Abstract
Background
Platelet-rich plasma (PRP) is an autologous concentration of human platelets contained in a small volume of plasma and has recently been shown to accelerate rejuvenate aging skin by various growth factors and cell adhesion molecules.
Objective
This study was conducted to evaluate the efficacy and safety of intradermal injection of PRP in the human facial rejuvenation.
Methods
This study was a prospective, single-center, single-dose, open-label, non-randomized controlled clinical study. PRP injected to the upper site of this right infra-auricular area and all face. Saline was injected to the left infra-auricular area. Histopathological examinations were performed before PRP treatment, 28 days after the PRP, and saline (control) treatments.
Results
Twenty women ranging in age from 40 to 49 years (mean age, 43.65±2.43 years) were enrolled in the study. The mean optical densities (MODs) of collagen in the pre-treatment, control, and PRP-treated area were measured. They were 539±93.2, 787±134.15, 1,019±178, respectively. In the MOD of PRP, 89.05 percent improvement was found when MOD of PRP was compared with MOD of pre-treatment. The mean MOD of collagen fibers was clearly highest on the PRP side (p<0.001). The PRP-to-saline improvement ratio (89.05% to 46.01%) was 1.93:1. No serious side effects were detected.
Conclusion
PRP increases dermal collagen levels not only by growth factors, but also by skin needling (the mesotherapy technique 'point by point'). PRP application could be considered as an effective (even a single application) and safety procedure for facial skin rejuvenation.
Keywords: Collagen, Mesotherapy, Platelet-rich plasma, Skin needling, Skin rejuvenation
INTRODUCTION
Platelet-rich plasma (PRP) has been used over the last several years as an effective treatment in various medical and surgical fields1. There are publication about the use of PRP in wound treatment, maxillofacial surgery, soft tissue injuries, periodontal and oral surgery, orthopedic and trauma surgery, gastrointestinal surgeries, burns, cosmetic and plastic surgery. PRP specifically has attracted the attention of dermatologists in the aesthetic field for skin rejuvenation1,2.
PRP contains a high concentration of thrombocytes (platelets). There are several growth factors in α-granules of platelets, secreted after the activation of platelets by aggregation initiators. Several growth factors and cytokines work in the stimulation process of fibroblast collagen synthesis3.
Up to date, nearly all of the studies investigating the effect of PRP on cell function such as fibroblast function, which will provide important data for clinical application, have obtained encouraging results4,5,6. However, most of them have been reported regarding the effect of the PRP on the proliferation of fibroblast, in vitro (in the cultured cells). To clarify the clinical efficacy of PRP, with the evaluation of collagen (in vivo), the effect of PRP on the proliferation of collagen needs to be investigated. The objective of this controlled clinical study was to investigate the effect of PRP on skin rejuvenation (and changes in collagen) by histological analysis of dermal collagen.
MATERIALS AND METHODS
This study was a prospective, single-center, single-dose, open-label, non-randomized controlled clinical study of the effects of PRP on dermal collagens. Patients who were diagnosed with skin aging in the Department of Dermatology, Eskisehir Military Hospital, Eskisehir, Turkey between September 2013 and December 2013 participated in the study. Ethical approval was obtained from Eskisehir Osmangazi University Clinical Research, Ethical Committee (August 29, 2012; protocol no., 2012/195). Institutional Review Board (IRB) approval was obtained from the Eskisehir Osmangazi University. The study protocol complied with the ethical guidelines of the Declaration of Helsinki of the World Medical Association.
Patients
Twenty healthy volunteer women who require facial skin rejuvenation were enrolled in the study and treated free of charge. They all had Fitzpatrick skin type I~III. None of the enrolled patients had been predisposed to hypertrophic/keloid scarring, had undergone any facial dermabrasion procedures or topical or systemic retinoid use, or had received dermal filler materials or facial botulinum toxin injections. The exclusion criteria for PRP were pregnant, breastfeeding, malignancy, autoimmune or blood diseases. Patients read a study overview description, were counseled as to the benefits and possible side effects of treatment and signed an informed consent form.
