Abstract
Doppler ultrasound has been extensively used in detecting reno-vascular diseases, showing to be a non-invasive, safe, low cost and repeatable tool. The Renal Resistive Index (RRI) [(peak systolic velocity − end diastolic velocity)/peak systolic velocity] is a semi-quantitative index derived by Doppler evaluation of renal vascular bed. Normally RRI is in the range of 0.47–0.70, it increases with aging and, usually, it shows a difference between the two kidneys less than 5–8 %. RRI is an important prognostic marker in chronic kidney diseases (CKD), both in diabetic and non-diabetic kidney diseases, because, in longitudinal prospective studies, it significantly correlated with hemodynamic (ABPM, SBP, DBP, pulse pressure) and histopathological parameters (glomerular sclerosis, arteriolosclerosis, interstitial fibrosis/tubular atrophy, interstitial infiltration). In acute kidney injury (AKI) RI is a valid tool in differentiating between pre-renal and renal failure and in predicting renal response to vaso-active agents. In addition a RRI >0.74 can predict the onset of AKI in septic patients. Renal Resistive Index is a useful marker in allograft diseases because it has been widely showed a correlation with histological lesions during worsening of renal function, both in acute rejection and in chronic allograft nephropathy. Recent studies suggest its role in the risk of new onset diabetes after transplantation and it could be one of the parameters to evaluate to shift or withdrawal immunological and/or hypertensive therapy.
Keywords: Renal resistive index, Diabetic nephropathy, Acute kidney injury, Renal transplantation, Chronic kidney disease
Riassunto
La Metodica Color Doppler è stata diffusamente utilizzata per evidenziare patologie nefrovascolari in relazione alle caratteristiche di non invasività, sicurezza, basso costo e ripetibilità della stessa. L'indice di resistenza renale (RRI) [(Picco di velocità sistolica - Velocità telediastolica)/ Picco di velocità sistolica] è un indice semiquantitativo derivato dalla valutazione Doppler del letto vascolare renale.
Normalmente il valore del RRI è nel range di 0,47- 0,70, aumentando con l'età, e usualmente, è presente una differenza tra i due reni inferiore al 5-8%. RRI è un importante marker prognostico nelle patologie renali croniche (CKD), sia diabetiche che non-diabetiche, poichè, in studi prospettici longitudinali, esso correla significativamente con parametri emodinamici (ABPM, Pressione Sistolica, pressione Diastolica, Pressione di Polso) e istopatologici (glomerulosclerosi, arteriolosclerosi, fibrosi interstiziale/atrofia tubulare e infiltrato interstiziale).
Nel Danno Renale Acuto (AKI) RI è un valido strumento nel distinguere l'insufficienza acuta pre-renale da quella renale e nel predire la risposta renale agli agenti vasoattivi. Inoltre un valore di RRI >0,74 può predire la comparsa di AKI nei pazienti affetti da sepsi.
RRI è un marker utile nelle patologie del rene trapiantato, perchè è stata ampiamente dimostrata una correlazione con lesioni istologiche nel contesto di un peggioramento della funzione renale, sia nel rigetto acuto che nella nefropatia cronica del trapianto. Studi recenti suggeriscono un suo ruolo nel predire la comparsa del diabete di nuova insorgenza dopo il trapianto (NODAT) e potrebbe essere uno dei parametri da valutare nella sostituzione o nella sospensione della terapia immunologica e/o ipertensiva.
Introduction
Doppler ultrasound has been extensively used in renal diseases both in diagnostic, prognostic and therapeutic assessments due to the non-invasive, safe and low cost method for the evaluation of the renal blood flow quantitative and semi-quantitative parameters. Renal Resistive Index (RRI) [(peak systolic velocity − end diastolic velocity)/peak systolic velocity] is a semi-quantitative index derived by Doppler evaluation of renal vascular bed. RRI value is in the range of 0.47–0.70, and, usually, it shows a difference between the two kidneys less than 5–8 %. Its role has been widely investigated among different kidney diseases as an early marker of endothelial dysfunction and arterial stiffness that may lead to severe end-organ dysfunction, due to its already known correlations with hystological parameters, like glomerulosclerosis and tubulointerstitial lesions. RRI ≥0.7 both in diabetic and in non-diabetic kidney diseases, predicts CKD progression to ESRD, even in the mild to moderate dysfunction. RRI could have a predictive role in future cardiovascular events in high-risk patients, such as in patients with chronic allograft function (CAN) and in diabetics. The aim of this review is to evaluate the already stabilized and the further potential applications of this haemodynamic derived parameter among different kidney diseases. In the last years a growing evidence of its prognostic role has been highlighted, especially in acute kidney injury and in renal allograft, as a reliable marker not only of sub-clinical organ damage, but also of patient survival.
