Abstract
Introduction
There is intense interest in identifying cerebrospinal fluid (CSF) biomarkers of Parkinson's disease (PD), both for early diagnosis and to track effects of putative treatments. Nigrostriatal dopamine depletion characterizes PD. Predictably, CSF levels of 3,4-dihydroxyphenylacetic acid (DOPAC), the main neuronal metabolite of dopamine, are decreased in PD, even in patients with recent onset of the movement disorder. Whether low CSF DOPAC is associated specifically with parkinsonism has been unclear. In the neuronal cytoplasm dopamine undergoes not only enzymatic oxidation to form DOPAC but also spontaneous oxidation to form 5-S-cysteinyl-dopamine (Cys-DA). Theoretically, oxidative stress or decreased activity of aldehyde dehydrogenase (ALDH) in the residual nigrostriatal dopaminergic neurons would increase CSF Cys-DA levels with respect to DOPAC levels. PD, parkinsonian multiple system atrophy (MSA-P), and pure autonomic failure (PAF) are synucleinopathies; however, PAF does not entail parkinsonism. We examined whether an elevated Cys-DA/DOPAC ratio provides a specific biomarker of parkinsonism in synucleinopathy patients.
Methods
CSF catechols were assayed in PD (n = 24), MSA-P (n = 32), PAF (n = 18), and control subjects (n = 32).
Results
Compared to controls, CSF DOPAC was decreased in PD and MSA-P (p < 0.0001 each). In both diseases Cys-DA/DOPAC ratios averaged more than twice control (0.14 ± 0.02 and 0.13 ± 0.02 vs. 0.05 ± 0.01, p < 0.0001 each), whereas in PAF the mean Cys-DA/DOPAC ratio was normal (0.05 ± 0.01).
Conclusions
CSF Cys-DA/DOPAC ratios are substantially increased in PD and MSA-P and are normal in PAF. Thus, in synucleinopathies an elevated CSF Cys-DA/DOPAC ratio seems to provide a specific biomarker of parkinsonism.
Keywords: Parkinson disease, Multiple system atrophy, Aldehyde dehydrogenase
Parkinson disease (PD), the most common neurodegenerative movement disorder, is a member of a family of disorders called synucleinopathies in which the protein, alpha-synuclein, is deposited in cytoplasmic inclusions [1]. The synucleinopathy family also includes multiple system atrophy [2] and pure autonomic failure (PAF) [3]. PD and the parkinsonian form of multiple system atrophy (MSA-P), referred to here as parkinsonian synucleinopathies, are characterized by dopaminergic neuronal loss in the nigrostriatal system [4] and severe putamen dopamine depletion [5,6]. In PAF, the patients do not have parkinsonism or neuroimaging evidence for loss of putamen dopaminergic terminals [7].
PD and MSA-P involve low cerebrospinal fluid (CSF) concentrations of 3,4-dihydroxyphenylacetic acid (DOPAC) [8]. One might expect this, since DOPAC is the main neuronal metabolite of dopamine [9]. Accumulating evidence supports the view that in synucleinopathies putamen dopamine depletion reflects not only denervation but also a variety of functional abnormalities in the residual terminals. These abnormalities include decreased activity of aldehyde dehydrogenase (ALDH) [10,11] or L-aromatic-amino-acid decarboxylase [12,13], decreased vesicular storage of cytoplasmic dopamine [14–16], and oxidative stress related to dopamine-based free radicals [17].
5-S-Cysteinyl-dopamine (Cys-DA) is produced from the spontaneous oxidation of dopamine to dopamine-quinone, followed by covalent bonding to glutathione or cysteine (glutathionyl-dopamine is converted to Cys-DA by cleavage of a peptide linkage). About 30 years ago Fornstedt, Carlsson, and colleagues described the occurrence of Cys-DA in several brain regions in mammalian species including humans [18]. These investigators proposed that measuring Cys-DA in CSF might provide a biomarker of early PD [19], based on the concept that the disease process in PD reflects a failure of anti-oxidative mechanisms to prevent spontaneous oxidation of dopamine. Consistent with their suggestion, CSF levels of Cys-DA are increased with respect to those of homovanillic acid (HVA), the main end-product of dopamine metabolism, in PD [20].
In this study we exploited the ability to measure CSF Cys-DA and DOPAC simultaneously to explore other determinants of low CSF DOPAC levels besides dopaminergic denervation. As reported here, we were able to measure Cys-DA and DOPAC simultaneously in human CSF. We then applied this CSF neurochemical approach to examine enzymatic and spontaneous oxidation of central dopamine in vivo in the three forms of synucleinopathy. We reasoned that central dopaminergic denervation alone should produce equivalent decreases in CSF levels of Cys-DA and DOPACs, because they have a shared source–cytoplasmic dopamine. On the other hand, if there were denervation-independent determinants of low DOPAC levels, such as a shift in the fate of cytoplasmic dopamine toward spontaneous oxidation or low ALDH activity, then the CSF ratio of Cys-DA/DOPAC would be increased.
Theoretically, Cys-DA might be derived from enzymatic decarboxylation of Cys-DOPA by L-aromatic-amino-acid decarboxylase (LAAAD), analogous to dopamine being derived from enzymatic decarboxylation of DOPA. If this were the case, then Cys-DA levels would not validly indicate spontaneous oxidation of cytoplasmic dopamine. Therefore, as part of this study we carried out experiments in test tubes and in cultured rat pheochromocytoma PC12 cells to show that Cys-DOPA is not a substrate for LAAAD.
To assess relationships between CSF Cys-DA/DOPAC ratios and putamen dopamine turnover, we also analyzed data from 18F-fluorodopa positron emission tomographic (PET) scanning of the brain in the same subjects. After 18F injection, the fractional loss (“washout fraction”) of 18F is related to dopamine turnover [21], and washout fractions of putamen 18F are increased in both PD and MSA [14]. By including the 18F-fluorodopa data from patients with PD, MSA-P, PAF, and controls, we could test whether in synucleinopathies elevated CSF fluid Cys-DA/DOPAC ratios are associated specifically with clinical parkinsonism and with increased dopamine turnover.
In summary, in this study we measured CSF levels of cysteinyl and parent catechols simultaneously in synucleinopathies with (PD, MSA-P) or without (PAF) parkinsonism, in order to assess whether in this family of diseases elevated Cys-DA/DOPAC ratios are associated specifically with parkinsonism.
