Abstract
Background
Femoral nerve blockade (FNB) provides effective postoperative analgesia in children undergoing arthroscopic knee surgery as evidenced by their opioid-sparing effects and decreased postoperative pain scores. Increasing the local anesthetic concentration in peripheral nerve blockade for adults undergoing orthopedic surgery has been shown to be beneficial, increasing block success rate, and providing a longer duration of analgesia. The effect of increasing the concentration of local anesthetic in extremity blocks in children remains largely unexplored.
Methods
We retrospectively evaluated the effectiveness of FNB using three concentrations of local anesthetic (ropivacaine 0.2%, bupivacaine 0.25%, and ropivacaine 0.5%) in children and adolescents undergoing arthroscopic knee surgery. The primary outcome evaluated was postoperative opioid consumption before discharge. Secondary outcomes included post-anesthesia care unit (PACU) and hospital discharge times, first pain score in PACU, and the incidence of adverse events.
Results
Two hundred and sixty-nine children and adolescents who received a FNB for arthroscopic knee surgery from January 2010 to December 2013 were included for analysis. Local anesthetic used in FNB was ropivacaine 0.2% in 116 (43%) cases, ropivacaine 0.5% in 75 (28%) cases, and bupivacaine 0.25% in 78 (29%) cases. Median postoperative opioid consumption (mg/kg intravenous morphine equivalents) in the ropivacaine 0.5% group was 0 mg/kg (interquartile ranges [IQR]: 0 mg, 0.03 mg/kg) compared to 0.02 mg/kg (IQR: 0, 0.08 mg/kg) in the ropivacaine 0.2% group and 0.01 mg/kg (IQR: 0, 0.08 mg/kg) in the bupivacaine 0.25% group (p=0.009). Median PACU time was shortest in the ropivacaine 0.5% group (47 min; IQR: 36, 68 min) compared to the ropivacaine 0.2% (58 min; IQR: 41, 77) and bupivacaine 0.25% (54 min; IQR: 35, 75 min) groups (p=0.040). Among groups, there were no significant differences in first postoperative pain scores or incidence of nausea and vomiting. No patient in any group experienced a serious adverse event.
Conclusion
The results suggest that ropivacaine 0.5% for FNB offers superior postoperative analgesia in the form of decreased postoperative opioid consumption and earlier PACU/hospital discharge, when compared to ropivacaine 0.2% and bupivacaine 0.25% in the pediatric population.
Level of evidence
III, Retrospective Comparative Study.
Keywords: anesthesia, regional, nerve block, pain, postoperative, local anesthetic, child, adolescent
Introduction
Assessment and management of postoperative pain in the pediatric population can be challenging, and as many studies indicate, remains suboptimal and poorly controlled.1–2 Prior to availability of ultrasound imaging for regional anesthesia, many providers were reluctant to perform peripheral nerve blockade (PNB) in the pediatric population. Regional techniques utilizing landmarks or nerve-stimulation methods were typically avoided in infants and children due to the difficulty in targeting neural structures that are often in close proximity to critical structures; the need for sedation or general anesthesia, which can potentially mask complications; and the need to use small volumes of local anesthetic to avoid toxicity, resulting in inadequate block coverage.3
Following the introduction of nerve localization under ultrasound guidance, regional anesthetic techniques have gained popularity as a method to improve pain management among children undergoing orthopedic procedures. The use of femoral nerve blockade (FNB) for orthopedic surgery in adults and children has been documented to reduce the use of intravenous opioids.4–9
Several studies have examined the effects of varying local anesthetic concentrations in PNB for adults.10–13 Yao et al examined the effect of different concentrations of ropivacaine on femoral nerve block for postoperative analgesia. They found a significant increase in pain scores and analgesic requirements with concentrations of ropivacaine at or below 0.16%, compared to ropivacaine 0.5%.10 In a separate study, PNB with a higher concentration of local anesthetic was associated with increased success rate, decreased postoperative pain scores, and prolonged duration of analgesia, compared to lower anesthetic concentration of the same volume.11 In addition, PNB with lower volume and higher concentration of local anesthetic was more efficacious than higher volumes and lower concentrations of local anesthetic of equivalent dose.12,13
In contrast to adults, the concentration of local anesthetic in PNB providing optimal postoperative analgesia has been rarely explored in children and adolescents. The relevant studies have primarily examined local anesthetic concentrations in truncal nerve blocks.14–16 In this study, we investigated the effects of three concentrations of local anesthetics (ropivacaine 0.2%, ropivacaine 0.5%, and bupivacaine 0.25%) on the postoperative course of pediatric patients undergoing FNB for knee arthroscopy. With a change of practice for FNB in our pediatric patients, we transitioned to an increased concentration of local anesthetic between 2010 and 2013: specifically, from ropivacaine 0.2% and bupivacaine 0.25% to ropivacaine 0.5%. Having performed this transition, we retrospectively evaluated postoperative block effectiveness via postoperative opioid consumption, with secondary analyses examining pain, safety, and length of stay outcomes.
