Abstract
Objective: Neurofilaments in CSF are promising biomarkers which might help in the diagnosis of motor neuron disease (MND). We aim to assess the diagnostic value of neurofilaments in CSF for MND.
Methods: Pubmed, Emabase, and Web of Science were searched for relevant studies systematically. Articles in English that evaluated the utility of neurofilaments in CSF in the diagnosis of MND were included. Data were extracted by two independent investigators. Diagnostic indexes for neurofilament light chain (NFL) and phosphorylated neurofilament heavy chain (pNFH) were calculated separately. Stata 12.0 software with a bivariate mixed-effects model was used to summarize the diagnostic indexes from eligible studies.
Results: Five studies on NFL and eight studies on pNFH met inclusion criteria. For NFL, the pooled sensitivity and specificity were 81% (95% confidence interval [CI], 72–88%) and 85% (95% CI, 76–91%), respectively; the positive likelihood ratio (PLR) and negative likelihood ratio (NLR) were 5.5 (95% CI, 3.1–9.8) and 0.22 (95% CI, 0.14–0.35), respectively; the summary diagnostic odds ratio (DOR) was 25 (95% CI, 9–70), and the area under summary receiver operator characteristic curve (AUC) was 0.90 (95% CI, 0.87–0.92). For pNFH, the pooled sensitivity, specificity, PLR and NLR were 85% (95% CI, 80–88%), 85% (95% CI, 77–90%), 5.5 (95% CI, 3.6–8.4), and 0.18 (95% CI, 0.13–0.25), respectively; the DOR was 30 (95% CI, 16–58), and the AUC was 0.91 (95% CI, 0.88–0.93).
Conclusion: Neurofilaments in CSF have a high value in the diagnosis of MND, though the optimal cutoff value remains to be further investigated.
Keywords: motor neuron disease, amyotrophic lateral sclerosis, CSF biomarkers, neurofilaments, diagnostic value, meta-analysis
Introduction
Motor neuron diseases (MND) are a group of progressive neurodegenerative disorders characterized by motor neuron loss in the motor cortex, brainstem, and spinal cord. The most common form is amyotrophic lateral sclerosis (ALS), which affects both upper motor neuron (UMN) and lower motor neuron (LMN). Early diagnosis of ALS remains to be a challenge worldwide. Population studies have shown that the average diagnosis latency is about 12 months (Zoccolella et al., 2006), and patients who fulfill the revised El Escorial criteria for clinically definite ALS are usually in the advanced stage of the disease (Turner and Talbot, 2013). Validated biomarkers that can facilitate earlier diagnosis of ALS are urgently needed in order to enable disease-modifying drugs to be administered at an earlier stage.
Neurofilaments are the most abundant neuronal cytoskeletal proteins and are essential to the structural integrity of neurons. Neurofilament subunits, mainly neurofilament light chain (NFL) and phosphorylated neurofilament heavy chain (pNFH), are actively involved in the pathogenesis of axonal injury and degeneration both as causative agents and progression markers for neurological diseases (Petzold, 2005). ALS is characterized by loss of large axons with abundant neurofilaments, and perikaryal accumulation of phosphorylated neurofilaments has been found to occur in ALS (Manetto et al., 1988). Several studies and meta-analysis have showed that concentrations of NFL and pNFH in CSF are significantly increased in patients with ALS (Xu et al., 2016), suggesting that they might be promising neurochemical diagnostic biomarkers for ALS. To fully understand the diagnostic performance of NFL and pNFH for MND, we performed the present meta-analysis to summarize their diagnostic indexes.
Materials and Methods
Inclusion of Studies
Pubmed, Embase databases and Web of Science were searched for studies published up to October 31st, 2016 that reported neurofilament concentrations in CSF in patients with MND. Search terms included (‘motor neuron disease’ or ‘MND’ or ‘amyotrophic lateral sclerosis’ or ‘ALS’) AND (‘NFL’ or ‘NEFL’ or ‘NFH’ or ‘NEFH’ or ‘pNFH’ or ‘neurofilament’ or ‘neurofilaments’ or ‘light chain’ or ‘heavy chain’) AND (‘cerebrospinal fluid’ or ‘cerebrospinal fluids’ or ‘CSF’ or ‘biomarker’ or ‘biomarkers’ or ‘biological marker’ or ‘biological markers’). Both text word and MeSH subject headings were used. Language was confined to English, and publication type of review, case reports and letter was excluded in the advanced search. The search strategy was supplemented by inspecting the reference lists of included articles. The studies were considered for inclusion if they (1) evaluated the utility of neurofilament concentrations in CSF for the diagnosis of MND; (2) enrolled healthy controls or patients with neurological disorders other than MND as controls; (3) provide enough data to construct a 2 × 2 table for the diagnostic accuracy. Studies were excluded if they were in line with the following criteria: (1) there was no control group; (2) measured neurofilament concentrations in biological samples other than CSF, including plasma, serum, spinal cords, or brain tissue from biopsy; (3) used non-quantitative methods such as western blot, or assessed the diagnostic accuracy of anti-neurofilament antibodies for MND; (4) had overlapped sample or the sample size <10; (5) could not provide valid data after contacting the authors.
Data Extraction and Quality Assessment
For each included study, the following data were extracted by two investigators independently using a standard form: country of origin, number of centers, number of cases, patient type, ALS diagnostic criteria, control type, study design, testing method, mean or median value of neurofilaments, cutoff value, and diagnostic indexes. The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was used to assess the quality of included studies and their risk of bias (Whiting et al., 2011). The figure of risk of bias and applicability concerns summary was produced using Revman 5.3 software. Any conflicts were resolved by a third party after discussion of each item.