Renal Resistive Index and diabetic nephropathy
Diabetes is a worldwide leading cause of kidney disease and both clinical and instrumental follow-up allow identifying micro- and macro-vascular complications. In the United States, microalbuminuria is found in 43 % and macroalbuminuria in 8 % of patients with a history of diabetes [1]. Moreover, diabetes accounts now for 50 % of prevalent kidney failure, starting from 18 % in 1980 [2].
The subclinical diagnosis of diabetic nephropathy, before the onset of micro-albuminuria, using renal ultrasound imaging and doppler technique could ameliorate its own management.
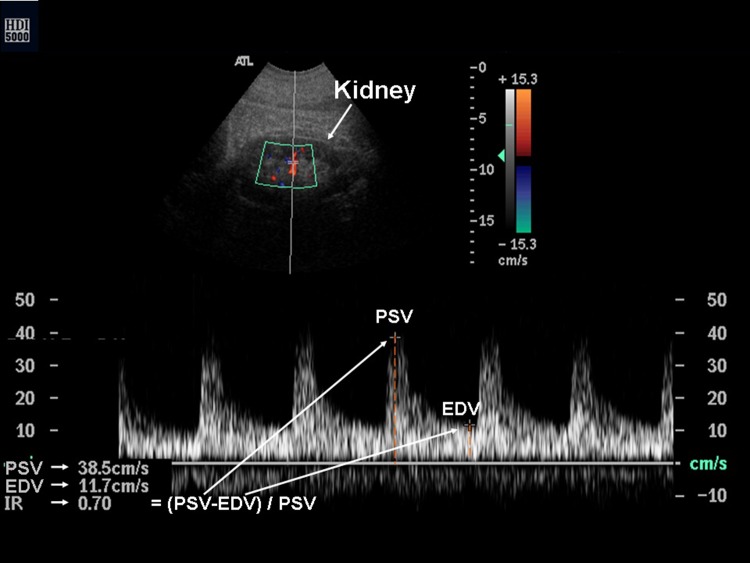
The doppler Renal Resistive Index (RRI) is a semi-quantitative index, derived by the ratio of the difference between peak systolic velocity (PSV) and enddiastolic velocity (EDV) divided by PSV, obtained from the Doppler spectrum of intrarenal segmental and interlobar arteries (Fig. 1). Normal RRI values in adults are in the range of 0.47–0.70 with a difference between two kidneys less than 5–8 % [3].
Fig. 1.
The Doppler Renal Resistive Index, labeled as IR in the figure, is equal to the ratio of the difference between peak systolic velocity (PSV) and enddiastolic velocity (EDV) divided by PSV, obtained from the doppler spectrum of intrarenal segmental and interlobar arteries
Mancini et al. [4] reported that diabetic patients with normal renal function and normo-albuminuria showed both a renal volume and RRI values significantly higher compared to non-diabetic controls without kidney disease (mean volume ± DS of 197.3 ± 47.6 mL in diabetic group vs 162.5 ± 35.2 mL in controls, P < 0.001; RRI of 0.70 ± 0.05 in diabetic group vs 0.59 ± 0.06 in controls, P < 0.001, respectively). This result suggests a potential role of Doppler ultrasound in the identification of morphologic and hemodynamic changes in the earlier stages of diabetic nephropathy [4]. It could reflect a reduction of the intra renal vessel surface due to the scarring process with an increased vascular resistance [5].
Bruno et al. [6] evaluated RRI among 32 type 2 diabetic patients without micro-albuminuria, hypertension, glucose-lowering medications and reduced renal function (GFR <60 mL/min 1.73 m2) and compared results with hypertensive and controls patients. RRI measurements were obtained at baseline and 5 min after pharmacological sublingual administration of 25 µg of glyceryltrinitrate (Dynamic Resistive Index, DRI). RRI at baseline in diabetic patients was significantly higher compared with hypertensive and control patients (0.65 ± 0.06; 0.59 ± 0.05 and 0.58 ± 0.05 respectively) and DRI was significantly reduced in diabetic patients (7.1 ± 6.1 % vs 9.0 ± 5.2 % and 11.1 ± 6.9 %). In multivariate analysis both RRI and DRI were independently related to fasting glucose, and this result highlights the close relation between subclinical alterations in intra-renal vasculature and metabolic control, even in the pre-micro-albuminuric phases. No correlation has been found between RI and DRI, suggesting that a high baseline RI could not reflect necessary a reduced renal vasodilatory response, while DRI measurement underline an impairment in renal microvasculature in type 2 diabetes.
