Abstract
Background
Although Becker's nevus (BN) is a relatively common disease, the systematic studies of clinicopathological and immunohistochemical results are poorly reported.
Objective
To investigate the clinicopathological features and immunohistochemical alterations of keratinocyte proliferation, melanocyte density, smooth muscle hyperplasia and nerve fiber distribution in BN.
Methods
Clinical and pathological data were collected in 60 newly-diagnosed BN cases. Immunohistochemical stain of Ki-67, Melan-A, keratin 15, smooth muscle actin and protein gene product 9.5 was performed in 21 cases.
Results
The median diagnostic and onset age was 17 and 12 years, respectively. Skin lesions usually appeared on the upper trunk and upper limbs. The pathological features included the rete ridge elongation and fusion and basal hyperpigmentation. Epidermal Ki-67, Melan-A and keratin 15 expression and dermal nerve fiber length were significantly higher in lesional and perilesional skin than in normal skin (p<0.05~0.01), while smooth muscle actin expression was upregulated only in skin lesion (p<0.05).
Conclusion
Although the clinical diagnosis of BN is often straightforward, histopathology is helpful to differentiate from other pigmentary disorders. The hyperproliferation of keratinocytes, melanocytes, arrector pili muscle and dermal nerve fibers could be involved in the pathogenesis of BN.
Keywords: Becker's nevus, Keratinocytes, Keratins, Melanocytes
INTRODUCTION
Becker's nevus (BN) is a relatively common disease characterized clinically by unilateral distribution of irregularly-bordered brown patches and pathologically acanthosis, basal hyperpigmentation, and rete ridge fusion and elongation1,2. BN generally begins during the second decade of life and preferentially involves the upper trunk3. It can be concomitant with various cutaneous anomalies. Although the etiopathogenesis of BN is still unknown, it may be an androgen-dependent and paradominantly inherited entity1.
The detailed data of histopathology and immunohistochemistry could be helpful for the diagnosis and pathogenetic study of BN. However, there are only a few systematic studies of its clinicopathologic features2,3,4. The immunohistochemical observations of other indexes are nearly unavailable in BN except androgen receptor and melanocyte2,5. Thus, in order to clarify the changes of epidermal and follicular keratinocytes, melanocytes, arrector pili muscle (APM) and nerve fibers, we summarized the clinicopathologic characteristics of 60 newly-diagnosed BN cases and investigated the immunohistochemical alterations of Ki-67, Melan-A, keratin 15 (K15), smooth muscle actin (SMA) and protein gene product 9.5 (PGP 9.5) in the lesional and perilesional normal-appearing skin of 21 BN patients.
MATERIALS AND METHODS
Patients
After approved by the Institutional Review Board of Affiliated Hospital of Guangdong Medical University (IRB no. PJ2013121), 35 patients with BN were prospectively collected during January 2014 and June 2015. Twenty-five archived cases of BN between January 2012 and December 2013 were also reviewed and followed up. The following data were collected: gender; diagnostic and onset age; morphology, distribution, and hairiness of lesions; subjective symptoms; associated diseases; and family history. All patients underwent the biopsies.
Histopathology
The biopsied samples were fixed in 10% neural formalin and embedded in paraffin. The 3-µm sections were stained with hematoxylin and eosin (H&E).
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded sections according to the manufacturers' instructions. The panel of primary antibodies included: mouse anti-K15 (clone LHK15, SC-47697, 1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA); mouse anti-Melan-A (MAB-0275) and SMA (KIT-0006) (Fuzhou Maixin Biotechnology Co. Ltd., Fuzhou, China); and mouse anti-Ki-67 (ZM-0166) monoclonal and rabbit anti-PGP 9.5 (ZA-0263) polyclonal antibodies (Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China). Citrate buffer (MVS-0066), EDTA buffer (MVS-0098), Elivision Plus kit (KIT-9901) and diaminobenzidine kit (DAB-2031) were purchased from Fuzhou Maixin Biotechnology Co. Ltd.