Data Analysis
We used STATA software (version 12.0, Stata Corporation, 93 College Station, TX, USA) to perform the meta-analysis. A bivariate mixed-effects model was used to analyze the estimates of sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) with 95% confidence interval (CI). The summary receiver operator characteristic (SROC) curve was constructed and the area under the SROC curve (AUC) was calculated to evaluate the overall performance of CSF neurofilaments in MND patients. P < 0.1 for Q-test or I2 ≥ 50% for I2 statistics indicated substantial heterogeneity, in which case heterogeneity test would be performed. The leave-one-out sensitivity analyses were conducted to test the replicability of the results, which consists of repeating the main analysis by removing each study one at a time to recalculate the stability of the remaining studies. Subgroup analyses for pNFH according to study design, testing method, cutoff value, patient type, diagnosis criteria, control type, and population were also carried out. Deeks’ funnel plots were used to test for the potential presence of publication bias, and P < 0.05 was considered statistically significant.
Results
Search Results and Characteristics of Included Studies
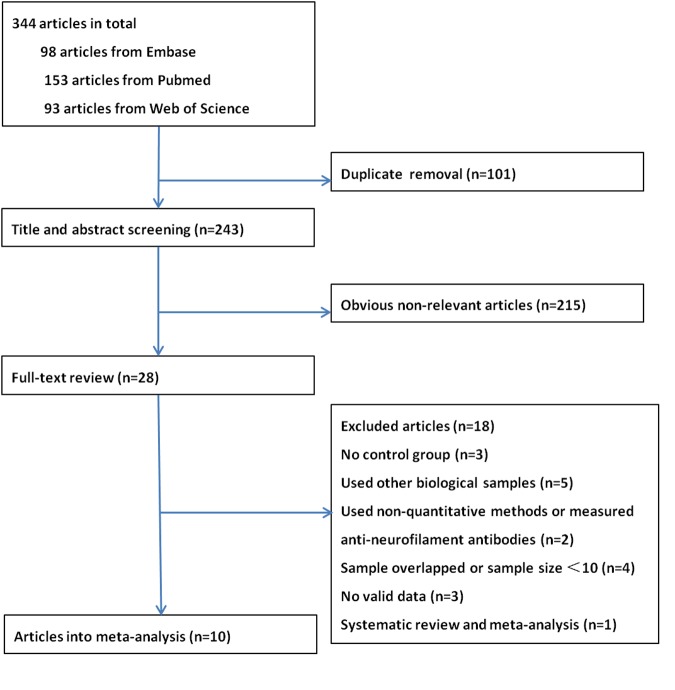
A total of 344 articles were identified. After removal of duplicate entries, 243 articles remained and then were screened by title and abstract. As a result, 28 full-text articles were assessed for eligibility, of which three had no control group (Boylan et al., 2013; Tortelli et al., 2015; Weydt et al., 2016), five investigated neurofilament levels in other biological samples (Troost et al., 1992; Strong et al., 2001; Mendonca et al., 2005; Puentes et al., 2014; McCombe et al., 2015), one utilized western blot (Mendonca et al., 2011) and one measured anti-neurofilament antibodies (Fialova et al., 2010), two had overlapping data sets (Brettschneider et al., 2006; Goncalves et al., 2015) and two had a sample size <10 (Norgren et al., 2003; Petzold et al., 2003), and three did not provide sufficient data to allow construct a 2 × 2 table (Kuhle et al., 2010; Gaiottino et al., 2013; Lehnert et al., 2014), one was a systematic review and meta-analysis (Xu et al., 2016). Finally, 10 articles were included in the meta-analysis, of which two reported data on NFL only (Tortelli et al., 2012; Lu et al., 2015), five reported on pNFH only (Ganesalingam et al., 2011, 2013; Chen et al., 2016; Goncalves et al., 2016; Li et al., 2016) and three reported both (Reijn et al., 2009; Steinacker et al., 2015; Oeckl et al., 2016). A flow chart of publication selection is presented in Figure 1. Of note, one study that focused on multicenter validation of CSF neurofilaments as diagnostic biomarkers for ALS enrolled participants from 15 centers across Europe and America (Oeckl et al., 2016), part of which might overlapped with other four studies (Ganesalingam et al., 2013; Lu et al., 2015; Steinacker et al., 2015; Goncalves et al., 2016). However, the multicenter study only recruited five ALS patients and five controls from each center; therefore, we reckoned that the multiple publication bias, if existed, could be ignored in view of the relative large total sample size. The basic characteristics of each study are shown in Table 1. More details please refer to the Supplementary Data Sheet 1.
FIGURE 1.
The flow chart of the literature search in the meta-analysis.
Table 1.
Basic characteristics of included studies.
