Abstract
Background
Cellulitis is a common bacterial infection of the superficial skin. Procalcitonin is one of the precursor proteins of calcitonin, its levels are elevated in bacterial infection, and it has been established as a diagnostic marker for severe bacterial infections.
Objective
This study evaluated the clinical usefulness of procalcitonin for predicting disease severity and prognosis of cellulitis.
Methods
We reviewed the medical records of 160 patients diagnosed with cellulitis in the past 3 years. Body temperature, procalcitonin, white blood cell (WBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels were measured on their first day of admission. The associations of procalcitonin, WBC, ESR, and CRP with the body temperature and the number of hospitalized days were assessed.
Results
Procalcitonin, WBC, and CRP showed a positive correlation with body temperature. In addition, procalcitonin, WBC, ESR, and CRP showed a positive correlation with number of hospitalized days (p<0.05).
Conclusion
In patients diagnosed with cellulitis, proclacitonin was a helpful parameter to indicate the severity of disease and also a useful predictor of prognosis.
Keywords: Cellulitis, Procalcitonin
INTRODUCTION
Procalcitonin, consisting of 116 amino acids, is one of the precursor proteins of calcitonin, although its biological role is unknown1,2. When there is a bacterial infection, CALC1 gene expression is increased, and the expression of the precursor of calcitonin, procalcitonin, is subsequently increased in all of the cells in the body3. Synthesis of procalcitonin is stimulated by bacterial endotoxins and the proinflammatory cytokines, interleukin (IL)-1β, IL-6 and TNF, and suppressed by the interferon-γ secreted during viral infection4.
White blood cell (WBC) count, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are often used as markers of bacterial infection severity. However, in some cases, there are difficulties in using these markers as indicators of diagnosis and treatment due to their low sensitivity and specificity5. A recent study reported the value of procalcitonin in bullous impetigo, staphylococcal scaled skin syndrome, localized skin infection, diabetic foot infection, septic arthritis and osteomyelitis6,7. In addition, procalcitonin may be a useful tool for the initial diagnosis and monitoring of cutaneous infection or cutaneous soft tissue infection8. Hence, we conducted this study on patients with cellulitis to identify whether serum procalcitonin, in addition to the WBC, ESR, and CRP, as used in conventional measurements, was useful for the evaluation of disease severity and prognosis.
MATERIALS AND METHODS
Patients
We enrolled 160 patients who were diagnosed with cellulitis and erysipelas at the Department of Dermatology of Wonkwang University Hospital, from March 2012 to February 2015. Patient's medical records were reviewed retrospectively. Age, sex, involved site(s), infectious route, complications, systemic manifestations, and number of hospitalized days were evaluated. The study was approved by the ethics committee of Wonkwang University Hospital (IRB no. WKUH 201505-HRE-037).
Clinical and biomarker assessment
The diagnosis of cellulitis and erysipelas was based on the physician's clinical diagnosis and histopathologic findings. We considered erysipelas a synonym for cellulitis because it is difficult to clinically distinguish cellulitis and erysipelas. In order to assess the severity of the disease, we measured body temperature for systemic symptoms. Fever was defined as a body temperature of more than 38.0℃, according to the systemic inflammatory response syndrome criteria.
WBC (XN-9000; Sysmex Co., Kobe, Japan), ESR (ALI FAX; Alere Inc., Waltham, MA, USA), CRP (Modular p800; Roche diagnostics, Basel, Switzerland) and Procalcitonin (Vidas; BioMerieux Co., Lyon, France) levels were measured at the first day of admission as part of the biomarker assessment. Procalcitonin was classified into 5 groups according to the reference levels: (1) group 1, normal limit (<0.05 ng/ml); (2) group 2, possible local bacterial infection (<0.5 ng/ml); (3) group 3, minor systemic inflammatory response (<2 ng/ml); (4) group 4, sever sepsis (<10 ng/ml); and (5) group 5, septic shock (≥10 ng/ml). Patients were discharged when their CRP level was reduced to below 10 mg/L, or the only symptom that remained was mild swelling.
Statistical analysis
To evaluate differences between groups, independent t-test analysis and ANOVA were used to analyze variables. We analyzed cross-sectional correlations between WBC, ESR, CRP, and procalcitonin, and body temperature and number of hospitalized days. Correlations between the biomarkers and variables were examined both graphically and using the Pearson correlation coefficient. All analyses were performed using statistical software IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). p<0.05 was considered significant.