Materials and methods
After obtaining approval from the IRB of Nationwide Children’s Hospital, the institutional electronic medical database at our tertiary pediatric hospital was retrospectively reviewed for all patients receiving FNB for arthroscopic knee surgery between January 1, 2010 and December 31, 2013. Patient consent was not required by the IRB given all data was collected retrospectively and was reported without patient identifiers. Patients having undergone knee arthroscopy were identified by querying the database for the associated common procedural terminology (CPT) codes used by the surgeons at our institution. Included procedures (CPT codes) were meniscus repair (29882 and 29883), meniscectomy (29880 and 29881), lateral release (29873), loose body removal (29874), drilling for osteochondritis dissecans (29885), drilling for intact osteochondritis dissecans (29886 and 29887), chondroplasty (29877), synovectomy (29875 and 29876), abrasion arthroplasty (29879), and diagnostic (29870). Cases were excluded if they involved anterior cruciate ligament (ACL) repair or reconstruction because of associated pain outside the femoral nerve distribution. Cases involving ACL repair or reconstruction were identified by the associated CPT code (29888). Patients receiving bupivacaine 0.5% were excluded due to the small size of this group. Finally, patients aged >18 years were excluded from the analysis. All cases meeting inclusion criteria were analyzed, and no power analysis was performed. Data for each case included patient demographics, intraoperative and postoperative opioid consumption, intraoperative and postoperative acetaminophen or ketorolac use, pain score at post-anesthesia care unit (PACU) arrival, postoperative nausea or vomiting, and PACU and hospital discharge times. Pain was assessed using the face, legs, activity, cry, consolability scale, or visual analog scale, as appropriate for patient age.
Three groups were defined by the local anesthetic agent used for FNB: ropivacaine 0.2%, ropivacaine 0.5%, and bupivacaine 0.25%. These groups were compared to examine the influence of local anesthetic agent and concentration on study outcomes. The primary outcome was total postoperative opioid consumption, converted to intravenous morphine equivalents. Secondary outcomes included pain score on PACU arrival, time to PACU discharge, time to hospital discharge, and incidence of postoperative nausea and vomiting. Data were presented as medians with interquartile ranges (IQR) for continuous variables and counts with percentages for categorical variables. Group characteristics were compared using chi-square tests for categorical variables (or Fisher’s exact tests where cell counts were <5) and Kruskal–Wallis tests for continuous variables. Multivariable regression analysis was performed to examine the influence of local anesthetic agent and concentration on opioid consumption, adjusting for patient gender, age, weight, and whether ketorolac or acetaminophen were administered intraoperatively. Cases with complete covariate data were included in multivariable models. PACU and hospital discharge were analyzed using Kaplan–Meier curves and multivariable Cox proportional hazards models, adjusting for covariates described above. The proportional hazards assumption of Cox regression was evaluated using the Grambsch–Therneau global test. Nausea and vomiting incidence were analyzed using univariate logistic regression. p<0.05 was considered statistically significant for all analyses. Data were analyzed using Stata/IC 13.1 (StataCorp LP, College Station, TX, USA).
Results
There were 167 males and 102 females of ages 4–18 years meeting inclusion criteria (median age =14 years; IQR: 12 and 16 years). One patient was excluded from analysis of PACU discharge due to missing PACU arrival time, and 21 patients were excluded from analyses of pain scores at PACU arrival due to missing pain score data. In the study cohort, 20 patients (7%) were admitted for overnight stay, with no statistically significant difference among ropivacaine 0.2% (10/116, 9%), ropivacaine 0.5% (5/75, 6%), or bupivacaine 0.25% (5/78, 6%) by chi-square test (p=0.810). Patients in each group did not differ in demographic characteristics (Table 1). Local anesthetic used in FNB was ropivacaine 0.2% in 116 (43%) cases, ropivacaine 0.5% in 75 (28%) cases, and bupivacaine 0.25% in 78 (29%) cases, with increasing use of ropivacaine 0.5% over the study period (Figure 1; Cochran–Armitage test for trend in proportion p<0.001). The weight-based volume of local anesthetic used for FNB did not significantly change over the study period. There were no significant differences across local anesthetic groups in the volume of local anesthetic solution on a per-weight basis. The median volume of ropivacaine 0.2% was 0.27 mL/kg (IQR 0.22, 0.35 mL/kg), bupivacaine 0.25% was 0.26 mL/kg (IQR 0.19, 0.32 mL/kg), and ropivacaine 0.5% was 0.29 mL/kg (IQR 0.23, 0.35 mL/kg).
