Abstract
Aim
To evaluate toxicity of high conformal image-guided radiotherapy of the prostate bed.
Background
Radiotherapy of the prostate bed has a pivotal role in the post-operative and salvage settings, but few clinical data are available on the use of daily image guidance in combination with highly conformal techniques, and data on long-term results are lacking.
Materials and methods
We analyzed 118 patients irradiated on the prostate bed using conformal plans processed with a micro-multileaf collimator, and daily checking treatment set-up with a cone-beam CT system. Correlation between toxicity and clinical-dosimetric parameters was assessed by the Cox regression model and log-rank test. Survival analyses were performed with the Kaplan–Meier method.
Results
Median follow-up was 54.08 months. Late grade ≥2 gastro-intestinal (GI) and genito-urinary (GU) toxicity were 3.4% and 4.2%, respectively. Actuarial 4-year late grade ≥2 GI and GU toxicities were 4% and 6%, respectively. Four-year relapse-free survival was 87%. At log-rank test, acute grade ≥2 GI toxicity is associated with the use of antihypertensives (p = 0.03), and there is a trend toward significance between the use of anticoagulants and late grade ≥2 GI toxicity (p = 0.07). At Cox analysis, acute grade ≥2 GU toxicity is correlated with the percentage of bladder volume receiving more than 65 Gy (p = 0.02, HR 1.87 CI 1.25–2.8), and the maximal dose to the rectum is correlated to the development of late grade ≥2 GI toxicity (p = 0.03, HR 2.75 CI 1.10–6.9).
Conclusions
Conformal volumetric image-guided radiotherapy of the prostate bed leads to low toxicity rates.
Keywords: Prostate bed, Image-guided radiotherapy, Conformal radiotherapy, Toxicity
1. Background
Radiotherapy of the prostate bed improves biochemical control in patients with adverse pathological features after radical prostatectomy,1, 2 increases overall survival and reduces the risk of metastasis.3 Several studies investigated acute and long-term toxicity after post-prostatectomy radiotherapy,4, 5, 6 but few clinical data are available on the use of daily image guidance and of highly conformal techniques, such as intensity modulated radiotherapy (IMRT).7, 8, 9, 10
2. Aim
In the current study we evaluated acute and late toxicity, and analyzed the impact of patient characteristics and rectal and bladder dose-volume parameters on the development of toxicity after highly conformal image-guided radiotherapy.
3. Materials and methods
From August 2007 to May 2015, 118 patients underwent adjuvant or salvage 3-dimensional conformal image-guided radiotherapy (IGRT) using an on-board cone beam computed tomography (CBCT) system, after radical prostatectomy (RP). Table 1 summarizes the clinical features of this patient population. With a median time of 6.45 months (range, 1.93–11.86) from RP, adjuvant radiotherapy was administered in 80 (67.8%) patients because of positive margins, seminal vesicle invasion, or extraprostatic extension (pT3). Thirty-eight patients (32.2%) received salvage radiotherapy for a rising PSA after RP, with a median time from RP of 51.11 months (range, 5.43–197.86). As there was no significant difference in the incidence of any grade ≥2 toxicity between patients treated in post-operative or salvage setting (Table 2), we performed a pooled analysis.
Table 1.
Patient characteristics (a), and co-morbidities status (b) (118 patients).
| Mean | Median | Range | No. of patients | ||
|---|---|---|---|---|---|
| (a) Patient characteristics | |||||
| Age (years) | 66.5 | 67 | 51–79 | ||
| PSA (ng/ml) before RP | 13.57 | 9 | 1.9–120 | ||
| Pathological stage | T2a | 6 | |||
| T2b | 7 | ||||
| T2c | 24 | ||||
| T3a | 43 | ||||
| T3b | 37 | ||||
| T4 | 1 | ||||
| Surgical margins | Positive | 52 | |||
| Negative | 66 | ||||
| Gleason score | 6 | 21 | |||
| 7 (3 + 4) | 31 | ||||
| 7 (4 + 3) | 30 | ||||
| 8 | 25 | ||||
| 9 | 11 | ||||
| PSA (ng/ml) before EBRT | 0.86 | 0.16 | 0–19.7 | ||
| Treatment setting | Post-operative | 80 | |||
| Salvage | 38 | ||||
| Pre-EBRT urinary symptomsa | Yes | 33 | |||
| No | 85 | ||||
| Androgen deprivation therapy | Yes | 46 | |||
| No | 72 | ||||
| (b) Co-morbidities status | |||||
| Diabetes | Yes | 9 | |||
| No | 109 | ||||
| Colitis | Yes | 2 | |||
| No | 116 | ||||
| Smoking abitude | Yes | 48 | |||
| No | 70 | ||||
| Abdominal surgery | Yes | 47 | |||
| No | 71 | ||||
| Antihypertensive medication | Yes | 46 | |||
| No | 72 | ||||
| Anticoagulants | Yes | 22 | |||
| No | 96 | ||||
Abbreviations: RP = radical prostatectomy; EBRT = external beam radiation therapy.