The correlation between RRI and progression of diabetic nephropathies has been widely evaluated and RI showed to be significantly related to pulse wave velocity (PWV), an already known marker of atherosclerosis and vascular stiffness [7] and with ABPM, SBP, DBP and pulse pressure [1, 5, 7].
RI significantly correlates with the degree of proteinuria resulting higher in patients with macroalbuminuria (>300 µg/mg creatinine) than in patients with normoalbuminuria and microalbuminuria, respectively 0.73 ± 0.04 and 0.69 ± 0.04, 0.69 ± 0.05 [1, 8].
RRI is higher in diabetic patients with one to three stages of CKD (mild to moderate) both in renal and interlobar vessels, in comparison with those affected by others kidney diseases, with an equivalent GFR [9, 10]. Patients with advanced stages of CKD (GFR <30 mL/min/1.73 m2) showed no differences in RRI values, underlying that the influence of advanced local alterations (vascular and interstitial) on RRI exceeds systemic factors like PWV and Pulse Pressure [9, 10] with a decreasing of GFR <30 mL/min/1.73 m2.
Sugiura et al. [11] proved that a IR >0.7 is an independent predictor of the risk of worsening renal function (defined as decrease in GFR of at least 20 mL/min/1.73 m2 or need for long-term dialysis therapy until 2 years), in patients with mild to moderate diabetic nephropathy both <60 and >60 years old and even if on RAS inhibitors therapy or not.
RRI is known to decrease with use of RAS inhibitors, due to hemodynamic changes related to these antihypertensive agents [12], even if it can be increased by others factors such as arrhythmia (bradycardia) and breath holding during Valsalva maneuver [13].
In conclusion renal imaging and RRI in diabetic nephropathy could be an useful tool for early diagnosis and long-term follow-up, as reported in Table 1.
Table 1.
RRI and diabetic nephropathy
| Renal imaging and RRI values: |
| Identifications of early morphologic and hemodynamic change in diabetic nephropathy |
| Correlation with ABPM, SBP, DBP, PP and proteinuria levels in mild to moderate CKD |
| Long-term follow-up of diabetic nephropathy and risk of ESRD |
RRI and chronic kidney disease (CKD)
Chronic kidney disease is universally considered a worldwide public health problem affecting more than 50 million people with a prevalence of 12 % in the USA [14] and Europe [15]. KDOQI criteria consider chronic kidney disease the kidney damage present for more than 3 months with or without a decrease in glomerular filtration rate (GFR), or a reduction in GFR for more than 3 months with or without kidney damage. The kidney damage is defined by either structural or functional abnormalities of the kidney manifest by markers of kidney damage, e.g. creatinine clearance and proteinuria.
Renal ultrasonography is usually the first-step among renal imaging technique due to wide availability, safety and low cost and this exam can easily identify a chronic kidney disease by measuring longitudinal length of the kidneys and evaluating cortical brightness.
Normal kidney length is about 11–12 cm (the left kidney being about 3 mm longer than the right kidney) in younger adults and a progressive atrophy with ageing (Table 2).
Table 2.
Normal renal values
| Normal renal values: |
| Renal length: 10–12 cm |
| Renal width: 4–6 cm |
| Respiratory mobility: 3–7 cm |
| Parenchymal width: 1.3–2.5 cm |
Normal cortical kidney is usually as bright as normal liver or spleen tissue, even if it could be physiologically brighter than normal liver or spleen.
Chronic kidney disease is often associated with increased brightness since fibrous tissue (e.g. interstitial fibrosis and glomerulosclerosis) increases echogenicity, even if this feature does not allow to differentiate AKI and CKD, considering that inflammatory states such glomerulonephritis, ATN, Acute Interstitial Nephritis) are associated with hyperechogenic aspect of the renal parenchyma. Small and echogenic kidneys suggest CKD instead of AKI.
Doppler Ultrasound could play an important role in defining CKD and its progression to ESRD.
RRI has shown to be related with glomerulosclerosis, arteriolosclerosis and tubulointerstitial lesions more than others morphologic parameters like renal length and cortex area [16]. In fact Patients with higher RRI (>0.7) generally show more severe arteriolosclerosis than others with normal (<0.65) or high normal RRI (0.65 ≤ RI < 0.7) [17]. Patients with High-Normal RRI showed good response to steroid therapy versus those with a RRI >0.7 [16], so it could be an useful tool to predict response to steroid therapy.