| Study | Country | Center (s) | Testing target (cutoff, pg/mL) | Patients/Controls | Patient type | Diagnostic criteria | Control type | Study design | Testing method | SEN | SPE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al., 2016 | China | 1 | pNFH (589) | 51/42 | ALS | R- El Escorial | NC and HC | Case-control | ELISA (BioVendor) | 82.4% | 73.8% |
| Chen et al., 2016 | China | 1 | pNFH (437) | 40/40 | ALS | R- El Escorial | NC | Cross-sectional | ELISA (BioVendor) | 97.3% | 83.8% |
| Oeckl et al., 2016 | Europe and America | 15 | NFL (1431) | 57/66 | ALS | R- El Escorial | NC and HC | Case-control | ELISA (IBL) | 79.0% | 86.4% |
| pNFH (568.5) | 75/75 | ELISA (BioVendor) | 78.7% | 93.3% | |||||||
| Goncalves et al., 2016 | Portugal | 1 | pNFH (554) | 36/15 | ALS | R- El Escorial | NC | Case-control | ELISA (BioVendor) | 75% | 60% |
| Lu et al., 2015 | UK | 1 | NFL (1781) | 38/20 | ALS | R- El Escorial | HC | Cross-sectional | ECL- immunoassay | 97% | 95% |
| Steinacker et al., 2015 | German | 1 | NFL (2200) | 253/202 | MND | R- El Escorial | NC | Case-control | ELISA (IBL) | 79% | 85% |
| pNFH (560) | ELISA (Biovendor) | 84% | 77% | ||||||||
| Ganesalingam et al., 2013 | Sweden | 1 | pNFH (1200) | 150/140 | ALS | Unknown | NC | Case-control | ELISA (BioVendor) | 90% | 87% |
| Tortelli et al., 2012 | Italy | 1 | NFL (1981) | 37/46 | ALS | El Escorial | NC | Cross-sectional | ELISA (Norgren et al., 2003) | 78.4% | 72.5% |
| Ganesalingam et al., 2011 | USA | 2 | pNFH (635) | 71/92 | ALS | El Escorial | NC and HC | Case-control | ELISA (BioVendor) | 87.7% | 93.7% |
| Reijn et al., 2009 | Netherland | 1 | NFL (22.6) | 28/14 | ALS | R- El Escorial | NC | Case-control | ELISA (Van Geel et al., 2005) | 75% | 79% |
| pNFH (502) | 29/15 | SMI35 pNfH Ab (Stenberger) | 72% | 80% |
ALS, amyotrophic lateral sclerosis; ECL, electrochemiluminescence; HC, healthy control; MND, motor neuron disease; NC, neurological control; NFL, neurofilament light chain; pNFH, phosphorylated neurofilament heavy chain; R- El Escorial, revised El Escorial; SEN, sensitivity; SPE, specificity.
Quality Assessment
Quality assessment results based on QUADAS-2 are shown in Figure 2. Concerning the domain of patient selection, only three studies were cross-sectional (Tortelli et al., 2012; Lu et al., 2015; Chen et al., 2016), while other seven studies were case-control designed; only one study reported that their patients were enrolled consecutively, and two studies reported their controls were age- and sex-matched to cases (Tortelli et al., 2012; Chen et al., 2016), and two studies reported the controls were only age-matched to the cases (Ganesalingam et al., 2011; Li et al., 2016), while other studies did not describe these information explicitly; four studies used healthy controls who “came from the community,” “were typically spouses and friends of patients,” “were initially presented with neurological symptoms and underwent lumber puncture as a part of the diagnostic examinations” but turned out to be without any neurological disease, or were not specified. (Ganesalingam et al., 2011; Lu et al., 2015; Li et al., 2016; Oeckl et al., 2016); neurological controls usually included the following: ALS mimics, other neurodegenerative diseases, inflammatory conditions, and other neurological diseases. More information about the neurological controls in each included study please refer to the Supplementary Data Sheet 1. As for the index test, all studies selected the test threshold to optimize sensitivity and specificity instead of using a pre-specified threshold. Two studies had a high risk of the flow and timing item, since some of the patients enrolled in the studies were not included in the final analysis (Reijn et al., 2009; Oeckl et al., 2016). One study did not report explicitly its diagnostic criteria for ALS, therefore its risk of reference standard remains unclear (Ganesalingam et al., 2013).
FIGURE 2.
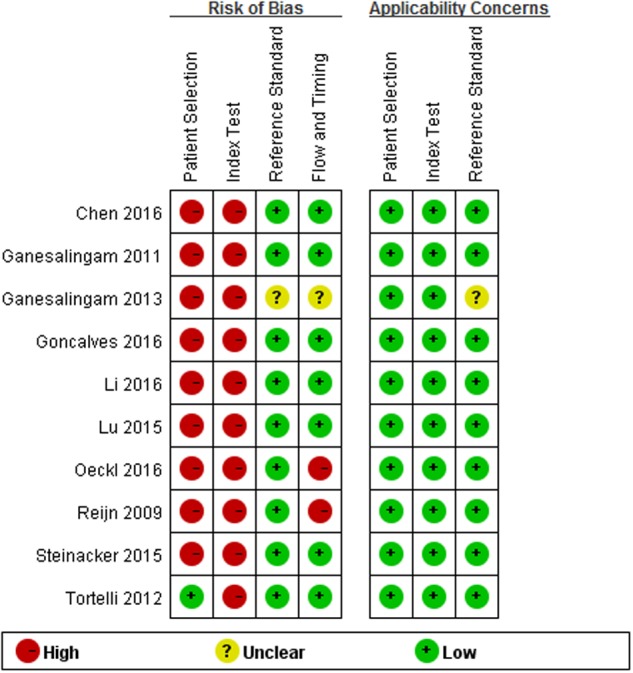
Methodological quality summary.