Table 1.
Characteristics of patients undergoing knee arthroscopy without ACL repair, by local anesthetic used in femoral nerve block
| Variable | Valid, Na | Ropivacaine 0.2% (n=116) |
Ropivacaine 0.5% (n=75) |
Bupivacaine 0.25% (n=78) |
p-Valueb |
|---|---|---|---|---|---|
| Gender, male (n; %) | 269 | 69; 59% | 52; 69% | 46; 59% | 0.312 |
| Age, years (median; IQR) | 269 | 14; 12, 16 | 15; 13, 16 | 14; 13, 17 | 0.279 |
| Weight, kg (median; IQR) | 269 | 66; 52, 81 | 67; 52, 85 | 65; 57, 86 | 0.640 |
| Local anesthetic volume, mL/kg (median; IQR) | 246 | 0.27; 0.22, 0.35 | 0.29; 0.23, 0.35 | 0.26; 0.19, 0.32 | 0.082 |
| Local anesthetic dose in mg/kg (median; IQR) | 246 | 0.54; 0.43, 0.70 | 1.46; 1.13, 1.77 | 0.64; 0.46, 0.80 | <0.001 |
| Morphine equivalent mg/kg in PACU (median; IQR) | 269 | 0.02; 0, 0.08 | 0; 0, 0.03 | 0.01; 0, 0.08 | 0.009 |
| Total morphine equivalent, mg/kg (median; IQR) | 269 | 0.12; 0.08, 0.19 | 0.16; 0.09, 0.21 | 0.13; 0.08, 0.18 | 0.208 |
| Fentanyl, µg/kg (median; IQR) | 268 | 0.5; 0, 1.1 | 1.0; 0, 1.4 | 0.7; 0, 1.3 | 0.004 |
| Ketorolac/acetaminophen used in OR (n; %) | 269 | 30; 26% | 31; 41% | 20; 26% | 0.045 |
| Any ketorolac used (n; %) | 269 | 25; 22% | 24; 32% | 19; 24% | 0.262 |
| Any acetaminophen used (n; %) | 269 | 5; 4% | 13 (17%) | 1 (1%) | <0.001 |
| Ketorolac/acetaminophen used in PACU (n; %) | 269 | 9; 8% | 6 (8%) | 5 (6%) | 0.918 |
| Any ketorolac used (n; %) | 269 | 7; 6% | 5 (7%) | 5 (6%) | 0.984 |
| Any acetaminophen used (n; %) | 269 | 2; 2% | 1 (1%) | 0 | 0.626 |
| PACU time in minutes (median; IQR) | 268 | 58; 41, 77 | 47; 36, 68 | 54; 35, 75 | 0.028 |
| Time until discharge in minutes (median; IQR) | 269 | 152; 125, 204 | 130; 103, 173 | 142;118, 203 | 0.011 |
| Pain score at PACU arrival | |||||
| FLACC (median; IQR) | 61 | 0; 0, 2 | 0; 0, 0 | 0; 0, 0 | 0.136 |
| VAS (median; IQR) | 187 | 1; 0, 5 | 0; 0, 3 | 0; 0, 6 | 0.224 |
| Side effects reported (n; %) | 269 | 4; 3% | 3 (4%) | 5 (6%) | 0.655 |
Notes:
Number of cases with complete data on each variable.
p-Value of chi-square test for categorical variables, Fisher’s exact test for categorical variables with one or more cell counts <5, or Kruskal–Wallis test for continuous variables, as applicable.
Abbreviations: ACL, anterior cruciate ligament; FLACC, face, legs, activity, cry, consolability; IQR, interquartile ranges; OR, odds ratio; PACU, post-anesthesia care unit; VAS, visual analog scale.
Figure 1.
