surgery.1–7 It is well documented that ERAS is clinically effective as well as cost-effective/cost-saving in that it reduces complications and health services utilization (HSU; e.g., length of stay in hospital [LOS]) without compromising patient safety and health-related quality of life (HRQoL).8–11 A recent meta-analysis of 16 randomized controlled trials shows that ERAS significantly decreases primary LOS by 2.28 days and risk of complications by 40% in patients undergoing colorectal surgery.10 Most recently, a cost-effectiveness study of ERAS for colorectal surgery showed that ERAS helped patients return to work sooner and lessened caregiver burden without lowering patients’ quality of life and that, societally, ERAS saved $2985 (range $373–$5753) per patient, with the probability for ERAS to be cost-effective at close to 100%.12 Most reports on implementation of enhanced recovery programs are single-centre studies and may or may not incorporate or audit for all ERAS elements. The Netherlands reported significant improvement using consensus guidance for a structured implementation program using a breakthrough methodology model from the Institute for Healthcare Improvement.13,14 In the United Kingdom, the National Health Service ran a national program of enhanced recovery in several disciplines based on lectures and protocols from leaders in enhanced recovery, leading to small but significant LOS savings.15
The current ERAS implementation program used in Alberta is the first experience for a health system (including many surgical sites/centres) in North America evolved from the initial Dutch experience using a structure and well-defined implementation model based on local multidisciplinary and multiprofessional teams.
Alberta Health Services (AHS) is Canada’s first province-wide, fully integrated health system, responsible for delivering health services to more than 4 million people. Provincial data reports that more than 275 000 surgical procedures are performed annually in 59 surgical facilities, with 11 of those performing 10 000–33 000 annual procedures. As part of its quality agenda, AHS uses Strategic Clinical Networks, groups of clinicians, managers, policy experts, researchers, patients and leaders, to drive innovation and research. Through the Diabetes, Obesity and Nutrition and the Surgery Strategic Clinical Networks, a demonstration project was funded to implement the ERAS Society colorectal guidelines16 and test them in the provincial health system with assistance from the ERAS Society’s endorsed ERAS implementation program. At the initial phase, between June 3, 2013, and Mar. 31, 2015, the implementation program included 6 hospitals/sites where more than 75% of all colorectal surgery in the province is performed. We conducted an economic evaluation of this initial phase of the Alberta ERAS implementation program to not only determine its cost-effectiveness, but also to inform strategy for synchronous structured implementation of ERAS expanding to multiple surgical specialties and sites across the province.
Methods
Within the ERAS implementation program, a database called “ERAS Interactive Audit System” had been developed for collecting pre-ERAS (conventional care) and post-ERAS data according to an evidence-based international guideline.17 The audit data scored compliance with the international guidelines to ensure adoption and limited variation among implementation sites across the health system.
We analyzed data from the pre- and post-ERAS patients recorded across the 6 early-adopter sites by the end of March 2015 together with the Alberta Health administrative databases,18 which provide information about LOS; readmissions; and visits to the emergency department (ED), specialists and general practitioners (GPs).
First, we assessed the impacts of ERAS on patients’ HSU within 30 days of hospital discharge by comparing pre- and post-ERAS patients. This time horizon allowed us to capture health system impacts in 5 areas: LOS during the time of surgery (primary LOS), inpatient readmissions occurring postdischarge (e.g., owing to a surgical complication), LOS for those readmitted, complications not requiring readmissions postdischarge presenting in an ambulatory setting (e.g., ED), and visits to a primary care provider in the community setting. Of note, these inpatient, outpatient and physician services also included rehabilitation services (if any). As these outcomes are count data and to account for random effects occurring among the 6 sites, we used multilevel (patients nested within sites) mixed-effects negative binomial regressions with random intercepts.
We measured the impacts of ERAS on HSU using incidence rate ratios (IRR) between post-ERAS patients (cases) and pre-ERAS patients (controls). We considered an IRR less than 1 to indicate that ERAS reduced HSU and vice versa. We considered results to be significant at p < 0.05. Patients’ demographic and perioperative characteristics were included as covariates in the negative binomial regression models analysis. These characteristics were age, sex, body mass index (BMI), smoking status, alcohol consumption, comorbidity, diagnosis, American Society of Anesthesiologists (ASA) physical status class, preoperative chemotherapy, surgical approach, surgical complexity (more complex group included abdominoperineal resection, anterior resection of rectum, total/subtotal colectomy, reversal of Hartmann procedure; less complex group included right hemicolectomy, left hemicolectomy, other large/small bowel resection, ileostomy reversal),19 blood loss, main procedure, year and hospital site. The χ2 test and t test were used for comparisons of proportion and mean, respectively.