RESULTS
Patient distribution
Patients' ages ranged from 3 to 84 years (mean, 48.0). Of the 160 subjects, 87 (54.4%) were male and 73 (45.6%) were female. The male-to-female ratio of the total patients was 1.2:1. Among the systemic symptoms, fever occurred in 27 (16.9%) cases. Complications were experienced in 13 (8.1%) cases; the complications included abscess, bursitis, necrotizing fasciitis and septic arthritis. In addition, recurrence occurred in 2 (1.25%) patients (Table 1).
Table 1. Patient characteristics.
| Characteristic | Value |
|---|---|
| Number of patients | 160 |
| Sex | |
| Male | 87 (54.4) |
| Female | 73 (45.6) |
| Age (yr) | 48.0±19.6 |
| ≤9 | 4 (2.5) |
| 10∼19 | 13 (8.1) |
| 20∼29 | 13 (8.1) |
| 30∼39 | 21 (13.1) |
| 40∼49 | 20 (12.5) |
| 50∼59 | 43 (26.9) |
| 60∼69 | 25 (15.6) |
| 70∼79 | 12 (7.5) |
| 80∼89 | 9 (5.6) |
| Systemic manifestation | |
| Yes (fever) | 27 (16.9) |
| None | 133 (83.1) |
| Complications | |
| None | 147 (91.9) |
| Abscess | 9 (5.6) |
| Bursitis | 2 (1.3) |
| Necrotizing fasciitis | 1 (0.6) |
| Septic arthritis | 1 (0.6) |
| Initial laboratory work up (mean) | |
| White blood cell (×103/µl) | 10.9 |
| Erythrocyte sedimentation rate | 26 |
| C-reactive protein (mg/L) | 67.5 |
| Procalcitonin (ng/ml) | 0.35 |
| Hospitalized days deviation | 9.3±4.8 |
| Recurrence | 2 (1.25) |
Values are presented as number only, number (%), or mean±standard deviation.
Anatomic location of the disease
The most common site of disease was the lower leg (n=66, 41.3%) followed by the head and neck (n=39, 24.4%), foot (n=36, 22.5%), hand (n=8, 5.0%), thigh (n=4, 2.5%), arm (n=4, 2.5%), and forearm (n=3, 1.9%) (Table 2). When viewed as a whole, 106 (66.3%) cases arose from the lower limbs, while 39 (24.4%) cases were from the head and neck and 15 (9.4%) cases were from the upper limbs.
Table 2. Involved sites and infectious routes.
| Anatomic site and infectious events | No. of patients (%) Site (n=160) |
|---|---|
| Site (n=160) | |
| Lower leg | 66 (41.3) |
| Head and neck | 39 (24.4) |
| Foot | 36 (22.5) |
| Hand | 8 (5.0) |
| Thigh | 4 (2.5) |
| Arm | 4 (2.5) |
| Forearm | 3 (1.9) |
| Preceding events (n=107) | |
| Tinea pedis | 49 (45.8) |
| Trauma | 41 (38.3) |
| Insect bite | 11 (10.3) |
| Furuncle | 4 (3.7) |
| Eczema | 2 (1.9) |
Infectious route
The route of infection could be inferred in 107 (66.9%) cases and 53 (33.1%) cases had cellulitis of unknown infectious origin. The most common possible route of infection was considered to be tinea pedis (n=49, 45.8%), followed by trauma (n=41, 38.3%), insect bites n=11, 10.3%), furuncle (n=4, 3.7%), and eczema (n=2, 1.9%) (Table 2).
Laboratory findings and relationships between body temperature and number of hospitalized days
WBC, ESR, and CRP levels were measured in a total of 160 patients, but procalcitonin level was measured in 144 (90.0%) patients, excluding 16 of the 160 patients. The average WBC level was 10.9×103/µl, and 80 of 160 patients (50.0%) demonstrated leukocytosis. The average ESR and CRP levels were 26.0 mm/h and 67.5 mg/L, respectively. Elevated ESR and CRP were seen in 89 (55.6%) and 141 of 160 patients (88.1%), respectively. The average procalcitonin value was 0.35 ng/ml, and 65 of 144 patients (45.1%) demonstrated procalcitonin ≥0.05 ng/ml. The number of hospitalized days was between 3 and 38. The average was 9.3.