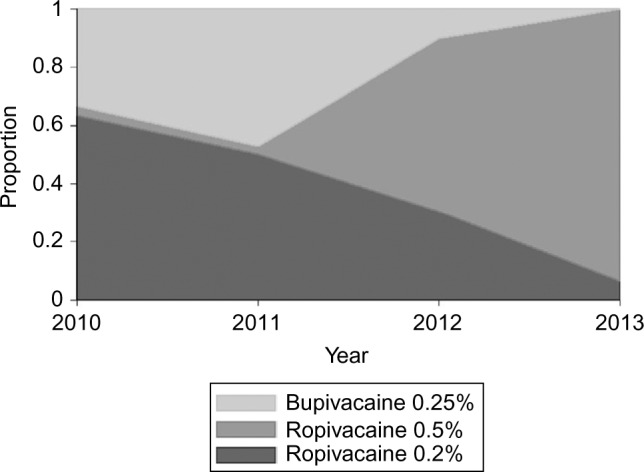
Annual proportions of femoral nerve blocks involving each of three local anesthetic agents and concentrations, 2010–2013 (N=269).
Postoperative opioid analgesia was not required in 51/116 (44%) cases in the ropivacaine 0.2% group, 52/75 cases (69%) in the ropivacaine 0.5% group, and 39/78 (50%) cases in the bupivacaine 0.25% group (chi-square test p=0.002). Consistent with this pattern, median postoperative opioid consumption in the PACU was lowest in the ropivacaine 0.5% group (0 morphine IV mg/kg; IQR: 0, 0.03 mg/kg), versus 0.02 mg/kg (IQR: 0, 0.08 mg/kg) morphine IV in the ropivacaine 0.2% group and 0.01 mg/kg (IQR: 0, 0.08 mg/kg) morphine IV in the bupivacaine 0.25% group, respectively (p=0.009; Figure 2). Intraoperative acetaminophen was used more commonly in the ropivacaine 0.5% group (17%) relative to the other groups (4% and 1% for ropivacaine 0.2% and bupivacaine 0.25%, respectively; p<0.001), related to the introduction of the intravenous preparation over the study period. Multivariable least-squares regression confirmed that opioid consumption in the PACU was 0.03 morphine IV mg/kg (95% confidence interval [CI]: 0.01, 0.05 mg/kg; p=0.003) lower in the ropivacaine 0.5% group compared to the ropivacaine 0.2% group, controlling for patient gender, age, weight, and intraoperative use of ketorolac or acetaminophen (Table 2). Based on the multivariable model, opioid consumption in the ropivacaine 0.5% group was also 0.03 mg/kg (95% CI: 0.004, 0.05 mg/kg; p=0.021) lower than in the bupivacaine 0.25% group.
Figure 2.
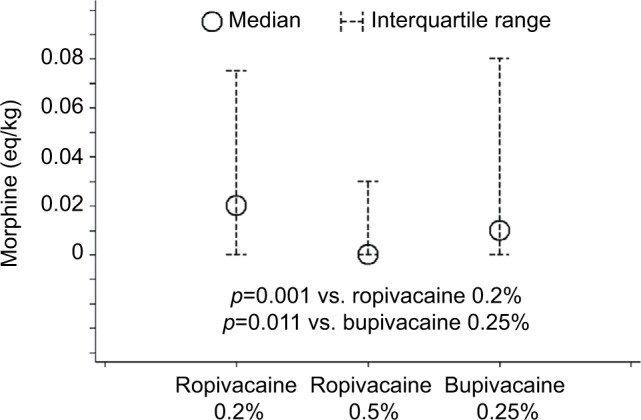
Medians and interquartile ranges of opioid consumption in the PACU, by local anesthetic agent and concentration (N=269).
Abbreviation: PACU, post-anesthesia care unit.
Table 2.
Multivariable ordinary least-squares regression of opioid consumption among patients undergoing knee arthroscopy without ACL repair, by local anesthetic used in femoral nerve block
| Variable | Model 1: total morphine equivalent mg/kg (N=269)
|
Model 2: morphine equivalent in PACU mg/kg (N=269)
|
||||
|---|---|---|---|---|---|---|
| Unstandardized coefficient | 95% CI | p-Value | Unstandardized coefficient | 95% CI | p-Value | |
| Local anesthetic | ||||||
| Ropivacaine 0.2% | Reference | Reference | ||||
| Ropivacaine 0.5% | 0.01 | (−0.03, 0.04) | 0.715 | −0.03 | (−0.05, −0.01) | 0.003 |
| Bupivacaine 0.25% | 0.02 | (−0.01, 0.05) | 0.280 | 0.00 | (−0.02, 0.01) | 0.611 |
| Gender | ||||||
| Female | Reference | Reference | ||||
| Male | 0.02 | (−0.01, 0.04) | 0.272 | 0.01 | (−0.01, 0.02) | 0.539 |
| Age (years/10) | −0.01 | (−0.07, 0.05) | 0.740 | −0.01 | (−0.04, 0.03) | 0.784 |
| Weight (kg/10) | −0.01 | (−0.01, 0.00) | 0.126 | 0.00 | (−0.01, 0.00) | 0.484 |
| Any ketorolac or acetaminophen in room | 0.03 | (0.01, 0.06) | 0.022 | 0.01 | (−0.01, 0.02) | 0.400 |
| Constant | 0.17 | (0.11, 0.24) | <0.001 | 0.06 | (0.02, 0.11) | 0.004 |
| R2 | 0.04 | 0.04 | ||||
Abbreviations: ACL, anterior cruciate ligament; CI, confidence interval; PACU, post-anesthesia care unit.
