Abstract
Aim
The objective of this study was determining if the use of products based in olive oil, betaine and xylitol are efficacious to decrease the impact of the dry mouth in the quality of life of the patients with xerostomia due to radiotherapy treatment.
Background
Following therapeutic irradiation of the head and neck, patients with profound xerostomia have complaints associated with oral dryness, speech, and taste. There is no strong evidence that any topical therapy is effective for relieving the symptom of dry mouth.
Material and methods
40 patients who had been treated with radiotherapy for head and neck carcinoma and reported symptoms of dry mouth were included in the study. A xerostomia-related quality of life questionnaire, visual analogue scale questionnaire for subjective assessment of salivary dysfunction and salivary flow were reported before and 15 days after the use of topical products based on olive oil, betaina and xylitol.
Results
The four primary quality of life areas demonstrated significantly greater improvement after the use of topical products and all eight VAS items had favourable changes. The reduction of symptoms was statistically significant in 7 of the 8 items. After the use of the products, there were improvements in salivary flow in 45%.
Conclusions
The use of products based on olive oil, betaine and xylitol, shaped like collutory, toothpaste, gel and spray significantly improved most symptoms and the quality of life limitations produced by dry mouth in patients treated with radiotherapy.
Keywords: Head and neck neoplasms, Quality of life, Questionnaires, Visual analogue scale, Xerostomia
1. Background
Xerostomia is the most frequent complication among patients who receive radiotherapy for oral cancers. A reduction in the salivary flow rate and the decrease of its pH is paralleled with a change in saliva competence and shifting of oral microflora to cariogenic bacterial species.1 Therefore, difficulties in speech, mastication, swallowing, changes in taste, dental caries, burning sensation, microbial infections and a compromised quality of life are associated with the presence of xerostomia.2
Studies have led to three therapeutic approaches for the treatment of xerostomia: prevention, stimulation and symptomatic treatment. Prevention is not always possible, although intensity modulated radiation therapy (IMRT) gives the ability to deliver lower doses of radiation to the parotid glands. According to published results, IMRT improves the toxicity profiles, but xerostomia does not disappear.3 Saliva stimulating agents such as cholinergic agonists have all demonstrated some ability to improve xerostomia; however, they all have side effects and are contraindicated for certain medical disorders.4 There is a huge variety of products to relieve dry mouth symptoms such as chewing gums, sugar free lozenges, salivary substitutes, moisturizers and toothpastes. A Cochrane review concluded that there is no strong evidence that any topical therapy is effective for relieving the symptom of dry mouth and more studies are required to provide evidence to guide clinical care.5
A previous study report that the daily use of topical dry mouth products containing olive oil, betaine and xylitol is safe and effective in relieving symptoms of xerostomia in a population with polypharmacy-induced xerostomia.6 Olive oil is a lubricant that decreases the effects in the mucous produced by a lack of saliva and inhibits bacterial growth.7 Betaine, a naturally-occurring amino acid, maintains humidity and protects from irritation.8 Xylitol is a valuable tool in combating dental caries.9 Other components of these topical products are vitamin E to reduce the irritation of the mucous, fluoride, calcium and allantoin. These products are sodium lauryl sulfate free.
2. Aim
The objective of this study was to test if the use of the products based on olive oil, betaine and xylitol are effective in decreasing the impact of dry mouth in the quality of life in patients with xerostomia due to radiotherapy. The secondary objective was to prove if there is an improvement in salivary flow after the topical application of the aforementioned products over 15 days.
3. Patients and methods
40 patients were included in the study. Some of them were recruited by phone and others during a routine visit. Topical dry mouth products used in this investigation were toothpaste, mouthrinse, spray and gel, containing olive oil, betaine and xylitol (Xerostom® products). All subjects had been treated with radiotherapy for head and neck carcinoma and reported symptoms of dry mouth. The patients were instructed to use the toothpaste 3 times a day after main meals, using the gel around the gums, rinsing with the mouthwash and the spray whenever they felt they needed them but at least 8 times in total.
The criteria of inclusion in the study were:
-
1.
Patients must be over 18 and have been treated with radiotherapy for head and neck carcinoma.