PRP preparation and application
A sterfile Conformité Européenne (CE) marked RegenLab® kit (Regen Lab., Le Mont-sur-Lausanne, Switzerland) was used for preparation of PRP. The kit was equipped with a butterfly 21 G needle; vacutainer kit; calcium chloride; 2 ml syringe and 30 G needle. Eight ml blood sample was aspirated from the patient's peripheral vein in tubes containing sodium citrate anticoagulant. The special 8 ml test tube was prepared. The tubes were equipped with a sepaseparator, which centrifugally separates red and white cells from PRP. The test tube was centrifuged at 3,000 rpm during 5 minutes. As the tubes contain a special gel separator, red blood cells were discarded from the plasma at the bottom of the gel. Platelets and white blood cells were pellet on top of the gel and re-suspended in plasma by gently mixing the tube. The 2 ml of cell suspension was called the PRP. A 30 G needle was used for superficial microinjections by the mesotherapy technique 'point by point'. Injections were spaced about 1 cm apart. The injections were administered into the papillary dermis (1.5~2.0 mm deep). Injection amount was 0.15 ml per injection. Approximately 2 ml of PRP was injected into the dermis of the face.
Skin biopsies
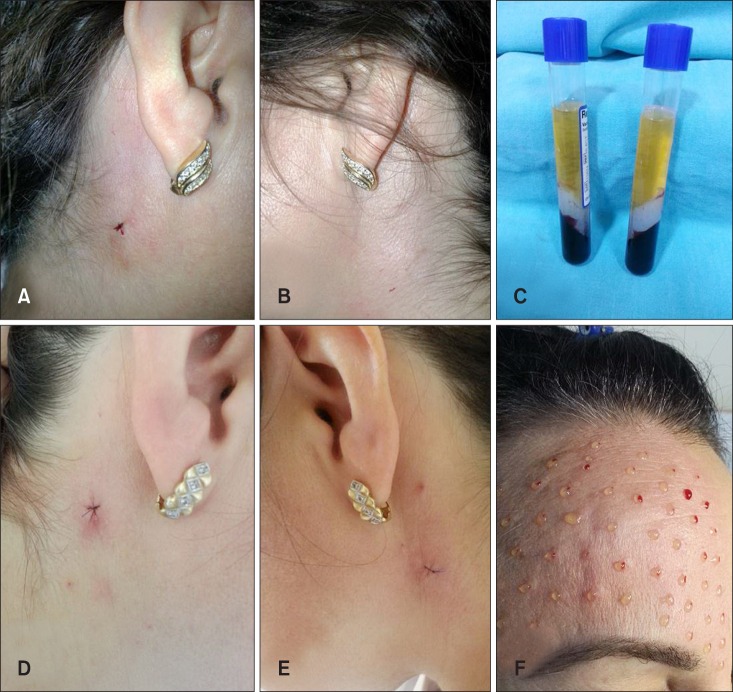
Three punch biopsies were obtained under local anesthesia from each patient. The first one was obtained from the right infra-auricular area before treatment (Fig. 1A). PRP was injected to the upper site of this right infra-auricular area and all face (Fig. 1A, F). Saline was injected into the left infra-auricular area (Fig. 1B). On the day 28 after PRP and saline injections, punch biopsy was performed on the PRP-treated (Fig. 1D) and control (saline injected area) site (Fig. 1E), followed by fixation with 10% paraformaldehyde.
Fig. 1. The first biopsy was obtained from the right infra-auricular area before treatment (A). Saline was injected to the left infra-auricular area (B). Platelet-rich plasma (PRP) (C) was injected to the upper site of this right infra-auricular area (A) and face (F). On day 28 after PRP and saline injections, punch biopsy was performed on PRP (C) injected to the upper site of this right infra-auricular area (D) and control (saline injected area) site (E).