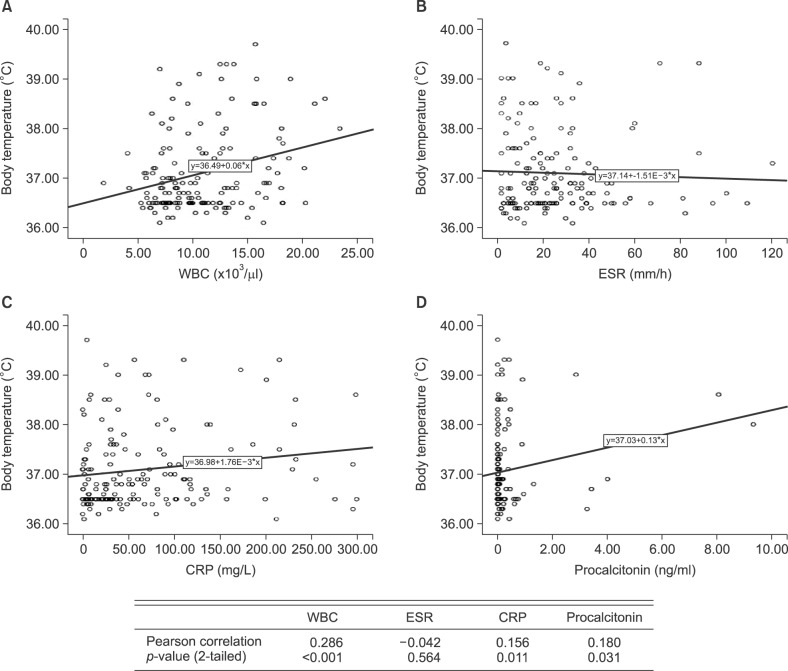
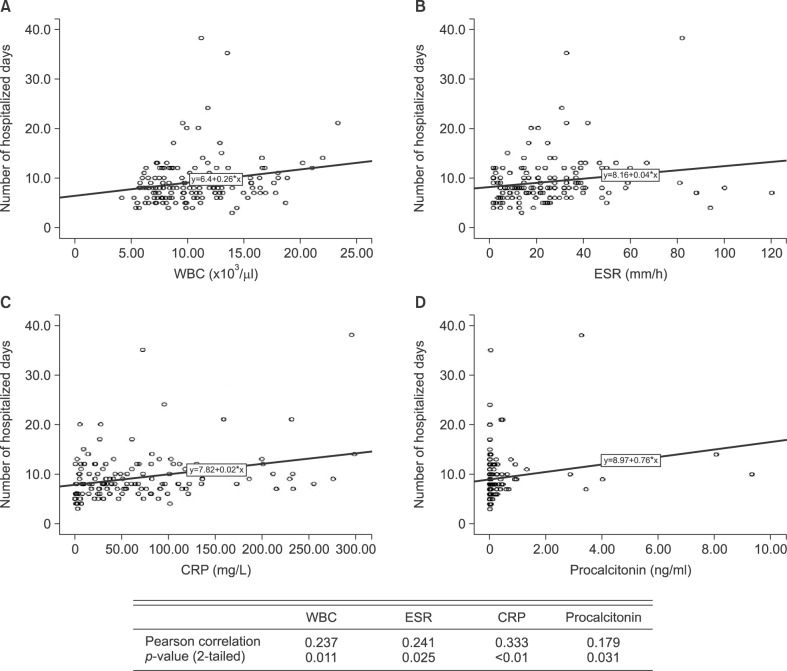
WBC, CRP, and procalcitonin showed a positive correlation with body temperature (p<0.05), but ESR had no relationship with body temperature (Fig. 1). WBC, ESR, CRP, and procalcitonin showed a positive correlation with number of hospitalized days (p<0.05; Fig. 2).
Fig. 1. Correlation of white blood cell (WBC; A), erythrocyte sedimentation rate (ESR; B), C-reactive protein (CRP; C), and procalcitonin (D) with body temperature.
Fig. 2. Correlation of white blood cell (WBC; A), erythrocyte sedimentation rate (ESR; B), C-reactive protein (CRP; C), and procalcitonin (D) with hospitalized days.
