Abstract
Objective
To evaluate the accuracy of contrast-enhanced ultrasound (CEUS) in comparison to CT angiography (CTA) to identify and classify endoleaks following abdominal aortic aneurism repair with endoprosthesis.
Materials and methods
A retrospective analysis of 181 patients treated with EVAR, from September 2009 to September 2014, was performed. Patients were evaluated with CEUS, CTA and angiography in the cases requiring treatment. Sac diameter, sac integrity, identification and classification of endoleaks were taken into consideration. Sensitivity, specificity, accuracy and negative predictive values were considered for each modality of endoleak identification.
Results
Forty-two endoleaks (23.2%; type II: 39 cases, type III: 3 cases) were documented. Sensitivity and specificity of CEUS and CT were, respectively, 97.6 and 90.5, 100 and 100%. In two cases, CEUS was able to better classify endoleaks compared to CT.
Conclusions
CEUS accuracy to identify endoleaks following EVAR is similar to CT. CEUS should be considered as an effective modality for the long-term surveillance of EVAR because of its capability to correctly classify endoleaks with no ionizing radiation exposure.
Keywords: Contrast-enhanced ultrasound, CT, Endoleak
Riassunto
Obiettivi dello studio
Valutare l’accuratezza dell’eco-contrastografia (CEUS), confrontandola con angiografia con tomografia computerizzata (CT) per l’identificazione degli endoleak dopo trattamento di aneurisma dell’aorta addominale con endoprotesi.
Materiali
Da Settembre 2008 a Dicembre 2014, 181 pazienti consecutivi trattati con EVAR sono stati valutati con CEUS, CTA, ed anche con angiografia nei casi da ritrattare. Sono stati valutati: diametro della sacca, valutazione dell’integrita della sacca, identificazione e classificazione degli endoleaks. Sensibilità, specificità, accuratezza e valore predittivo negativo sono stati valutati per ogni modalita nell’identificazione degli endoleak.
Risultati
Quarantadue endoleak (23.2%; tipo II: 39 casi, tipo III: 3 casi) sono stati documentati. La Sensibilita della CEUS e della TC e stata rispettivamente del 97.6, 90.5%, mentre la specificita per entrambe e stata del 100%. In due casi la CEUS e stata in grado di classificare meglio gli endoleak rispetto alla CT. La sacca aneurismatica presentava alla CEUS e CDUS un diametro massimo compreso tra 39-82 mm, mentre alla TC tra 38 e 78 mm, senza significativa differenza tra le due metodiche.
Conclusioni
L’accuratezza della CEUS nell’identificazione degli endoleak e nella misurazione della sacca dopo EVAR, e simile alla TC con maggiore sensibilita ma analoga specificita. La CEUS e da considerarsi una modalita efficace per la sorveglianza a lungo termine degli EVAR in quanto capace di classificare correttamente gli endoleak senza esposizione a radiazioni ionizzanti.
Parolechiave: Contrast-enhanced ultrasound, CT, Endoleak
Introduction
Endovascular abdominal aortic aneurysm repair (EVAR) has become particularly significant as an alternative to traditional surgery, due to its less aggressiveness and invasiveness, the lesser hospitalization time and early mortality [1, 2].
The most frequent complication, observed in about 20% of cases, is represented by the incomplete exclusion of the aneurysm sac from circulation with persistent arterial flow communication between the aneurysm sac and systemic circulation, i.e., the endoleak. In 10–45% of cases, such complication can be associated with a dilation of the aneurysm sac and rupture [3, 4]. Such a risk makes it necessary to carefully survey patients who have undergone EVAR to identify endoleaks and plan future treatment [5].
Endoleak types, in accordance with the Society for Vascular Surgery and the American Association for Vascular Surgery [5], can be classified into five categories, according their origin. Moreover, according to the time of appearance, endoleaks can be divided into early endoleaks which develop during the operation or within 30 days post-op and late endoleaks which appear after more than 30 days post-op.
Early detection and classification of an endoleak represent the primary aim of the follow-up of patients treated with aortic endoprostheses. Currently, non-invasive imaging has replaced diagnostic angiography, which has now been given a therapeutic role. The ideal imaging modality should be inexpensive, repeatable, safe and accurate [5]. Currently, the correct follow-up modality and timing is still unknown [5].