Stress incontinence.
Table 2.
Toxicity per grade for all patients and per treatment group separately.
| Toxicity | Grade | Post-operative (N = 80) | Salvage (N = 38) | p-value |
|---|---|---|---|---|
| Gastro-intestinal | Acute < 2 | 79 (98.7%) | 36 (94.7%) | 0.4 |
| Acute ≥ 2 | 1 (1.2%) | 2 (5.2%) | ||
| Late < 2 | 78 (97.5%) | 36 (94.7%) | 0.39 | |
| Late ≥ 2 | 2 (2.5%) | 2 (5.2%) | ||
| Genito-urinary | Acute < 2 | 75 (93.7%) | 35 (92.1%) | 0.51 |
| Acute ≥ 2 | 5 (6.2%) | 3 (7.8%) | ||
| Late < 2 | 77 (96.2%) | 36 (94.7%) | 0.52 | |
| Late ≥ 2 | 3 (3.7%) | 2 (5.2%) | ||
All patients underwent CT (2.5 slice thickness) under radiotherapy planning conditions in the supine position; bowel and bladder preparation were prescribed11 in order to have an empty rectum and a full bladder during the CT scan and the treatment course. For each patient the clinical target volume (CTV), consisting of the prostate bed, was defined as suggested by Poortmans et al.12 Rectum and bladder were defined as solid organs; the rectum was considered from recto-sigmoid junction to the lowest level of the ischial tuberosities, and the bladder was contoured in its entirety. Planning target volumes (PTVs) were generated by an asymmetric expansion of CTVs (6 mm at the posterior margin, and 8 mm in all other directions). Conformal treatment plans were obtained on Pinnacle3 version 8.0 m (Philips Medical System, Andover, MA). For the whole patient population, a total median dose of 66 Gy (range, 66–76 Gy) at 2 Gy per fraction was prescribed to the PTV (Table 3). Radiotherapy treatment was delivered using Elekta Synergy® S linear accelerator equipped with the Beam ModulatorTM, which is a high definition multileaf collimator (4 mm leaf width at the isocenter),13 and with a kV-CBCT system for daily image-guidance. CBCTs images were used for on-line comparison with planning CT.11 Rectal and bladder volumes were checked, and on-line corrections were performed before the treatment session (set-up errors greater than 3 mm were corrected).
Table 3.
Dose–volume-histogram parameters (118 conformal plans).
| Mean organ volume (cc) (range) | Mean volume (%) (range) | Maximum dose (Gy) (range) | Mean dose (Gy) (range) | Minimum dose (Gy) (range) | |
|---|---|---|---|---|---|
| Rectum | 39.2 (15.3–82.8) | 68.6 (50–76.7) | 42.5 (29–60) | 7.9 (5.3–32.4) | |
| V65 | 20 (1–35) | ||||
| V60 | 26.8 (6–55) | ||||
| V50 | 39.5 (17–65) | ||||
| Bladder | 115.6 (25.8–375) | 70.1 (51–71.7) | 40.1 (11.2–58.8) | 6.3 (1.2–28.5) | |
| V65 | 22 (1–52) | ||||
| V60 | 31 (3–63) | ||||
| V50 | 40 (9–68) | ||||
| PTV | 115.9 (27.5–238.8) | 67.8 (66–76) | |||
Acute (within 90 days from the start of radiotherapy), and late toxicities were scored by the radiation oncologist, according to the RTOG/EORTC toxicity scale.14 Toxicity was reported as the highest toxicity in each patient.