In the second step, we estimated health care costs/savings associated with impacts of ERAS calculated in the first step for the post-ERAS patients using a decision analytic modelling technique (Fig. 1). The costs/savings were estimated by multiplying the difference in HSU between cases and controls with the respective unit cost. We estimated the difference in HSU using the following formula: D = HSU1 −HSU1 × IRR, where D is the difference in HSU, HSU1 is the number of HSU of the controls, and IRR is the impact of ERAS estimated by multilevel negative binomial regressions described in the first step. The unit cost can be cost per hospital day of primary LOS, cost per hospital day of readmission LOS, cost per ED visit, cost per specialist visit, or cost per GP visit, which was estimated using the data of pre-ERAS patients. We specifically used the pre-ERAS study cohort unit costs because if ERAS had not existed, the unit costs of post-ERAS patients would have been the same as those of pre-ERAS patients. To estimate the net costs/savings, we subtracted the ERAS intervention costs, including labour/coordination and licensing fees, which amounted to $826 210 or $638 per patient. Of note, there was no pay increase for doctors, surgeons and other medical staff participating in the ERAS implementation program. To estimate the return on investment ratio, we divided the total cost savings by the ERAS intervention cost.
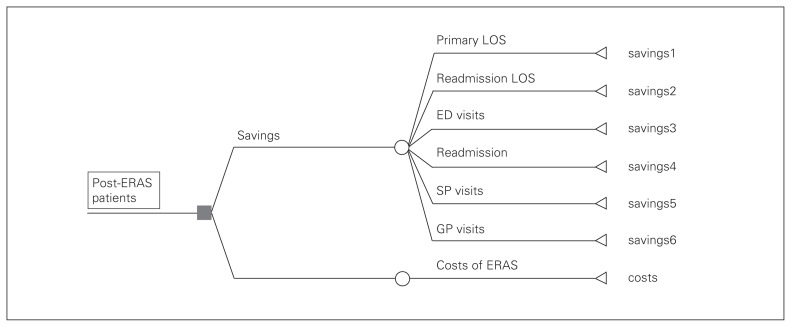
Fig. 1.
Decision tree to estimate health system savings. Savings1 = (LOS1 – LOS1 × IRR1) × c1, where LOS1 is primary LOS of pre-ERAS patients, IRR1 is the impact of ERAS on primary LOS measured by an IRR, and c1 is the marginal cost per hospital day of the primary LOS shortened by ERAS. Savings2 = (LOS2 – LOS2 × IRR2) × c2, where LOS2 is readmission LOS of pre-ERAS patients, IRR2 is the impact of ERAS on readmission LOS measured by an IRR and c2 is the marginal cost per hospital day of the readmission LOS shortened by ERAS. Savings3 = (ED – ED × IRR3) × c3, where ED is the number of ED visits of pre-ERAS patients, IRR3 is the impact of ERAS on ED visits measured by an IRR and c3 is cost per ED visit. Savings4 = (readmit – readmit × IRR4) × LOS2 × c4, where readmit is the number of readmissions of pre-ERAS patients, IRR4 is the impact of ERAS on readmissions measured by an IRR, LOS2 is readmission LOS of pre-ERAS patients, and c4 is the average cost per hospital day of readmissions prevented by ERAS. Savings5 = (SP – SP × IRR5) × c5, where SP is the number of specialist visits of pre-ERAS patients, IRR5 is the impact of ERAS on specialist visits measured by an IRR and c5 is the cost per specialist visit. Saving6 = (GP – GP × IRR6) × c6, where GP is the number of GP visits of pre-ERAS patients, IRR6 is the impact of ERAS on GP visits measured by an IRR and c6 is the cost per GP visit. ED = emergency department; ERAS = enhanced recovery after surgery; GP = general practitioner; IRR = incidence rate ratio; LOS = length of stay; SP = specialist.