Semiquantitative analysis of immunohistochemistry
Histomorphometry was performed using Image-Pro® Plus v6.0 (Media Cybernetics Inc., Silver Spring, MD, USA). Epidermal Ki-67+ and Melan-A+ cells were counted in 5 different fields at ×200 magnification and expressed as per mm of the basement membrane zone. K15 expression was assessed semi-quantitatively in 5 different fields at ×200 magnification based on the percentage of stained cells (0%~1%=0; 2%~25%=1; 26%~50%=2; 51%~75%=3; 76%~100%=4). SMA+ APM were measured within the whole dermis at ×40 magnification (excluding microvessels, hair follicles, and eccrine glands), and expressed as per square millimeter of dermal tissue.
Dermal nerve fiber morphometry of PGP 9.5 staining was referred to the previous report6. Five different fields were chosen from each section at ×200 magnification. After delineating the dermal-epidermal junction, multiple orthogonal lines were marked at a distance of 200 µm below every rete ridge and dermal papilla, and their distal ends were joined. The dermal nerve fiber length (DNFL) was obtained (DNFL/mm2) after validating the dermal area of interest. Nerve fibers running into the dermal annexes, APM and nerve plexus were not included.
Statistical analysis
Because all immunoreactive results showed non-normal distribution, Ki-67, Melan-A, SMA and PGP 9.5 results were analyzed using Welch test with Dunnett T3 posthoc test, while K15 data were calculated using Kruskal-Wallis test with Mann-Whitney U test. Statistical analysis was performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic and clinical data
Of 60 cases, 49 (81.7%) were male and 11 (18.3%) were female. The diagnostic age ranged from 11 to 38 years (median 17 years) and onset age ranged from birth to 36 years (median 12 years). The adolescent (10~20 years), childhood (<10 years), congenital and late (>20 years) onset accounted for 70%, 20%, 6.7%, and 3.3%, respectively. The lesions manifested as light to dark brown patches with irregular borders, and their sizes varied from 5×1.5 cm to 56×25 cm (Fig. 1A). Scapular region, four limbs and chest were the predilection sites, while head, neck, abdomen, waist and buttock were uncommon (Table 1). Single lesion (93.3%) and unilateral distribution (95.0%) were the most common. Hypertrichosis was observed in 33 males and 8 females, of which there was no significant difference in gender (χ2=0.12, p=0.729) and diagnostic age (t=0.77, p=0.939) in patients with or without hypertrichosis. Multiple papules were seen within BN lesions in 20% cases, but acneform eruptions were absent. Acne vulgaris, neurofibromatosis type 1 and ovarian teratoma were concomitant in 17, 2, and 1 cases, respectively. There were no symptoms, mucosal involvement and family history of BN.
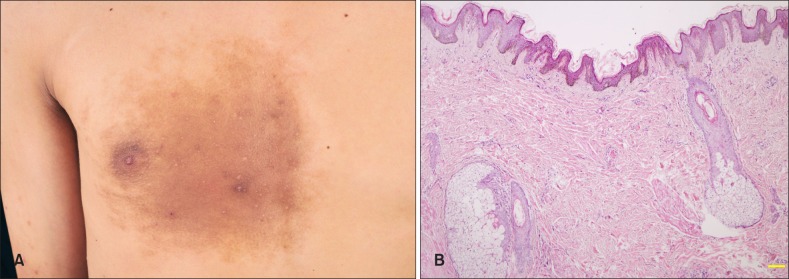
Fig. 1. Clinicopathologic images of Becker's nevus in an 18-year-old man. (A) A brown hypertrichotic patches with multiple papules on the right chest. (B) Keratotic plugging, acanthosis, mild rete ridge elongation and fusion, basal hyperpigmentation, as well as smooth muscle and sebaceous hyperplasia and superficial perivascular lymphohistiocytic infiltration in the dermis (H&E, ×40).