1. Methods
1.1. DA and Cys-DOPA as sources of intracellular Cys-DA
The use of DOPAC/Cys-DA ratios to indicate spontaneous oxidation of cytoplasmic DA requires excluding enzymatic decarboxylation of Cys-DOPA as the source of Cys-DA (Fig. 1). In test tubes, incubation of Cys-DOPA with LAAAD and pyridoxal phosphate did not yield Cys-DA, whereas incubation of DOPA with LAAAD and pyridoxal phosphate yielded abundant dopamine (data not shown). To examine whether Cys-DA is produced intracellularly by the enzymatic action of LAAAD on Cys-DOPA, PC12 cells were incubated with Cys-DOPA or DOPA (0, 10, and 100 μM, 4 replicates at each concentration) at 37 °C for 3 h, and the cell contents of Cys-DOPA and Cys-DA were assayed. Under these conditions incubation with DOPA is known to produce substantial amounts of DA and its deaminated metabolites [22]; however, whether Cys-DOPA produces Cys-DA has not been reported.
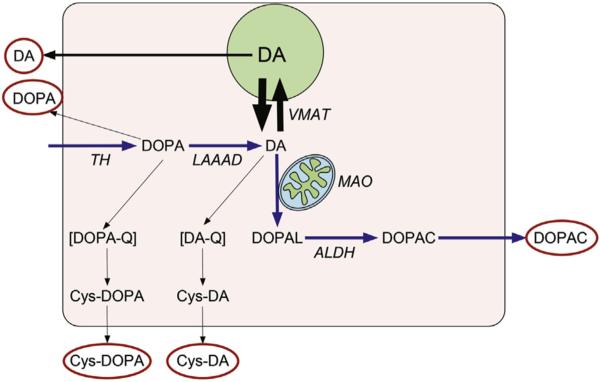
Fig. 1. Parent and Cys-catechols.
Cytoplasmic dopamine (DA) synthesis depends on conversion of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) via tyrosine hydroxylase (TH) and decarboxylation of DOPA via L-aromatic-amino-acid decarboxylases. Cytoplasmic DA is taken up into vesicles via the vesicular monoamine transporter (VMAT), is metabolized by monoamine oxidase (MAO), or oxidizes spontaneously to form cysteinyl-dopamine (Cys-DA). MAO converts DA to toxic 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is detoxified by aldehyde dehydrogenase (ALDH) to form 3,4-dihydroxyphenylacetic acid (DOPAC). DOPA oxidizes spontaneously to cysteinyl-DOPA (Cys-DOPA) and DA to Cys-DA. Red ovals indicate CSF analytes assayed in this study. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
PC12 cells were from the American Type Culture Collection (ATCC, Manassas, VA; PC12 cells catalog no. CRL-1721). The cell culture medium, F12K, was from Gibco Life Technologies (Grand Islands, NY); tolcapone (to block catechol-O-methyltransferase) from Orion Pharma (Espoo, Finland). The cells were kept frozen in liquid nitrogen until passaged for experiments. The experiments reported here involved non-adherent, non-differentiated cells. Non-adherent PC12 cells were grown in F12K medium with 15% horse serum and 2.5% fetal bovine serum. The cells were incubated at 37 °C in an atmosphere of 5% carbon dioxide. Media were changed several times per week and cells passaged once per week.
Since PC12 cells express catechol-O-methyltransferase, the cells in this study were pre-incubated for 24 h in medium containing the catechol-O-methyltransferase inhibitor, tolcapone. After the 24 h, the cells were collected, suspended in the same medium, and counted using a Cellometer device (Nexcelom Bioscience, Lawrence, MA). About 500,000 cells/well were plated in 12 well plates. The experiments began after 24 h of incubation with tolcapone-containing medium.
1.2. Subjects
Patients with PD, MSA-P, or PAF were referred to the Clinical Neurocardiology Section for evaluation of autonomic function at the Clinical Center of the National Institutes of Health. All the patients participated in Institutional Review Board-approved protocols of the National Institute of Neurological Disorders and Stroke after giving written informed consent.
1.3. Diagnostic assignments
For each patient a diagnosis was assigned based on the medical and neurological history and physical examination and by a set of tests conducted at the National Institutes of Health Clinical Center.
A diagnosis of PD was assigned based on at least 3 of the following 4 clinical criteria: bradykinesia, resting “pill roll” tremor, “cogwheel” rigidity, and excellent response of the movement disorder to levodopa treatment. Supportive clinical laboratory findings included cardiac sympathetic denervation as indicated by low interventricular septal myocardial 18F-dopamine-derived radioactivity [23] and anosmia [24].
According to consensus criteria, MSA is divided into two groups, parkinsonian (MSA-P) and cerebellar (MSA-C), with MSA-P involving parkinsonian features and autonomic dysfunction and MSA-C involving signs of cerebellar failure and autonomic dysfunction [25]. The present study included MSA-P patients as defined by these criteria. Among patients with parkinsonism, the findings of normal myocardial 18F-dopamine-derived radioactivity (>5000 nCi-kg/cc-mCi) and normal or mildly decreased olfactory function were used to help identify MSA-P [24] as opposed to PD.
A diagnosis of PAF was assigned based on chronic, persistent, consistent orthostatic hypotension, identified as neurogenic by clinical laboratory testing [26], without a known cause (e.g., diabetic autonomic neuropathy, autoimmune autonomic ganglionopathy) and by clinical laboratory evidence of loss of sympathetic noradrenergic innervation (low myocardial 18F-dopamine-derived radioactivity, low plasma levels of 3,4-dihydroxyphenylglycol (DHPG), or both [27].
The control group comprised 10 healthy volunteers, 10 patients referred for autonomic testing who did not have autonomic failure or parkinsonism, and 12 people who were tested because of risk factors for PD, had insufficient clinical evidence to diagnose PD, and were followed for at least 3 years without developing PD.
1.4. Data selection and exclusions
All the patients in this study were off levodopa, as documented by a CSF DOPA concentration less than 1000 pg/mL, and none was taking a monoamine oxidase (MAO) inhibitor.