Color Doppler ultrasound (CDUS) was initially the first choice due to its low cost, repeatability and the patient’s compliance, but it has a variable sensitivity (between 43 and 97%) and it is operator and patient dependent [6, 7].
To date, CT angiography (CTA) is the reference diagnostic method, because of its wide availability, diagnostic value, acquisition speed, resolution and uniformity of protocols. However, it is expensive, it uses ionizing radiation and potentially allergenic and nephrotoxic contrast agents. Moreover, CTA allows only multiphase imaging and not dynamic acquisitions of the flow inside the aneurysm sac.
As reported in the literature, some valid alternatives to CT are contrast-enhanced ultrasound (CEUS) and magnetic resonance angiography (MRA) [8–11].
We retrospectively review our database aiming to identify the role of CEUS in the follow-up of patients treated with EVAR in comparison with multislice computed tomography (64 slices).
Materials and methods
In the period between September 2009 and September 2014, 181 patients with EVAR (123 males, 48 females, with average age of 65 years) were evaluated by means of CDUS, CEUS and CTA. They had previously undergone EVAR through the positioning of aortic bifurcated endoprostheses: 103 patients had the Excluder type implanted (W. L. Gore & Associates, Flagstaff, AZ, USA), whereas 78 the Talent-Endurant (Medtronic, Santa Rosa, CA, USA). The average diameter of the aneurysm sac was 54 mm (range: 39–87 mm).
All imaging, clinical and laboratory data derived from follow-up through CDUS, CEUS and CTA at a distance of maximum 6 days and within a week with CT were retrospectively reviewed.
Angiography was carried out in patients for whom the two methods gave contrasting results or because of the presence of endoleaks.
Ultrasonographic technique
Patients were advised to follow a low-residual diet the day before the exam and to fast in the morning of the day of the investigation. The CEUS study was conducted by one radiologist with 15 years of ultrasound experience, respectively, and high-end machines (Aplio XV, XG, Aplio 500, Toshiba, Zoetermeer, NL and Tecnos MPX, Mylab 70, Esaote Genova, Italy), equipped with convex probes (3–5 MHz), using software for scanning gray-scale images in real time, with a low Mechanical Index (MI) between 0.05 and 0.8 (corresponding to about 35–40 kPa).
The first part of the exam consists of a complete study of the abdominal aorta in B-mode, from the diaphragm to the iliac arteries, including a color and power Doppler evaluation of the vessel and most importantly of the aneurysm sac. Hemodynamics was documented through the measurement of speed with Doppler spectrum; the aneurysm sac was measured both in its longitudinal and transversal size in the segment with the largest dimension. Later a quick bolus injection with 2.4 ml of second-generation contrast agent (SonoVue®, Bracco, Milan, Italy—composed of gas microbubbles of sulfur hexafluoride encapsulated by phospholipid shells) followed by 5 cc of saline was given.
The whole abdominal aorta (up to the iliac branches) was examined for 10 min following the injection of SonoVue® and the presence of contrast enhancement within the aneurysm sac was evaluated, by monitoring the time of appearance (if synchronous or delayed with respect to prosthesis enhancement) and persistence in inflow and outflow vessels.
The exams were digitally recorded in the form of cine-loops and all cases were analyzed for the two operators to characterize lesions in “consensus reading”.
CTA technique
All patients underwent within 1 week a CT exam carried out with Somatom 64 (Siemens, Erlangen, Germany). A triphasic CT protocol was put in place with a pre-contrast phase, an arterial phase (started with bolus tracking) and a late phase at 120 s., using 130 mL of non-ionic contrast agent: Iomeron (Bracco, Milan, Italy) at 4 mL/s. The other scanning parameters were: 1.2 mm acquisition; reconstruction with a soft-margin kernel algorithm (B30) at 1.5 and 3 mm with a reconstruction increase of 1.5 mm; pre-contrast scans at a low-power tube (120 mAs); the other phases at 120 kVp and 200 mAs. CT images were analyzed on a dedicated workstation (Aquarius, TeraRecon, San Matteo, Ca) using traditional post-processing techniques. Images were revised in consensus by two radiologists with 15 and 8 years of experience in the field. The size of the aneurysm sac, the integrity of the prosthesis and the presence or absence and type of endoleak were evaluated.