In the base-case analysis, we conservatively included the significant impact (impact of ERAS on primary LOS) only. Furthermore, we did not use the average but the “marginal cost”20 per hospital day for the stays that were shortened by ERAS. The rationale was that during a hospital stay, the cost of health services in the last days of the stay is usually lower than the average and close or equal to the “hotel cost” because of the high treatment cost in the first few days when major procedures (i.e., surgery) are done. As actual data were not available, we applied the “hotel cost” as a percentage of the average cost (43.5%, range 32.9%–58.8%), which we estimated based on micro-costing data of pre-ERAS colorectal surgeries in Montreal reported by Lee and colleagues,12 to the average cost of pre-ERAS patients ($3600) estimated from our data in order to estimate the marginal cost. Accordingly, the marginal cost estimated at $1566 (range $1184–$2117) was used in this study to estimate the cost savings of ERAS for shortening LOS (both primary and readmission). Of note, this unit cost is comparable to those used in recent publications of the Alberta ERAS implementation program ($1114–$2106 in 2014, equivalent to $1127–$2131 in 2015 Canadian dollars)19,21 and to the marginal cost ($1237 in 2014, equivalent to $1271 in 2015 Canadian dollars) of patients undergoing hip and knee replacements in Alberta estimated by Alberta Bone and Joint Health Institute (unpublished data, but available upon request).
In a scenario analysis, we also included the statistically nonsignificant impacts of ERAS on other HSUs (readmission, readmission LOS, ED visits, specialist visits and GP visits) because the costs of these outcomes may still be substantial, as suggested by Stowers and colleagues.11 For the number of readmissions prevented by ERAS, as the whole LOS of each readmission was prevented, the average cost per day for readmission was used. The average cost per hospital day of readmission LOS ($2696), cost per ED visit ($904), cost per specialist visit ($352) and cost per GP visit ($196) were estimated from the data of pre-ERAS patients. As mentioned earlier, we used the pre-ERAS study cohort unit costs because if ERAS had not existed, the unit costs of post-ERAS patients would have been the same as those of pre-ERAS patients.
Sensitivity analysis
For the base-case scenario, we performed sensitivity analyses on 3 variables: the primary LOS of pre-ERAS patients, the impact of ERAS on this LOS and the marginal cost. In a deterministic 1-way sensitivity analysis, which allows 1 variable to vary at a time, the variation range was the 95% confidence interval (CI).
In a probabilistic sensitivity analysis that allows all variables to vary simultaneously, we analyzed 2 scenarios: 1) including only significant impact of ERAS on primary LOS (base-case scenario) and 2) including all statistically significant and nonsignificant impacts of ERAS on HSU. We assumed a normal distribution for impacts of ERAS (IRRs) and a γ distribution for LOS, numbers of HSU, and costs. We ran 100 000 samples and reported the probabilities for ERAS to be cost-saving.
We used Stata MP 13 (www.stata.com) for the impact analysis in the first step and TreeAge Pro 2015 (www.treeage.com) for the cost analysis in the second step. All costs were adjusted to 2015 Canadian dollars using the consumer price index.22
Results
In total, 1295 post-ERAS patients (cases) and 331 pre-ERAS patients (controls) who had colorectal surgery between 2013 and 2015 in 6 hospitals/sites across Alberta were included in our analyses. There was no significant difference in mortality between the 2 groups during the study period (0.23% of cases v. 0.30% of controls, p = 0.82). The demographic and perioperative characteristics of cases (post-ERAS) and controls (pre-ERAS) are shown in Table 1. There were no significant differences between the 2 groups in terms of demographic characteristics. We found some significant differences in pre- and intraoperative characteristics, including diagnosis, number of comorbidities, number of procedures, proportion of open surgery and amount of intraoperative blood loss. Specifically, post-ERAS patients had fewer malignant neoplasms of the rectum (12.28% v. 16.92%) but more benign neoplasms of the colon, rectum, anus and anal canal (8.42% v. 4.53%); slightly fewer comorbidities (mean 2.86 v. 3.25) and procedures (mean 2.01 v. 2.20); fewer open surgeries (35.75% v. 46.22%); and less intraoperative blood loss (mean 190.4 mL v. 216.1 mL).
Table 1.