-
2.
Patients with dry mouth symptoms, regardless of their salivary flow.
-
3.
Patients willing to use the products as instructed.
-
4.
Patients willing to answer xerostomia-related quality of life and visual analogue scale questionnaires.
-
5.
Patients who signed the informed consent form.
The criteria of exclusion were:
-
1.
Patients with Sjogren's syndrome.
-
2.
Patients unable to administrate appropriately the products, and or unable to understand the consent form.
-
3.
Patients that have taken pilocarpine or cevimeline in 7 days preceding the study.
-
4.
Patients that make provisions for an oral surgery in the course of study.
-
5.
Patients with medical pathologies that require changes in medication.
-
6.
Patients excluded during the study by some medical problem.
4. Basal measures
4.1. Salivary flow
In order to measure salivary flow rate, unstimulated saliva was collected by direct emission into a plastic glass. Saliva was weighed with high precision scales. Patients did not drink, perform oral hygiene, or smoke for at least 90 min before. Salivary flow above 0.25 ml/min was considered normal. Different grades of salivary flow are recorded in Table 1.
Table 1.
Grades of salivary flow.
| Grade | Salivary flow (ml/min) |
|---|---|
| 1 | 0.25–0.2 |
| 2 | 0.2–0.1 |
| 3 | <0.1 |
4.2. Xerostomia-related quality of life questionnaire
A 15-question validated xerostomia-related quality of life questionnaire was used. The questions refer to how dry mouth affects the quality of life in four major domains:
Physical function:
-
1.
My mouth/throat dryness limits the kinds or amounts of food I eat.
-
6.
My mouth/throat dryness makes me uncomfortable speaking in front of other people.
-
10.
My mouth/throat dryness keeps me from enjoying life.
-
12.
My mouth/throat dryness had a bad effect on tasting food.
Pain:
-
2.
My mouth/throat dryness causes discomfort.
-
3.
My mouth/throat dryness causes a lot of worry or concern.
-
7.
My mouth/throat dryness makes me nervous.
-
9.
My mouth/throat dryness keeps me from enjoying life.
Psychological function:
-
8.
My mouth/throat dryness makes me concerned about the looks of my teeth and mouth.
-
13.
My mouth/throat dryness reduces my general happiness with life.
-
14.
My mouth/throat dryness affects all aspects of my life.
-
15.
If you were to spend the rest of your life with your mouth/throat dryness just the way it is now, how would you feel about this?
Social function:
-
4.
My mouth/throat dryness keeps me from socializing (going out).
-
5.
My mouth/throat dryness makes me uncomfortable when eating in front of other people.
-
11.
My mouth/throat dryness interferes with my intimate relationships.
Each question has 5 possible answers: not at all, a little, somewhat, quite a bit and, very much. For item 15, five different responses were used: delighted, mostly satisfied, mixed satisfied/dissatisfied, mostly dissatisfied and terrible.
4.3. Visual analogue scale (VAS) questionnaire for subjective assessment of salivary dysfunction
This questionnaire has been developed and validated in previous studies and it have been demonstrated to be helpful in the diagnosis of salivary dysfunction.10 It consists of 8 questions about major aspects of salivary output: dryness of oral mucosa (lips, mouth, tongue, or throat) and oral functional ability caused by dryness (difficulty in swallowing and speaking). Two global items were included, too (amount of saliva in the mouth and level of thirst). Patients were asked to mark their responses to each item by placing a vertical line on the 100 mm horizontal scale to indicate their level of dryness. The questions of this questionnaire are:
-
1.
Rate the difficulty you experience in speaking due to dryness.
-
2.
Rate the difficulty you experience in swallowing due to dryness.
-
3.
Rate how much saliva is in your mouth.
-
4.
Rate the dryness of your mouth.
-
5.
Rate the dryness of your throat.
-
6.
Rate the dryness of your lips.
-
7.
Rate the dryness of your tongue.
-
8.
Rate the level of your thirst.
4.4. Statistical analysis
Data were analysed with Statistical Package for the Social Sciences (SPSS v16 Inc, Chicago, IL). A descriptive study of means, medians and rates of the demographic characteristics or the patients and of the scales previously referred to, as well as salivary flow. Comparison was performed for values before and after the utilization of the topical products using paired t-tests.