Digital subtraction angiography
Digital subtraction angiography (DSA) was carried out through a digital angiographer (Integris 5000, Philips Medical System, The Netherlands). Aortography was carried out through transfemoral access with a 4-Fr pigtail catheter (Cordis Endovascular, Miami Lakes, FL, USA) positioned above the renal arteries by injecting 20 mL of iodinated contrast at a speed of 20 mL/sec to evaluate the flow inside endoprostheses, the patency of the splanchnic arteries and of the iliofemoral run-off and the presence of endoleaks. The following selective catheterizations were also carried out: of the superior mesenteric artery, by means of a Sim 1–4-Fr catheter (Cordis Endovascular, Miami Lakes, FL, USA) to evaluate collateral flow of the arc of Riolan and the complete exclusion of the inferior mesenteric artery; of the internal iliac arteries bilaterally to evaluate revascularization through the iliolumbar arteries. In case of an endoleak with a progressive growth of the aneurysm sac, treatment with definitive embolic agents or prosthesis segments (aortic cuffs) was performed.
Statistical analysis
Statistical analysis was carried out with SPSS software (SPSS v. 16.01, SPSS Inc., Chicago IL, USA). The maximum transversal diameter of the sac was measured with all diagnostic methods and data were expressed with ±standard deviation (SD). The variables taken into account were: changes in the maximum diameter of the aneurysm sac, presence and type of the endoleak, if present. To identify the endoleak, sensitivity, specificity, accuracy and negative predictive value were evaluated. The Spearman’s rank correlation coefficient was used to evaluate the correlation between the measurements in the different imaging methods. The Wilcoxon’s Mann–Whitney test was used to estimate the reduction of the aneurysm sac. A p < 0.05 was considered statistically significant.
Results
The average follow-up time after EVAR was 19 months (1–48-month interval) when evaluating the prosthesis.
No side effects due to the diagnostic methods were registered. All the exams showed the patency and integrity of the prosthesis in all patients.
Amongst the 181 patients, 42 endoleaks were detected (23.2%), 39 were of type II (28 due to the flow in the sac through the lumbar arteries and 11 through the mesenteric artery) and 2 were type 1 and 1 late type 3 endoleaks (i.e., appeared after 30 days). CEUS evidenced 41 cases of endoleak out of 42 (40 true positives, 1 false negative). The remaining part of the subjects studied gave negative results with both methods. According to CDUS and CEUS, diameters (summarized in Table 1) were 52 ± 12 with a range of 39–82 mm, showing a significant correlation with the measurements of CT (ρ = 0.903 and ρ = 0.813, respectively): according to CT, sizes were 51 ± 11 cm in a range between 38 and 78 mm.
Table 1.
Comparison between CEUS and CTA: Aneurism Size
| No. of patients | Aneurysm size | ||
|---|---|---|---|
| Mean ± SD (cm) | Interval (cm) | ||
| CEUS | 181 | 5.2 ± 1.2 | 3.9–8.0 |
| CTA | 181 | 5.1 ± 1.1 | 3.8–7.8 |
The sac reduced itself in 127 patients (70.2%)—with a reduction of 0.3 ± 0.4 cm (interval 1.4–0.8 cm, Wilcoxon test: p < 0.0001). It did not change in 40 patients (22.1%), it grew in 14 patients (7.7%), who had also endoleaks and were treated. The size change was of −0.4 ± 0.3 cm (range 1.4–0.0 cm) in patients with no endoleaks, while it was of 0.0 ± 0.5 cm (range 1.2–0.8 cm, Mann–Whitney test: p = 0.002) in the 42 patients with endoleaks. In all patients with no endoleaks, the aneurysm sac always reduced itself or did not change, with no complications and, therefore, not endotension diagnosis.