1.5. CSF sampling conditions, storage, and assay
To obtain CSF for neurochemical assays, subjects underwent lumbar puncture under fluoroscopic guidance at the National Institutes of Health Clinical Center. Aliquots of 1 mL of fluid were collected into chilled 1.5 mL plastic sample tubes, which were frozen immediately in dry ice and then stored at or below −70 °C until the samples were assayed. All the assays were by the same person (C.H.), who was blinded as to clinical diagnosis, using batch alumina extraction followed by liquid chromatography with electrochemical detection [8]. Limits of detection for catechols were about 10 pmol/L, or 10 fmol per assayed mL of CSF.
Dopamine, norepinephrine, DOPAC, and DHPG were purchased from Sigma. In order to have standards for quantifying Cys-DA and Cys-DOPA levels, 10 mg of each were obtained from the Chemical Synthesis and Drug Supply Program of the National Institute of Mental Health (catalog numbers C-805 and C-702). The identity of the standards was confirmed by liquid chromatography with electrochemical detection in our laboratory.
CSF DOPAC data from most of the subjects have already been reported [8]. For this study previously unthawed aliquots were used that had been frozen continuously at or below −70 °C, often for several years. Comparison of CSF DOPAC levels in the present study with those previously obtained in different aliquots from the same subjects demonstrated excellent reproducibility of the assay results (r = 0.92, p < 0.0001, n = 125).
1.6. Putamen dopaminergic neuroimaging
After i.v. administration of 18F-fluorodopa, peak putamen 18F-fluorodopa-derived radioactivity is attained at about 30 min after intravenous injection of the tracer. Subsequently, radioactivity in the putamen normally declines more slowly than does radioactivity in the occipital cortex, a control region virtually lacking dopaminergic innervation. The PUT/OCC ratio of 18F-fluorodopa-derived radioactivity therefore increases over time. By about 2 h after 18F-fluorodopa administration the mean PUT/OCC ratio normally averages about 3.0 [24].
Increased dopamine turnover, whether from a vesicular storage defect [14] or compensatorily increased exocytosis and decreased reuptake of released dopamine [28] in the residual terminals would increase the rate of decline in 18F-fluorodopa-derived radioactivity. The fractional loss of putamen radioactivity (washout fraction) was measured between the midpoint of the 15-min static image beginning at 30’ (mean about 38′) and the midpoint of the last 15-min static image ending at 120’ (mean about 113′). All the imaging analyses were done by personnel who were blinded as to the assigned clinical diagnosis.
In essentially all subjects the same 18F-fluorodopa scanning protocol was used. Dynamic scanning was done in a conventional scanner for 30 min followed by a 15-min static scan; the patient was transferred to a high resolution research tomograph for a 15-min static scan; and the patient was then transferred back to the conventional scanner for a final 15-min static scan timed to end at about 120 min from the time of initiation of the 3-min injection of 18F-fluorodopa injection. For analyzing the images, regions of interest were placed on the MRI of each patient. No carbidopa pre-treatment was used. The patients received 7 mCi of 18F-fluorodopa; the control subjects received 7 or 10 mCi, depending on the particular protocol.
1.7. Statistics
Data for PC12 cell contents of catechols after incubation with DOPA or Cys-DOPA were compared by factorial analyses of variance, with post-hoc comparisons vs. no added DOPA or Cys-DOPA by Dunnett's post-hoc test, using KaleidaGraph 4.5 (Synergy Software, Reading, PA).
Because of skewed distributions for CSF levels of catechols (higher concentrations associated with greater inter-individual variability), all the clinical neurochemical data were log-transformed before statistical testing was done. Data for CSF neurochemical and putamen neuroimaging data across the 4 diagnostic groups were compared by factorial analyses of variance followed by Fisher's Least Significant Difference test. Post-hoc comparisons with the control group used Dunnett's post-hoc test. Associations between individual values for neurochemical and neuroimaging data were analyzed by calculation of Pearson correlation coefficients. Frequencies of findings in compared groups were analyzed by calculation of χ2, with the Yates correction. Mean values were expressed ±1 SEM, and a p value < 0.05 defined statistical significance.
2. Results
2.1. Cytoplasmic dopamine as the source of Cys-DA
The neurochemical assay method enabled simultaneous measurement of 10 endogenous catechols of interest–the catecholamines dopamine, norepinephrine, and epinephrine, the deaminated metabolites of dopamine (DOPAL, DOPAC, and DOPET) and of norepinephrine (DHPG), the catecholamine precursor DOPA, and the spontaneous oxidation products of DOPA and dopamine, Cys-DOPA and Cys-DA (Fig. 2A). In untreated PC12 cells dopamine, norepinephrine, DOPAC, DOPET, DOPA, Cys-DOPA and Cys-DA were consistently detected (Fig. 2B).
Fig. 2. Cell and medium contents of catechols in rat pheochromocytoma (PC12) cells.
The upper panel (A) shows a chromatograph of standards for DHPG = 3,4-dihydroxyphenylglycol, 1000 pg; NE = norepinephrine, 250 pg; DOPA = 3,4-dihydroxyphenylalanine, 1000 pg; EPI = epinephrine, 250 pg; DOPAL, 1000 pg; Cys-fluorodopa, 812 pg; DOPET = 3,4-dihydroxyphenylethanol, 1000 pg; DA, 250 pg; DOPAC, 1000 pg; Cys-DA, 787 pg; I.S. = internal standard (isoproterenol). The 10 catechols were baseline-resolved. The middle panel (B) shows a chromatograph corresponding to 400 μL of medium at 3 h of incubation. Note detection of endogenous DOPAL, DOPAC, and Cys-DA. The lower left panel (C) shows cell contents of Cys-DA after incubation with various concentrations of DOPA in the medium. Brown represents Cys-DOPA and blue Cys-DA; there are large increases in cell contents of Cys-DA but no change in Cys-DOPA. The lower right panel (D) shows cells contents of Cys-DOPA and Cys-DA after incubation with various concentrations of Cys-DOPA in the medium. There is no change in Cys-DA content despite large increases in Cys-DOPA content, indicating little if any conversion of Cys-DOPA to Cys-DA in the cells. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Incubation of PC12 cells with Cys-DOPA resulted in concentration-dependent increases in cell Cys-DOPA contents but no changes in cell Cys-DA contents (Fig. 2C). In contrast, incubation with DOPA resulted in concentration-dependent increases in cell contents of Cys-DA (Fig. 2D).