Histological evaluation
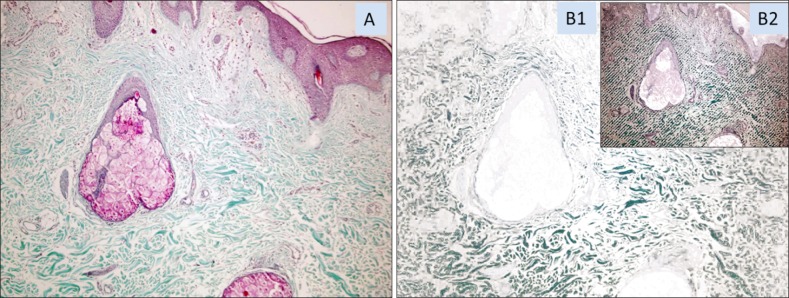
Sections were stained with hematoxylin and eosin and Masson's thichrome stains. Areas in blue color spectrum in Masson's thichrome stained section were accepted as collagen rich (collagenous) areas (Fig. 2A). The proportion of the blue stained area within the skin biopsy was measured by the Samba 4000 image analysis system (Samba Technologies, Meylan, France) for each skin biopsy pieces. In addition, at least 50% of blue stained collagenous areas were quantified in terms of blue staining intensity and these measurements were used to compare the induction effects of testing methods on collagen production. For collagen intensity measurements, target areas were randomly selected with the blue spectrum with an attention to yield minimum 5 mm2 areas per case (Fig. 2B1). This randomly selected blue spectrum stained collagenous areas of each case were submitted to optical density measurements. Color related luminescence for each pixel in selected area, the integrated optical density (IOD) was calculated and divided into a total pixel number to reach the mean optical density (MOD for each skin biopsy by a computerized image analysis system. In the image analysis system, the continuous tone of blue color pixels, which show the presence of collagen, was chosen in the target area. All of the pixels were converted into numeric values from zero to 15 in the blue spectrum (16 sensitivity values). The sum of the values in the target area displayed the IOD, which showed the collagen area (Fig. 2B2). The IOD had been divided into the number of pixels which number used to compare collagen density for each biopsy in order to get the MOD7.
Fig. 2. (A) Photography of papillary and reticular dermis of the face skin, stained by Masson's trichrome, collagen fibers stained blue, ×100. (B1) In the image analysis system, the continuous tone of blue color pixels, which show the presence of collagen, was chosen in the target area. (B2) All of the pixels were converted into numeric values from zero to 15 in the blue spectrum. The sum of the values in the target area represents the integrated optical density, which displayed the collagen area (B1, B2: Masson's trichrome, ×100).
Statistical analysis
The obtained data were evaluated using the PASW Statistics ver. 18.0 for Windows (IBM Co., Armonk, NY, USA). The numerical figures obtained from the measurements were expressed as mean±standard deviation and the data obtained by counting were expressed as frequency (%). The normal distribution of the quantitative data was tested by using the Shapiro-Wilk Test. Levene's test was used to analyze the homogeneous of variance. Paired t-test was used in comparison of each group's pre-treatment and post-treatment. Student's t-test was used in analyzing the significance between the groups. The results were evaluated within a 95% confidence limit, assuming p<0.05 as the significance level.
RESULTS
Twenty women ranging in age from 40 to 49 years (mean age, 43.65±2.43 years) were enrolled into the study. The age distribution of the group was normal (Shapiro-Wilk Test). The age of the group was homogeneous (Levene's test).
Of the 20 patients, 12 (60.0%) were Fitzpatrick skin types II, five (25.0%) were Fitzpatrick skin types III and three (15.0%) were Fitzpatrick skin types I.
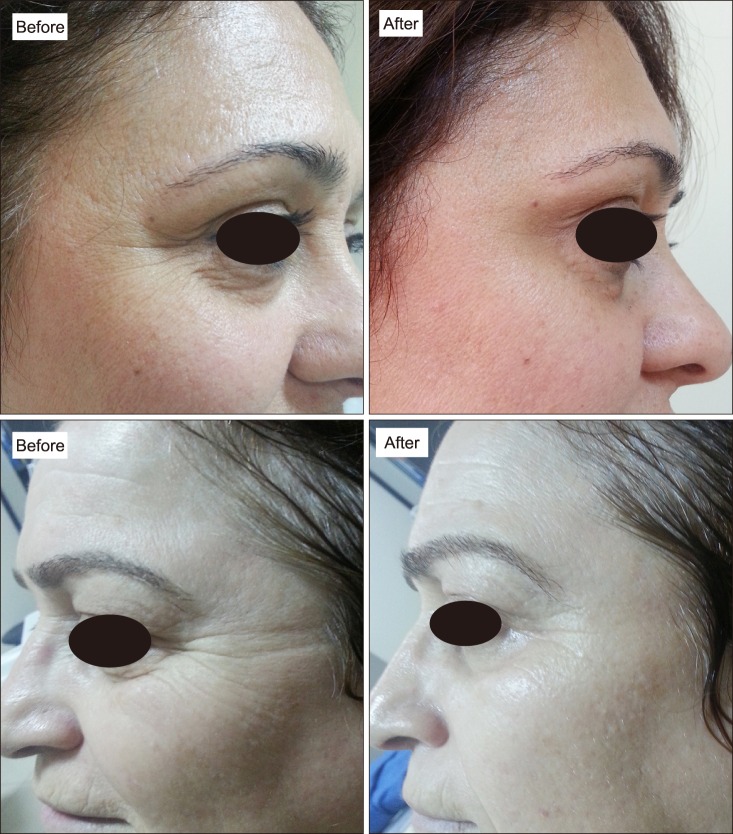
All patients' preoperative and postoperative (at one month) photographs were documented (Fig. 3), but we did not evaluate the efficiency of PRP treatment with these photographs. We think that histophathologic evaluation of collagen is more important, reliable and objective method than photographic evaluation.