As it was said previously, the quality of life questionnaire evaluates four major domains. A mean of the punctuations for each domain was made, as well as a total mean of the four domains. Changes on the quality of life questionnaire were tested with analyses of variance for repeated measures. The independent variable was time and the dependent variables were the total index which was the average score of 15 items and the mean of the four separate domains.
5. Results
40 patients were recruited for this study, some of them by phone, other were invited to take part in the study when they came to a programmed appointment. Out of 40 patients, 25 were male and 15 female. Their mean age was 65 years. The most frequent carcinoma was of the larynx. In addition, to radiotherapy treatment, 25 patients were taking prescription medications associated with salivary hypofunction or xerostomia. 20 patients did not present any salivary flow without stimulation. Patient's characteristics are presented in Table 2.
Table 2.
Population characteristics.
| Characteristic | N (%; max–min) |
|---|---|
| Gender | |
| Male | 25 (62.5%) |
| Female | 15(37.5%) |
| Age | 65 (47–99) |
| Tumour location | |
| Farynx | 12 (30%) |
| Larynx | 14 (35%) |
| Oral cavity | 12 (30%) |
| Others | 2 (5%) |
| N° of xerostomic medications | |
| 0 | 15 (37%) |
| 1 | 9 (22.5%) |
| 2 | 12 (30%) |
| 3 | 3 (2.5%) |
| 5 | 1 (2.5%) |
| Time from the end of RT | 3 (1 month–9 years) |
Thirty-eight patients completed the study. One of the two that did not finish the study had complete aphagia and a nasogastric tube, he stated during the second visit that after three days of using the products he had to discontinue them because they produced such a great quantity of saliva that he could not swallow. The other patient did not complete the two weeks of use of the products because he did not notice an improvement of the salivary flow and decided to use the previous products again. The answers of both patients in the second visit were included in the final analysis.
5.1. Quality of life
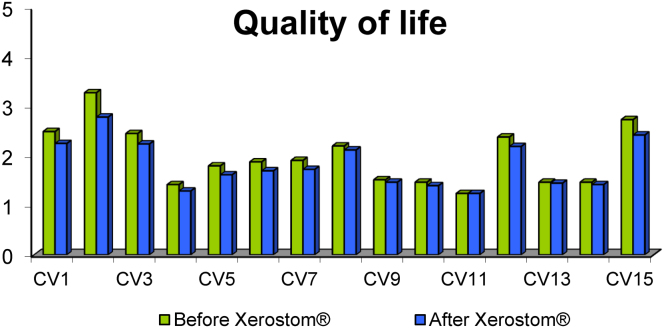
14 of 15 items improved with Xerostom®. Seven items showed statistical significance (Fig. 1). These questions were:
-
1.
The dryness of mouth limits the quantity of food that I like (p: 0.05).
-
2.
The dryness of mouth turns out to be uncomfortable to me (p: 0.0).
-
3.
I worry about the dryness of mouth (p: 0.01).
-
5.
The dryness of mouth inconveniences me when I eat in front of other people (p: 0.03).
-
6.
The dryness of mouth makes it difficult for me to talk in front of other people (p: 0.006).
-
7.
The dryness of mouth gets on my nerves to (p: 0.006).
-
12.
The dryness of mouth makes it difficult to enjoy her food (p: 0.01).
Fig. 1.
Quality of life before and after Xerostom®.