Demographic and clinical characteristics of patients in the pre-ERAS and post-ERAS groups
| Characteristic | Group; % or mean ± SD | p value | |
|---|---|---|---|
| Pre-ERAS (n = 331) | Post-ERAS (n = 1295) | ||
| Male | 52.27 | 55.98 | 0.23 |
| Age, yr | 61.6 ± 13.8 | 61.1 ± 14.8 | 0.26 |
| BMI | 28.5 ± 6.6 | 27.9 ± 5.9 | 0.07 |
| Smoker | 0.48 | ||
| Yes | 17.52 | 17.22 | |
| Stopped because of surgery | 0.91 | 1.85 | |
| No or unknown | 81.57 | 80.93 | |
| Drinker | 0.85 | ||
| Yes | 6.04 | 6.18 | |
| Stopped because of surgery | 0.3 | 0.15 | |
| No or unknown | 93.66 | 93.67 | |
| ASA physical status class | 0.39 | ||
| 1 | 6.65 | 6.95 | |
| 2 | 58.61 | 60 | |
| 3 | 29.61 | 29.88 | |
| Other | 5.14 | 3.17 | |
| Diabetes | 16.62 | 15.06 | 0.48 |
| Chemo therapy | 11.18 | 11.12 | 0.98 |
| Primary diagnosis | |||
| Malignant neoplasm of colon | 23.56 | 25.79 | 0.41 |
| Malignant neoplasm of rectosigmoid junction | 9.37 | 11.12 | 0.36 |
| Malignant neoplasm of rectum | 16.92 | 12.28 | 0.026 |
| Benign neoplasm of colon, rectum, anus and anal canal | 4.53 | 8.42 | 0.017 |
| Crohn disease (regional enteritis) | 6.04 | 6.41 | 0.81 |
| Attention to artificial openings | 18.43 | 15.06 | 0.13 |
| Other | 21.15 | 20.93 | 0.93 |
| No. of comorbidities | 3.25 ± 3.55 | 2.86 ± 3.30 | 0.030 |
| No. of procedures | 2.20 ± 1.92 | 2.01 ± 1.81 | 0.047 |
| Main procedure | 0.12 | ||
| Intestine | 46.83 | 50.73 | |
| Rectal | 30.82 | 31.81 | |
| Revision | 22.36 | 17.45 | |
| Open surgery | 46.22 | 35.75 | < 0.001 |
| Intraoperative blood loss, mL | 216.1 ± 226.7 | 190.4 ± 230.9 | 0.042 |
| More complex surgery | 40.48 | 40.46 | 0.10 |
| Mortality | 0.3 | 0.23 | 0.87 |
| Year of surgery | < 0.001 | ||
| 2013 | 45.32 | 10.19 | |
| 2014 | 54.68 | 67.34 | |
| 2015 | 0 | 22.47 | |
| Hospital/site | < 0.001 | ||
| Foothills Medical Centre | 16.31 | 10.58 | |
| Grey Nuns Hospital | 13.9 | 19 | |
| Misericordia Community Hospital | 14.8 | 7.88 | |
| Peter Lougheed Centre | 22.66 | 43.01 | |
| Royal Alexandra Hospital | 17.52 | 7.57 | |
| University of Alberta Hospital | 14.8 | 11.97 | |
ASA = American Society of Anesthesiologists; ERAS = Enhanced Recovery After Surgery.
Observed differences in years and hospitals/sites were owing to differences in the start time of ERAS implementation and in volume of patients among sites. The 2 sites with the largest volumes of colorectal surgery (Peter Lougheed Centre, Calgary, and Grey Nuns Hospital, Edmonton) were the first to start ERAS.
The HSU of controls (pre-ERAS), impacts of ERAS on HSU, average change per patient and total change for all post-ERAS patients are shown in Table 2. On average, a control patient stayed 9.04 days in hospital for the surgery (primary LOS). Within 30 days of discharge, control patients had an average of 0.55 ED visits, 2.65 specialist visits and 2.1 GP visits and were readmitted an average of 0.14 times with a mean readmission LOS of 10.1 days.
Table 2.