In mild to moderate renal dysfunction, RRI predicts CKD progression and poor outcome especially when RRI ≥0.7, independently from others measures like eGFR, maybe due to advanced glomerulosclerosis [16–18].
RRI examinations don’t recognize among different renal medical pathologies, so that RI cannot be used as a diagnostic marker [19]. Ultrasound may be a useful tool to identify the degree of disease progression and secondary response to immunological and antihypertensive therapy, even if further studies are needed to have clear recommendations of follow-up.
In haemolytic-uremic syndrome RRI usually exceed 0.7 and renal cortex appears hyperechotic with a marked cortical-medullary differentiation as a result of platelet aggregates and fibrin thrombi in the lumen of glomerular capillaries [20]. In vasculitides such LES, Wegener Granulomatosis and PAN, RRI shows significant correlation with creatinine level and presence of interstitial disease and normal RRI value is considered a good prognostic factor [19].
In conclusion RRI has different applications in CKD, as summarized in Table 3.
Table 3.
RRI and CKD
| RRI values in CKD: |
| Predict response to immunological and hypertensive therapy |
| Estimate renal lesions (vascular, glomerular and tubulo-interstitial) |
| Predict CKD outcome in mild to moderate renal dysfunction |
RRI and AKI
The incidence of acute kidney injury in in-hospital individuals varies according to the used definition, the type of hospital and the active specialities. The most cited definitions include RIFLE, AKIN and KDIGO classifications. The different classifications use different criteria. For example AKIN (acute kidney injury network) [21] classification consider AKI stage 1 an increase of more than or equal to 0.3 mg/dL (26.5 mmol/L) or increase from baseline to more than or equal to 1.5- to 2-fold within a 48 h period. Instead this result could be not considered as AKI in the RIFLE classification, in which the first class considers only serum creatinine increase of 0.5 mg/dL [22] within a period of 1–7 days and sustained (for more than 24 h) (Table 4).
Table 4.
AKIN (acute kidney injury network) classification consider AKI stage 1 an increase of more than or equal to 0.3 mg/dL (26.5 mmol/L) or increase from baseline to more than or equal to 1.5- to 2-fold within a 48 h period
| Rifle classification | Urinary output | AKIN classification |
|---|---|---|
| Risk increase in serum creatinine × 1.5 or GFR decreased >25 % | <0.5 mL/kg/h for more than 6 h | Stage 1 increase ≥0.3 mg/dL or ≥1.5 from baseline |
| Injury Increase in serum creatinine × 2 or GFR decreased >50 % | <0.5 mL/kg/h for more than 12 h | Stage 2 Increase >2- to 3-fold from baseline |
| Failure Increase in serum creatinine x 3 or GFR decreased >75 % | <0.3 mL/kg/h for 24 h or anuria for 12 h | Stage 3 increase >3-fold from baseline or on RRT |
| Loss persistent acute renal failure >4 weeks | ||
| End-stage kidney disease ESRD >3 months |
The RIFLE classification, in which the first class considers only serum creatinine increase of 0.5 mg/dL within a period of 1–7 days
In a recent review, Zeng et al. [23] showed a different incidence of AKI among hospitalized patients and it was highest according to KDIGO classification (18.3 %), followed by AKIN (16.6 %) and RIFLE (16.1 %) and CK (7.0 %).
The creatinine kinetics (CK)—based definition of AKI include absolute increase in sCr ≥0.3 mg/dL within 24 h or ≥0.5 mg/dL within 48 h [23]. The in-hospital mortality related to AKI varies from 20 to 50 % according to the stage or class of AKI [24, 25].
Several biomarkers have been investigated to predict the onset and the prognosis of AKI like NGAL, KIM-1, Cystatin C, IL-18 but all of them are not reliable and precise enough.
Renal ultrasound imaging and Doppler technique could offer the possibility to fill the gap at least in part. B-mode allows to point out variations in kidney parenchyma brightness and in longitudinal length that are easy reproducible, rapid and non-invasive that may identify renal preclinical dysfunction or vascular damages, to prevent the onset of clinical renal dysfunction.
Increased renal parenchymal echogenicity could be due to inflammatory states (acute glomerulonephritis, acute tubular necrosis, acute interstitial nephritis, HIV nephropathy) or infiltrative diseases (lymphoma, myeloma and monoclonal gammopathies) [26], with thickness of renal parenchyma (including the cortex and the medulla which is about 1.5 cm thick), while decreased echogenicity is due to renal edema. Color Doppler identifies vessels localization and allows to calculate RRI to monitor renal perfusion in critically ill patients and vasoactive agents’ impact on renal circulation, like low-dose dopamine agent or renal arterial response to norepinephrine infusion [27, 28].