Table 1. Lesional distribution of Becker's nevus (BN) (n=60).
| Site | n (%) |
|---|---|
| Head | 4 (6.7) |
| Neck | 2 (3.3) |
| Scapular region* | 19 (31.7) |
| Chest | 6 (10.0) |
| Waist and abdomen | 5 (8.3) |
| Buttock† | 4 (6.7) |
| Upper limbs‡ | 7 (11.7) |
| Lower limbs | 13 (21.7) |
*BN extended to the ipsilateral chest in 2 cases and arms in 4 cases. †BN extended to the ipsilateral thigh in 1 case. ‡Concomitant with BN on the contralateral thigh in 1 case.
Histopathologic findings
The rete ridge elongation (100.0%) and fusion (91.7%), keratotic plugging (85.0%), basal pigmentation (98.3%), and mild perivascular lymphohistiocytic infiltration in the superficial dermis (100.0%) were the most common, followed by hyperkeratosis (61.7%), acanthosis (58.3%), bottom flattening of rete ridge (78.3%), dermal fibrosis (78.3%), and smooth muscle (75.0%) and sebaceous hyperplasia (70.0%), while papillomatosis (6.7%) and dermal melanophages (35.0%) were uncommon (Fig. 1B).
Immunohistochemical evaluation
Skin lesions and perilesional samples were collected in 21 patients (18 males and 3 females; mean age 19.7±6.6 years, range 12~38 years). Normal skin samples were obtained from 10 healthy subjects (8 males and 2 females; mean age 20.4±4.9 years, range 13~30 years) who underwent aesthetic surgery.
Ki-67 and Melan-A
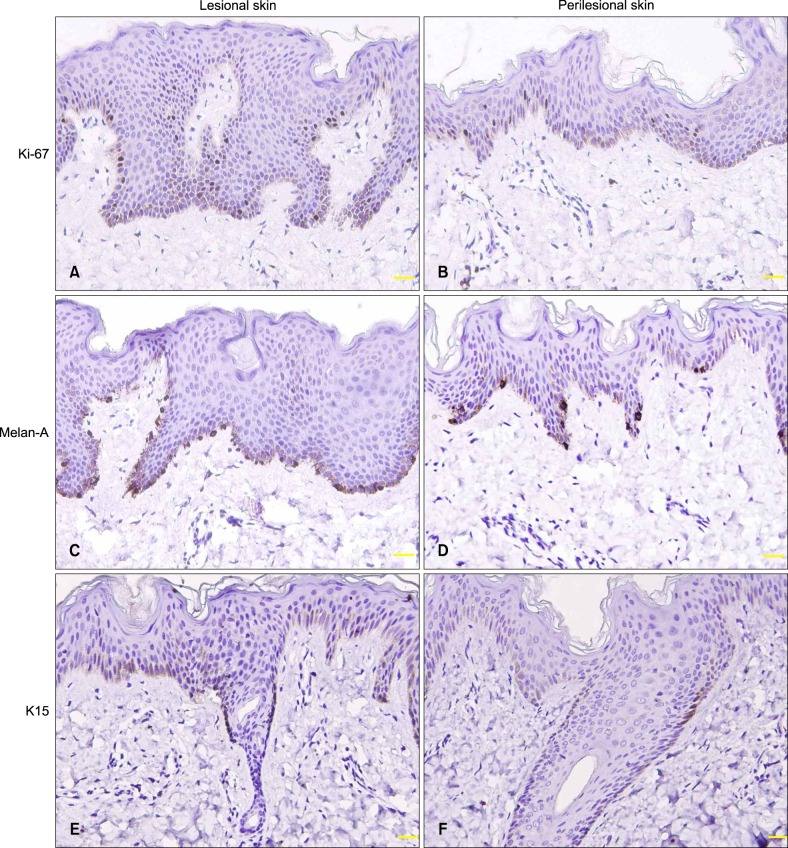
Nuclear Ki-67 stain was observed in the basal and parabasal keratinocytes of the epidermis, hair matrix cells and a few outermost layer cells of the outer root sheath (ORS) in the hair follicles, and some eccrine duct cells (Fig. 2A, B). Cytoplasmic Melan-A expession was seen in the basal melanocytes of the epidermis and some hair follicles (Fig. 2C, D). Compared with normal skin, epidermal Ki-67+ and Melan-A+ cells were increased in lesional and perilesional skin (p<0.05~0.01), especially in lesional skin (Table 2).