Impacts of ERAS on health services utilization within 30 days of discharge
| Outcome | Pre-ERAS | IRR (95%CI) | Average change (95%CI) | No. of patients | Total change, d (95%CI)* |
|---|---|---|---|---|---|
| Primary LOS, d | 9.04 | 0.83 (0.77 to 0.90) | −1.537 (−2.079 to −0.994) | 1295 | −1990 (−2693 to −1288) |
| No. of ED visits | 0.55 | 0.92 (0.66 to 1.28) | −0.044 (−0.185 to 0.152) | 1295 | −57 (−240 to 197) |
| No. of specialist visits | 2.65 | 1.04 (0.84 to 1.29) | 0.106 (−0.432 to 0.756) | 1295 | 137 (−560 to 979) |
| No. of GP visits | 2.1 | 0.96 (0.79 to 1.17) | −0.084 (−0.432 to 0.361) | 1295 | −109 (−560 to 467) |
| No. of readmissions | 0.14 | 0.99 (0.66 to 1.48) | −0.014 (−0.047 to 0.067) | 1295 | −18 (−61 to 87) |
| Readmission LOS. d | 10.1 | 0.71 (0.46 to 1.10) | −2.929 (−5.417 to 1.001) | 143 | −419 (−775 to 143) |
CI = confidence interval; ED = emergency department; ERAS = Enhanced Recovery After Surgery; GP = general practicioner; IRR = incidence rate ratio; LOS = length of stay in hospital.
Number of readmission was multiplied by the readmission LOS to get the total change in days.
With the exception of specialist visits, all IRRs were less than 1, indicating that ERAS reduced all the HSU; however, only the reduction in primary LOS was significant. The average primary LOS of a post-ERAS patient was equal to 83% (95% CI 77%–89%) of a pre-ERAS patient. In other words, ERAS reduced 17% (95% CI 11%–23%) of primary LOS, equating to 1.5 (95% CI 0.99–2.1) days per patient or 1990 (95% CI 1288–2693) days for all 1295 patients.
Table 3 shows the numbers of days or visits reduced by ERAS, the unit cost for each health service, and the associated cost savings of ERAS in both the base-case and scenario analyses. In the base-case analysis, ERAS saved about $3 116 000 (range $2 017 000–$4 217 000) in HSU costs. Given the ERAS cost was approximately $826 000, the net cost savings of ERAS were estimated at $2 290 000 (range $1 191 000–$3 391 000) or $1768 (range $920–$2619) per patient. The return on investment ratio of ERAS was 3.8 (range 2.4–5.1) meaning that every $1 invested in ERAS would bring $3.8 (range $2.4–$5.1) in return.
Table 3.
Health care cost savings with ERAS (2015 Canadian dollars)
| Outcome | Total change, d | Unit cost | Base-case analysis | Scenario analysis | ||
|---|---|---|---|---|---|---|
| Base case | Low | High | ||||
| Primary LOS, d* | −1990 | $1566 | $3 116 340 | $2 017 008 | $4 217 238 | $3 116 340 |
| Number of ED visits | −57 | $904 | 0 | 0 | 0 | $51 528 |
| Number of specialist visits | 137 | $352 | 0 | 0 | 0 | −$48 224 |
| Number of GP visits | −109 | $196 | 0 | 0 | 0 | $21 364 |
| Prevented readmissions | −18 | $2696 | 0 | 0 | 0 | $48 528 |
| Readmission LOS, d | −419 | $1566 | 0 | 0 | 0 | $656 154 |
| Total cost | $3 116 340 | $2 017 008 | $4 217 238 | $3 845 690 | ||
| Cost of ERAS† | $826 210 | $826 210 | $826 210 | $826 210 | ||
| Total net cost savings | $2 290 130 | $1 190 798 | $3 391 028 | $3 019 480 | ||
| Net cost savings per patient | $1,768 | $920 | $2,619 | $2332 | ||
| Return on investment ratio | 3.8 | 2.4 | 5.1 | 4.7 | ||
ED = emergency department; ERAS = Enhanced Recovery After Surgery; GP = general practitioner; LOS = length of stay in hospital.
p < 0.000.
$638 per patient × 1295 patients.
In the scenario analysis where all outcomes (statistically significant and nonsignificant impacts of ERAS on HSU) were included, the net cost savings of ERAS was $3 019 000 or $2332 per patient. The return on investment ratio was 4.7, meaning that every $1 invested in ERAS would bring $4.7 in return.
The deterministic sensitivity analysis showed that the most sensitive variable was the impact of ERAS on the primary LOS. When the impact of ERAS on the primary LOS varied by the 95% CI (0.77–0.89), the net cost savings varied from $1 191 000 to $3 391 000 or $920 to $2619 per patient, and the return on investment ratio varied from 2.4 to 5.1, as mentioned earlier.