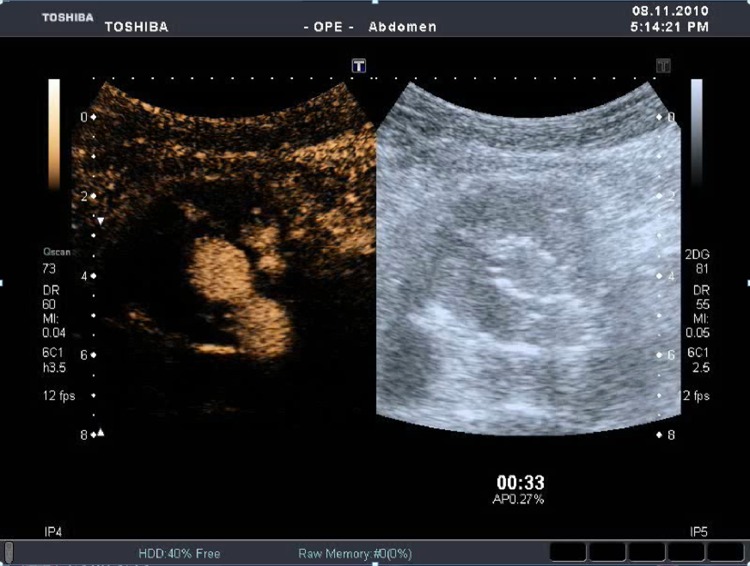
CT showed 38 endoleaks; therefore, it did not identify 4 type II endoleaks. Among them only 2 underwent angiography which confirmed the endoleak diagnosis shown by CEUS (Fig. 1) as an increase of the aneurysm sac. 30 type II endoleaks detected by CTA had low flow because they were detected only in the late phase.
Fig. 1.
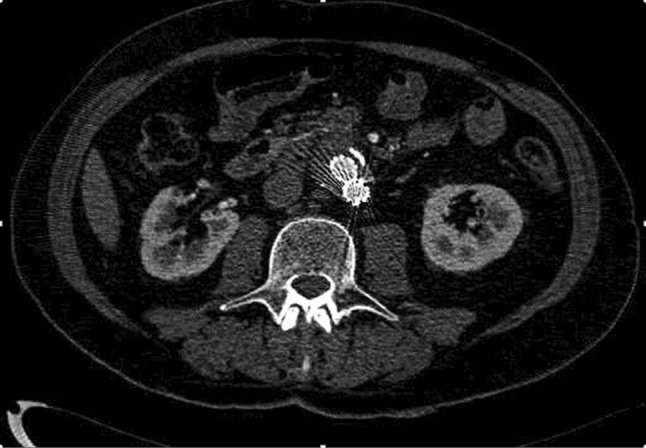
Contrast-enhanced CT (CTA) did not reveal any clear sign of endoleak. CEUS showed endoleak (Fig. 2)
CT did not classify correctly two endoleaks: two type 1 which were a type II with CEUS and DSA. Accuracy of the different exams has been summarized in Table 2.
Table 2.
Comparison between CEUS and CTA: Statistic analysis
| CTA (%) | CEUS (%) | |
|---|---|---|
| Sensitivity | 90.5 | 97.6 |
| Specificity | 100 | 100 |
| Neg. predict. val. | 97.2 | 99.3 |
According to these results, CEUS was slightly more accurate compared to CTA for identifying endoleaks. Moreover, in two cases CEUS allowed to better classify endoleak type compared to CTA. The reasons why four endoleak cases were not identified by CTA could be found in artifacts due to the metal endoprosthesis and the small size of the endoleaks.
DSA was performed on 19 patients, 17 of whom underwent treatment. Three patients with type 1 (N = 2) and III endoleaks were treated successfully with an endoprosthesis extension. Fourteen patients with an important type II endoleak, associated with a progressive growth of the aneurysm sac, were treated with spirals and other embolic agents. Two patients, who evidenced minor type II endoleaks, were kept under strict observation (Table 3).
Table 3.
Comparison between CEUS and CTA: Results
| FP | FN | TP | TN | |
|---|---|---|---|---|
| CTA | 0 | 4 | 38 | 139 |
| CEUS | 0 | 1 | 41 | 139 |
FP false positives, FN false negatives, TP true positives, TN true negatives
Discussion
Endovascular abdominal aortic aneurysm repair with metal endoprosthesis (EVAR) has become particularly significant as an alternative to traditional surgery, due to less early mortality and complications and a low rate of aneurysm rupture [1, 2, 12–15].