Analyses of secondary outcomes demonstrated no statistically significant differences in pain scores across local anesthetic groups (Table 1). Relative to the ropivacaine 0.2% group, the odds of nausea or vomiting were not significantly different in the ropivacaine 0.5% (odds ratio [OR]=1.17; 95% CI: 0.25, 5.37; p=0.843) or bupivacaine 0.25% (OR=1.91; 95% CI: 0.50, 7.38; p=0.344) groups in univariate logistic regression. Comparisons of length of stay in Table 1 revealed that PACU time was shortest in the ropivacaine 0.5% group, at a median of 47 min (IQR: 36, 68 min) versus the ropivacaine 0.2% (58 min; IQR: 41, 77 min) and bupivacaine 0.25% (54 min; IQR: 35, 75 min) groups (p=0.040). Kaplan–Meier curves demonstrated earlier discharge from the PACU (Figure 3) and from the hospital (Figure 4) among patients receiving ropivacaine 0.5%. Multivariable Cox proportional hazards regression, adjusted for gender, age, weight, and intraoperative use of ketorolac or acetaminophen, confirmed that the hazard of discharge from the PACU was lower (implying longer length of stay) for patients receiving ropivacaine 0.2% (hazard ratio [HR]=0.71; 95% CI: 0.52, 0.96; p=0.026). The lower hazard of PACU discharge in the bupivacaine 0.25% group, compared to the ropivacaine 0.5% group, was of similar magnitude but did not reach statistical significance (HR=0.76; 95% CI: 0.55, 1.05; p=0.097). A separate multivariable Cox model demonstrated that the hazard of discharge from the hospital was lower in the ropivacaine 0.2% group (HR=0.67; 95% CI: 0.49, 0.90; p=0.008), compared to the ropivacaine 0.5% group. The hazard of hospital discharge was statistically indistinguishable between the bupivacaine 0.25% and ropivacaine 0.5% groups (HR=0.77; 95% CI: 0.55, 1.06; p=0.106). The proportional hazards assumption of the Cox models was supported by statistically nonsignificant Grambsch–Therneau global tests (p=0.147 for PACU discharge and p=0.356 for hospital discharge).
Figure 3.
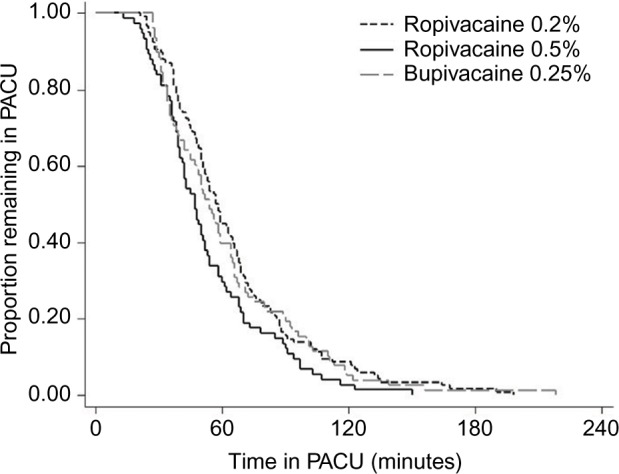
Kaplan–Meier survival curves of time until PACU discharge, by local anesthetic agent and concentration (N=268).
Abbreviation: PACU, post-anesthesia care unit.
Figure 4.