2.2. Characteristics of subject groups
The subject groups had similar ages and sex makeups (Table 1), and the clinical movement and autonomic findings were consistent with the diagnostic assignments. Among the PD patients, 12 of 17 were anosmic by the University of Pennsylvania Smell Identification Test, whereas among the MSA-P patients, 2 of 16 were anosmic (χ2 = 10.9, p = 0.001). Eighteen of 24 PD patients had low interventricular septal 18F-dopamine-derived radioactivity, whereas only 1 of 31 MSA-P patients had low septal 18F-dopamine-derived radioactivity (χ2 = 39, p < 0.0001).
Table 1.
Clinical characteristics of patient groups with synucleinopathy and control subjects.
| Parameter | MSA-P | PD | PAF | Control |
|---|---|---|---|---|
| Age | 59.7 ± 1.7 | 61.4 ± 2.3 | 63.2 ± 3.1 | 52.6 ± 1.9 |
| Sex (M/F) | 21/11 | 14/10 | 12/6 | 17/15 |
| Motor Onset Age, years | 53.8 ± 1.9 | 58.2 ± 2.3 | N/A | N/A |
| Anosmia | 13% | 71% | 22% | 20% |
| Dec. myocardial 18F-DA | 6.5% | 75% | 94% | 3% |
| Bradykinesia | 90% | 92% | 20% | 28% |
| Cogwheel Rigidity | 56% | 78% | 0% | 18% |
| Constipation | 68% | 70% | 83% | 50% |
| Decreased Sweating | 33% | 38% | 67% | 33% |
| Dementia | 12% | 44% | 0% | 28% |
| Depression | 43% | 58% | 33% | 50% |
| Erectile Failure in Men | 91% | 62% | 75% | 56% |
| Levodopa Responsive | 50% | 64% | N/A | 33% |
| Resting Tremor | 19% | 58% | 0% | 28% |
| Slurred Speech | 63% | 24% | 0% | 17% |
| Urinary Symptoms | 92% | 91% | 67% | 83% |
2.3. Simultaneous measurement of CSF DOPAC, Cys-DA, and other catechols
Normal CSF levels of catechols varied widely, with DHPG having the highest concentration (about 11 pmol/mL) and epinephrine not detected (Table 2). DOPAC and Cys-DA were detected in all the samples.
Table 2.
Mean (±SEM) cerebrospinal fluid concentrations (pmol/mL) of Catechols in patient groups with synucleinopathy and control subjects.
| Catechol | MSA-P | PD | PAF | Control |
|---|---|---|---|---|
| DOPA | 2.97 ± 0.13 | 3.20 ± 0.20 | 2.82 ± 0.19 | 3.49 ± 0.11 |
| (32) | (24) | (18) | (32) | |
| 0.006 | n.s. | 0.0019 | ||
| Cys-DOPA | 1.03 ± 0.08 | 1.14 ± 0.10 | 1.02 ± 0.08 | 0.92 ± 0.06 |
| (32) | (24) | (18) | (32) | |
| n.s. | n.s. | n.s. | ||
| DA | 0.06 ± 0.01 | 0.07 ± 0.02 | 0.12 ± 0.05 | 0.08 ± 0.02 |
| (32) | (24) | (18) | (32) | |
| n.s. | n.s. | n.s. | ||
| Cys-DA | 0.11 ± 0.02 | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.08 ± 0.01 |
| (32) | (24) | (18) | (32) | |
| n.s. | n.s. | n.s. | ||
| DOPAC | 0.89 ± 0.08 | 0.94 ± 0.09 | 1.43 ± 0.10 | 1.60 ± 0.12 |
| (32) | (24) | (18) | (32) | |
| <0.0001 | <0.0001 | n.s. | ||
| NE | 0.47 ± 0.05 | 0.64 ± 0.07 | 0.65 ± 0.13 | 0.87 ± 0.08 |
| (32) | (24) | (18) | (32) | |
| <0.0001 | 0.0562 | 0.0023 | ||
| DHPG | 7.57 ± 0.36 | 8.88 ± 0.54 | 7.40 ± 0.73 | 10.92 ± 0.56 |
| (32) | (24) | (18) | (32) | |
| 0.0001 | 0.0286 | <0.0001 | ||
| EPI | N.D. | N.D. | N.D. | N.D. |
Notes:
Numbers in italics are p values for the difference from Control (Fisher's Least Significant Difference post-hoc test applied to log-transformed data).
Numbers in parentheses are numbers of data points.
Abbreviations: Cys-DA = cysteinyl-dopamine; Cys-DOPA = cysteinyl-DOPA; DA = dopamine; DHPG = 3,4-dihydroxyphenylglycol; DOPAC = 3,4-dihydroxyphenylacetic acid; EPI = epinephrine; MSA-P = parkinsonian multiple system atrophy; NE = norepinephrine; N.D. = not detected; n.s. = not statistically significant; PAF = pure autonomic failure; PD=Parkinson.
2.4. CSF catechols
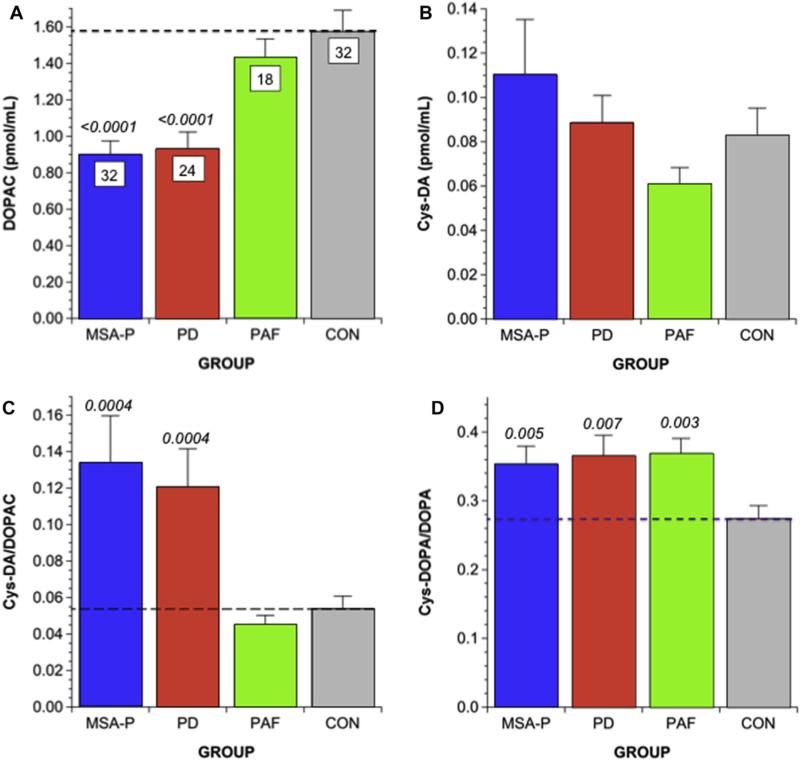
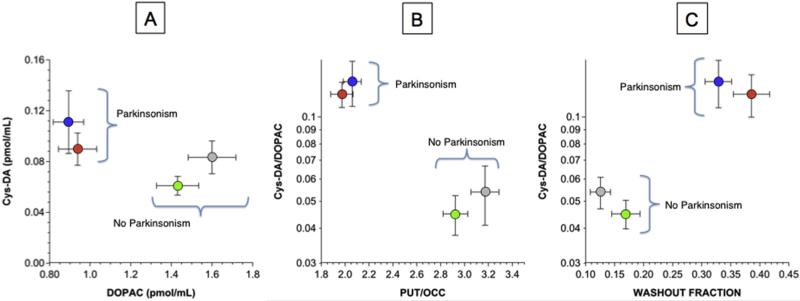
Mean CSF DOPAC was decreased in the PD and MSA-P groups compared to the PAF and control groups (Fig. 3A). Mean Cys-DA did not differ between the controls and any of the synucleinopathy groups (Fig. 3B); however, in the PD and MSA-P groups, CSF Cys-DA was markedly higher than expected for CSF DOPAC (Fig. 4). CSF Cys-DA/DOPAC ratios in the PD and MSA-P groups averaged more than twice those in the PAF and control groups (p = 0.0004 each; Figs. 3C and 4B,C).