An appropriate surveillance follow-up of patients who have undergone EVAR is necessary to evaluate the integrity, good positioning of the graft and possible complications, such as endoleaks, which have an incidence between 2.4 and 45.5% and must be treated to avoid rupture [16, 17].
Endoleak types can be classified into five categories [15, 16]. Type 1 endoleaks are caused by incomplete proximal or distal adhesion of the endoprosthesis and require immediate repair. Type 2 endoleaks (Fig. 2) are due to the presence of collateral flow from lumbar arteries and the inferior mesenteric artery. They can resolve spontaneously, so they are monitored through imaging. Type 3 endoleaks regard structural defects or disconnections of parts of the graft and require immediate repair. Type 4 endoleaks are rare and are caused by the porosity of the graft. Aneurysms can enlarge without endoleak evidence. Such phenomenon has been named endotension or type 5 endoleak, which cannot be detected because of its low flow and fast thrombosis [18] (Table 4).
Fig. 2.
CEUS clearly depicts a case of endoleak
Table 4.
Contrast-enhanced ultrasound
| Advantages |
| Safe, repeatable, inexpensive and more appreciated by the patient |
| Possibility to study patients with renal failure |
| Dynamic analysis of the flow with real time acquisition imaging, over up to 10 min |
| Possible identification of late and low-flow endoleaks, usually not detected by spiral CT |
| Possible use in the systematic follow-up of type II endoleaks with cost and radiation exposure reduction |
| Non-toxic contrast agents, with no side effects |
| Disadvantages |
| Operator-dependent procedure, it needs special equipment and good expertise |
| Ultrasound procedure, with its traditional limits (severe obesity, intestinal gas, extensive calcification and metal artifacts) |
| Poor and non-standardized iconography, therefore, lacking immediate readability |
Type I and II endoleaks are the most frequent in the literature [3, 14, 18]. Type I endoleaks are easy to treat, while type II endoleaks are more difficult to treat because vascular branches are usually small and multiple. The correct identification of the type of endoleak through imaging is useful. Types I and III must always undergo DSA and treatment because of the high risk of aneurysm rupture. Type II endoleaks are usually followed up with a “wait and see” approach without any need for treatment since they can resolve spontaneously within six months, as reported in literature [3, 14].
This retrospective study for evaluating the success of endovascular treatment with the positioning of metal endoprosthesis (EVAR) was conducted because the present follow-up modalities still represent a controversial issue [9, 17, 19].
An alternative could be baseline ultrasound which would show an enhancement of the residual aneurysmal sac. Amongst potentially useful exams to identify an endoleak, CDUS (Color-Doppler Ultrasound) can be considered safe, inexpensive, repeatable and praised as a good choice. However, as it has been shown in our previous experience [20] and in many other studies [5–7, 21], even this method is not reliable enough to identify endoleaks, with a high rate of false negatives and positives. This is due to the large amount of artifacts, such as the metal of the endoprosthesis, the shadow cone of possible calcification, the presence of low-flow endoleaks and of course intestinal gas or severe obesity (which would affect any type of ultrasound). According to some authors, CDUS would have the advantage, compared to CTA, of providing hemodynamic information about the blood flow and its direction, allowing a better classification of endoleaks. However, it is not sensitive enough, unless it is performed by an expert in perfect anatomical conditions.
Referring to our previous experience [20], in the present work we focused on the CEUS data, compared to the ones obtained through second-level imaging. At the moment, triphasic CT is considered the reference procedure in the follow-up of patients who have undergone EVAR, because it allows a good identification of endoleaks [18, 19, 22]. Pre- and post-contrast scans in the arterial phase allow the identification of small endoleaks adjacent to the metal parts and calcifications, and low-flow endoleaks, visible in delayed scans. The unsuccessful directional identification of the flow makes the origin of the endoleak more difficult to detect [14, 23]. The emergent role of CEUS and its growing indications compared to CT are also shown by several studies in different fields [24–27], which prove its applications in the body, for liver, kidney, testis, lymph nodes, thyroid, prostate and small bowel.