Diagnostic Accuracy
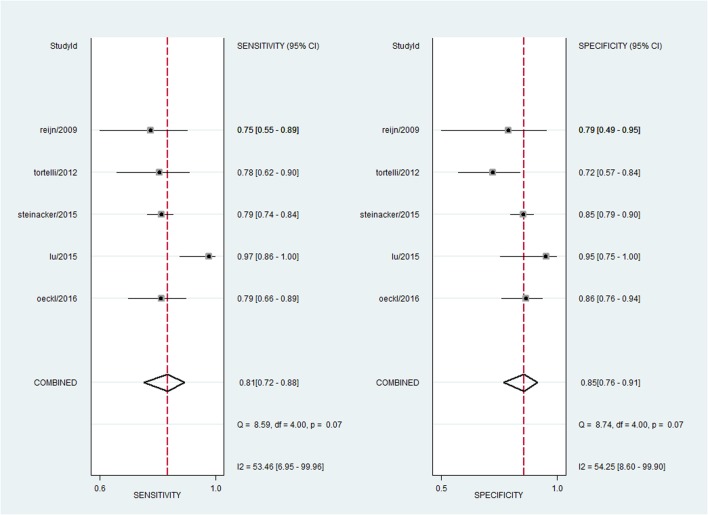
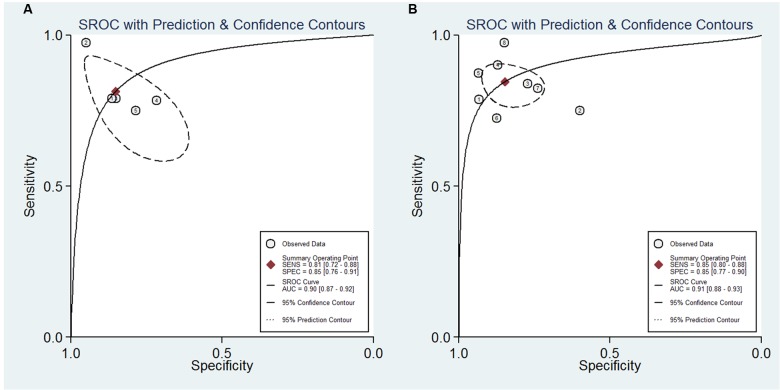
For NFL, the pooled sensitivity, specificity, PLR and NLR were 81% (95% CI, 72–88%), 85% (95% CI, 76–91%), 5.5 (95% CI, 3.1–9.8), and 0.22 (95% CI, 0.14–0.35), respectively; the summary DOR was 25 (95% CI, 9–70), and the AUC was 0.90 (95% CI, 0.87–0.92) (Figures 3 and 4A).
FIGURE 3.
Forest plots showing the sensitivity and specificity of NFL in the diagnosis of MND.
FIGURE 4.
Summary receiver operator characteristic of the accuracy of NFL (A) and pNFH (B) in the diagnosis of MND.
For pNFH, the pooled sensitivity, specificity, PLR and NLR were 85% (95% CI, 80–88%), 85% (95% CI, 77–90%), 5.5 (95% CI, 3.6–8.4), and 0.18 (95% CI, 0.13–0.25), respectively; the summary DOR was 30 (95% CI, 16–58), and the AUC was 0.91 (95% CI, 0.88–0.93) (Figures 4B and 5).
FIGURE 5.
Forest plots showing the sensitivity and specificity of pNFH in the diagnosis of MND.
Exploration of Heterogeneity and Publication Bias
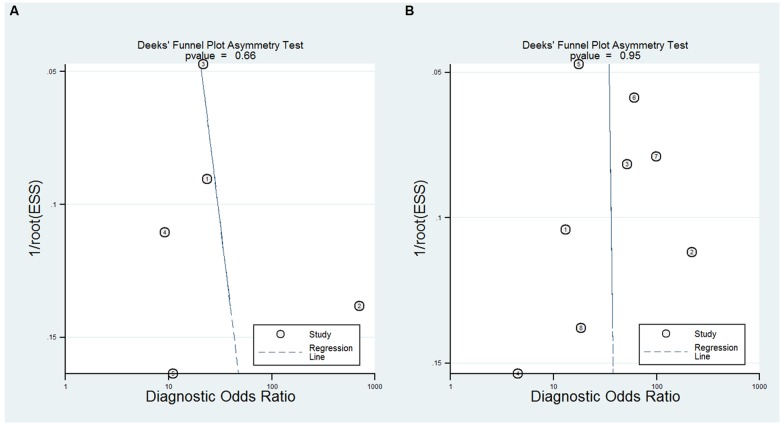
A bivariate mixed-effects model was used in this meta-analysis. No significant heterogeneity was found among studies on NFL (P = 0.493, I2 = 0), and moderate heterogeneity was detected among studies on pNFH (P = 0.077, I2 = 47). Sensitivity analyses indicated that results of the main meta-analysis were stable for NFL (Table 2). However, in the leave-one-out analysis for pNFH, the moderate heterogeneity disappeared when Ganesalingam et al. (2011; I2= 0) or Oeckl et al. (2016; I2 = 8) was removed, suggesting they might be the sources of heterogeneity. In the subgroup analyses for pNFH, when studies were confined to those used neurological controls only, the heterogeneity also disappeared (Table 3). The results of Deeks’ test did not reveal significant publication bias in this study (Figure 6).
Table 2.
Results of sensitivity analyses.