Fig. 3. Mean (±SEM) CSF levels of parent and cysteinyl catechols in parkinsonian and non-parkinsonian synucleinopathies.
(A) DOPAC; (B) Cys-DA; (C) Cys-DA/DOPAC; (D) Cys-DOPA/DOPA. Shown are data from groups with Parkinson disease (PD, red), parkinsonian multiple system atrophy (MSA-P, blue), pure autonomic failure (PAF, green), and controls (CON, gray). Numbers in white boxes are the numbers of subjects. Numbers in italics indicate p values for comparison with CON. The parkinsonian synucleinopathies (PD, MSA-P) are associated with decreased DOPAC levels and increased Cys-DA/DOPAC ratios; PAF is not. The data in (D) show that all three synucleinopathies are associated with increased Cys-DOPA/DOPA ratios. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4. Mean (±SEM) CSF Cys-DA as a function of DOPAC (A) and Cys-DA/DOPAC ratios as functions of putamen/occipital cortex (PUT/OCC) ratios (B) and of washout fractions of putamen 18F-DOPA-derived radioactivity (C).
Grouped data are shown for patients with MSA-P (blue), PD (red), PAF (green), and controls (gray). In the parkinsonian synucleinopathies elevated CSF Cys-DA/DOPAC ratios are associated with decreased PUT/OCC ratios and elevated 18F-DOPA washout fractions, in contrast with normal values in the non-parkinsonian synucleinopathy PAF. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the synucleinopathy groups, mean CSF levels of NE and DHPG were less than in the controls (Table 2). CSF DOPA was low in MSA-P and PAF and tended to be decreased in PD. Mean Cys-DOPA/DOPA ratios were increased similarly in all 3 synucleinopathy groups (Fig. 3D).
2.5. PUT/OCC ratios and putamen washout fractions of 18F-DOPA-derived radioactivity
A total of 81 subjects (39 parkinsonian synucleinopathy,14 PAF, 28 controls) had data for both CSF Cys-DA/DOPAC ratios and PUT/OCC ratios and putamen washout fractions of 18F-DOPA-derived radioactivity. The parkinsonian groups had lower mean PUT/OCC ratios of 18F-DOPA-derived radioactivity (1.97 ± 0.09 in PD, 2.06 ± 0.10 in MSA-P) than did the PAF (2.92 ± 0.12) and control (3.17 ± 0.14) groups (p < 0.0001 each; Fig. 4B). Putamen washout fractions of 18F-DOPA-derived radioactivity were higher in the parkinsonian synucleinopathies (0.329 ± 0.023 in PD, 0.386 ± 0.031 in MSA-P) than in the PAF group (0.169 ± 0.024) and the control group (0.126 ± 0.017, p < 0.0001 each; Fig. 4C). Across synucleinopathy patients, Cys-DA/DOPAC ratios were correlated negatively with PUT/OCC ratios (r = −0.40, p = 0.003) and positively with washout fractions of 18F-fluorodopa-derived radioactivity (r = 0.41, p = 0.002).
3. Discussion
It is widely presumed that profound striatal dopamine depletion in PD and related disorders directly reflects loss of substantia nigra dopaminergic neurons. Since DOPAC is the main intra-neuronal metabolite of dopamine [9], nigrostriatal denervation can explain low CSF DOPAC levels in PD and MSA-P [8]. As explained below, the present results indicate that low CSF DOPAC levels in these diseases have other determinants in addition to denervation.
Cys-DA and DOPAC have a shared source–cytoplasmic dopamine. Because of this shared source, denervation would be expected to produce proportionately equal decreases in Cys-DA and DOPAC levels, and the Cys-DA/DOPAC ratio would be unchanged. Instead, CSF Cys-DA/DOPAC ratios were increased in the parkinsonian synucleinopathies. From these results we infer that denervation-independent factors decrease DOPAC levels without decreasing Cys-DA levels. There are multiple possible causes of elevated CSF Cys-DA/DOPAC ratios, and the present data do not identify those factors; however, from previous post-mortem studies, decreased anti-oxidant capacity and decreased ALDH are reasonable possibilities, as discussed below.
MAO inhibition would be expected to build up cytoplasmic dopamine and consequently increase spontaneous oxidation of dopamine. Consistent with this view, MAO inhibition increases striatal Cys-DA content in guinea pigs [29] and endogenous Cys-DA production in PC12 cells [30]. None of the patients in this study was on an MAO inhibitor. This did not rule out the possibility of decreased MAO activity as part of the disease process; however, in PD striatal MAO activity has been reported to be normal [31].