Fig. 2. Epidermal Ki-67, Melan-A, and keratin 15 (K15) expression in lesional skin (A, C, E) was higher than in perilesional skin (B, D, F). Follicular K15 expression was located in the outermost layer of outer root sheath at the isthmus level (E, F) (immunostaining, ×200).
Table 2. Immunohistochemical results of Ki-67+, Melan-A+, keratin 15 (K15), smooth muscle actin (SMA) and dermal nerve fiber length (DNFL) in lesional, perilesional and normal skin.
| Group | n | Ki-67+ | Melan-A+ | K15 | SMA+ | DNFL |
|---|---|---|---|---|---|---|
| Lesional skin | 21 | 3.71±1.36 | 1.85±0.90 | 0.96±0.05 | 1.25±1.35 | 0.94±0.48 |
| Perilesional skin | 21 | 2.77±0.96** | 1.54±0.81* | 0.88±0.09** | 0.56±0.59 | 0.57±0.32** |
| Normal skin | 10 | 1.15±0.50**,†† | 1.08±0.54*,† | 0.80±0.09**,†† | 0.33±0.42* | 0.28±0.22**,†† |
Values are presented as number only or mean±standard deviation. *p<0.05; **P<0.01, compared with lesional skin; †P<0.05; ††p<0.01, compared with perilesional skin.
K15
Cytoplasmic K15 immunostaining was seen in the basal layer of the epidermis and secretory cells of some eccrine glands, and its epidermal expression was higher in lesional and perilesional skin than in normal skin (p<0.01; Table 2). In the hair follicles, K15 was expressed mainly in the outermost ORS layer at the isthmus level and sometimes in the bulbar and suprabulbar cells (Fig. 2E, F).
SMA
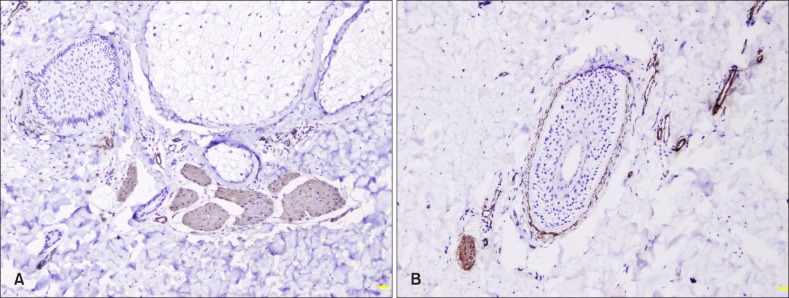
Cytoplasmic staining of SMA was observed in APM, vessel walls, dermal sheath of hair follicles, and myoepithelial cells of eccrine glands (Fig. 3). SMA+ APM area was higher in skin lesion than in normal skin (p<0.05; Table 2).
Fig. 3. Smooth muscle actin expression in arrector pili muscle, vessel walls, dermal sheath of hair follicles in lesional (A) and perilesional (B) skin (immunostaining, ×100).
PGP 9.5
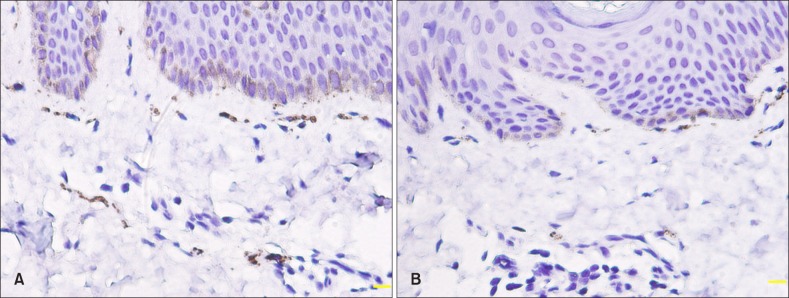
Intraepidermal nerve fibers were not counted due to non-specific staining of the basal keratinocytes and a small number of nerve fibers in the epidermis. PGP 9.5+ nerve fibers were abundantly distributed in the nerve plexus and APM and around eccrine ducts (Fig. 4). DNFL was higher in lesional and perilesional skin compared with normal skin (p<0.01; Table 2).
