Abstract
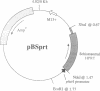
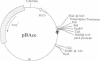
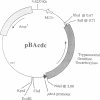
The bacterial alkaline phosphatase (phoA) promoter and signal peptide have been used previously to control recombinant expression and secretion of eukaryotic proteins in Escherichia coli. Other reports have shown that this expression system can generate relatively modest levels of active hypoxanthine/guanine phosphoribosyltransferase (HPRT; hypoxanthine phosphoribosyltransferase; IMP:pyrophosphate phosphoribosyltransferase, EC 2.4.2.8), which carries part of the signal peptide but remains in the cytosol of the bacteria. Herein, the phoA promoter without its associated signal peptide is used to regulate expression of the HPRT of Schistosoma mansoni and the ornithine decarboxylase (ODC; L-ornithine carboxy-lyase, EC 4.1.1.17) of Trypanosoma brucei, two enzymes that have been identified as potential targets for antiparasitic chemotherapy. The levels of recombinant expression range from 20% to 60% of the total bacterial protein, and the majority of both recombinant enzymes was soluble. The specific activity for the recombinant trypanosomal ODC was one-third to two-thirds that of the authentic native enzyme and yields were predicted to be 15-30 mg of active enzyme per liter of bacterial culture. The specific activity for the recombinant schistosomal HPRT was equivalent to that for the native enzyme purified from schistosomes and up to 10 mg of enzymatically active HPRT has been purified from a 0.5-liter culture of treated bacteria. These results represent a break-through in recombinant expression of HPRT and ODC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Bacchi C. J., Garofalo J., Mockenhaupt D., McCann P. P., Diekema K. A., Pegg A. E., Nathan H. C., Mullaney E. A., Chunosoff L., Sjoerdsma A. In vivo effects of alpha-DL-difluoromethylornithine on the metabolism and morphology of Trypanosoma brucei brucei. Mol Biochem Parasitol. 1983 Mar;7(3):209–225. doi: 10.1016/0166-6851(83)90022-1. [DOI] [PubMed] [Google Scholar]
- Bacchi C. J., Nathan H. C., Hutner S. H., McCann P. P., Sjoerdsma A. Polyamine metabolism: a potential therapeutic target in trypanosomes. Science. 1980 Oct 17;210(4467):332–334. doi: 10.1126/science.6775372. [DOI] [PubMed] [Google Scholar]
- Balbas P., Bolivar F. Design and construction of expression plasmid vectors in Escherichia coli. Methods Enzymol. 1990;185:14–37. doi: 10.1016/0076-6879(90)85005-9. [DOI] [PubMed] [Google Scholar]
- Bradshaw R. A., Cancedda F., Ericsson L. H., Neumann P. A., Piccoli S. P., Schlesinger M. J., Shriefer K., Walsh K. A. Amino acid sequence of Escherichia coli alkaline phosphatase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3473–3477. doi: 10.1073/pnas.78.6.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie G. E., Farnham P. J., Platt T. Synthetic sites for transcription termination and a functional comparison with tryptophan operon termination sites in vitro. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4180–4184. doi: 10.1073/pnas.78.7.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. P., 3rd, McKerrow J. H., Newport G. R., Wang C. C. Analysis of cDNA encoding the hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) of Schistosoma mansoni; a putative target for chemotherapy. Nucleic Acids Res. 1988 Jul 25;16(14B):7087–7101. doi: 10.1093/nar/16.14.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovey H. F., McKerrow J. H., Aldritt S. M., Wang C. C. Purification and characterization of hypoxanthine-guanine phosphoribosyltransferase from Schistosoma mansoni. A potential target for chemotherapy. J Biol Chem. 1986 Jan 15;261(2):944–948. [PubMed] [Google Scholar]
- Free M. L., Gordon R. B., Keough D. T., Beacham I. R., Emmerson B. T., de Jersey J. Expression of active human hypoxanthine-guanine phosphoribosyltransferase in Escherichia coli and characterisation of the recombinant enzyme. Biochim Biophys Acta. 1990 Oct 23;1087(2):205–211. doi: 10.1016/0167-4781(90)90206-h. [DOI] [PubMed] [Google Scholar]
- Gold L. Expression of heterologous proteins in Escherichia coli. Methods Enzymol. 1990;185:11–14. doi: 10.1016/0076-6879(90)85004-8. [DOI] [PubMed] [Google Scholar]