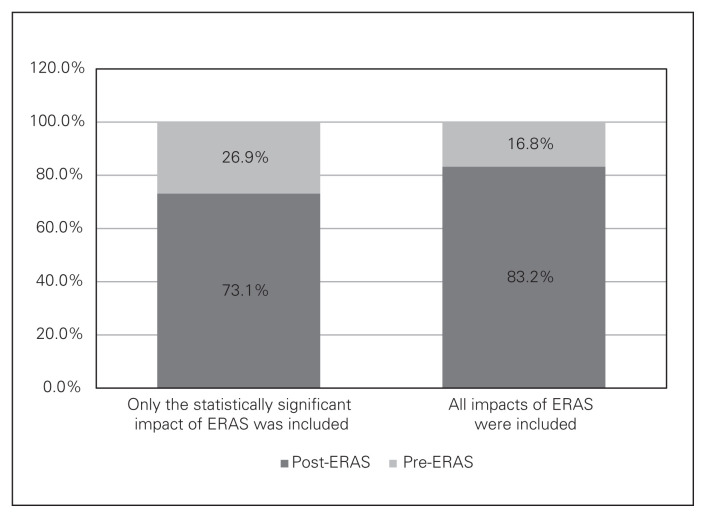
The probabilistic sensitivity analysis results show the probability for ERAS to be cost saving (Fig. 2). If only significant impact of ERAS on the primary LOS was included, the probability for ERAS to be cost saving was 73%. If all significant and nonsignificant impacts of ERAS on HSU were included, the probability was 83%.
Fig. 2.
Probability of Enhanced Recovery After Surgery (ERAS) to be cost saving.
Discussion
Alberta Health Services provided a large health system that embraced rapid implementation of ERAS driven by the Strategic Clinical Networks. Alignment of system leadership and executive support of the frontline efforts required by the ERAS implementation program helped stimulate rapid uptake and interest across the province.23,24 The present study economically evaluated the ERAS implementation program and its adoption of the ERAS Society colorectal guidelines within AHS from June 2013 to March 2015 in 6 sites that perform more than 75% of all colorectal surgeries in the province. Our results show that ERAS significantly reduced the primary LOS, resulting in health care cost savings. In terms of return on investment, every $1 invested in ERAS would bring $3.8 in return. This finding is consistent with the results of 17 other studies, as reported in a review by Stowers and colleagues,11 and with the results of a recent cost-effectiveness study of ERAS for colorectal surgery in Montreal by Lee and colleagues.12 These findings have produced important economic evidence to support a strategy for synchronous structured implementation of ERAS expanding to multiple surgical specialties and sites across the province.
Our results also show that within 30 days of surgical discharge, ERAS reduced the rate of readmission, readmission LOS, ED visits and GP visits; these changes were not statistically significant, a result that is consistent with a meta-analysis of 16 randomized controlled trials.10 Currently, compliance with the ERAS guideline has increased to 73%, and focusing on more consistent application of the guideline may improve the benefits described. Although we did not find statistical evidence of changes in these outcomes, we still included these costs in the scenario analysis, as suggested by Stowers and colleagues,11 and found that they accounted for 19% of the total health care cost savings of the ERAS implementation program.
Stengths and limitations
The inclusion of HSU within 30 days postdischarge, especially of GP visits, is a strength of our study because little is known about how cost is transferred from the hospital to the community setting and because it can show if there are unintended impacts of shortened primary LOS.11 Our results indicate that ERAS not only reduced primary LOS, but also reduced HSU within 30 days after surgical discharge. The increase (4%; Table 2) in the number of specialist visits can be explained by the inclusion of visits made by specialists in hospital during the primary LOS of patients undergoing surgery, which cannot be separated from visits to specialists made by patients after surgical discharge because of data limitation.
Another strength of this study is the use of marginal cost for analyses. It is well known that during a hospital stay, the cost of the last few days is close or equal to the “hotel cost,” which is often lower than the average cost per day because of the high treatment cost in the first few days.20 By taking this into consideration, we ensured that cost savings of ERAS are not overestimated.