| Variables | P for Q-test | I2 statistics | Sensitivity (95% CI) | Specificity (95% CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Sensitivity analysis for NFL | ||||||||
| Oeckl et al., 2016 | 0.496 | 0 | 0.82 (0.69–0.91) | 0.86 (0.73–0.93) | 5.7 (2.6–12.7) | 0.21 (0.10–0.41) | 28 (6–119) | 0.91 (0.88–0.93) |
| Lu et al., 2015 | 0.492 | 0 | 0.79 (0.74–0.83) | 0.83 (0.76–0.88) | 4.6 (3.2–6.5) | 0.26 (0.21–0.32) | 18 (11–30) | 0.83 (0.79–0.86) |
| Steinacker et al., 2015 | 0.489 | 0 | 0.83 (0.71–0.91) | 0.84 (0.71–0.92) | 5.3 (2.5–11.4) | 0.20 (0.10–0.39) | 26 (7–101) | 0.90 (0.88–0.93) |
| Tortelli et al., 2012 | 0.426 | 0 | 0.84 (0.71–0.91) | 0.87 (0.80–0.92) | 6.5 (3.7–11.5) | 0.19 (0.10–0.36) | 35 (11–109) | 0.92 (0.89–0.94) |
| Reijn et al., 2009 | 0.497 | 0 | 0.83 (0.71–0.90) | 0.87 (0.76–0.93) | 6.2 (3.0–12.9) | 0.20 (0.10–0.37) | 32 (8–118) | 0.92 (0.89–0.94) |
| Sensitivity analysis for pNFH | ||||||||
| Li et al., 2016 | 0.065 | 51 | 0.85 (0.79–0.89) | 0.86 (0.79–0.91) | 6.1 (3.8–9.6) | 0.18 (0.12–0.25) | 35 (17–70) | 0.92 (0.89–0.94) |
| Chen et al., 2016 | 0.041 | 60 | 0.83 (0.79–0.87) | 0.85 (0.76–0.90) | 5.4 (3.3–8.7) | 0.20 (0.15–0.26) | 28 (14–55) | 0.89 (0.86–0.91) |
| Oeckl et al., 2016 | 0.361 | 0 | 0.86 (0.81–0.89) | 0.82 (0.75–0.88) | 4.9 (3.2–7.5) | 0.17 (0.12–0.25) | 28 (13–60) | 0.91 (0.88–0.93) |
| Goncalves et al., 2016 | 0.064 | 51 | 0.85 (0.81–0.89) | 0.86 (0.80–0.91) | 6.3 (4.2–9.4) | 0.17 (0.12–0.23) | 37 (21–67) | 0.92 (0.89–0.94) |
| Steinacker et al., 2015 | 0.112 | 33 | 0.85 (0.78–0.90) | 0.86 (0.78–0.91) | 6.1 (3.7–9.8) | 0.18 (0.12–0.26) | 34 (16–73) | 0.92 (0.89–0.94) |
| Ganesalingam et al., 2013 | 0.032 | 64 | 0.83 (0.79–0.87) | 0.84 (0.75–0.90) | 5.3 (3.3–8.7) | 0.20 (0.15–0.26) | 27 (14–53) | 0.88 (0.85–0.91) |
| Ganesalingam et al., 2011 | 0.169 | 8 | 0.84 (0.78–0.89) | 0.83 (0.75–0.88) | 4.9 (3.32–7.2) | 0.19 (0.13–0.27) | 26 (13–49) | 0.90 (0.87–0.93) |
| Reijn et al., 2009 | 0.091 | 41 | 0.85 (0.81–0.89) | 0.84 (0.76–0.90) | 5.5 (3.4–8.7) | 0.17 (0.13–0.24) | 32 (16–65) | 0.91 (0.88–0.93) |
AUC, area under curve; DOR, diagnostic odd ratio; NFL, neurofilament light chain;NLR, negative likelihood ratio; PLR, positive likelihood ratio; pNFH, phosphorylated neurofilament heavy chain.
Table 3.
Results of subgroup analyses for pNFH.
| Variables | P for Q-test | I2 statistics | Sensitivity (95% CI) | Specificity (95% CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Case-control studies | 0.041 | 60 | 0.83 (0.79–0.87) | 0.85 (0.76–0.90) | 5.4 (3.3–8.7) | 0.20 (0.15–0.26) | 28 (14–55) | 0.89 (0.86–0.91) |
| Cutoff <1000 pg/mL | 0.032 | 64 | 0.83 (0.79–0.87) | 0.84 (0.75–0.90) | 5.3 (3.3–8.7) | 0.20 (0.15–0.26) | 27 (14–53) | 0.88 (0.85–0.91) |
| ELISA method | 0.091 | 41 | 0.85 (0.81–0.89) | 0.84 (0.76–0.90) | 5.5 (3.4–8.7) | 0.17 (0.13–0.24) | 32 (16–65) | 0.91 (0.88–0.93) |
| ALS only | 0.112 | 33 | 0.85 (0.78–0.90) | 0.86 (0.78–0.91) | 6.1 (3.7–9.8) | 0.18 (0.12–0.26) | 34 (16–73) | 0.92 (0.89–0.94) |
| R-El Escorial only | 0.08 | 45 | 0.82 (0.79–0.86) | 0.82 (0.74–0.89) | 4.6 (4.0–7.0) | 0.21 (0.17–0.26) | 22 (13–36) | 0.85 (0.82–0.88) |
| Europe and America | 0.04 | 60 | 0.83 (0.78–0.88) | 0.86 (0.77–0.92) | 6.0 (3.5–10.2) | 0.19 (0.14–0.26) | 31 (15–66) | 0.90 (0.87–0.92) |
| NC only | 0.465 | 0 | 0.86 (0.78–0.91) | 0.81 (0.73–0.87) | 4.5 (3.0–6.7) | 0.18 (0.11–0.29) | 25 (10–62) | 0.90 (0.87–0.92) |
ALS, amyotrophic lateral sclerosis; AUC, area under curve; DOR, diagnostic odd ratio; NC, neurological control; NLR, negative likelihood ratio; PLR, positive likelihood ratio; pNFH, phosphorylated neurofilament heavy chain, R-El Escorial, revised El Escorial.
FIGURE 6.
The Deeks’ test of the diagnostic meta-analysis [(A) NFL and (B) pNFH].
Discussion
Neurofilament proteins are essential for maintaining normal axonal caliber and conduction velocity. Elevated levels of NFL and pNFH are reflections of ongoing destruction of axons, as have been shown in MND and other neurodegenerative diseases (Brettschneider et al., 2006). Our study provides a comprehensive meta-analysis of the CSF neurofilaments as diagnostic biomarkers in MND. A high AUC for both biomarkers qualified them as discriminative markers for suspected MND, and the results of sensitivity analyses demonstrated the overall results were stable. However, negative results for these markers cannot rule out MND diagnosis.