Elevated Cys-DA/DOPAC ratios in parkinsonian synucleinopathies might reflect decreased anti-oxidant capacity in central dopaminergic neurons, as suggested originally by Fornstedt, Carlsson, and co-workers [19,32]. Decreased anti-oxidant capacity would shift the balance from the native catechols to catecholquinones and consequently to cysteinyl-catechols. There is substantial interest in oxidative stress in the pathogenesis of synucleinopathies [33–35]. Since Cys-DOPA is a product of spontaneous oxidation of cytoplasmic DOPA, just as Cys-DA is a product of spontaneous oxidation of cytoplasmic dopamine, the finding of increased CSF Cys-DOPA/DOPA ratios in the synucleinopathies fits with the oxidative stress concept.
Third, increased CSF Cys-DA/DOPAC ratios would be consistent with decreased ALDH activity. We previously reported postmortem neurochemical evidence for decreased putamen ALDH activity in PD [36] and MSA [10]. Moreover, decreased ALDH activity could be pathophysiologic, because animals with genetic ALDH deficiency have aging-related nigrostriatal neurodegeneration and PD-like locomotor abnormalities [37], and ALDH over-expression is neuroprotective in cellular and animal models of parkinsonism induced by the pesticide rotenone [38]. Cellular and epidemiologic/studies have implicated an interplay between pesticide exposure and decreased ALDH activity in predisposing to PD [39,40].
Oxidative stress and decreased ALDH activity may be linked, in that lipid peroxidation products generated from reactions of free radicals with membranes inhibit ALDH and consequently build up the toxic dopamine metabolite, 3,4-dihydroxyphenylacetaldehyde (DOPAL) [41–43]. DOPAL in turn can react with hydrogen peroxide (produced on an equimolar basis with DOPAL upon enzymatic oxidation of dopamine) to generate hydroxyl radicals [44]. DOPAL also potently oligomerizes alpha-synuclein [44], especially in the setting of divalent metal cations [45], and alpha-synuclein oligomers are also thought to be pathogenic [46].
It was reported previously that PD patients have increased CSF Cys-DA/HVA ratios [20]. Cys-DA/DOPAC has advantages over Cys-DA/HVA as a biomarker of parkinsonism, because CSF DOPAC is a more sensitive and specific biomarker of central dopamine deficiency than is CSF HVA. CSF DOPAC is derived from intra-neuronal metabolism of cytoplasmic dopamine, whereas CSF HVA is determined complexly by both extra-neuronal metabolism of released dopamine and by extra-neuronal metabolism of released DOPAC. Regarding DOPAC, virtually all of intra-neuronal dopamine is within vesicles, vesicular dopamine leaks continuously into the cytoplasm as a first order process, and cytoplasmic dopamine is subject to enzymatic oxidation to form DOPAC. This sequence explains why in PD putamen DOPAC content is as severely decreased as is putamen dopamine content [47]. DOPAC is actively extruded from dopaminergic cells into the extracellular fluid [48], and CSF DOPAC is directly related to brain DOPAC content [49]. Central dopamine deficiency therefore would be expected to result in low CSF DOPAC levels. Regarding HVA, as dopamine stores become depleted, traffic to the residual terminals increases compensatorily, and there is decreased reuptake of the released dopamine [50]. In this setting CSF HVA could be at least partly maintained despite decreased dopamine stores [51]. Thus, in PD striatal HVA content is not as decreased as is dopamine content [52], and investigators have questioned whether CSF HVA provides an adequate biomarker for PD [53]. Since CSF DOPAC is more sensitive and specific than CSF HVA as an index of central dopamine depletion, CSF Cys-DA/DOPAC is more sensitive and specific than CSF Cys-DA/HVA as an index of spontaneous oxidation of cytoplasmic dopamine for a given amount of central dopamine depletion.
Patients with PD or MSA-P also had evidence for increased dopamine turnover in the residual nigrostriatal terminals, based on increased washout fractions of putamen 18F-DOPA-derived radioactivity [10]. Denervation alone also cannot explain increased dopamine turnover. Potential functional explanations for increased turnover of vesicular dopamine include decreased vesicular storage and increased exocytotic release combined with escape of neuronal reuptake via the cell membrane dopamine transporter [21].
Since CSF Cys-DA/DOPAC ratios were correlated with washout fractions of 18F-DOPA-derived radioactivity, across subject groups and individual patients, the denervation-independent abnormalities of dopamine metabolism seem to be related to each other. Importantly, patients with the non-parkinsonian synucleinopathy, PAF, had normal PUT/OCC ratios, washout fractions, and CSF Cys-DA/DOPAC ratios. Thus, in synucleinopathies elevated Cys-DA/DOPAC ratios are linked specifically with parkinsonism, loss of putamen dopaminergic terminals, and increased dopamine turnover in the residual terminals.
In conclusion, based on measurements of CSF levels of cysteinyl and parent catechols and on 18F-DOPA brain scanning data in the same patients, we report that in synucleinopathies elevated CSF Cys-DA/DOPAC ratios are associated specifically with parkinsonism and with increased dopamine turnover in residual dopaminergic terminals. These in vivo abnormalities may reflect a combination of decreased vesicular storage, decreased ALDH activity, and oxidative stress. Because of the patient groups studied, the present data do not apply to the issue of whether elevated CSF Cys-DA/DOPAC ratios characterize other diseases manifesting with parkinsonism.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, NINDS.
Abbreviations
- ALDH
aldehyde dehydrogenase
- Cys-DA
5-S-cysteinyl-dopamine
- Cys-fluorodopa
5-S-cysteinyl-fluorodopa
- DHPG
3,4-dihydroxyphenylglycol
- DOPAC
3,4-dihydroxyphenylacetic acid
- DOPAL
3,4-dihydroxyphenylacetaldehyde
- HVA
homovanillic acid
- LAAAD
L-aromatic-amino-acid decarboxylase
- MAO
monoamine oxidase
- MSA-P
parkinsonian form of multiple system atrophy
- PAF
pure autonomic failure
- PD
Parkinson's disease
- PUT/OCC
putamen/occipital cortex
Footnotes
Author contributions
DSG: study conception, designing the research studies, analyzing the data, writing the manuscript, manuscript editing.
CH: acquiring data, analyzing data, manuscript editing.
PS: acquiring data, analyzing data, manuscript editing.
YJ: acquiring data, analyzing data, manuscript editing.
IJK: study conception, designing the research studies, manuscript editing.
YS: study conception, designing the research studies, writing the manuscript, manuscript editing.
Potential conflicts of interest
DSG: Nothing to report.