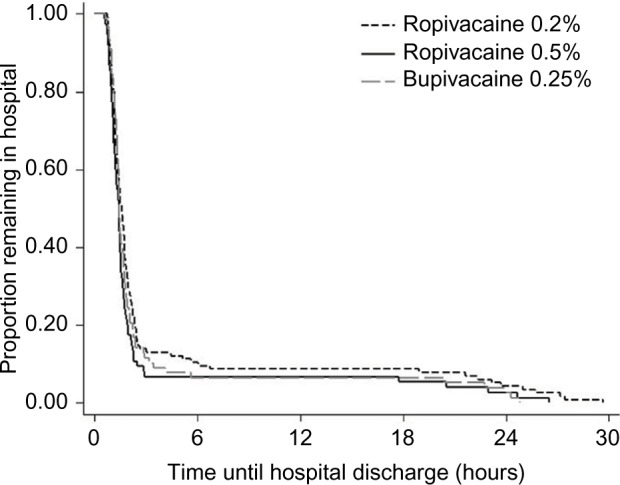
Kaplan–Meier survival curves of time from PACU discharge until hospital discharge, by local anesthetic agent and concentration (N=269).
Abbreviation: PACU, post-anesthesia care unit.
Discussion
FNB is an effective and safe method of providing postoperative analgesia to children and adolescents undergoing orthopedic knee surgery.9 In many pediatric tertiary care hospitals, FNB is now routinely used for arthroscopic knee surgery. Despite this, questions remain regarding the optimal local anesthetic solution for FNB, particularly in children where local anesthetic toxicity remains a heightened concern.
In children, increasing local anesthetic concentration had several benefits on the effects of PNB of the trunk. Lower pain scores and longer time to first rescue analgesia were observed in children receiving 0.25% versus 0.125% levobupivacaine for ilioinguinal/iliohypogastric (IL/IH) blocks for inguinal herniorrhaphy.14 Suresh et al reported bupivacaine 0.25% improved duration of analgesia compared to bupivacaine 0.125% in transverse abdominal plane blocks of the same volume.15 Additionally, lower volume/higher concentration local anesthetic IL/IH blocks improved postoperative analgesia compared to higher volume/lower concentration IL/IH block of equivalent dose in children undergoing outpatient surgery.16 No serious adverse events were reported at any local anesthetic concentration in these studies. Notwithstanding these findings, the pediatric literature lacks data on effects of increased local anesthetic concentration in PNB for the limbs.
In this retrospective analysis, we evaluated the effects of increasing local anesthetic concentration in FNBs for arthroscopic knee surgery in children and adolescents. The study period, spanning January 1, 2010 through December 31, 2013, corresponds to a transition in our department’s practice from bupivacaine 0.25% and ropivacaine 0.2% to nearly exclusive use of ropivacaine 0.5% for FNB, providing a setting for evaluating the efficacy of these local anesthetic solutions. The change was largely precipitated by the arrival of staff who had clinical experience with the use of ropivacaine 0.5% in pediatric regional anesthesia, documented safe use of ropivacaine 0.5% in the pediatric literature, and discontent with the intensity and duration of anesthesia achieved with the lower concentrations of local anesthetics.17 All FNBs were performed or directly supervised by a member of the regional pain service with extensive experience in PNB techniques. Although there was occasional use of bupivacaine 0.5% for FNB over the 4-year period, ropivacaine 0.5% was generally adopted because of its perceived greater safety over bupivacaine.18
Our results indicate superior analgesia with ropivacaine 0.5% compared to lower concentration of local anesthetics, ropivacaine 0.2% and bupivacaine 0.25%, based on lower postoperative opioid consumption. Furthermore, the percentage of patients not requiring any opioid rescue was significantly higher in the ropivacaine 0.5% group compared to the others, indicating highly effective regional blockade in the ropivacaine 0.5% group. Although postoperative opioid consumption was lowest in the ropivacaine 0.5% group, total (intraoperative + postoperative) opioid consumption in morphine equivalents was similar among the 3 groups. Differences in total opioid consumption among these groups may have been obscured by the greater use of intravenous fentanyl in the ropivacaine 0.5% group. Fentanyl, with its high potency and short context-sensitive half-life, may overestimate opioid equivalents when compared to morphine and hydromorphone, which were used more often intraoperatively in the ropivacaine 0.2% and bupivacaine 0.25% local anesthetic groups. Intravenous acetaminophen was administered intraoperatively to the ropivacaine 0.5% group more frequently than to patients in the other groups, as this medication was added to our hospital formulary while the use of ropivacaine 0.5% FNB was increasing. Controlling for intraoperative acetaminophen use, multivariable analysis confirmed that opioid consumption in the PACU was lowest in the ropivacaine 0.5% group. Postoperative use of acetaminophen and intraoperative and postoperative use of ketorolac were similar among the 3 groups, and these analgesics were not considered to confound our findings.