Fig. 3. Periorbital wrinkles before platelet-rich plasma (PRP) treatment and clinical improvement of periorbital wrinkles after PRP treatment.
Masson's trichrome stain results of the skin samples (control vs. saline vs. PRP injection site) were represented in Fig. 4. In the saline and PRP sites, the collagen fiber bundles in the dermis were increased than pre-treatment site. There was an increase in the number and thickness of elastic fibers in the PRP and saline sites. We used the MOD in order to measure and show these increases.
Fig. 4. Photography of collagen fibers, stained by Masson's trichrome, collagen fibers stained blue, ×40. (A) Pre-treatment site. (B) Saline injected site. (C) Platelet-rich plasma injected site.
MOD of the pre-treatment side was 539±93.2 (ranging from 406 to 697). In the saline side, the MOD increased 787±134.15 (ranging from 591 to 1,081). On the other side where we used PRP treatment, the MOD increased to 1,019±178 (ranging from 674 to 1,344). The MOD of collagen fibers was clearly greatest on the PRP side (Table 1). Paired t-test was used for comparison of each group's pre-treatment and post-treatment. The increases in the MOD of collagen fibers after saline and PRP injections were compared with baseline. The increase of collagen was statistically significant (p<0.001).
Table 1. The MODs of collagen in the pre-treatment, control, and PRP-treated area are presented.
| Patient No. | Age (yr) | Measured total area (×103 μm2) |
Pre-treatment MOD | After saline MOD | After PRP MOD |
|---|---|---|---|---|---|
| 1 | 43 | 1,086 | 630 | 936 | 1,302 |
| 2 | 45 | 1,245 | 508 | 791 | 848 |
| 3 | 40 | 1,023 | 420 | 683 | 911 |
| 4 | 46 | 986 | 417 | 701 | 1,187 |
| 5 | 49 | 1,124 | 484 | 812 | 978 |
| 6 | 42 | 894 | 671 | 806 | 982 |
| 7 | 46 | 1,256 | 507 | 880 | 962 |
| 8 | 42 | 1,245 | 471 | 655 | 863 |
| 9 | 45 | 1,362 | 406 | 741 | 983 |
| 10 | 42 | 1,187 | 644 | 705 | 1,120 |
| 11 | 41 | 1,002 | 468 | 801 | 982 |
| 12 | 42 | 1,007 | 603 | 805 | 1,121 |
| 13 | 43 | 1,020 | 513 | 591 | 814 |
| 14 | 42 | 1,045 | 501 | 593 | 674 |
| 15 | 43 | 1,035 | 625 | 711 | 825 |
| 16 | 41 | 1,023 | 697 | 884 | 1,078 |
| 17 | 44 | 1,156 | 644 | 990 | 1,120 |
| 18 | 42 | 929 | 538 | 1,081 | 1,344 |
| 19 | 47 | 980 | 609 | 943 | 1,301 |
| 20 | 48 | 1,115 | 427 | 639 | 991 |
| Mean±SD | 43.65±2.43 | 1,086±123 | 539±93.2 | 787±134.15 | 1,019±178 |
MOD: mean optical density, PRP: platelet-rich plasma, SD: standard deviation.
The MOD of collagen fibers after saline treatment was compared with the MOD of collagen fibers after PRP treatment by Student's t-test. The increase in PRP treatment side was found statistically highly significant (p<0.001).
We compared to MOD of the pre-treatment with the MOD of saline treatment. In the saline site, 46.01 percent improvement was found. Then, we compared the pre-treatment MOD with the MOD of the PRP. In the MOD of the PRP, 89.05 percent improvement was seen. Finally, we compared improvement of PRP the PRP-to-saline improvement ratio (89.05% to 46.01%) was 1.93:1.