CEUS with low-mechanical index technique, evaluated in some studies for the EVAR follow-up [8, 9, 28, 29], used second-generation ultrasound contrast agents (e.g., SonoVue®) which employ gas microbubbles of sulfur hexafluoride encapsulated by phospholipid shells. They circulate with a good concentration up to 10 min offering, with harmonic imaging, pure vascular enhancement. In the literature, CEUS has given promising results both for the identification of endoleaks and their correct classification, as we have already underlined in our previous work [20]. This is due to the possibility to follow in real time the blood flow and origin of type II endoleaks.
In the present study, 181 patients were evaluated with non-invasive methods. In 19 out of 42 endoleak cases, DSA was also performed. Ultrasound with second-generation contrast agents was able to identity 41 endoleaks (39 type II, 1 type 1 and 1 type III) out of 181 examinations carried out, while CT identified 38 out of 42. Patients who also reported a growth of the aneurysm sac, underwent angiography, which confirmed CEUS results, and they were treated. Therefore, contrast-enhanced ultrasound has a 97.6% sensitivity and 100% specificity.
As far as endoleak types, CEUS managed to solve two cases not correctly identified by CT. In both cases, classified by CT as a type I endoleak, CEUS clearly showed the hypertrophic lumbar artery as an afferent vessel, classifying a type II endoleak. Indeed, in addition to morphologic evaluation, CEUS has the advantage of offering dynamic information of the blood flow and its direction, showing on the same screen in real time, native and contrast images.
CEUS was carried out with a bolus of 2.4 mL of second-generation contrast agent, followed by 5 mL of saline, as suggested by Iezzi et al. [28], who documented how 2.4 mL is more convenient than 1.2 mL, since they produce better images as far as intensity and contrast enhancement are concerned. A second injection should be limited to more difficult cases to better characterize the endoleak. However, the optimal dosage is under evaluation since other authors suggest especially with the new equipment to use no more than 1 ml [25].
The secondo-generation contrast we used was safe with no side effects, in accordance with the literature [30–32].
We also noted that CT did not identify 4 cases of endoleak probably because of artifacts due to beam hardening and small endoleak size. Aneurysm sac diameter measurements were very close in the two procedures with a strong correlation in Spearman test. A correct measurement of the sac allows to identify possible growths which would require a careful analysis of the endoleak.
Conclusion
Following the results of our work and the more recent literature [33], CEUS exam results are better than CT to identify endoleaks, since the relatively long time of permanence of the contrast agent (more than 5 min) in the blood flow and real-time study allows the study of patients also in delayed phases.
However, CT, with all its limits regarding classification and identification of endoleaks, cannot be replaced because it allows a more precise evaluation of the aneurysm sac and provides information about anchorage, integrity and morphology of the graft, which cannot be obtained with ultrasound.
Contrast-enhanced ultrasound can be, therefore, used as an aid to CT in cases of suspected endoleaks, giving more information about their origin and extension and allowing a more precise classification. It could be suggested for those patients who have a growing aneurysm sac, but CT did not reveal a reperfusion of the aneurysm sac. In addition, type II endoleaks which need constant monitoring can be kept under observation with ultrasound, reducing the use of CT and, therefore, expenses and radiation exposure.
Finally, further evaluations on the effectiveness of contrast-enhanced ultrasound with second-generation contrast agents must be put in place before its routine use in patients who have undergone endovascular abdominal aortic aneurism repair.
Compliance with ethical standards
Funding
This study was not funded.