Dose–volume-histograms (DVHs) were used to provide a quantitative analysis. Maximum and mean dose, and a set of appropriate Vx (percent of OAR volume receiving the x dose) were evaluated for the rectum and bladder. Statistical analysis was carried out using a commercial statistical software package (SPSS 9.0; SPSS Inc., Chicago, IL). Correlation between dose volume parameters considered as continuous variables and grade ≥2 toxicity was assessed by the Cox regression model. Correlation between grade ≥2 late toxicity and clinical parameters was performed using the log-rank test for categorical variables. The survival analysis and the cumulative incidence of late toxicity were performed with the Kaplan–Meier method. The Cox-model was used for multivariate analysis.
4. Results
With a median follow-up of 54.08 months (range, 4.9–103.1 months) from the end date of radiotherapy, 93 (78.8%) patients were free from biochemical recurrence, 18 (15.2%) had a biochemical recurrence (of whom 11 also with metastatic disease), 2 (1.7%) patients were dead from prostate cancer, 1 (0.8%) patient was dead from another cause. Four patients (3.4%) were lost at follow-up. Four-year relapse-free survival was 87%.
Table 4 reports the frequency of genito-urinary (GU) and gastro-intestinal (GI) toxicity. Taking into account GI toxicity, no patient experienced G3 acute or late toxicity; 3 (2.5%) patients had G2 acute toxicity (urgency and proctitis requiring medication), and 25 (21.2%) patients had G1 acute toxicity (urgency, mucus loss). Grade 2 late GI toxicity was registered in 4 (3.3%) patients (bleeding in 3 cases, and proctitis in 1), G1 late toxicity in 4 patients (proctitis in 3 cases, mucus loss in 1). Actuarial 3 and 4 years late grade ≥2 GI toxicity were 2% and 4%, respectively (Fig. 1a).
Table 4.
Radiation Therapy Oncology Group (RTOG) toxicity in 118 patients.
| Grade 0–1 | Grade ≥2 | Grade 3 | |
|---|---|---|---|
| Acute | |||
| GI toxicity | 97.5% (115/118) | 2.5% (3/118) | 0% (0/118) |
| GU toxicity | 93.3% (110/118) | 6.7% (8/118) | 2.5% (3/118) |
| Late | |||
| GI toxicity | 96.6% (114/118) | 3.4% (4/118) | 0% (0/118) |
| GU toxicity | 95.8% (113/118) | 4.2% (5/118) | 3.3% (4/118) |
Fig. 1.
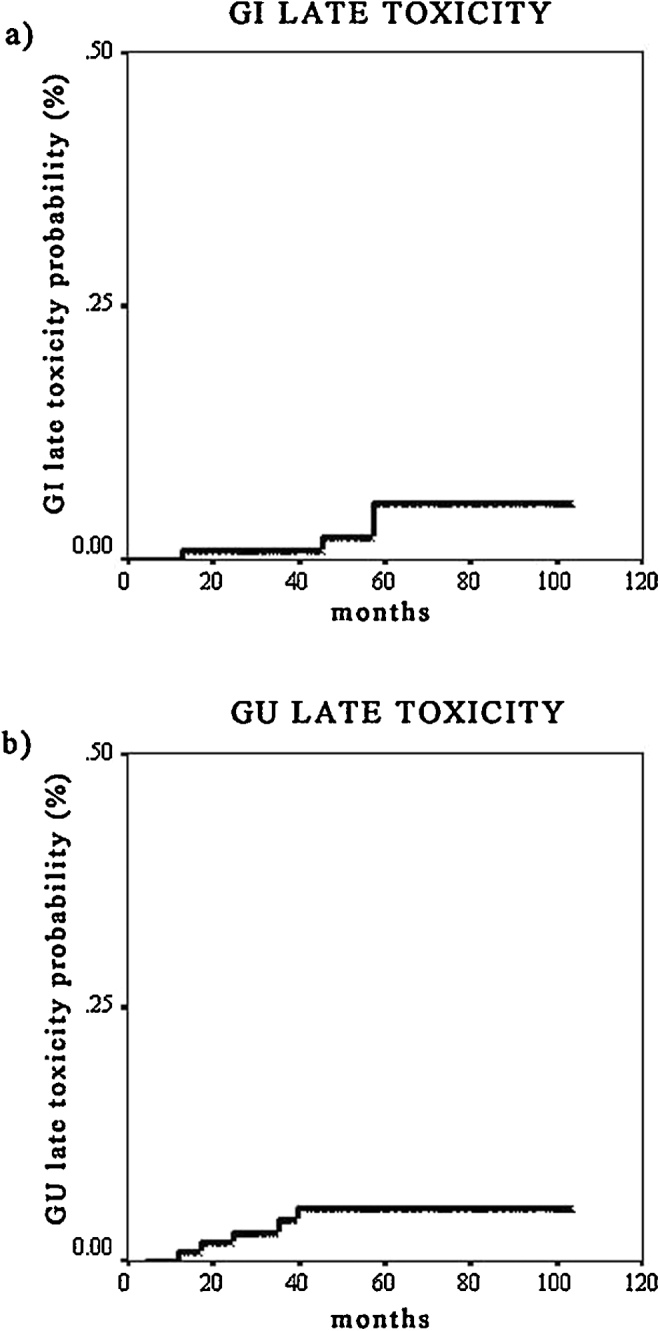
Kaplan–Meier curves. (a) Late gastro-intestinal toxicity. (b) Late genito-urinary toxicity.