Though CSF NFL and pNFH concentrations have been shown to be consistently increased in ALS patients, the cutoff value for diagnosis among studies varies considerably, and the pNFH measurement appears to be more robust than the NFL assay (Li et al., 2016). For pNFH, its concentrations were comparable among different centers and the inter-laboratory variation of measurements was acceptable, partly because it seems to be stable against preanalytical variables and is less affected by blood contamination, repeated freeze-thaw cycles, delayed processing, or storage temperature (Koel-Simmelink et al., 2014). On the contrary, stability remains to be a particular problem for NFL (Koel-Simmelink et al., 2011), which limits its practicability and challenges it as a biomarker to be used in clinical trials. Concerning the assay methods, besides the purchasable ELISA methods, two studies (Reijn et al., 2009; Tortelli et al., 2012) utilized independent specific enzyme immunoassay for the detection of NFL (Norgren et al., 2003; Van Geel et al., 2005), and one study used electrochemiluminescence immunoassay (Lu et al., 2015). Beyond the preanalytical and analytical aspects as reasons for neurofilament cutoff variability among centers, effects of clinical differences also played an important role, especially disease duration, rate of progress and the neurological controls with a variety of syndromes. Studies have shown that CSF pNFH levels are inversely correlated with disease duration (Ganesalingam et al., 2013), while NFL levels changed only minimally throughout most of the disease course in ALS (Lu et al., 2015); CSF pNFH levels were higher in patients with predominantly UMN involvement (Brettschneider et al., 2006), while NFL levels did not differ between patients with predominantly UMN involvement and patients with predominantly LMN involvement (Tortelli et al., 2012); ALS-fast tend to have higher CSF NFL and pNFH levels than ALS-slow (Tortelli et al., 2012; Goncalves et al., 2015; Lu et al., 2015; Chen et al., 2016).
The broad range of neurofilament values might accentuate its general applicability in MND diagnosis. Thus, standardization of the above-mentioned factors in combination with personnel training and regular quality control are now recommended and commonly accepted in order to give reproducible results in different laboratories and to successfully implement neurofilaments determination as diagnostic criteria for ALS (Lehnert et al., 2014; Oeckl et al., 2016). In addition, neurofilament measurements should be ideally undertaken as close as to reported disease onset, when ALS is suspected or at diagnosis.
An important concern for neurofilaments as diagnostic biomarkers is the timing of their increase. Weydt et al. (2016) found that asymptomatic individuals carrying an ALS mutation did not show any trend toward increased levels of NFL and pNFH, even when they were close to the assumed disease onset. However, there was an increase in CSF neurofilament levels between asymptomatic carriers and symptomatic ALS patients and this increase might turn out to be a rather sudden event, possibly reflecting the onset of irreversible structural damage of motor neurons. Their results indicate that neurofilaments are state markers closely related to the symptomatic phase. Longitudinal studies are needed to help determine when the surge in neurofilament levels occurs exactly in relation to the phenotype conversion.
Despite the potential diagnostic accuracy of CSF neurofilaments, serial lumbar punctures for longitudinal neurofilaments monitoring are far less practical than blood sampling, which makes blood-derived neurofilaments level a more favorable surrogate marker for disease staging and prognosis in ALS (Puentes et al., 2014; McCombe et al., 2015). Three studies investigating the correlation between CSF and serum neurofilaments revealed that NFL levels in matched CSF and serum samples were highly correlated (Lu et al., 2015), while the correlation between plasma and CSF levels of pNFH is controversial (Ganesalingam et al., 2011; Li et al., 2016). CSF and blood matrices may act differently on neurofilament homeostasis and clearance depending on its concentrations (Lu et al., 2015). Future studies are required to further explore the relationship between CSF and blood levels of neurofilament and to optimize the detection of neurofilaments in the blood as well.
This meta-analysis had some limitations. First, we only included 10 studies, most of which had a relatively small sample size. Thus, though no significant heterogeneity or publication bias was found, we were unable to conduct meta-regression to further investigate the variability between studies, and to conclude the optimal cutoff value. Second, certain concerns were raised during quality assessment of the included studies. Most studies are case-control designed, which warrants well-designed prospective cohort studies with larger sample size in the future. Furthermore, most studies did not explicitly report whether a consecutive or random sample of patients was enrolled, and four studies used healthy controls, and ROC was used to determine a cutoff to give the optimum sensitivity and specificity in all studies. All of above might lead to overestimation of the test performance. It should also be noted that increased neurofilament level are not specific for ALS and have been reported in a number of other neurological diseases, including multiple sclerosis, dementia and brain trauma (Norgren et al., 2003; Skillback et al., 2014; Arrambide et al., 2016; Kirkcaldie and Collins, 2016; Xu et al., 2016). An ideal study should include other neurodegenerative disorders and ALS mimics as controls and use a pre-specified threshold. Finally, most studies included in the meta-analysis were from western countries, while only two studies were from China and there is no relevant report about other Asian population or African population. This becomes important because ALS patients from Asia are very different and they survive substantially longer than western patients (Nalini et al., 2008; Liu et al., 2014).
Conclusion
This meta-analysis demonstrated that CSF neurofilaments may currently serve as diagnostic biomarkers for MND. Our data support future prospective studies of neurofilament levels in CSF to determine its clinical utility. Standardized procedures for obtaining and processing CSF, and an improved understanding of how neurofilaments change with the pathology will further strengthen the case for neurofilaments in the diagnosis and therapeutic trials of ALS.
Author Contributions
DL: literature search, statistical analysis, writing of the first draft. DS: literature search, statistical analysis, writing of the first draft. HT: statistical analysis review and critique. LC: research organization, manuscript review, and critique.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fnagi.2016.00290/full#supplementary-material
More detail information of included studies.