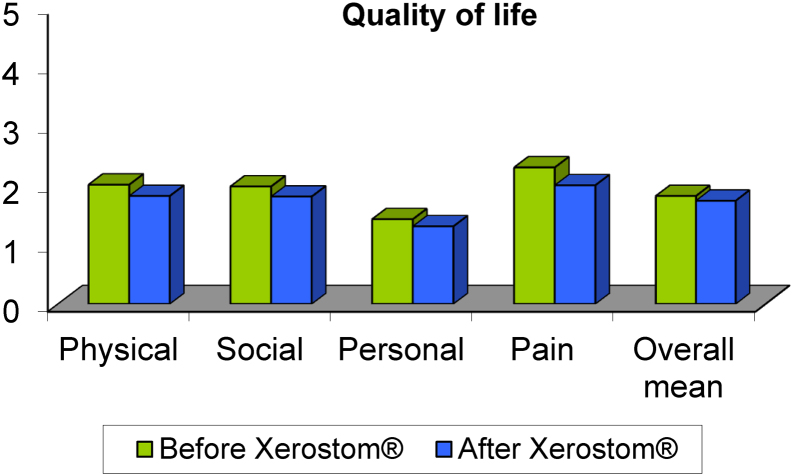
The four quality of life areas demonstrated significant improvement after the use of Xerostom® (Fig. 2): physical function (p: 0.0), psychological function (p: 0.01), social function (p: 0.01) and pain (p: 0.0).
Fig. 2.
Quality of life areas before and after Xerostom®.
5.2. Visual analogue scale questionnaire
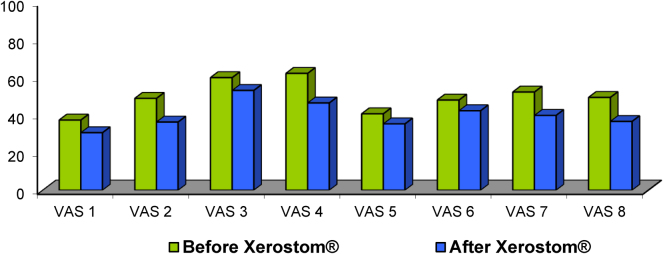
All eight VAS items had a favourable change, and the reduction of symptoms was statistically significant in 7 of the 8 items (Fig. 3). The sensation of dryness of mouth and tongue improved in 75% of the patients and the degree of thirst improved in 57% of the patients. The rate of dryness of throat was the only symptom in which differences were not statistically significant.
Fig. 3.
Visual analogue scale questionnaire before and after Xerostom®.
5.3. Salivary flow
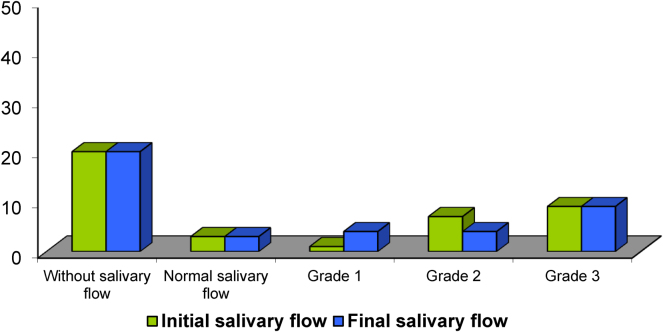
Salivary flow measurement was not possible in 20 patients, 18 of them did not have any saliva without stimulation, and the other 2 suffered surgery which made them mechanically incapable of swallowing the saliva. Of the other 20 patients (Fig. 4), 4 had a normal salivary flow, but the appearance of the saliva was very viscous and thick. Although the composition of saliva was not analysed, it could be said that the saliva was altered. The salivary flow mean was 0.17 ml/min, but if we excluded those 4 patients previously referred to, it was 0.09 ml/min. After using Xerostom® products, the salivary flow increased in 9 (45%) of the 20 patients. Three out of these 9 patients, shifted from grade 2 to grade 1. The salivary flow mean from the 20 patients was 0.25 ml/min, and 0.14 ml/min. if we excluded the 4 patients with altered saliva. These differences were not statistically significant.
Fig. 4.
Salivary flow before and after Xerostom®.