Acute genito-urinary toxicity was as follows: there were 3 (2.5%) cases of G3 toxicity (2 obstructions, and 1 gross hematuria), 5 (4.2%) cases of G2 toxicity (4 with dysuria and 1 with spasm, requiring medication), and 37 (31.3%) cases of G1 (dysuria, frequency, and urgency). Four patients (3.3%) experienced grade 3 late GU toxicity (3 urethral strictures, and 1 gross hematuria), 1 (0.8%) patient had G2 late toxicity (frequency and urgency requiring medication), and 19 (16.1%) patients G1 late toxicity (frequency, or microscopic hematuria). Actuarial 3 and 4 years late grade ≥2 GU toxicity were 5% and 6%, respectively (Fig. 1b). Univariate analysis (log-rank test for categorical variables) for the correlations between acute and late toxicity and clinical variables (Table 5) evidenced that acute grade ≥2 GI toxicity is strongly associated with the use of antihypertensive medication (p = 0.03), and with concomitant androgen deprivation therapy (protective; weak correlation, p = 0.08). Finally, there is a trend toward significance between the use of anticoagulants and late grade ≥2 GI toxicity (p = 0.07). Cox proportional hazard regression model for continuous variables (Table 6) showed that acute grade ≥2 GU toxicity is strongly correlated with the bladder-V65 DVH parameter (p = 0.02, HR 1.87 CI 1.25–2.8); finally, the maximal dose to the rectum correlated with developing late grade ≥2 GI toxicity (p = 0.03, HR 2.75 CI 1.10–6.9). The multivariate analysis showed no statistically significant variable.
Table 5.
Correlation between toxicity and clinical variables (logrank test).
| Clinical variable | Acute GI grade ≥2 | Late GI grade ≥2 | Acute GU grade ≥2 | Late GU grade ≥2 |
|---|---|---|---|---|
| p-value | p-value | p-value | p-value | |
| Prescription dose | 0.40 | 0.11 | 0.35 | 0.49 |
| Hormonal therapy | 0.08 | 0.92 | 0.13 | 0.50 |
| Nicotine consumption | 0.89 | 0.15 | 0.53 | 0.32 |
| Use of antihypertensives | 0.03 | 0.11 | 0.51 | 0.29 |
| Diabetes | 0.61 | 0.53 | 0.73 | 0.45 |
| Previous abdominal surgery | 0.77 | 0.43 | 0.79 | 0.80 |
| Presence of colitis | 0.77 | 0.71 | – | – |
| Use of anticoagulants/antiaggregants | 0.40 | 0.07 | 0.83 | 0.37 |
| Pre-RT symptoms | – | – | 0.29 | 0.62 |
Table 6.
Correlation between toxicity and clinical variables (Cox proportional hazards regression).