PACU and hospital discharge times are important metrics in monitoring hospital efficiency and costs.19,20 In our study, FNB with ropivacaine 0.5% had both shorter PACU and post-PACU stay, compared to lower concentration local anesthetics. This finding suggests the potential for cost savings with the use of higher-concentration local anesthetic in this patient population.
Special concern exists for local anesthetic toxicity in children undergoing regional anesthesia, given their reduced body mass and consequently reduced maximum allowed doses of local anesthetic. Yet, no serious adverse events were detected in the study cohort, including evidence of local anesthetic toxicity (seizure, arrhythmia, cardiac arrest), or nerve injury. While this cohort was too small to define any increase in the risk of serious adverse events attributable to the increased concentration of local anesthetic, our findings suggest that ropivacaine 0.5% can be used safely in children for FNB.
Several study limitations precluded a more comprehensive evaluation of the effects of increased local anesthetic concentration for pediatric FNB in arthroscopic surgery. As most patients were discharged on the day of surgery, there was no record of the duration of analgesia or motor block. Pain assessments were infrequent, and the only consistent pain evaluation was upon PACU arrival. Additionally, no postoperative records were made of the degree of the patient’s motor block. As the average duration of these blocks in adults is typically <12 hours, and physical rehabilitation does not usually begin until postoperative day 1, the significance of the degree of motor block is unclear.21 However, persistent weakness in knee extension and flexion after FNB for ACL reconstruction have been recently reported.22 Any contribution of the density of motor block and local anesthetic concentration to this issue is unclear. No follow-up component was included in this study to determine differences in long-term recovery, including functional criteria. The volumes of local anesthetic used for FNBs did not significantly change as a result of increasing concentration over the 4-year study period. As a result, total local anesthetic doses increased over this time period and the effects of total local anesthetic dosage cannot be separated from effects of concentration. The sample size of this study is too small to determine the incidence of rare adverse effects, such as local anesthetic toxicity, in any of the local anesthetic groups. Large prospective studies are required to evaluate the overall safety of various concentrations of perineural ropivacaine in children.
Our retrospective analysis demonstrates the increased effectiveness of higher concentration local anesthetic in FNB compared to lower concentration local anesthetic in the pediatric population undergoing arthroscopic knee surgery. Specifically, ropivacaine 0.5% decreased postoperative opioid consumption compared to ropivacaine 0.2% and bupivacaine 0.25% after knee arthroscopy. In addition, earlier PACU and hospital discharges were seen in the ropivacaine 0.5% group versus the other groups. There were no differences in first postoperative pain scores or incidence of nausea and vomiting, and no major adverse events were observed.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Groenewald CB, Rabbitts JA, Schroeder DR, Harrison TE. Prevalence of moderate-severe pain in hospitalized children. Paediatr Anaesth. 2012;22(7):661–668. doi: 10.1111/j.1460-9592.2012.03807.x. [DOI] [PubMed] [Google Scholar]
- 2.Rony RYZ, Fortier MA, MacLaren J, Chorney JM, Perret D, Kain ZN. Parental postoperative pain management: attitudes, assessment, and management. Pediatrics. 2010;125(6):e1373–e1378. doi: 10.1542/peds.2009-2632. [DOI] [PubMed] [Google Scholar]
- 3.Tsui N, Suresh S. Ultrasound imaging for regional anesthesia in infants, children, and adolescents. Anesthesiology. 2010;112:473–492. doi: 10.1097/ALN.0b013e3181c5dfd7. [DOI] [PubMed] [Google Scholar]
- 4.Simion C, Suresh S. Lower extremity peripheral nerve blocks in children. Tech Reg Anesth Pain Manag. 2007;11:222–228. [Google Scholar]