5.4. Satisfaction and side effects
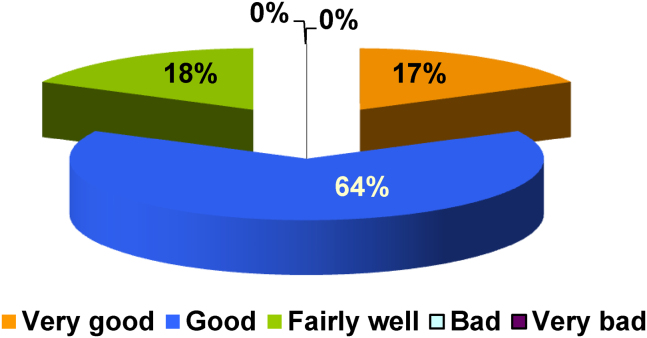
The degree of satisfaction of the patients was very high. The great majority of patients, 82%, stated that the products were good or very good (Fig. 5), 8% considered the products fairly good and no one rated them as bad or very bad. The product most highly appraised was the mouthwash, followed by the spray and the gel.
Fig. 5.
Satisfaction with the products.
No important adverse events were reported that caused treatment interruptions. Only an initial itching that disappeared rapidly was reported by 7% of the patients.
6. Discussion
Xerostomia is a very frequent side effect with high impact on the quality of life of patients after radiotherapy treatment. The decrease of saliva production causes symptoms such as oral soreness, difficulty in speaking, chewing and swallowing as well as distortion of taste and sleep.
As a result of these symptoms, patients can feel physical, psychological and social consequences.
Prevention of salivary glands damage during radiotherapy is sometimes impossible and the pharmacologic treatment with muscarinic receptor agonist, to increase the salivary flow, it is not free of side effects.
In contrast to systemic therapy, topical treatments have excellent tolerance and have shown varying degrees of success.11, 12 Current oral hygiene products can include ingredients that can worsen xerostomia symptoms. In general, topical products for patients with xerostomia should be free from irritating substances (sodium lauryl sulphate, strong aromas, alcohol), and include ingredients that will improve the symptoms.13 Xerostom® products contain olive oil that has lubricating, anti-inflammatory and anti-caries properties, they also contain betaine with osmoprotectant capabilities and xylitol with anticaries and salivary stimulation activity.
A previous study have proven that the use of Xerostom® products is safe without side effects and improves dry mouth symptoms due to polypharmacy.6 Our study also shows that these products are useful in patients with xerostomia caused by irradiation.
The use of these products improved most of the 15 items of quality of life questionnaire, 7 with statistical significance, and in the four groups of functions: social, physical, psychological and personal. In the aforementioned study with patients with xerostomia of pharmacologic cause, statistically significant improvement was obtained in 4 of the questions and in 3 of the functions, psychological, physical and personal, but not in the social.
Subjects also showed improvements in xerostomia issues as assessed by VAS. Seventy-five percent of patients reported a decrease in the sensation of dryness of mouth and tongue and 56% in the sensation of thirst. This last fact is very important because, although this question was not included in the scale used, these patients reported less interruptions during the sleep due to the dryness of mouth. Seven items showed statistically significant improvements after the use of Xerostom® products, only the rate of dryness of throat did not change, probably because the effect of these products is local and they did not reach the throat. For patients with drug-induced xerostomia the improvement was achieved only in two items.
The levels of salivary flow do not always correlate with the symptoms.14 During this study, most of the patients improved the symptoms without changes in saliva production, but surprisingly, the salivary flow also got better in a high percentage of patients.
7. Conclusion
Xerostomia is very frequent in patients after radiotherapy treatment in the area ORL with serious physical and psychological complications.
Simple measures without side effects, like the use of products based on olive oil, betaine and xylitol, in a mouthwash, toothpaste, gel and spray preparations improve most of the symptoms and the quality of life produced by salivary flow reduction.
This study highlights the importance of optimal oral hygiene with suitable products, not only to avoid complications but also to relieve xerostomia symptoms in patients with severe injury of salivary glands due to radiotherapy treatment.
Conflict of interest
Biocosmetics laboratories paid the products used in the study.
A speaker's fee was paid for Biocosmetics laboratories to Margarita Martín for comment the study results in a meeting in Barcelona Spain.
Financial disclosure
None declared.
Contributor Information
Margarita Martín, Email: margarita.martin@salud.madrid.org.
Alicia Marín, Email: alicia.marin@salud.madrid.org.
Mario López, Email: mlopezrgez@yahoo.es.
Olga Liñán, Email: olga.linan@salud.madrid.org.
Felipe Alvarenga, Email: drfevial@gmail.com.