Conflict of interest
Vito Cantisani lectured for Bracco, Samsung, Toshiba and Fabrizio Calliada lectured for Hitachi and Mindray but they did not receive funding. The other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.United Kingdom EVAR Trial Investigators. Greenhalgh RM, Brown LC, Powell JT, et al. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362(20):1863–1871. doi: 10.1056/NEJMoa0909305. [DOI] [PubMed] [Google Scholar]
- 2.Biancari F, Catania A, D’Andrea V. Elective endovascular vs. open repair for abdominal aortic aneurysm in patients aged 80 years and older: systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2011;42(5):571–576. doi: 10.1016/j.ejvs.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Cao P, De Rango P, Verzini F, Parlani G. Endoleak after endovascular aortic repair: classification, diagnosis and management following endovascular thoracic and abdominal aortic repair. J Cardiovasc Surg. 2010;51(1):53–69. [PubMed] [Google Scholar]
- 4.Mehta M, Sternbach Y, Taggert JB. Long-term outcomes of secondary procedures after endovascular aneurysm repair. J Vasc Surg. 2010;52(6):1442–1449. doi: 10.1016/j.jvs.2010.06.110. [DOI] [PubMed] [Google Scholar]
- 5.Corriere MA, Feurer ID, Becker SY. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg. 2004;239(6):800–805. doi: 10.1097/01.sla.0000128300.60156.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raman KG, Missig-Carroll N, Richardson T, Muluk SC, Makaroun MS. Color-flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. J Vasc Surg. 2003;38(4):645–651. doi: 10.1016/S0741-5214(03)00909-1. [DOI] [PubMed] [Google Scholar]
- 7.Manning BJ, O’Neill SM, Haider SN. Duplex ultrasound in aneurysm surveillance following endovascular aneurysm repair: a comparison with computed tomography aortography. J Vasc Surg. 2009;49(1):60–65. doi: 10.1016/j.jvs.2008.07.079. [DOI] [PubMed] [Google Scholar]
- 8.Carrafiello G, Laganà D, Recaldini C. Comparison of contrast-enhanced ultrasound and computed tomography in classifying endoleaks after endovascular treatment of abdominal aorta aneurysms: preliminary experience. Cardiovasc Intervent Radiol. 2006;29(6):969–974. doi: 10.1007/s00270-005-0267-x. [DOI] [PubMed] [Google Scholar]
- 9.Dill-Macky MJ, Wilson SR, Sternbach Y, Kachura J, Lindsay T. Detecting endoleaks in aortic endografts using contrast-enhanced sonography. AJR Am J Roentgenol. 2007;188(3):W262–W268. doi: 10.2214/AJR.05.0532. [DOI] [PubMed] [Google Scholar]
- 10.van der Laan MJ, Bartels LW, Viergever MA, et al. Computed tomography versus magnetic resonance imaging of endoleaks after EVAR. Eur J Vasc Endovasc Surg. 2006;32(4):361–365. doi: 10.1016/j.ejvs.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Wieners G, Meyer F, Halloul Z, et al. Detection of type II endoleak after endovascular aortic repair: comparison between magnetic resonance angiography and blood-pool contrast agent and dual-phase computed tomography angiography. Cardiovasc Intervent Radiol. 2010;33(6):1135–1142. doi: 10.1007/s00270-010-9984-x. [DOI] [PubMed] [Google Scholar]
- 12.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5(6):491–499. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- 13.Blum U, Voshage G, Lammer J, et al. Endoluminal stent-grafts for infrarenal abdominal aortic aneurysms. N Engl J Med. 1997;336(1):13–20. doi: 10.1056/NEJM199701023360103. [DOI] [PubMed] [Google Scholar]
- 14.Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, van Marrewijk C, Laheij RJ. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32(4):739–749. doi: 10.1067/mva.2000.109990. [DOI] [PubMed] [Google Scholar]
- 15.White GH, Yu W, May J, Chaufour X, Stephen MS. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. J Endovasc Surg. 1997;4(2):152–168. doi: 10.1583/1074-6218(1997)004<0152:EAACOE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Bernhard VM, Mitchell RS, Matsumura JS. Ruptured abdominal aortic aneurysm after endovascular repair. J Vasc Surg. 2002;35(6):1155–1162. doi: 10.1067/mva.2002.123758. [DOI] [PubMed] [Google Scholar]
- 17.Toya N, Fujita T, Kanaoka Y, Ohki T. Endotension following endovascular aneurysm repair. Vasc Med. 2008;13(4):305–311. doi: 10.1177/1358863X08094850. [DOI] [PubMed] [Google Scholar]