- 5.Stewart B, Smith CT, Teebay L, Cunliffe M, Low B. Emergency department use of a continuous femoral nerve block for pain relief for fractured femur in children. Emerg Med J. 2007;24:113–114. doi: 10.1136/emj.2006.041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu R, Browne G, Cheng N, Lam LT. Femoral nerve block for femoral shaft fractures in a paediatric emergency department: can it be done better? Eur J Emerg Med. 2003;10(4):258–263. doi: 10.1097/00063110-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Mutty CE, Jensen EJ, Manka MA, Anders MJ, Bone LB. Femoral nerve block for diaphyseal and distal femoral fractures in the emergency department. J Bone Joint Surg Am. 2007;89:2599–2603. doi: 10.2106/JBJS.G.00413. [DOI] [PubMed] [Google Scholar]
- 8.Tobias JD. Continuous femoral nerve block to provide analgesia following femur fracture in a paediatric ICU population. Anaesth Intens Care. 1994;22(5):616–618. doi: 10.1177/0310057X9402200524. [DOI] [PubMed] [Google Scholar]
- 9.Schloss B, Bhalla T, Klingele K, Phillips D, Prestwich B, Tobias JD. A retrospective review of femoral nerve block for postoperative analgesia after knee surgery in the pediatric population. J Pediatr Ortho. 2014;34(4):459–461. doi: 10.1097/BPO.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 10.Yao J, Zeng Z, Jiao ZH, Wang AZ, Wang J, Yu A. Optimal effective concentration of ropivacaine for postoperative analgesia by single-shot femoral-sciatic nerve block in outpatient knee arthroscopy. J Int Med Res. 2013;41(2):395–403. doi: 10.1177/0300060513476427. [DOI] [PubMed] [Google Scholar]
- 11.El-Sharrawy E, Yagiela JA. Anesthetic efficacy of different ropivacaine concentrations for inferior alveolar nerve block. Anesth Prog. 2006;53(1):3–7. doi: 10.2344/0003-3006(2006)53[3:AEODRC]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taboada MM, Rodriguez J, Bermudez M, et al. Low volume and high concentration of local anesthetics is more efficacious than high volume and low concentration in Labat’s sciatic nerve block: a prospective, randomized comparison. Anesth Analg. 2008;107(6):2085–2088. doi: 10.1213/ane.0b013e318186641d. [DOI] [PubMed] [Google Scholar]
- 13.Fredrickson MJ, Abeysekera A, White R. Randomized study of the effect of local anesthetic volume and concentration on the duration of peripheral nerve blockade. Reg Anesth Pain Med. 2012;37(5):495–501. doi: 10.1097/AAP.0b013e3182580fd0. [DOI] [PubMed] [Google Scholar]
- 14.Disma N, Tuo P, Pellegrino S, Astuto M. Three concentrations of levobupivacaine for ilioinguinal/iliohypogastric nerve block in ambulatory pediatric surgery. J Clin Anesth. 2009;21(6):389–393. doi: 10.1016/j.jclinane.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Suresh S, Taylor LJ, De Oliveira GS., Jr Dose effect of local anesthetics on analgesic outcomes for the transversus abdominis plane (TAP) block in children: a randomized, double-blind, clinical trial. Paediatr Anaesth. 2015;25(5):506–510. doi: 10.1111/pan.12550. [DOI] [PubMed] [Google Scholar]
- 16.Trifa M, Chaabane Z, Dridi S, Sebai B, Missaoui A, Fekih Hassen A, Ben Khalifa S. The analgesic effects of ropivacaine in ilioinguinal-iliohypogastric nerve block in children – concentration or volume? Middle East J Anaesthesiol. 2009;20:83–87. [PubMed] [Google Scholar]
- 17.Felfernig M, Marhofer P, Weintraud M, Huber G, Duma A, Nosa A, Kapral S. Use of ropivacaine and lidocaine for axillary plexus blockade. Afr J Paediatr Surg. 2010;7(2):101–104. doi: 10.4103/0189-6725.62860. [DOI] [PubMed] [Google Scholar]
- 18.Mazoit JX, Dalens BJ. Ropivacaine in infants and children. Curr Opin Anaesthesiol. 2003;16(3):305–307. doi: 10.1097/00001503-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8(3):257–263. doi: 10.1007/s11832-014-0587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan CM, Moeschler SM, Horlocker TT, Hanssen AD, Hebl JR. A self-paired comparison of perioperative outcomes before and after implementation of a clinical pathway in patients undergoing total knee arthroplasty. Reg Anesth Pain Med. 2013;38:533–538. doi: 10.1097/AAP.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 21.Weber A, Fournier R, Riand N, Gamulin Z. Duration of analgesia is similar when 15, 20, 25 and 30 ml of ropivacaine 0.5% are administered via a femoral catheter. Can J Anaesth. 2005;52(4):390–396. doi: 10.1007/BF03016282. [DOI] [PubMed] [Google Scholar]
- 22.Luo TD, Ashraf A, Dahm DL, Stuart MJ, McIntosh AL. Femoral nerve block is associated with persistent strength deficits at 6 months after anterior cruciate ligament reconstruction in pediatric and adolescent patients. Am J Sports Med. 2015;43(2):331–336. doi: 10.1177/0363546514559823. [DOI] [PubMed] [Google Scholar]