Fig. 4. Protein gene product 9.5+ nerve fibers in the arrector pili muscle and neuroplexus in lesional (A) and perilesional (B) skin (immunostaining, ×400).
DISCUSSION
BN lesions usually appeared on the upper trunk and upper limbs in this series, especially on scapular region, while the frequency of lower limb involvement was higher than the previous reports (21.7% vs. 3.2%~12.8%)4,7. This discrepancy may be due to the case recruitment and misdiagnosis and/or undiagnosis of atypical cases. In addition, the frequency of hypertrichosis ranged from 17% to 70%3, while it was prevalent (68.3%) in this study. Although the diverse age may be a credible explanation for this difference3,4, no statistical difference of gender and diagnostic age in this study seems not to support this hypothesis. Furthermore, multiple papules within the lesion might be a clue to the clinical diagnosis of BN based on the present and previous observations4,8. On the other hand, we did not found positive family history, breast hypoplasia and increased prevalence of acne vulgaris, while the concomitance of neurofibromatosis type 1 in 2 cases may be interesting. Although neurofibromatosis may be a component of BN syndrome9, the underlying mechanism is poorly understood.
The diagnosis of BN mainly hinges on the clinical features, but histopathology may be sometimes necessary to differentiate from other pigmentary disorders such as melanocytic nevus, café-au-lait macules, congenital smooth muscle hamartoma and postinflammatory hyperpigmentation8. Compared with the previous studies2,8, our results revealed the epidermal changes (elongated and fused rete ridges, keratotic plugging and basal pigmentation) were similar except acanthosis, but dermal alterations (mild perivascular lymphohistiocytic infiltration, fibrosis, and smooth muscle and sebaceous hyperplasia) were more common. Whether these histopathologic differences might be related to the diagnostic age, biopsied sites and judgement criteria, the rete ridge elongation and fusion and basal hyperpigmentation seems to be the typical features of BN2,8.
H&E stain demonstrates normal or increased number of melanocytes in BN8,10, while Dopa and immunohistochemical stain displays a significant increment2,11. We also found the incremental counts of Melan-A+ melanocyte in the lesional and perilesional skin compared with normal skin. Although the increment of melanocyte density may partly explain the cause of basal hyperpigmentation in BN, its pathogenetic role remains to be further investigated.
Since hypertrichosis and acanthosis are common features of BN, We examined the keratinocytic proliferation using Ki-67 and K15 immunostaining. Because the hair follicles had various growth cycles and profiles in each section, Ki-67+ and K15+ follicular cells were not compared in this study. The number of Ki-67+ and K15+ epidermal keratinocytes was significantly higher in lesional and perilesional skin than in normal skin, especially in lesional skin. In line with previous report12, we also found Ki-67 immunoreactivity was mainly present in hair matrix cells in the anagen hair follicles, and a few cells in the outermost layer of ORS. Meanwhile, the predominant expression of K15 in the upper part of ORS cells in our study is analogue to the previous studies13,14. K15+ cells in the bulge zone and interfollicular epidermis have self-renewal and multipotent features of stem cells, and K15 may involve in the regulation of early keratinocyte differentiation15. Thus, our results not only implicate the high proliferative rate of epidermal keratinocytes in the development of BN, but also foresee its progression.
In conformity with the histopathological results, our immunohistochemical observation disclosed the increased number of SMA+ APM in skin lesion compared with normal skin. APM can protect the bulge stem cells and maintain the follicular integrity16, while the bulge stem cells in turn may induce APM differentiation and anchorage to the bulge via producing a specialized basement membrane17. The APM-bulge connection plays a crucial role in the follicular morphogenesis and renewal16. Therefore, APM hyperplasia in BN might originate from bulge stem cells and then promote follicular renewal, which may partly explain the cause of the hairy presentation in BN. In addition, immunostaining pattern of SMA in the dermal sheath of hair follicles is coincident with the previous reports18,19. SMA expression in the dermal sheath cells may play a role in the shortening and curly morphology of hair follicles18, and suggest an epithelial-mesenchymal interaction for hair follicle development19.