References
- Arrambide G., Espejo C., Eixarch H., Villar L. M., Alvarez-Cermeno J. C., Picon C., et al. (2016). Neurofilament light chain level is a weak risk factor for the development of MS. Neurology 87 1076–1084. 10.1212/WNL.0000000000003085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan K. B., Glass J. D., Crook J. E., Yang C., Thomas C. S., Desaro P., et al. (2013). Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 84 467–472. 10.1136/jnnp-2012-303768 [DOI] [PubMed] [Google Scholar]
- Brettschneider J., Petzold A., Sussmuth S. D., Ludolph A. C., Tumani H. (2006). Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 66 852–856. 10.1212/01.wnl.0000203120.85850.54 [DOI] [PubMed] [Google Scholar]
- Chen X., Chen Y., Wei Q., Ou R., Cao B., Zhao B., et al. (2016). Assessment of a multiple biomarker panel for diagnosis of amyotrophic lateral sclerosis. BMC Neurol. 16:173 10.1186/s12883-016-0689-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialova L., Svarcova J., Bartos A., Ridzon P., Malbohan I., Keller O., et al. (2010). Cerebrospinal fluid and serum antibodies against neurofilaments in patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 17 562–566. 10.1111/j.1468-1331.2009.02853.x [DOI] [PubMed] [Google Scholar]
- Gaiottino J., Norgren N., Dobson R., Topping J., Nissim A., Malaspina A., et al. (2013). Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 8:e75091 10.1371/journal.pone.0075091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesalingam J., An J., Bowser R., Andersen P. M., Shaw C. E. (2013). pNfH is a promising biomarker for ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 14 146–149. 10.3109/21678421.2012.729596 [DOI] [PubMed] [Google Scholar]
- Ganesalingam J., An J., Shaw C. E., Shaw G., Lacomis D., Bowser R. (2011). Combination of neurofilament heavy chain and complement C3 as CSF biomarkers for ALS. J. Neurochem. 117 528–537. 10.1111/j.1471-4159.2011.07224.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves M., De Carvalho M., Peixoto C., Alves P., Barreto C., Oliva A., et al. (2016). Phosphoneurofilament heavy chain and vascular endothelial growth factor as cerebrospinal fluid biomarkers for ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 10.1080/21678421.2016.1212894 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Goncalves M., Tillack L., de Carvalho M., Pinto S., Conradt H. S., Costa J. (2015). Phosphoneurofilament heavy chain and N-glycomics from the cerebrospinal fluid in amyotrophic lateral sclerosis. Clin. Chim. Acta 438 342–349. 10.1016/j.cca.2014.09.011 [DOI] [PubMed] [Google Scholar]
- Kirkcaldie M. T., Collins J. M. (2016). The axon as a physical structure in health and acute trauma. J. Chem. Neuroanat. 76 9–18. 10.1016/j.jchemneu.2016.05.006 [DOI] [PubMed] [Google Scholar]
- Koel-Simmelink M. J., Teunissen C. E., Behradkia P., Blankenstein M. A., Petzold A. (2011). The neurofilament light chain is not stable in vitro. Ann. Neurol. 69 1066–1067. 10.1002/ana.22438 [DOI] [PubMed] [Google Scholar]
- Koel-Simmelink M. J., Vennegoor A., Killestein J., Blankenstein M. A., Norgren N., Korth C., et al. (2014). The impact of pre-analytical variables on the stability of neurofilament proteins in CSF, determined by a novel validated SinglePlex Luminex assay and ELISA. J. Immunol. Methods 402 43–49. 10.1016/j.jim.2013.11.008 [DOI] [PubMed] [Google Scholar]
- Kuhle J., Regeniter A., Leppert D., Mehling M., Kappos L., Lindberg R. L., et al. (2010). A highly sensitive electrochemiluminescence immunoassay for the neurofilament heavy chain protein. J. Neuroimmunol. 220 114–119. 10.1016/j.jneuroim.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Lehnert S., Costa J., de Carvalho M., Kirby J., Kuzma-Kozakiewicz M., Morelli C., et al. (2014). Multicentre quality control evaluation of different biomarker candidates for amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 15 344–350. 10.3109/21678421.2014.884592 [DOI] [PubMed] [Google Scholar]
- Li S., Ren Y., Zhu W., Yang F., Zhang X., Huang X. (2016). Phosphorylated neurofilament heavy chain levels in paired plasma and CSF of amyotrophic lateral sclerosis. J. Neurol. Sci. 367 269–274. 10.1016/j.jns.2016.05.062 [DOI] [PubMed] [Google Scholar]
- Liu M. S., Cui L. Y., Fan D. S. (2014). Age at onset of amyotrophic lateral sclerosis in China. Acta Neurol. Scand. 129 163–167. 10.1111/ane.12157 [DOI] [PubMed] [Google Scholar]
- Lu C. H., Macdonald-Wallis C., Gray E., Pearce N., Petzold A., Norgren N., et al. (2015). Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 84 2247–2257. 10.1212/WNL.0000000000001642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetto V., Sternberger N. H., Perry G., Sternberger L. A., Gambetti P. (1988). Phosphorylation of neurofilaments is altered in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 47 642–653. 10.1097/00005072-198811000-00007 [DOI] [PubMed] [Google Scholar]
- McCombe P. A., Pfluger C., Singh P., Lim C. Y., Airey C., Henderson R. D. (2015). Serial measurements of phosphorylated neurofilament-heavy in the serum of subjects with amyotrophic lateral sclerosis. J. Neurol. Sci. 353 122–129. 10.1016/j.jns.2015.04.032 [DOI] [PubMed] [Google Scholar]