CH: Nothing to report.
PS: Nothing to report.
YJ: Nothing to report.
IJK: Nothing to report.
YS: Nothing to report.
References
- 1.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 2.Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci. Lett. 1998;249:180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann H, Hague K, Perl D. Accumulation of alpha-synuclein in autonomic nerves in pure autonomic failure. Neurology. 2001;56:980–981. doi: 10.1212/wnl.56.7.980. [DOI] [PubMed] [Google Scholar]
- 4.Kume A, Takahashi A, Hashizume Y. Neuronal cell loss of the striatonigral system in multiple system atrophy. J. Neurol. Sci. 1993;117:33–40. doi: 10.1016/0022-510x(93)90151-n. [DOI] [PubMed] [Google Scholar]
- 5.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N. Engl. J. Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 6.Tong J, Wong H, Guttman M, Ang LC, Forno LS, Shimadzu M, Rajput AH, Muenter MD, Kish SJ, Hornykiewicz O, Furukawa Y. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation. Brain. 2010;133:172–188. doi: 10.1093/brain/awp282. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein DS, Holmes C, Sato T, Bernson M, Mizrahi N, Imrich R, Carmona G, Sharabi Y, Vortmeyer AO. Central dopamine deficiency in pure autonomic failure. Clin. Auton. Res. 2008;18:58–65. doi: 10.1007/s10286-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein DS, Holmes C, Sharabi Y. Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson's disease and other synucleinopathies. Brain. 2012;135:1900–1913. doi: 10.1093/brain/aws055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Sharabi Y, Mash DC. Decreased vesicular storage and aldehyde dehydrogenase activity in multiple system atrophy. Park. Relat. Disord. 2015;21:567–572. doi: 10.1016/j.parkreldis.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunblatt E, Riederer P. Aldehyde dehydrogenase (ALDH) in Alzheimer's and Parkinson's disease. J. Neural Transm. (Vienna) 2016;123:83–90. doi: 10.1007/s00702-014-1320-1. [DOI] [PubMed] [Google Scholar]
- 12.Gjedde A, Leger GC, Cumming P, Yasuhara Y, Evans AC, Guttman M, Kuwabara H. Striatal L-dopa decarboxylase activity in Parkinson's disease in vivo: implications for the regulation of dopamine synthesis. J. Neurochem. 1993;61:1538–1541. doi: 10.1111/j.1471-4159.1993.tb13651.x. [DOI] [PubMed] [Google Scholar]
- 13.Nagatsu T, Sawada M. Biochemistry of postmortem brains in Parkinson's disease: historical overview and future prospects. J. Neural Transm. Suppl. 2007:113–120. doi: 10.1007/978-3-211-73574-9_14. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DS, Holmes C, Sullivan P, Mash DC, Sidransky E, Stefani A, Kopin IJ, Sharabi Y. Deficient vesicular storage: a common theme in catecholaminergic neurodegeneration. Park. Relat. Disord. 2015;21:1013–1022. doi: 10.1016/j.parkreldis.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein DS, Sullivan P, Holmes C, Miller GW, Sharabi Y, Kopin IJ. A vesicular sequestration to oxidative deamination shift in myocardial sympathetic nerves in Parkinson disease. J. Neurochem. 2014;131:219–228. doi: 10.1111/jnc.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumakura Y, Gjedde A, Danielsen EH, Christensen S, Cumming P. Dopamine storage capacity in caudate and putamen of patients with early Parkinson's disease: correlation with asymmetry of motor symptoms. J. Cereb. Blood Flow. Metab. 2006;26:358–370. doi: 10.1038/sj.jcbfm.9600202. [DOI] [PubMed] [Google Scholar]
- 17.Hornykiewicz O. Mechanisms of neuronal loss in Parkinson's disease: a neuroanatomical-biochemical perspective. Clin. Neurol. Neurosurg. 1992;(94 Suppl):S9–S11. doi: 10.1016/0303-8467(92)90008-q. [DOI] [PubMed] [Google Scholar]
- 18.Fornstedt B, Rosengren E, Carlsson A. Occurrence and distribution of 5-S-cysteinyl derivatives of dopamine, dopa and dopac in the brains of eight mammalian species. Neuropharmacology. 1986;25:451–454. doi: 10.1016/0028-3908(86)90242-x. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson A, Fornstedt B. Catechol metabolites in the cerebrospinal fluid as possible markers in the early diagnosis of Parkinson's disease. Neurology. 1991;41:50–51. doi: 10.1212/wnl.41.5_suppl_2.50. discussion 52. [DOI] [PubMed] [Google Scholar]
- 20.Cheng FC, Kuo JS, Chia LG, Dryhurst G. Elevated 5-S-cysteinyldopamine/homovanillic acid ratio and reduced homovanillic acid in cerebrospinal fluid: possible markers for and potential insights into the pathoetiology of Parkinson's disease. J. Neural Transm. 1996;103:433–446. doi: 10.1007/BF01276419. [DOI] [PubMed] [Google Scholar]
- 21.Sossi V, de la Fuente-Fernandez R, Schulzer M, Troiano AR, Ruth TJ, Stoessl AJ. Dopamine transporter relation to dopamine turnover in Parkinson's disease: a positron emission tomography study. Ann. Neurol. 2007;62:468–474. doi: 10.1002/ana.21204. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Sullivan R, Gross DJ, Holmes C, Kopin IJ, Sharabi Y. Vesicular uptake blockade generates the toxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 Cells: relevance to the pathogenesis of Parkinson disease. J. Neurochem. 2012;123:932–943. doi: 10.1111/j.1471-4159.2012.07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Ann. Intern. Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein DS, Holmes C, Bentho O, Sato T, Moak J, Sharabi Y, Imrich R, Conant S, Eldadah BA. Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Park. Relat. Disord. 2008;14:600–607. doi: 10.1016/j.parkreldis.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein DS, Sharabi Y. Neurogenic orthostatic hypotension: a pathophysiological approach. Circulation. 2009;119:139–146. doi: 10.1161/CIRCULATIONAHA.108.805887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein DS, Holmes C, Sharabi Y, Brentzel S, Eisenhofer G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology. 2003;60:1327–1332. doi: 10.1212/01.wnl.0000058766.46428.f3. [DOI] [PubMed] [Google Scholar]