There are several limitations to be acknowledged. First, the study would have been strengthened if the patients had been followed for a longer time period (e.g., 1 yr after surgical discharge). Second, one may argue that this study was not a full economic evaluation, as outcomes, such as mortality and HRQoL, were not included. However, we believe that this study is sufficient because there was no significant difference in mortality between pre- and post-ERAS groups (0.3% v. 0.23%, p = 0.82), and other studies have demonstrated that HRQoL does not differ between pre- and post-ERAS patients.12 Third, the health system savings of ERAS would be greater if benefits associated with “free capacity” were included. That is, there will be more space (hospital/ward/bed) and staff to serve other patients as ERAS shortens hospital LOS, saving health system resources, reducing wait times and thereby improving patients’ outcomes and satisfaction. Fourth, taking a health care rather than a societal perspective, as indirect cost (e.g., lost productivity) was not included, our study likely underestimated the total benefits of ERAS for society because by shortening hospital LOS, ERAS enables patients to return to work sooner. Finally, there may have been a selection bias as there were differences between pre- and post-ERAS patients (Table 1). However, we believe that multivariate and sensitivity analyses minimize this bias. Also, the multilevel regression analysis can control for random effects among hospitals/sites.
Conclusion
The initial phase of the ERAS implementation program for colorectal surgery in Alberta was cost saving. The net health system savings were estimated at $2 290 000 (range $1 191 000–$3 391 000) or $1768 (range $920–$2619) per patient. The probability of the program being cost saving was estimated to be 73%–83%. In terms of return on investment, every $1 invested in ERAS would bring $3.8 (range $2.4–$5.1) in return. The total savings or return on investment may be more substantial when ERAS is spread to other surgical specialties and sites.
Acknowledgements
The authors acknowledge the work and contribution of the provincial implementation team in this work. Organizational partners: Alberta Health Services, Covenant Health. ERAS provincial implementation leadership: Diabetes, Obesity and Nutrition, Provincial Nutrition and Food Services, Dr. Tom Noseworthy and Dr. Alun Edwards. Implementation site partners and unit teams: Peter Lougheed Centre, Grey Nuns Community Hospital, Royal Alexandra Hospital, University of Alberta Hospital, Misericordia Community Hospital, Foothills Medical Centre. ERAS surgeon leadership: Drs. Douglas Hedden, John Kortbeek, John Heine, Michael Chatenay, Art Plewes, Haili Wang, Anna Borowiec, Tony MacLean, Don Buie, Dale Berg, Ron Brisebois and Cliff Sample. ERAS anesthesiology leadership: Drs. Marelise Kruger, Bart Godlewski, Dean Jordon, James Chin, Neil Klassen, Derek Dillane, Bernard Sowa and Michael Chong. Provincial site coordinator and data collection team: Melissa Mucenski, Kevin Connolly, Katrina Percival, Miranda Klein, Christine Garland, Danielle Stevenson, Shawna Gallagher. Data analysts: Edwin Rogers, Lawrence Kiyang. Research consultant: Kelvin Mok, Ellen Crumley.
Footnotes
Competing interests: T. Wasylak declares travel assistance from ERAS World Congress. O. Ljungqvist is the current Chairman of the ERAS Society. He founded and owns stock in Encare AB that runs the ERAS Society Interactive Audit System. He also declares speaker fees and travel assistance from Merck. No other competing interests declared.
Contributors: N. Thanh, A. Chuck, T. Wasylak, P. Faris, O. Ljungqvist, G. Nelson and L. Gramlich designed the study. N. Thanh, A. Chuck, T. Wasylak, J. Lawrence, P. Faris and G. Nelson acquired the data, which N. Thanh, A. Chuck, O. Ljungqvist and G. Nelson analyzed. N. Thanh, A. Chuck, T. Wasylak, J. Lawrence, O. Ljungqvist and G. Nelson wrote the article, which all authors reviewed and approved for publication.
Funding: This study was funded by Alberta Health Services and the Partnership for Research and Innovation in the Health System (PRIHS) Research Grant from Alberta Innovates Health Solutions.