- Mendonca D. M., Chimelli L., Martinez A. M. (2005). Quantitative evidence for neurofilament heavy subunit aggregation in motor neurons of spinal cords of patients with amyotrophic lateral sclerosis. Braz. J. Med. Biol. Res. 38 925–933. 10.1590/S0100-879X2005000600015 [DOI] [PubMed] [Google Scholar]
- Mendonca D. M., Martins S. C., Higashi R., Muscara M. N., Neto V. M., Chimelli L., et al. (2011). Neurofilament heavy subunit in cerebrospinal fluid: a biomarker of amyotrophic lateral sclerosis? Amyotroph. Lateral Scler. Frontotemporal Degener. 12 144–147. 10.3109/17482968.2010.542002 [DOI] [PubMed] [Google Scholar]
- Nalini A., Thennarasu K., Gourie-Devi M., Shenoy S., Kulshreshtha D. (2008). Clinical characteristics and survival pattern of 1,153 patients with amyotrophic lateral sclerosis: experience over 30 years from India. J. Neurol. Sci. 272 60–70. 10.1016/j.jns.2008.04.034 [DOI] [PubMed] [Google Scholar]
- Norgren N., Rosengren L., Stigbrand T. (2003). Elevated neurofilament levels in neurological diseases. Brain Res. 987 25–31. 10.1016/S0006-8993(03)03219-0 [DOI] [PubMed] [Google Scholar]
- Oeckl P., Jardel C., Salachas F., Lamari F., Andersen P. M., Bowser R., et al. (2016). Multicenter validation of CSF neurofilaments as diagnostic biomarkers for ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 17 404–413. 10.3109/21678421.2016.1167913 [DOI] [PubMed] [Google Scholar]
- Petzold A. (2005). Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J. Neurol. Sci. 233 183–198. 10.1016/j.jns.2005.03.015 [DOI] [PubMed] [Google Scholar]
- Petzold A., Keir G., Green A. J., Giovannoni G., Thompson E. J. (2003). A specific ELISA for measuring neurofilament heavy chain phosphoforms. J. Immunol. Methods 278 179–190. 10.1016/S0022-1759(03)00189-3 [DOI] [PubMed] [Google Scholar]
- Puentes F., Topping J., Kuhle J., van der Star B. J., Douiri A., Giovannoni G., et al. (2014). Immune reactivity to neurofilament proteins in the clinical staging of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 85 274–278. 10.1136/jnnp-2013-305494 [DOI] [PubMed] [Google Scholar]
- Reijn T. S., Abdo W. F., Schelhaas H. J., Verbeek M. M. (2009). CSF neurofilament protein analysis in the differential diagnosis of ALS. J. Neurol. 256 615–619. 10.1007/s00415-009-0131-z [DOI] [PubMed] [Google Scholar]
- Skillback T., Farahmand B., Bartlett J. W., Rosen C., Mattsson N., Nagga K., et al. (2014). CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 83 1945–1953. 10.1212/WNL.0000000000001015 [DOI] [PubMed] [Google Scholar]
- Steinacker P., Feneberg E., Weishaupt J., Brettschneider J., Tumani H., Andersen P. M., et al. (2015). Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J. Neurol. Neurosurg. Psychiatry 2015:311387 10.1136/jnnp-2015-311387 [DOI] [PubMed] [Google Scholar]
- Strong M. J., Strong W. L., Jaffe H., Traggert B., Sopper M. M., Pant H. C. (2001). Phosphorylation state of the native high-molecular-weight neurofilament subunit protein from cervical spinal cord in sporadic amyotrophic lateral sclerosis. J. Neurochem. 76 1315–1325. 10.1046/j.1471-4159.2001.00094.x [DOI] [PubMed] [Google Scholar]
- Tortelli R., Copetti M., Ruggieri M., Cortese R., Capozzo R., Leo A., et al. (2015). Cerebrospinal fluid neurofilament light chain levels: marker of progression to generalized amyotrophic lateral sclerosis. Eur. J. Neurol. 22 215–218. 10.1111/ene.12421 [DOI] [PubMed] [Google Scholar]
- Tortelli R., Ruggieri M., Cortese R., D’Errico E., Capozzo R., Leo A., et al. (2012). Elevated cerebrospinal fluid neurofilament light levels in patients with amyotrophic lateral sclerosis: a possible marker of disease severity and progression. Eur. J. Neurol. 19 1561–1567. 10.1111/j.1468-1331.2012.03777.x [DOI] [PubMed] [Google Scholar]
- Troost D., Sillevis S. P., de Jong J. M., Swaab D. F. (1992). Neurofilament and glial alterations in the cerebral cortex in amyotrophic lateral sclerosis. Acta Neuropathol. 84 664–673. 10.1007/BF00227744 [DOI] [PubMed] [Google Scholar]
- Turner M. R., Talbot K. (2013). Mimics and chameleons in motor neurone disease. Pract. Neurol. 13 153–164. 10.1136/practneurol-2013-000557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Geel W. J., Rosengren L. E., Verbeek M. M. (2005). An enzyme immunoassay to quantify neurofilament light chain in cerebrospinal fluid. J. Immunol. Methods 296 179–185. 10.1016/j.jim.2004.11.015 [DOI] [PubMed] [Google Scholar]
- Weydt P., Oeckl P., Huss A., Müller K., Volk A. E., Kuhle J., et al. (2016). Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann. Neurol. 79 152–158. 10.1002/ana.24552 [DOI] [PubMed] [Google Scholar]
- Whiting P. F., Rutjes A. W., Westwood M. E., Mallett S., Deeks J. J., Reitsma J. B., et al. (2011). QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155 529–536. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- Xu Z., Henderson R. D., David M., McCombe P. A. (2016). Neurofilaments as biomarkers for amyotrophic lateral sclerosis: a systematic review and meta-analysis. PLoS ONE 11:e164625 10.1371/journal.pone.0164625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolella S., Beghi E., Palagano G., Fraddosio A., Samarelli V., Lamberti P., et al. (2006). Predictors of delay in the diagnosis and clinical trial entry of amyotrophic lateral sclerosis patients: a population-based study. J. Neurol. Sci. 250 45–49. 10.1016/j.jns.2006.06.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
More detail information of included studies.