| DVH parameter | Gastro-intestinal late toxicity |
Genito-urinary acute toxicity |
||||
|---|---|---|---|---|---|---|
| p-value | Exp (B) | 95% CI Exp (B) | p-value | Exp (B) | 95% CI Exp (B) | |
| V50 | 0.55 | 0.63 | 0.14–2.79 | 0.69 | 1.25 | 0.98–1.58 |
| V60 | 0.44 | 2.80 | 0.21–3.73 | 0.06 | 0.46 | 0.26–0.79 |
| V65 | 0.57 | 0.68 | 0.19–2.53 | 0.02 | 1.87 | 1.25–2.80 |
| Dmax | 0.03 | 2.75 | 1.10–6.94 | 0.89 | 1.01 | 0.85–1.20 |
| Dmean | 0.65 | 0.95 | 0.75–1.19 | 0.15 | 0.97 | 0.93–1.01 |
5. Conclusion
Radiotherapy of the prostate bed has a pivotal role in post-operative and salvage settings1, 2, 3, 15, but the clinical benefit of new technologies, such as IMRT and IGRT, in these patients is not clear yet16 and few data are available in the literature.7, 8, 9, 17 Some phase II studies investigated acute toxicity in hypofractionated IMRT of the prostate bed using image-guidance,18, 19, 20 but data on long-term results are lacking. In the pelvic region, 3-D prostate radiation therapy treatment plans processed with a micro-multileaf collimator provide high conformal treatments that are dosimetrically comparable with IMRT plans, but are less demanding in terms of equipment, personnel and time.21 Finally, daily IGRT for the pelvis seems to be determinant, allowing precise targeting and organs at risk sparing.20 On the other hand, concerns have been raised about the additional dose from daily image-guided procedures such as kV-CBCT,22 and many works have been published up to now investigating the dose delivered with CBCT during radiotherapy.23 In particular, Spezi et al.24 calculated the concomitant dose received by patients undergoing Elekta XVI kV-CBCT for pelvic IGRT, using the Monte Carlo method. A single CBCT delivers a mean dose of 1–3 cGy to the target volume, 1–2 cGy to the rectum, and 2–6 cGy to the femoral heads. In our series, we did not take into account the dose coming from daily kV-CBCT. We believe that information for image guidance dose to organs is important as we are in the IGRT era, but the probabilistic risk associated with the additional image-guided procedures is difficult to assess, and the low additional dose of kV-CBCT technique represents a small risk to the patient, especially if compared with the benefits provided by image guidance.
We retrospectively analyzed patients irradiated on the prostate bed using highly conformal shaped fields with a micro-multileaf collimator (median total dose, 66 Gy; 2 Gy per fraction), and daily checking treatment set-up with volumetric image-guidance. Limitations of our study are the number of patients and the absence of patient self-assessed toxicity. The strengths are the homogeneity of the patient population and treatment modality, together with the long median follow-up. Our analysis showed a good toxicity profile. With a median follow-up of 54.08 months, we reported a late grade ≥2 GI and GU toxicities of 3.4% and 4.2%, respectively. Actuarial 4 years late grade ≥2 GI and GU toxicities were 4% and 6%, respectively.
A recent retrospective study by Nath et al.7 analyzed 50 patients treated with adjuvant or salvage IMRT to a median dose of 68 Gy (range, 62–68 Gy). The prostate bed localization was obtained via planar kV imaging, performed on a daily basis using existing surgical clips as a surrogate for the prostate bed. With a median follow-up of 24 months (range, 13–38 months), acute grade 2 GI and GU toxicities were 8% and 14%, respectively. No acute grade 3 toxicity was reported. Late grade ≥2 GI and GU toxicities were 2% and 18%, respectively. The 2-year cumulative incidence of late grade ≥2 GI and GU toxicities were 2% and 16%, respectively.
Using 3D-conformal radiotherapy (median dose, 66 Gy), Bellavita et al.4 reported a late grade ≥2 GU and GI toxicities of 13.2% and 6% in 182 patients, respectively (median follow-up of 55.6 months).
It is to say that comparison between studies is quite difficult because of differences in total prescribed dose, radiation technique, and toxicity scales (Table 7). Nevertheless, differences exist in contouring between studies, and between different consensus guidelines.25 Maybe the introduction of MRI in the target definition phase will contribute to perform a better delineation of CTV and OARs.26, 27
Table 7.
RT technique, fractionation scheme, and toxicity rate from different studies.
| Author | Patients (n) | RT technique | Total dose (Gy) | Fractions (n) | Toxicity scoring | G ≥ 2 acute toxicity |
G ≥ 2 late toxicity |
||
|---|---|---|---|---|---|---|---|---|---|
| GI (%) | GU (%) | GI (%) | GU (%) | ||||||
| Bolla1, 33 | 457 | 2D-simulation | 60 | 30 | WHO/RTOG | 23.2 | 33.3 | 2.5 | 21.3 |
| Wiegel2 | 148 | 3DCRT | 60 | 30 | RTOG | – | – | 1.4 | 2.7 |
| Thompson3, 34 | 214 | 2D-simulation | 60–64 | 30–32 | (QoL questionnaires) | – | – | 3.3 | 24.3 |
| Katayama19 | 39 | IMRT, daily image guidance | 54 | 18 | CTCAE v4.0 | 18 | 0 | – | – |
WHO = World Health Organization; RTOG = Radiation Therapy Oncology Group; QoL = quality of life; CTCAE = Common Terminology Criteria for Adverse Events.