- 28.Sossi V, de La Fuente-Fernandez R, Holden JE, Doudet DJ, McKenzie J, Stoessl AJ, Ruth TJ. Increase in dopamine turnover occurs early in Parkinson's disease: evidence from a new modeling approach to PET 18 F-fluorodopa data. J. Cereb. Blood Flow. Metab. 2002;22:232–239. doi: 10.1097/00004647-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Fornstedt B, Carlsson A. Effects of inhibition of monoamine oxidase on the levels of 5-S-cysteinyl adducts of catechols in dopaminergic regions of the brain of the Guinea pig. Neuropharmacology. 1991;30:463–468. doi: 10.1016/0028-3908(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein DS, Jinsmaa Y, Sullivan P, Holmes C, Kopin IJ, Sharabi Y. Comparison of monoamine oxidase inhibitors in decreasing production of the autotoxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 cells. J. Pharmacol. Exp. Ther. 2016;356:484–493. doi: 10.1124/jpet.115.230201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riederer P, Youdim MB. Monoamine oxidase activity and monoamine metabolism in brains of parkinsonian patients treated with l-deprenyl. J. Neurochem. 1986;46:1359–1365. doi: 10.1111/j.1471-4159.1986.tb01747.x. [DOI] [PubMed] [Google Scholar]
- 32.Carlsson A, Fornstedt B. Possible mechanisms underlying the special vulnerability of dopaminergic neurons. Acta Neurol. Scand. Suppl. 1991;136:16–18. doi: 10.1111/j.1600-0404.1991.tb05014.x. [DOI] [PubMed] [Google Scholar]
- 33.Perfeito R, Cunha-Oliveira T, Rego AC. Revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse. Free Radic. Biol. Med. 2013;62:186–201. doi: 10.1016/j.freeradbiomed.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 34.Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson's disease. Mov. Disord. Off J. Mov. Disord. Soc. 2011;26:1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 35.Hastings TG. The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson's disease. J. Bioenerg. Biomembr. 2009;41:469–472. doi: 10.1007/s10863-009-9257-z. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ, Sharabi Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson's disease. J. Neurochem. 2013;126:591–603. doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wey M, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson's disease. PLoS One. 2012;7:e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu CC, Yeh TH, Lai SC, Wu-Chou YH, Chen CH, Mochly-Rosen D, Huang YC, Chen YJ, Chen CL, Chang YM, Wang HL, Lu CS. Neuro-protective effects of aldehyde dehydrogenase 2 activation in rotenone-induced cellular and animal models of parkinsonism. Exp. Neurol. 2015;263:244–253. doi: 10.1016/j.expneurol.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Kopin IJ, Sharabi Y. Rotenone decreases intracellular aldehyde dehydrogenase activity: implications for the pathogenesis of Parkinson's disease. J. Neurochem. 2015;133:14–25. doi: 10.1111/jnc.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitzmaurice AG, Rhodes SL, Cockburn M, Ritz B, Bronstein JM. Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease. Neurology. 2014;82:419–426. doi: 10.1212/WNL.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rees JN, Florang VR, Anderson DG, Doorn JA. Lipid peroxidation products inhibit dopamine catabolism yielding aberrant levels of a reactive intermediate. Chem. Res. Toxicol. 2007;20:1536–1542. doi: 10.1021/tx700248y. [DOI] [PubMed] [Google Scholar]
- 42.Florang VR, Rees JN, Brogden NK, Anderson DG, Hurley TD, Doorn JA. Inhibition of the oxidative metabolism of 3,4-dihydroxyphenylacetaldehyde, a reactive intermediate of dopamine metabolism, by 4-hydroxy-2-nonenal. Neurotoxicology. 2007;28:76–82. doi: 10.1016/j.neuro.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 43.Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE. The neurotoxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS One. 2010;5:e15251. doi: 10.1371/journal.pone.0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O'Dell M, Li SW, Pan Y, Chung HD, Galvin JE. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 45.Jinsmaa Y, Sullivan P, Gross D, Cooney A, Sharabi Y, Goldstein DS. Divalent metal ions enhance DOPAL-induced oligomerization of alpha-synuclein. Neurosci. Lett. 2014;569:27–32. doi: 10.1016/j.neulet.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. U. S. A. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Basile MJ, Mash DC. Catechols in post-mortem brain of patients with Parkinson disease. Eur. J. Neurol. 2011;18:703–710. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamensdorf I, Hrycyna C, He LP, Nechushtan A, Tjurmina O, Harvey-White J, Eisenhofer G, Rojas E, Kopin IJ. Acidic dopamine metabolites are actively extruded from PC12 cells by a novel sulfonylurea-sensitive transporter. Naunyn Schmiedeb. Arch. Pharmacol. 2000;361:654–664. doi: 10.1007/s002100000246. [DOI] [PubMed] [Google Scholar]
- 49.Palfreyman MG, Huot S, Wagner J. Value of monoamine metabolite determinations in CSF as an index of their concentrations in rat brain following various pharmacological manipulations. J. Pharmacol. Methods. 1982;8:183–196. doi: 10.1016/0160-5402(82)90073-0. [DOI] [PubMed] [Google Scholar]
- 50.Nandhagopal R, Kuramoto L, Schulzer M, Mak E, Cragg J, McKenzie J, McCormick S, Ruth TJ, Sossi V, de la Fuente-Fernandez R, Stoessl AJ. Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson's disease. Brain. 2011;134:3290–3298. doi: 10.1093/brain/awr233. [DOI] [PubMed] [Google Scholar]
- 51.Loeffler DA, LeWitt PA, DeMaggio AJ, Juneau PL, Milbury PE, Matson WR. Markers of dopamine depletion and compensatory response in striatum and cerebrospinal fluid. J. Neural Transm. Park. Dis. Dement. Sect. 1995;9:45–53. doi: 10.1007/BF02252962. [DOI] [PubMed] [Google Scholar]
- 52.Lloyd KG, Davidson L, Hornykiewicz O. The neurochemistry of Parkinson's disease: effect of L-dopa therapy. J. Pharmacol. Exp. Ther. 1975;195:453–464. [PubMed] [Google Scholar]
- 53.LeWitt PA, Galloway MP, Matson W, Milbury P, McDermott M, Srivastava DK, Oakes D. Markers of dopamine metabolism in Parkinson's disease. The Parkinson study group. Neurology. 1992;42:2111–2117. doi: 10.1212/wnl.42.11.2111. [DOI] [PubMed] [Google Scholar]