In acute renal injury AKIN stage 3 or RIFLE class F, RRI usually exceed 0.7, and a threshold of 0.75 is reported as optimal in recognizing between renal and prerenal disease. In fact in prerenal ARF, RRI values lower than 0.7 are related to a good recovery after fluid rehydration, while RRI >0.7 suggest a developing ischemic ATN and worse prognosis [20].
RRI values obtained at the admission in ICU in septic shock patients was an useful tool to predict renal dysfunction 5 days later. Lerolle et al. [29] showed that patients who developed renal dysfunction exhibited on the admission higher RRI values than those who did not develop it (0.77 vs 0.68, respectively, P < 0.001). A RRI >0.74 at inclusion had a sensitivity of 78 % (95 % CI 52–94 %), a specificity of 77 % (95 % CI 50–93 %) for severe renal dysfunction (Class I or F according to RIFLE classification).
Granata et al. [30] reported that RRI and especially ΔRI (the difference between left and right kidney mean RI) measurements can predict renal obstruction in patients with unilateral renal colic with a sensitivity of 94 %, a specificity of 95 % and an accuracy of 94 %. In this study functional exclusion of the kidney after intravenous pyelography and delayed excretion of the contrast medium were significantly related to a higher RRI from the side of renal colic in respect to the other side.
In conclusion RRI could play an important role in different aspects of AKI as summarized in Table 5.
Table 5.
RRI in AKI
| RRI and AKI: |
| Evaluating renal perfusion |
| Predicting renal response to vasoactive agents |
| Recognize between pre-renal and renal acute disease |
| Predicting AKI onset and recovery in septic shock patients |
| Predicting renal obstruction |
RRI and renal allograft survival
Kidney transplantation is the gold standard therapy for ESRD and a careful follow-up of the graft is essential for graft and patient survival.
Chronic allograft nephropathy (CAN) remains the leading cause of poor graft outcome, even though improving graft allocation strategies with the expanded criteria donor (ECD) and the optimization of immunosuppressive protocol therapies. The prevalence of CAN is as high as 60–70 % on protocol biopsies after the first year [31–33]. However, CAN—defined by interstitial fibrosis and tubular atrophy—is probably the result of several different immunologic and non-immunologic processes.
The main clinical aspects of CAN include slow but variable loss of function, often in combination with proteinuria and hypertension. The histopathological changes in renal biopsies are not specific and involve vascular, glomerular and tubulo-interstitial compartments of the kidney [34]. Histologic findings show tubular atrophy, interstitial fibrosis, fibrous intimal thickening in the arteries and a variety of glomerular lesions.
Protocol renal biopsies represent invasive procedures that allow to find sub-clinical chronic rejection, with the aim of treating it and obtaining the best management of immunosuppressive therapy. However these procedures are not performed in any Kidney Transplant Unit and the biopsy timing schedule often differs between Transplant Units according to the protocol used.
Color Doppler ultrasonography and RRI are valid tools in evaluating vascular and not vascular graft compartments and its evolution in the follow-up.
Radermacher et al. [35], measuring RRI in 601 patients at least three months after transplantation, showed that a RRI higher than 0.8 was associated with a 9.1-fold increase in the risk of graft loss compared with patients with a lower RRI value. The combined end point, defined by a decrease of 50 % or more in the creatinine clearance rate, allograft failure indicated by the need for dialysis or death, was reached by 88 % of patients with a RRI value higher then 0.8 and by only 17 % of patients with a lower RRI value (P < 0.001).