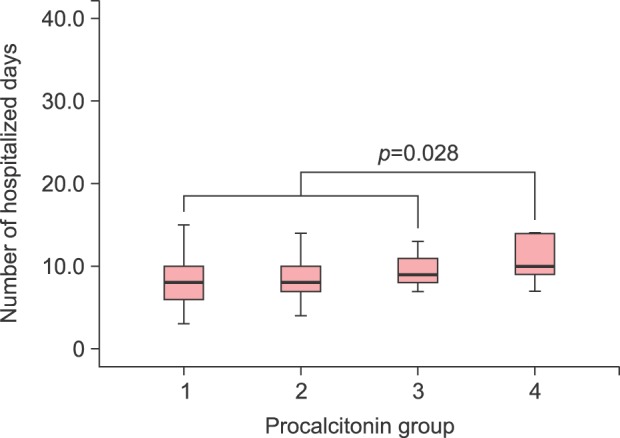
Procalcitonin could be divided into five groups according to its level: (1) group 1 (n=79, 54.9%), (2) group 2 (n=50, 34.7%), (3) group 3 (n=9, 6.2%), (4) group 4 (n=6, 4.2%), and (5) group 5 (n=0, 0%). Using Duncan post-hoc analysis, we found no differences in the first 3 groups and their relationship to the number of hospitalized days. However, there was a statistically significant difference between the first 3 groups and group 4 (p=0.028; Fig. 3).
Fig. 3. In the post-hoc analysis, group 4 had a statistically significant increase in the number of hospitalized days compared to the other groups.
Relationship between laboratory markers and severity of cutaneous symptoms
The most common cutaneous symptoms were erythema, tenderness, and swelling (100%) followed by pus drainage (n=21, 13.1%), bullae (n=12, 7.5%), and hemorrhagic swelling (n=8, 5.0%). There was a relationship between bulla formation and CRP and ESR (p=0.008 and 0.044, respectively). But differences between the others cutaneous symptoms and laboratory markers were not significant (Table 3).
Table 3. Relationship between procalcitonin, WBC, CRP, and ESR and initial presentation of cutaneous symptoms (n=160).
| n | Procalcitonin (ng/ml) | WBC (×103/µl) | CRP (mg/L) | ESR (mm/h) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | p-value | Mean±SD | p-value | Mean±SD | p-value | Mean±SD | p-value | ||
| Bulla formation | 0.178 | 0.773 | 0.008 | 0.044 | |||||
| Present | 12 | 1.33±2.46 | 9.98±5.54 | 120.16±124.78 | 38.50±30.78 | ||||
| Absent | 148 | 0.26±0.95 | 10.94±3.97 | 63.21±64.72 | 25.03±21.35 | ||||
| Hemorrhage | 0.630 | 0.813 | 0.303 | 0.197 | |||||
| Present | 8 | 0.15±0.18 | 9.13±3.69 | 93.10±78.10 | 36.00±31.47 | ||||
| Absent | 152 | 0.35±1.19 | 10.96±4.10 | 66.13±71.63 | 25.52±21.79 | ||||
| Pus drainage | 0.381 | 0.706 | 0.299 | 0.610 | |||||
| Present | 21 | 0.12±0.28 | 12.24±5.02 | 52.23±61.37 | 23.71±13.60 | ||||
| Absent | 139 | 0.37±1.23 | 10.66±3.90 | 69.79±73.06 | 26.40±23.33 | ||||
WBC: white blood cell, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, SD: standard deviation.
DISCUSSION
In 1993, Assicot et al.9, first reported the clinical use of procalcitonin to differentiate between bacterial and viral meningitis. Recently, several studies have shown increased procalcitonin in patients with infectious disease10. An increased blood procalcitonin level reflects the severity of infection and can be helpful to predict the prognosis of patients11,12. The procalcitonin level is also useful in the differential diagnosis of infections caused by bacteria and viruses, because it does not increase in viral infection13. Procalcitonin does not increase in patients with a severe inflammatory response unless the cause is infection9.
Procalcitonin is detected 2 hours after injection of Escherichia coli-derived endotoxin, reaches its highest concentration after 6 hours, and is maintained at the level for 9~24 hours. In contrast, CRP begins to increase after 12 hours and does not reach its peak level for 30 hours. Therefore, procalcitonin has the advantage of more rapid detection than CRP14.