References
- 1.Fearon KCH, Ljungqvist O, Meyenfeldt MV, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24:466–77. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Roulin D, Donadini A, Gander S, et al. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg. 2013;100:1108–14. doi: 10.1002/bjs.9184. [DOI] [PubMed] [Google Scholar]
- 3.Bakker N, Cakir H, Doodeman HJ, et al. Eight years of experience with Enhanced Recovery After Surgery in patients with colon cancer: Impact of measures to improve adherence. Surgery. 2015;157:1130–6. doi: 10.1016/j.surg.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Ljungqvist O, Jonathan E. Rhoads lecture 2011: Insulin resistance and enhanced recovery after surgery. J Parenter Enteral Nutr. 2012;36:389–98. doi: 10.1177/0148607112445580. [DOI] [PubMed] [Google Scholar]
- 5.Melnyk M, Casey RG, Black P, et al. Enhanced recovery after surgery (ERAS) protocols: Time to change pactice? Can Urol Assoc J. 2011;5:342–8. doi: 10.5489/cuaj.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ. 2001;322:473–6. doi: 10.1136/bmj.322.7284.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921–8. doi: 10.1016/S0140-6736(03)14966-5. [DOI] [PubMed] [Google Scholar]
- 8.Varadhan KK, Neal KR, Dejong CHC, et al. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434–40. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. Clin Nutr. 2012;31:783–800. doi: 10.1016/j.clnu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Greco M, Capretti G, Beretta L, et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38:1531–41. doi: 10.1007/s00268-013-2416-8. [DOI] [PubMed] [Google Scholar]
- 11.Stowers MDJ, Lemanu DP, Hill AG. Health economics in Enhanced Recovery After Surgery programs. Can J Anaesth. 2015;62:219–30. doi: 10.1007/s12630-014-0272-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee L, Mata J, Ghitulescu GA, et al. Cost-effectiveness of enhanced recovery versus conventional perioperative management for colorectal surgery. Ann Surg. 2015;262:1026–33. doi: 10.1097/SLA.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 13.Institute for Healthcare Improvement. IHI Innovation Series white paper. Boston: 2003. [accessed 2016 Feb. 9]. The breakthrough series: IHI’s collaborative model for achieving breakthrough improvement. Available: www.IHI.org. [Google Scholar]
- 14.Gillissen F, Hoff C, Maessen JM, et al. Structured synchronous implementation of an enhanced recovery program in elective colonic surgery in 33 hospitals in The Netherlands. World J Surg. 2013;37:1082–93. doi: 10.1007/s00268-013-1938-4. [DOI] [PubMed] [Google Scholar]
- 15.Simpson JC, Mooneshinghe SR, Grocott MP, et al. Enhanced recovery from surgery in the UK: an audit of the enhanced recovery partnership programme 2009 – 2012. Br J Anaesth. 2015;115:560–8. doi: 10.1093/bja/aev105. [DOI] [PubMed] [Google Scholar]
- 16.ERAS Society. ERAS Society Guidelines. [accessed 2016 Jan. 14]. Available: www.erassociety.org/index.php/eras-guidelines/eras-society-guidelines.
- 17.ERAS Society. ERAS Interactive Audit System — Data entry and analysis & reports. [accessed 2016 Jan. 15]. Available: www.erassociety.org/index.php/eras-care-system/eras-interactive-audit-system.
- 18.Alberta Health. Health research in Alberta. [accessed 2016 Jan. 14]. Available: www.health.alberta.ca/initiatives/health-research.html.
- 19.Nelson G, Kiyang LN, Crumley ET, et al. Implementation of Enhanced Recovery After Surgery (ERAS) across a provincial healthcare system: the ERAS Alberta Colorectal Surgery Experience. World J Surg. 2016;40:1092–103. doi: 10.1007/s00268-016-3472-7. [DOI] [PubMed] [Google Scholar]
- 20.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. Third Edition. Oxford Medical Publication. Oxford University Press; 2005. [Google Scholar]
- 21.Nelson G, Kiyang LN, Crumley ET, et al. Cost impact analysis of Enhanced Recovery After Surgery (ERAS) program implementation in Alberta colon cancer patients. Curr Oncol. 2016;23:e221–7. doi: 10.3747/co.23.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Statistics Canada. Consumer Price Index, by province. [accessed 2016 Jan. 8]. Available: www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/econ09a-eng.htm.
- 23.Smith C, Christiansen T, Dick D, et al. Performance management tools motivate change at the frontlines. Healthc Manage Forum. 2014;27:15–9. doi: 10.1016/j.hcmf.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Werle J, Dobbelsteyn L, Feasel AL, et al. A study of the effectiveness of performance-focused methodology for improved outcomes in Alberta public healthcare. Healthc Manage Forum. 2010;23:169–74. doi: 10.1016/j.hcmf.2010.08.007. [DOI] [PubMed] [Google Scholar]