In contrast in early protocol renal allograft biopsies between the third and the sixth month in patients with stable renal function (serum creatinine <300 µmol/L), RRI failed to show a role in predicting graft function deterioration [36]. Therefore the authors of this Catalan monocentric study did not consider RRI a parameter able to predict chronic histological damage and thus graft function deterioration, at least in patients with stable renal function. The discrepancy of these two studies can be due to a different study design and sample size: Radermarcher study [35] is performed on a large prevalent sample: the time since transplantation, in which the RRI evaluation was performed, was 6.6 ± 5.5 in patient group with a RRI ≥0.8 and 4.6 ± 4.6 years in the patient group with a RRI <0.8. Note that the wide standard deviation indicated that the enrolled patients could be transplanted from few months to many years. Thus the study sample is large but very heterogeneous. Vallejos study [36] is performed on an incident cohort of patients, given that all enrolled patients were evaluated in a narrow period of time between 3 and 6 months after transplantation. This good aspect was counterbalanced by a low sample size (87 patients) and by a stable renal function in the 2 week period around the protocol biopsy. All these aspects can explain why this study concluded with the lack of a role of RRI given that its evaluation was made early after transplantation. The Nankivell study [33] suggest also that two distinctive phases of injury were evident as chronic allograft nephropathy evolved. In the early 1 year phase after transplantation the renal damage is mainly tubulointerstitial and derived from ischemic injury and/or prior severe or subclinical rejection. Beyond 1 year after transplantation, a later phase of chronic allograft nephropathy was characterized by a predominant microvascular injury. For this reason, the measure of RRI in the late period of post-transplant follow-up, as it is the case in many patients of Radermarcher study, can give a major prognostic role. This point was stressed by the study of Kramann et al. [37]: this author clearly showed that measure of RRI within 6 month after transplantation fail to predict renal allograft failure or death in patient with stable renal function (serum creatinine 1.7 ± 0.9 mg/dL) while measuring RRI later, 12–18 months after transplantation, is useful as a prognostic marker of long-term clinical outcomes (renal allograft failure or death), mainly when RRI >0.75.
Naesens et al. [38] measured RRI at 0–3–6–12 and 24 months after renal transplantation in 321 patients prospectively followed for at least 4.5 years. Protocol renal biopsies were also performed at 3–12 and 24 month follow-up. No correlations were observed between histological parameters at protocol biopsy and doppler ultrasound parameters. RRI values at 3 months after transplantation were related only to the recipient age and to recipient death, without any predictive power on graft survival or on the need of dialysis. The authors discovered a direct correlation between acute tubular necrosis and IRR, when biopsy was performed because of graft dysfunction (P = 0.008). The Naesens study suggest the catastrophic consequence of choosing a bad and heterogeneous composite end-point (renal function deterioration, need for dialysis or death) and an arbitrary cut off value of RRI (equal to 0.8) when the predictor is intrinsically continuous. Note that patients with a RRI value higher then 0.8 were likely oldest and thus, obviously prone to death.
All the previous studies point out that RRI cannot predict graft function in protocol biopsies, while it could be useful in case of graft dysfunction where it correlates with graft diseases (vascular and tubule-interstitial lesions). In addition RRI at the 3 month by the time of transplantation has been associated with increased risk of new onset diabetes after transplantation (NODAT), especially when RRI >0.75 (HR for >0.75 vs ≤0.75: 3.29 95 % IC 1.91–5.67 P < 0.0001), even after multiple adjustments [for age, BMI, glucose, initial nephropathy and pulse pressure: HR 3.29 (1.50–7.54) P = 0.0030] [39]. However, this study has several limitations. Although it is based on a relevant sample size (656 recipient kidney transplant patients), this study is retrospective, monocentric, spanned on a long calendar period (since 1985 to 2009), in which many treatment protocols were subsequently administrated. Thus, a calendar period effect, that was not investigated, cannot be excluded. Moreover, the tested models were applied in an arbitrary sequence without a systematic strategy. The predictor role of RRI should have been tested additionally after the inclusion of all other effects. The authors suggest that the relationship between RRI and NODAT could be due to micro vascular damage of pancreas related to aortic stiffness associated with increased central pressure.
In conclusion, RI could be useful in kidney transplanted patients in different aspects as summarized in Table 6.
Table 6.
RRI and graft
| RRI and graft: |
| Correlation with the age of patients |
| Correlation with histopathological lesions only in patients with worsening of renal function |
| Predicting risk of new onset diabetes after transplantation (NODAT) when IR >0.75 |
Conclusion
Ultrasound imaging and Color Doppler permit to define morphological and functional parameters to reach a better prognostic evaluation among kidney diseases. Considering that different nephropathies could show the same US appearance, it is still difficult to define the role of ultrasound in the diagnosis of kidney diseases. Moreover, the usefulness of RRI as a prognostic marker has been clearly and successful highlighted in the follow-up of diabetic and non-diabetic nephropathies, acute kidney injury and kidney transplantation.
It already well known that RI is related to disease markers in patients with cardiovascular disease (IMT, PP, PWV, albuminuria) or CKD (GFR).
This Doppler index requires skilled physicians and high-quality instrumentations to obtain plausible and repeatable evaluations. The correct identification and monitoring RRI can help to determine long-term prognosis and guide therapy in patients with medical renal diseases.
Compliance with ethical standards
Conflict of interests
The authors declare that they have no competing or conflicting interests.
Informed consent
For this type of study formal consent is not required.
Human and animal studies
The study described in this article does not contain studies with animal and human subjects performed by any of the authors.