We found the increase of DNFL in lesional and perilesional skin compared with normal skin, and prominent nerve fibers interspersed in APM. The significance of dermal hyperinnervation in BN is unknown in view of the lack of itching. This hyperinnervation might be due to nerve growth factors produced by keratinocytes, mast cells, eosinophils and fibroblasts20, and expression of two important myelin proteins induced by androgen21. The hair follicles are major components of the cutaneous nerve network, and play a pivotal role in modulation of skin innervation. The transplanted adult hair follicle unit including functional APM can spontaneously induce the recovery of the neurofollicular and neuromuscular junctions22. Therefore, the incremental DNFL might contribute to the follicle cycling and APM function.
In summary, this study demonstrates the hyperproliferation of keratinocytes, melanocytes, APM and dermal nerve fibers could be involved in the pathogenesis of BN. More research is needed to determine the relationship between their proliferation and sex hormone expression.
ACKNOWLEDGMENT
This study was supported by Scientific and Technological Project of Zhanjiang City (grant no. 2014B101) and Outstanding Master Cultivation Programme of Guangdong Medical University (grant no. 4CX14091G).
References
- 1.Patel P, Malik K, Khachemoune A. Sebaceus and Becker's nevus: overview of their presentation, pathogenesis, associations, and treatment. Am J Clin Dermatol. 2015;16:197–204. doi: 10.1007/s40257-015-0123-y. [DOI] [PubMed] [Google Scholar]
- 2.Kim YJ, Han JH, Kang HY, Lee ES, Kim YC. Androgen receptor overexpression in Becker nevus: histopathologic and immunohistochemical analysis. J Cutan Pathol. 2008;35:1121–1126. doi: 10.1111/j.1600-0560.2008.00988.x. [DOI] [PubMed] [Google Scholar]
- 3.Rasi A, Berenji Ardestani H, Tabaie SM. Hypertrichosis is not so prevalent in Becker's nevus: analysis of 47 cases. ISRN Dermatol. 2014;2014:953747. doi: 10.1155/2014/953747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrizi A, Medri M, Raone B, Bianchi F, Aprile S, Neri I. Clinical characteristics of Becker's nevus in children: report of 118 cases from Italy. Pediatr Dermatol. 2012;29:571–574. doi: 10.1111/j.1525-1470.2012.01734.x. [DOI] [PubMed] [Google Scholar]
- 5.Grande Sarpa H, Harris R, Hansen CD, Callis Duffin KP, Florell SR, Hadley ML. Androgen receptor expression patterns in Becker's nevi: an immunohistochemical study. J Am Acad Dermatol. 2008;59:834–838. doi: 10.1016/j.jaad.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Lauria G, Cazzato D, Porretta-Serapiglia C, Casanova-Molla J, Taiana M, Penza P, et al. Morphometry of dermal nerve fibers in human skin. Neurology. 2011;77:242–249. doi: 10.1212/WNL.0b013e318225ab51. [DOI] [PubMed] [Google Scholar]
- 7.de Almeida HL, Jr, Duquia RP, Souza PR, Breunig Jde A. Prevalence and characteristics of Becker nevus in Brazilian 18-year-old males. Int J Dermatol. 2010;49:718–720. doi: 10.1111/j.1365-4632.2009.04224.x. [DOI] [PubMed] [Google Scholar]
- 8.Alfadley A, Hainau B, Al Robaee A, Banka N. Becker's melanosis: a report of 12 cases with atypical presentation. Int J Dermatol. 2005;44:20–24. doi: 10.1111/j.1365-4632.2004.02077.x. [DOI] [PubMed] [Google Scholar]