David Büchser, Email: davidbuchser@gmail.com.
Laura Cerezo, Email: lcerezo@salud.madrid.org.
References
- 1.Almstahl A., Wikstrom M. Electrolytes in stimulated whole saliva in individuals with hyposalivation of different origins. Arch Oral Biol. 2003;48(May (5)):337–344. doi: 10.1016/s0003-9969(02)00200-5. [DOI] [PubMed] [Google Scholar]
- 2.Jham B., da Silva Freire A. Oral complications of radiotherapy in the head and neck. Braz J Otorhinolaryngol. 2006;72(5):704–708. doi: 10.1016/S1808-8694(15)31029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kouloulias V., Thalassinou S., Platoni K. The treatment outcome and radiation-induced toxicity for patients with head and neck carcinoma in the IMRT era: a systematic review with dosimetric and clinical parameters. Biomed Res Int. 2013;2013:401261. doi: 10.1155/2013/401261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbasi F., Farhadi S., Esmaili M. Efficacy of pilocarpine and bromhexine in improving radiotherapy-induced xerostomia. Dent Res Dent Clin Dent Prospects. 2012;7(2):86–90. doi: 10.5681/joddd.2013.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furness S., Worthington H.V., Bryan G., Birchenough S., McMillan R. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev. 2011;(12):CD008934. doi: 10.1002/14651858.CD008934.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Ship J.A., McCutcheon J.A., Spivakovsky S., Kerr A.R. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. J Oral Rehabil. 2007;34(October (10)):724–732. doi: 10.1111/j.1365-2842.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 7.Pretty I.A., Gallagher M.J., Martin M.V., Edgar W.M., Higham S.M. A study to assess the effects of a new detergent-free, olive oil formulation dentifrice in vitro and in vivo. J Dent. 2003;31(July (5)):327–332. doi: 10.1016/s0300-5712(03)00052-6. [DOI] [PubMed] [Google Scholar]
- 8.Rantanen I., Nicander I., Jutila K., Ollmar S., Tenovuo J., Soderling E. Betaine reduces the irritating effect of sodium lauryl sulfate on human oral mucosa in vivo. Acta Odontol Scand. 2002;60(October (5)):306–310. doi: 10.1080/00016350260248292. [DOI] [PubMed] [Google Scholar]
- 9.Makinen K.K., Makinen P.L., Pape H.R., Jr. Conclusion and review of the Michigan Xylitol Programme (1986–1995) for the prevention of dental caries. Int Dent J. 1996;46(February (1)):22–34. [PubMed] [Google Scholar]
- 10.Pai S., Ghezzi E.M., Ship J.A. Development of a Visual Analogue Scale questionnaire for subjective assessment of salivary dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(March (3)):311–316. doi: 10.1067/moe.2001.111551. [DOI] [PubMed] [Google Scholar]
- 11.Nagy K., Urban E., Fazekas O., Thurzo L., Nagy E. Controlled study of lactoperoxidase gel on oral flora and saliva in irradiated patients with oral cancer. J Craniofac Surg. 2007;18(September (5)):1157–1164. doi: 10.1097/scs.0b013e3180de6311. [DOI] [PubMed] [Google Scholar]
- 12.Epstein J.B., Emerton S., Kolbinson D.A. Quality of life and oral function following radiotherapy for head and neck cancer. Head Neck. 1999;21(January (1)):1–11. doi: 10.1002/(sici)1097-0347(199901)21:1<1::aid-hed1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Gil-Montoya J., Guardia-Lopez I., Gonzalez-Moles M. Evaluation of the clinical efficacy of a mouthwash and oral gel containing the antimicrobial proteins lactoperoxidase, lysozyme and lactoferrin in elderly patients with dry mouth – a pilot study. Gerodontology. 2008;25:3–9. doi: 10.1111/j.1741-2358.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- 14.López-Jornet P., Camacho-Alonso F. Quality of life in patients with Sjögren's syndrome and sicca complex. J Oral Rehabil. 2008;35(12):875–881. doi: 10.1111/j.1365-2842.2008.01919.x. [DOI] [PubMed] [Google Scholar]