None of our patients were lost during the follow-up period. PRP application was well tolerated by all patients. No serious side effects due to PRP were detected. Mild and transient side effects were observed. The frequently seen complication during the course of treatment were mild erythema (75%, n=15) and burning sensation (70%, n=14). All these complications disappeared spontaneously in the two days follow up period. Bruising/ecchymosis (15%, n=3) and severe erythema (10%, n=2) were also seen. Bruising/ecchymosis disappeared spontaneously in the seven days follow up period. The pain of mesotherapy technique 'point by point' was tolerated by all patients with anesthetic EMLA cream 5% (~2.5%).
DISCUSSION
The use of PRP has been known in aesthetic medicine, as well, although very few of the studies specifically attest to benefits in the face and neck skin rejuvenation. PRP is an autologous preparation of platelets in concentrated plasma. Optimal PRP platelet concentration is unclear1. PRP contains a mixture of bioactive agents derived from both platelets and plasma6. Various growth factors, including platelet-derived growth factor (PDGF), transforming growth factor (TGF), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF), are secreted from the α-granules of concentrated platelets activated by aggregation inducers4. There are more than 30 bioactive substances in these α-granules8. Fibroblasts express numerous surface receptors and can simultaneously sense multiple molecules that trigger behavioral responses6. Various growth factors and cytokines that facilitate extracellular matrix (ECM) accumulation and improve cell proliferation and differentiation are activated after injection into the target tissue. Tissue regeneration results from cell proliferation, angiogenesis and cell migration. Matrix metalloproteinase proteins (MMP) are involved in the ageing process by degradation of collagen and ECM proteins2.
Kim et al.4 investigated on the remodeling of the ECM, a process that requires activation of dermal fibroblast, which is essential for rejuvenation of aged skin. They found that PRP increased the expression of type I collagen, MMP-1, and mRNA in human dermal fibroblasts. PRP induces the synthesis of new collagen by fibroblasts4. Kakudo et al.3 showed that adding activated platelet-rich or platelet poor plasma significantly promoted the proliferation of human adipose-derived stem cells and human dermal fibroblast in the cell culture. They suggested that PRP can enhance the proliferation of human dermal fibroblast and adipose-derived stem cells. Cho et al.5 evaluated the effect of PRP. They suggest that PRP induces increased expression of type I collagen, MMP-1 and MMP-2 in human skin fibroblasts. In the other study, Kakudo et al.9 investigated a side-by-side (half-side) test between the PRP-treated and control (untreated) side of a split-thickness skin graft donor site, and compared the number of days until epithelialization and pain during gauze change. They revealed that PRP promotes epithelialization and angiogenesis of split thickness skin graft donor sites. These experimental (in vitro) studies suggested that PRP improves wound healing by fibroblast proliferation, collagen synthesis, angiogenesis, and epithelialization. Shin et al.10 assessed combined PRP with fractional laser therapy and compared with the control group. They reported that PRP combined with fractional laser increased subject satisfaction and skin elasticity, the amount of collagen, and the number of fibroblasts. Yuksel et al.11 investigated the effect of PRP on the human facial skin. They applied to PRP with dermaroller and intradermal injections. They did not evaluate the patients histologically. They concluded that PRP was effective and safe. In the present study, we compared the collagen of patients with baseline collagen levels and control side. In the PRP side, great improvement from the baseline data was observed in the week 4. PRP was considered effective, significantly improving collagen. Our results were comparable with the previous studies. These results are important because the study represents the formal controlled clinical (in vivo) study of aging skin. To the best of our knowledge, histological evaluation of the independent effect of the PRP in facial rejuvenation was not asses in the previous study, in vivo.
There is yet no definitive method for clinical use of PRP4. Some authors have done three treatments for the best results10,11,12. In this study, PRP was applied only once. On day 28 after PRP and saline injections, punch biopsy was performed on the PRP-treated and control (saline injected area) side and they were evaluated. The results of this study represent only one application of PRP. It seems that even a single application is effective. Repeated treatment of PRP may result to increase collagen synthesis.