- 9.Kar S, Preetha K, Yadav N, Madke B, Gangane N. Becker's nevus with neurofibromatosis type 1. Ann Indian Acad Neurol. 2015;18:90–92. doi: 10.4103/0972-2327.144281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AlGhamdi KM, AlKhalifah AI, AlSheikh AM, AlSaif FM. Clinicopathologic profile of Becker's melanosis with atypical features. J Drugs Dermatol. 2009;8:745–748. [PubMed] [Google Scholar]
- 11.Tate PR, Hodge SJ, Owen LG. A quantitative study of melanocytes in Becker's nevus. J Cutan Pathol. 1980;7:404–409. doi: 10.1111/j.1600-0560.1980.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsujita-Kyutoku M, Kiuchi K, Danbara N, Yuri T, Senzaki H, Tsubura A. p63 expression in normal human epidermis and epidermal appendages and their tumors. J Cutan Pathol. 2003;30:11–17. doi: 10.1034/j.1600-0560.2003.300102.x. [DOI] [PubMed] [Google Scholar]
- 13.Poblet E, Jiménez F, Godínez JM, Pascual-Martín A, Izeta A. The immunohistochemical expression of CD34 in human hair follicles: a comparative study with the bulge marker CK15. Clin Exp Dermatol. 2006;31:807–812. doi: 10.1111/j.1365-2230.2006.02255.x. [DOI] [PubMed] [Google Scholar]
- 14.Kanoh M, Amoh Y, Sato Y, Katsuoka K. Expression of the hair stem cell-specific marker nestin in epidermal and follicular tumors. Eur J Dermatol. 2008;18:518–523. doi: 10.1684/ejd.2008.0485. [DOI] [PubMed] [Google Scholar]
- 15.Abbas O, Richards JE, Yaar R, Mahalingam M. Stem cell markers (cytokeratin 15, cytokeratin 19 and p63) in in situ and invasive cutaneous epithelial lesions. Mod Pathol. 2011;24:90–97. doi: 10.1038/modpathol.2010.180. [DOI] [PubMed] [Google Scholar]
- 16.Torkamani N, Rufaut NW, Jones L, Sinclair RD. Beyond goosebumps: does the arrector pili muscle have a role in hair loss? Int J Trichology. 2014;6:88–94. doi: 10.4103/0974-7753.139077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thibaut S, Gaillard O, Bouhanna P, Cannell DW, Bernard BA. Human hair shape is programmed from the bulb. Br J Dermatol. 2005;152:632–638. doi: 10.1111/j.1365-2133.2005.06521.x. [DOI] [PubMed] [Google Scholar]
- 19.Aneiros-Fernández J, Husein-ElAhmed H, Arias-Santiago S, Campos A, Carriel V, Sánchez-Montesinos I, et al. Expression of smoothelin and smooth muscle actin in the skin. Histol Histopathol. 2011;26:673–678. doi: 10.14670/HH-26.673. [DOI] [PubMed] [Google Scholar]
- 20.Taneda K, Tominaga M, Negi O, Tengara S, Kamo A, Ogawa H, et al. Evaluation of epidermal nerve density and opioid receptor levels in psoriatic itch. Br J Dermatol. 2011;165:277–284. doi: 10.1111/j.1365-2133.2011.10347.x. [DOI] [PubMed] [Google Scholar]
- 21.Melcangi RC, Ballabio M, Cavarretta I, Gonzalez LC, Leonelli E, Veiga S, et al. Effects of neuroactive steroids on myelin of peripheral nervous system. J Steroid Biochem Mol Biol. 2003;85:323–327. doi: 10.1016/s0960-0760(03)00228-0. [DOI] [PubMed] [Google Scholar]
- 22.Sato A, Toyoshima KE, Toki H, Ishibashi N, Asakawa K, Iwadate A, et al. Single follicular unit transplantation reconstructs arrector pili muscle and nerve connections and restores functional hair follicle piloerection. J Dermatol. 2012;39:682–687. doi: 10.1111/j.1346-8138.2012.01505.x. [DOI] [PubMed] [Google Scholar]