References
- 1.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl 1):266. [PubMed] [Google Scholar]
- 2.Tuttle KR, Bakris GL, Bilous RW. Diabetic kidney disease: a report from an ADA consensus conference. Am J Kidney Dis. 2014;64(4):510–533. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Darmon M, Schnell D, Zeni F. Doppler-based renal resistive index: a comprehensive review. In: Vincent JL, editor. Yearbook of intensive care and emergency medicine. Heidelberg: Springer; 2010. pp. 331–338. [Google Scholar]
- 4.Mancini M, Masulli M, Liuzzi R, et al. Renal duplex sonographic evaluation of type 2 diabetic patients. J Ultrasound Med. 2013;32(6):1033–1040. doi: 10.7863/ultra.32.6.1033. [DOI] [PubMed] [Google Scholar]
- 5.Pape L, Offner G, Ehrich JH. Renal arterial resistance index. N Engl J Med. 2003;349(16):1573–1574. doi: 10.1056/NEJM200310163491616. [DOI] [PubMed] [Google Scholar]
- 6.Bruno RM, Daghini E, Landini L. Dynamic evaluation of renal resistive index in normoalbuminuric patients with newly diagnosed hypertension or type 2 diabetes. Diabetologia. 2011;54:2430–2439. doi: 10.1007/s00125-011-2148-y. [DOI] [PubMed] [Google Scholar]
- 7.Ohta Y, Fujii K, Arima H, et al. Increased renal resistive index in atherosclerosis and diabetic nephropathy assessed by Doppler sonography. J Hypertens. 2005;23(10):1905–1911. doi: 10.1097/01.hjh.0000181323.44162.01. [DOI] [PubMed] [Google Scholar]
- 8.Hamano K, Nitta A, Ohtake T, Kobayashi S. Association of renal vascular resistance with albuminuria and other macroangiopathy in type 2 diabetic patients. Diabetes Care. 2008;31(9):1853–1857. doi: 10.2337/dc08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai T, Kamide K, Onishi M, et al. Usefulness of the resistive index in renal Doppler ultrasonography as an indicator of vascular damage in patients with risks of atherosclerosis. Nephrol Dial Transplant. 2011;26(10):3256–3262. doi: 10.1093/ndt/gfr054. [DOI] [PubMed] [Google Scholar]
- 10.Heine GH, Reichart B, Ulrich C, et al. Do Ultrasound renal resistance indices reflect systemic rather than renal vascular damage in chronic kidney disease? Nephrol Dial Transplant. 2007;22:163–170. doi: 10.1093/ndt/gfl484. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura T, Wada A. A Resistive index predicts renal prognosis in chronic kidney disease. Nephrol Dial Transplant. 2009;24(9):2780–2785. doi: 10.1093/ndt/gfp121. [DOI] [PubMed] [Google Scholar]
- 12.Leoncini G, Martinoli C, Viazzi F. Change in renal resistive index and urinary albumin excretion in Hypertensive patients under long-term treatment with lisinopril and Nifedipine GITS. Nephron. 2002;90(2):169–173. doi: 10.1159/000049038. [DOI] [PubMed] [Google Scholar]
- 13.Radermacher J. Resistive Index: an ideal test frorenovascular disease or ischemic nephropathy? Nat Clin Pract Nephrol. 2006;2(5):232–233. doi: 10.1038/ncpneph0177. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Zeeuw D, Hillege HL, de Jong PE. The kidney, a cardiovascular risk marker, and a new target for therapy. Kidney Int Suppl. 2005;98:S25–S29. doi: 10.1111/j.1523-1755.2005.09805.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanamura K, Tojo A, Knugasa S, Asaba K, Fujita TT. The resistive index is a marker of renal function, pathology, prognosis, and responsiveness to steroid therapy in chronic kidney disease patients. Int J Nephrol. 2012;2012:139565. doi: 10.1155/2012/139565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikee R, kobayashi S, Hemmi N, et al. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J kidney dis. 2005;46(4):603–609. doi: 10.1053/j.ajkd.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Parolini C, Noce A, Staffolani E, Giarrizzo GF, Costanzi S, Splendiani G. Renal resistive index and long-term outcome in chronic nephropathies. Radiology. 2009;252(3):888–896. doi: 10.1148/radiol.2523080351. [DOI] [PubMed] [Google Scholar]
- 19.Quaia E, Bertolotto M. Renal parenchymal diseases: is characterization feasible with ultrasound? Eur Radiol. 2002;12(8):2006–2020. doi: 10.1007/s00330-002-1360-z. [DOI] [PubMed] [Google Scholar]