- 18.Rand T, Uberoi R, Cil B, Munneke G, Tsetis D. Quality improvement guidelines for imaging detection and treatment of endoleaks following endovascular aneurysm repair (EVAR) Cardiovasc Intervent Radiol. 2012;36(1):35–45. doi: 10.1007/s00270-012-0439-4. [DOI] [PubMed] [Google Scholar]
- 19.Stavropoulos SW, Charagundla SR. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology. 2007;243(3):641–655. doi: 10.1148/radiol.2433051649. [DOI] [PubMed] [Google Scholar]
- 20.Cantisani V, Ricci P, Grazhdani H, et al. Prospective comparative analysis of colour-Doppler ultrasound, contrast-enhanced ultrasound, computed tomography and magnetic resonance in detecting endoleak after endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2011;41(2):186–192. doi: 10.1016/j.ejvs.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Bakken AM, Illig KA. Long-term follow-up after endovascular aneurysm repair: is ultrasound alone enough? Perspect Vasc Surg Endovasc Ther. 2010;22(3):145–151. doi: 10.1177/1531003510382664. [DOI] [PubMed] [Google Scholar]
- 22.Uthoff H, Peña C, Katzen BT, et al. Current clinical practice in postoperative endovascular aneurysm repair imaging surveillance. J Vasc Interv Radiol. 2012;23(9):1152e6–1159e6. doi: 10.1016/j.jvir.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Napoli V, Bargellini I, Sardella SG, et al. Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology. 2004;233(1):217–225. doi: 10.1148/radiol.2331031767. [DOI] [PubMed] [Google Scholar]
- 24.Cantisani V, Bertolotto M, Weskott HP, et al. Growing indications for CEUS: The kidney, testis, lymph nodes, thyroid, prostate, and small bowel. Eur J Radiol. 2015;84(9):1675–1684. doi: 10.1016/j.ejrad.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Cantisani V, Grazhdani H, Clevert DA, et al. EVAR: Benefits of CEUS for monitoring stent-graft status. Eur J Radiol. 2015;84(9):1658–1665. doi: 10.1016/j.ejrad.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 26.D’Onofrio M, Vecchiato F, Cantisani V, et al. Intrahepatic peripheral cholangiocarcinoma (IPCC): comparison between perfusion ultrasound and CT imaging. Radiol Med. 2008;113(1):76–86. doi: 10.1007/s11547-008-0225-1. [DOI] [PubMed] [Google Scholar]
- 27.Cantisani V, Ricci P, Erturk M, Pagliara E, et al. Detection of hepatic metastases from colorectal cancer: prospective evaluation of gray scale US versus SonoVue® low mechanical index real time-enhanced US as compared with multidetector-CT or Gd-BOPTA-MRI. Ultraschall Med. 2010;31(5):500–505. doi: 10.1055/s-0028-1109751. [DOI] [PubMed] [Google Scholar]
- 28.Iezzi R, Basilico R, Giancristofaro D, Pascali D, Cotroneo AR, Storto ML. Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2009;49(3):552–560. doi: 10.1016/j.jvs.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Gilabert R, Buñesch L, Real MI, et al. Evaluation of abdominal aortic aneurysm after endovascular repair: prospective validation of contrast-enhanced US with a second-generation US contrast agent. Radiology. 2012;264(1):269–277. doi: 10.1148/radiol.12111528. [DOI] [PubMed] [Google Scholar]
- 30.Bokor D, Chambers JB, Rees PJ, Mant TG, Luzzani F, Spinazzi A. Clinical safety of SonoVue, a new contrast agent for ultrasound imaging, in healthy volunteers and in patients with chronic obstructive pulmonary disease. Invest Radiol. 2001;36(2):104–109. doi: 10.1097/00004424-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Torzilli G. Adverse effects associated with SonoVue use. Expert Opin Drug Saf. 2005;4(3):399–401. doi: 10.1517/14740338.4.3.399. [DOI] [PubMed] [Google Scholar]
- 32.Piscaglia F, Bolondi L, Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32(9):1369–1375. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Chung J, Kordzadeh A, Prionidis I, et al. Contrast-enhanced ultrasound (CEUS) versus computed tomography angiography (CTA) in detection of endoleaks in post-EVAR patients. Are delayed type II endoleaks being missed? A systematic review and meta-analysis. J Ultrasound. 2015;18(2):91–99. doi: 10.1007/s40477-014-0154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]