Meanwhile, CRP, one of the acute phase proteins, is a useful measure of the inflammatory response. Even if there are no systemic symptoms, it is sensitive enough to still detect an inflammatory condition. This is useful in making diagnosis, but in some cases, it is difficult to interpret the results because an increased CRP level is non-specific, and cannot identify the cause of inflammation15,16. ESR alone is also not sufficient for a diagnosis, although it too increases with inflammation17,18.
In our study, analyses were performed with respect to the clinical usefulness of the procalcitonin level in cellulitis. Erysipelas is an inflammatory disease of the superficial dermis including the superficial lymphatics, and mainly occurs on the face and cellulitis is inflammation of the reticular dermis and subcutaneous fat, and presents with unclear margins. However, erysipelas is often considered a synonym for cellulitis affecting any skin area because of difficulties in accurately differentiating between the two diseases19,20. Thus, patients with erysipelas are included in this study.
The procalcitonin level was shown to have a positive correlation with body temperature. Fever, body temperature above 38℃, is one of the criteria for systemic inflammatory response syndrome, and can be considered a factor indicating the severity of disease. Therefore, we thought that a higher procalcitonin level would correlate with higher disease severity. Pearson correlation coefficients calculated in this study between body temperature and parameters were WBC, 0.286; ESR, −0.042; CRP, 0.156); and procalcitonin, 0.180. WBC, CRP, and procalcitonin were all correlated with disease severity, and WBC appeared to have the highest Pearson correlation coefficient. These results suggest that the WBC level was the most valuable inflammatory indicator, but, procalcitonin was useful in evaluating the severity of disease.
Lazzarini et al.21 found that CRP and ESR levels were elevated in patients hospitalized for more than 10 days, but WBC had no relationship to duration of hospitalization. In this study, the Pearson correlation coefficients between the number of hospitalized days and parameters were WBC, 0.237; ESR, 0.241; CRP, 0.333; and procalcitonin, 0.179. Therefore, all parameters could be used as indicators to predict prognosis, especially, CRP level, which had the highest correlation coefficient and was the most useful predictor of prognosis.
Procalcitonin was divided into five groups depending on its level: group 1 had 79 patients (54.9%), group 2 had 50 (34.7%), group 3 had 9 (6.2%), group 4 had 6 (4.2%), and group 5 had 0 (0%). Interestingly, the number of hospitalized days in group 4 was significantly higher than that in the other groups. In a recent study, procalcitonin had a better diagnostic value when compared with CRP and WBC in erysipelas, and its concentration was relatively low in localized inflammatory state22. Our study was consistent with this previous study.
At a procalcitonin threshold of 0.05 ng/ml or more, the sensitivity and specificity for cellulitis were 45.1% (95% confidence interval [CI], 42.2%~45.1%) and 100% (95% CI, 97.0%~100%), respectively. The result revealed that a low procalcitonin level (<0.05 ng/ml) did not ultimately rule out cellulitis. And the procalcitonin level can be normal in cellulitis. However, clinicians should be highly cautious in the case of a procalcitonin level high enough to indicate poor prognosis.
In conclusion, procalcitonin reflected severity of disease in cellulitis and high levels suggested a poor prognosis. However, compared to procalcitonin, WBC and CRP are most closely associated with severity of disease and prognosis, respectively.
ACKNOWLEDGMENT
This work was supported by Wonkwang University in 2016.