Lu13 suggested that surrounding needling can change the aging state of the skin, possibly by strengthening the activity of fibroblast in the skin and increasing the content of soluble collagen. Skin needling (micro needling) is a technique that involves using a sterile dermaroller that punctures the skin with a series of fine sharp needles. The skin develops multiple micro bruises in the dermis that initiate the complex cascade of wound healing and growth factor release, and finally results in collagen production12. There was another important point of our study. In this study, the levels of collagen in the control side (saline injected side) were compared with the pre-treatment side. The differences were statistically significant between saline injected side and baseline (untreated) collagen levels. The reason of elevated collagen levels of the saline injected side is skin needling effect.
Side-effects such as mild bruising/ecchymosis/hematoma, occasional swelling, mild or prolonged erythema, burning sensation, and rarely infections were reported. No serious or persistent side effects were reported1,2,10,11. Redaelli et al.1 suggested that mild erythema occurred probably due to calcium chloride. Mild and transient side effect such as bruising/ecchymosis, burning sensation, mild erythema, and severe erythema were observed in the present study. We did not observe any serious side effects due to PRP. We considered that PRP is a safe choice as a cosmetic procedure for facial skin rejuvenation.
In conclusion, PRP increases dermal collagen levels not only by growth factors, but also by skin needling (the mesotherapy technique 'point by point'). PRP application could be considered as an effective (even a single application) and safety procedure for facial skin rejuvenation.
References
- 1.Redaelli A, Romano D, Marcianó A. Face and neck revitalization with platelet-rich plasma (PRP): clinical outcome in a series of 23 consecutively treated patients. J Drugs Dermatol. 2010;9:466–472. [PubMed] [Google Scholar]
- 2.Banihashemi M, Nakhaeizadeh S. An introduction to application of platelet rich plasma (PRP) in skin rejuvenation. Rev Clin Med. 2014;1:38–43. [Google Scholar]
- 3.Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122:1352–1360. doi: 10.1097/PRS.0b013e3181882046. [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Je YJ, Kim CD, Lee YH, Seo YJ, Lee JH, et al. Can platelet-rich plasma be used for skin rejuvenation? evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol. 2011;23:424–431. doi: 10.5021/ad.2011.23.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho JW, Kim SA, Lee KS. Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int J Mol Med. 2012;29:32–36. doi: 10.3892/ijmm.2011.803. [DOI] [PubMed] [Google Scholar]
- 6.Anitua E, Sánchez M, Zalduendo MM, de la Fuente M, Prado R, Orive G, et al. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009;42:162–170. doi: 10.1111/j.1365-2184.2009.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karabudak O, Dogan B, Baloglu H. Histologic evidence of new collagen formation using a Q-switched Nd:YAG laser in periorbital rhytids. J Dermatolog Treat. 2008;19:300–304. doi: 10.1080/09546630801961075. [DOI] [PubMed] [Google Scholar]
- 8.Cayirli M, Calışkan E, Açıkgöz G, Erbil AH, Ertürk G. Regression of melasma with platelet-rich plasma treatment. Ann Dermatol. 2014;26:401–402. doi: 10.5021/ad.2014.26.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakudo N, Kushida S, Minakata T, Suzuki K, Kusumoto K. Platelet-rich plasma promotes epithelialization and angiogenesis in a splitthickness skin graft donor site. Med Mol Morphol. 2011;44:233–236. doi: 10.1007/s00795-010-0532-1. [DOI] [PubMed] [Google Scholar]
- 10.Shin MK, Lee JH, Lee SJ, Kim NI. Platelet-rich plasma combined with fractional laser therapy for skin rejuvenation. Dermatol Surg. 2012;38:623–630. doi: 10.1111/j.1524-4725.2011.02280.x. [DOI] [PubMed] [Google Scholar]
- 11.Yuksel EP, Sahin G, Aydin F, Senturk N, Turanli AY. Evaluation of effects of platelet-rich plasma on human facial skin. J Cosmet Laser Ther. 2014;16:206–208. doi: 10.3109/14764172.2014.949274. [DOI] [PubMed] [Google Scholar]
- 12.Nofal E, Helmy A, Nofal A, Alakad R, Nasr M. Platelet-rich plasma versus CROSS technique with 100% trichloroacetic acid versus combined skin needling and platelet rich plasma in the treatment of atrophic acne scars: a comparative study. Dermatol Surg. 2014;40:864–873. doi: 10.1111/dsu.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y. Effects of "surrounding needling" on hydroxyproline content and ultrastructures in the dermis of aged rats. Zhongguo Zhen Jiu. 2008;28:61–64. [PubMed] [Google Scholar]