- 20.Pozzi Mucelli R, Bertolotto M, Quaia E. Imaging techniques in acute renal failure. Contrib Nephrol. 2001;132:76–91. doi: 10.1159/000060076. [DOI] [PubMed] [Google Scholar]
- 21.Mehta RL, Kellum JA, Shah SV. Acute kidney injury network report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.KDIGO (Kidney Disease Improving Global Outcomes) Clinical practical guidelines for acute kidney injury. Kidney Int Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [Google Scholar]
- 23.Zeng X, McMahon G, Brunelli SM. Incidence outcomes, and comparisons across definitions of AKI in Hospitalized individuals. Clin J Am Soc Nephrol. 2014;9:12–20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singbartl K, et al. AKI in ICU: definition, epidemiology, risk stratification and outcomes. Kidney Int. 2012;81(9):819–825. doi: 10.1038/ki.2011.339. [DOI] [PubMed] [Google Scholar]
- 25.Li P, et al. Acute kidney injury: global health alert. Transplantation. 2013;95(5):653–657. doi: 10.1097/TP.0b013e31828848bc. [DOI] [PubMed] [Google Scholar]
- 26.Legrand MM, Darmon M. Renal imaging in acute kidney injury. Acute Nephrol Crit Care Phys. 2015 [Google Scholar]
- 27.Lauschke A, Teichgraber UKM, Frei U, et al. “Low-Dose” dopamine worsens renal perfusion in patients withacute renal failure. Kidney Int. 2006;69:1669–1674. doi: 10.1038/sj.ki.5000310. [DOI] [PubMed] [Google Scholar]
- 28.Deruddre S, Cheisson G, Mazoit JX, et al. Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med. 2007;33(9):1557–1562. doi: 10.1007/s00134-007-0665-4. [DOI] [PubMed] [Google Scholar]
- 29.Lerolle N, Guerot E, Faisy C, et al. Renal failure in septic shock: predictive value of Doppler based renal arterial resistive index. Intensive Care Med. 2006;32(10):1553–1559. doi: 10.1007/s00134-006-0360-x. [DOI] [PubMed] [Google Scholar]
- 30.Granata A, Andrulli S, Bigi MQ, et al. Predictive role of duplex Doppler ultrasonography in the diagnosis of acute renal obstruction in patients with unilateral renal colic. Clin Nephrol. 2009;71(6):680–686. doi: 10.5414/CNP71680. [DOI] [PubMed] [Google Scholar]
- 31.Solez K, Vincenti F, Filo R. Histopathologic findings from 2-year protocol biopsies from a U:S: multicenter kidney transplant trial comparing tacrolimus versus cyclosporine: a report of the FK506. Kidney Transplant Study Group. Transplantation. 1998;66:1736–1740. doi: 10.1097/00007890-199812270-00029. [DOI] [PubMed] [Google Scholar]
- 32.Paul LC. Chronic allograft nephropathy: an update. Kidney Int. 1999;56:783–793. doi: 10.1046/j.1523-1755.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- 33.Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 34.Solez K, Colvin RB, Racusen LC, et al. Banff’05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7:518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 35.Radermacher J, Mengel M, Ellis S, et al. The renal resistance index and renal allograft survival. N Engl J Med. 2003;349(2):115–124. doi: 10.1056/NEJMoa022602. [DOI] [PubMed] [Google Scholar]
- 36.Vallejos A, Alperovich G, Moreso F, et al. Resistive Index and chronic allograft nephropathy evaluated in protocol biopsies as predictor of graft outcome. Nephrol Dial Transplant. 2005;20(11):2511–2516. doi: 10.1093/ndt/gfi041. [DOI] [PubMed] [Google Scholar]
- 37.Kramann R, Frank D, Brandeburg VM, et al. Prognostic impact of renal arterial resistance index upon renal allograft survival: the time point matters. Nephrol Dial Transplant. 2012;27(10):3958–3963. doi: 10.1093/ndt/gfr772. [DOI] [PubMed] [Google Scholar]
- 38.Naesens M, Heylen L, Lerut H, et al. Intrarenal resistive index after renal transplantation. N Engl J Med. 2013;369(19):1797–1806. doi: 10.1056/NEJMoa1301064. [DOI] [PubMed] [Google Scholar]
- 39.Mutinelli-Szymanski P, Caille A, Tranquart F, et al. Renal resistive index as a new independent risk factor for new onset diabetes mellitus after kidney transplantation. Transpl Int. 2012;25(4):464–470. doi: 10.1111/j.1432-2277.2012.01445.x. [DOI] [PubMed] [Google Scholar]