- Graf L., Craik C. S., Patthy A., Roczniak S., Fletterick R. J., Rutter W. J. Selective alteration of substrate specificity by replacement of aspartic acid-189 with lysine in the binding pocket of trypsin. Biochemistry. 1987 May 5;26(9):2616–2623. doi: 10.1021/bi00383a031. [DOI] [PubMed] [Google Scholar]
- Grumont R., Sirawaraporn W., Santi D. V. Heterologous expression of the bifunctional thymidylate synthase-dihydrofolate reductase from Leishmania major. Biochemistry. 1988 May 17;27(10):3776–3784. doi: 10.1021/bi00410a039. [DOI] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S., Loeb L. A. Structure-function relationships in Escherichia coli promoter DNA. Prog Nucleic Acid Res Mol Biol. 1990;38:137–164. doi: 10.1016/s0079-6603(08)60710-2. [DOI] [PubMed] [Google Scholar]
- Jochimsen B., Nygaard P., Vestergaard T. Location on the chromosome of Escherichia coli of genes governing purine metabolism. Adenosine deaminase (add), guanosine kinase (gsk) and hypoxanthine phosphoribosyltransferase (hpt). Mol Gen Genet. 1975 Dec 30;143(1):85–91. doi: 10.1007/BF00269424. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Greene M. L., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency in gout. Ann Intern Med. 1969 Jan;70(1):155–206. doi: 10.7326/0003-4819-70-1-155. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Garboczi D. N., Thomas P. J., Pedersen P. L. Mitochondrial ATP synthase. cDNA cloning, amino acid sequence, overexpression, and properties of the rat liver alpha subunit. J Biol Chem. 1990 Mar 15;265(8):4664–4669. [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Sakamoto S., Miyoshi K., Fuwa T., Yoda K., Yamasaki M., Tamura G., Miyake T. Synthesis and secretion of human epidermal growth factor by Escherichia coli. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7212–7216. doi: 10.1073/pnas.82.21.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. A., Coffino P., Wang C. C. Trypanosoma brucei ornithine decarboxylase: enzyme purification, characterization, and expression in Escherichia coli. J Biol Chem. 1988 Dec 5;263(34):17933–17941. [PubMed] [Google Scholar]
- Queen S. A., Vander Jagt D. L., Reyes P. Characterization of adenine phosphoribosyltransferase from the human malaria parasite, Plasmodium falciparum. Biochim Biophys Acta. 1989 Jul 6;996(3):160–165. doi: 10.1016/0167-4838(89)90242-2. [DOI] [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Labeling and quantitation of ornithine decarboxylase protein by reaction with alpha-[5-14C]difluoromethylornithine. Methods Enzymol. 1983;94:206–209. doi: 10.1016/s0076-6879(83)94034-x. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Measurement of the number of ornithine decarboxylase molecules in rat and mouse tissues under various physiological conditions by binding of radiolabelled alpha-difluoromethylornithine. Biochem J. 1982 Aug 15;206(2):311–318. doi: 10.1042/bj2060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft A. W., Miech R. P., Brown P. R., Senft D. G. Purine metabolism in Schistosoma mansoni. Int J Parasitol. 1972 Jun;2(2):249–260. doi: 10.1016/0020-7519(72)90013-6. [DOI] [PubMed] [Google Scholar]
- Shahabuddin M., Scaife J. The gene for hypoxanthine phosphoribosyl transferase of Plasmodium falciparum complements a bacterial HPT mutation. Mol Biochem Parasitol. 1990 Jun;41(2):281–288. doi: 10.1016/0166-6851(90)90191-n. [DOI] [PubMed] [Google Scholar]
- Shuttleworth H., Taylor J., Minton N. Sequence of the gene for alkaline phosphatase from Escherichia coli JM83. Nucleic Acids Res. 1986 Nov 11;14(21):8689–8689. doi: 10.1093/nar/14.21.8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerdsma A., Schechter P. J. Chemotherapeutic implications of polyamine biosynthesis inhibition. Clin Pharmacol Ther. 1984 Mar;35(3):287–300. doi: 10.1038/clpt.1984.33. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Craig S. P., McKerrow J. H., Wang C. C. The hypoxanthine-guanine phosphoribosyltransferase of Schistosoma mansoni. Further characterization and gene expression in Escherichia coli. J Biol Chem. 1990 Aug 15;265(23):13528–13532. [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]