References
- 1.van Rossum AM, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis. 2004;4:620–630. doi: 10.1016/S1473-3099(04)01146-6. [DOI] [PubMed] [Google Scholar]
- 2.Russwurm S, Wiederhold M, Oberhoffer M, Stonans I, Zipfel PF, Reinhart K. Molecular aspects and natural source of procalcitonin. Clin Chem Lab Med. 1999;37:789–797. doi: 10.1515/CCLM.1999.119. [DOI] [PubMed] [Google Scholar]
- 3.Müller B, White JC, Nylén ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001;86:396–404. doi: 10.1210/jcem.86.1.7089. [DOI] [PubMed] [Google Scholar]
- 4.Schuetz P, Albrich W, Christ-Crain M, Chastre J, Mueller B. Procalcitonin for guidance of antibiotic therapy. Expert Rev Anti Infect Ther. 2010;8:575–587. doi: 10.1586/eri.10.25. [DOI] [PubMed] [Google Scholar]
- 5.Kim SI, Shim BY, You HY, Jung J, Wie SH, Kim YR, et al. Clinical usefulness of procalcitonin in febrile Patients; comparison with erythrocyte sedimentation rate and C-reactive protein. Korean J Infect Dis. 2000;32:129–134. [Google Scholar]
- 6.Zeng M, Guo Z, Shen S, Liu S. Value of serum procalcitonin and interleukin-6 in patients with bullous impetigo and staphylococcal scalded skin syndrome. J Dermatol. 2014;41:1028–1029. doi: 10.1111/1346-8138.12638. [DOI] [PubMed] [Google Scholar]
- 7.Saeed K, Ahmad N, Dryden M. The value of procalcitonin measurement in localized skin and skin structure infection, diabetic foot infections, septic arthritis and osteomyelitis. Expert Rev Mol Diagn. 2014;14:47–54. doi: 10.1586/14737159.2014.864238. [DOI] [PubMed] [Google Scholar]
- 8.Eder J, Hlavin G, Haushofer A, Trubert-Exinger D, Trautinger F. Correlation of serum procalcitonin with the severity of skin and skin structure infections-a pilot study. J Dtsch Dermatol Ges. 2012;10:564–571. doi: 10.1111/j.1610-0387.2011.07858.x. [DOI] [PubMed] [Google Scholar]
- 9.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gendrel D, Bohuon C. Procalcitonin, a marker of bacterial infection. Infection. 1997;25:133–134. doi: 10.1007/BF02113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schröder J, Staubach KH, Zabel P, Stüber F, Kremer B. Procalcitonin as a marker of severity in septic shock. Langenbecks Arch Surg. 1999;384:33–38. doi: 10.1007/s004230050170. [DOI] [PubMed] [Google Scholar]
- 12.Ugarte H, Silva E, Mercan D, De Mendonça A, Vincent JL. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999;27:498–504. doi: 10.1097/00003246-199903000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Viallon A, Zeni F, Lambert C, Pozzetto B, Tardy B, Venet C, et al. High sensitivity and specificity of serum procalcitonin levels in adults with bacterial meningitis. Clin Infect Dis. 1999;28:1313–1316. doi: 10.1086/514793. [DOI] [PubMed] [Google Scholar]
- 14.Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24:888–889. doi: 10.1007/s001340050683. [DOI] [PubMed] [Google Scholar]
- 15.Jaye DL, Waites KB. Clinical applications of C-reactive protein in pediatrics. Pediatr Infect Dis J. 1997;16:735–746. doi: 10.1097/00006454-199708000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Korppi M, Heiskanen-Kosma T, Leinonen M. White blood cells, C-reactive protein and erythrocyte sedimentation rate in pneumococcal pneumonia in children. Eur Respir J. 1997;10:1125–1129. doi: 10.1183/09031936.97.10051125. [DOI] [PubMed] [Google Scholar]
- 17.Grønlie M, Hjortdahl P. The erythrocyte sedimentation rate; its use and usefulness in primary health care. Scand J Prim Health Care. 1991;9:97–102. doi: 10.3109/02813439109026591. [DOI] [PubMed] [Google Scholar]
- 18.Melbye H, Straume B, Brox J. Laboratory tests for pneumonia in general practice: the diagnostic values depend on the duration of illness. Scand J Prim Health Care. 1992;10:234–240. doi: 10.3109/02813439209014067. [DOI] [PubMed] [Google Scholar]
- 19.Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. Lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.e1-e12. doi: 10.1016/j.jaad.2012.03.024. quiz 175-176. [DOI] [PubMed] [Google Scholar]
- 20.Na SY, Lee HY, Baek JO, Roh JY, Lee JR. A case of cellulitis associated with coral injury. Ann Dermatol. 2008;20:212–215. doi: 10.5021/ad.2008.20.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazzarini L, Conti E, Tositti G, de Lalla F. Erysipelas and cellulitis: clinical and microbiological spectrum in an Italian tertiary care hospital. J Infect. 2005;51:383–389. doi: 10.1016/j.jinf.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Rast AC, Knobel D, Faessler L, Kutz A, Felder S, Laukemann S, et al. Use of procalcitonin, C-reactive protein and white blood cell count to distinguish between lower limb erysipelas and deep vein thrombosis in the emergency department: a prospective observational study. J Dermatol. 2015;42:778–785. doi: 10.1111/1346-8138.12922. [DOI] [PubMed] [Google Scholar]