Analyzing our toxicity data in correlation with patients’ clinical variables, the log-rank test evidenced a protective effect of hormonal therapy on the intestinal tissue (weak correlation), as reported in other studies28, 29 and demonstrated in animal models.30 We found that antihypertensive medication increases the risk of acute GI toxicity, whereas it seems to have a protective effect in the series by Fellin et al.31 Finally, the use of anticoagulants or antiaggregants is weakly associated to late GI toxicity, as reported by Choe et al.29 and Takeda et al.32
Our Cox proportional hazards regression confirms the relation between dose–volume parameters (maximal dose to the rectum, and bladder V65) and toxicity. Hence, new treatment strategies addressed to reduce the irradiation of normal tissues might allow dose escalation and hypofractionation,20 which are becoming common in the post-prostatectomy setting. However, the recent population-based study by Goldin et al.16 evidences a relative lack of comparative effectiveness with data demonstrating the superiority of IMRT in respect to 3DCRT in terms of outcomes (disease recurrence, and late toxicity).
The role of new technologies in the post-prostatectomy radiotherapy needs to be further investigated. Our results confirm the importance of high conformal radiotherapy, and image-guidance in this setting of patients. In particular, 3D-plans processed with a micro-multileaf collimator providing highly conformal treatments, and the use of daily CBCT to check radiotherapy delivery lead to low acute and late toxicity rates.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Bolla M., van Poppel H., Tombal B. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380(9858):2018–2027. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 2.Wiegel T., Bartkowiak D., Bottke D. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66(2):243–250. doi: 10.1016/j.eururo.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Thompson I.M., Tangen C.M., Paradelo J. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term follow up of a randomized clinical trial. J Urol. 2009;181(3):956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellavita R., Massetti M., Abraha I. Conformal postoperative radiotherapy in patients with positive resection margins and/or pT3–4 prostate adenocarcinoma. Int J Radiat Oncol Biol Phys. 2012;84(3):e299–e304. doi: 10.1016/j.ijrobp.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Fonteyne V., Ost P., Vanpachtenbeke F. Rectal toxicity after intensity modulated radiotherapy for prostate cancer: which rectal dose volume constraints should we use? Radiother Oncol. 2014;113(3):398–403. doi: 10.1016/j.radonc.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Daly T., Hickey B.E., Lehman M., Francis D.P., See A.M. Adjuvant radiotherapy following radical prostatectomy for prostate cancer. Cochrane Database Syst Rev. 2011;(12):CD007234. doi: 10.1002/14651858.CD007234.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Nath S.K., Sandhu A.P., Rose B.S. Toxicity analysis of postoperative image-guided intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78(2):435–441. doi: 10.1016/j.ijrobp.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Wong G.W., Palazzi-Churas K.L., Jarrard D.F. Salvage hypofractionated radiotherapy for biochemically recurrent prostate cancer after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2008;70(2):449–455. doi: 10.1016/j.ijrobp.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Cheng J.C., Schultheiss T.E., Nguyen K.H., Wong J.Y. Acute toxicity in definitive versus postprostatectomy image-guided radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;71(2):351–357. doi: 10.1016/j.ijrobp.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 10.Zhu M., Bharat S., Michalski J.M., Gay H.A., Hou W.H., Parikh P.J. Adaptive radiation therapy for postprostatectomy patients using real-time electromagnetic target motion tracking during external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(4):1038–1044. doi: 10.1016/j.ijrobp.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingrosso G., Carosi A., Ponti E. Acute and late toxicity after three-dimensional conformal image-guided radiotherapy for localized prostate cancer. Cancer Invest. 2014;32(10):526–532. doi: 10.3109/07357907.2014.970193. [DOI] [PubMed] [Google Scholar]
- 12.Poortmans P., Bossi A., Vandeputte K. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother Oncol. 2007;84(2):121–127. doi: 10.1016/j.radonc.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Patel L., Glendinning A.G., Kirbi M.C. Dosimetric characteristics of the Elekta Beam ModulatorTM. Phys Med Biol. 2005;50:5479–5492. doi: 10.1088/0031-9155/50/23/004. [DOI] [PubMed] [Google Scholar]
- 14.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 15.Apicella G., Beldì D., Marchioro G. Postoperative radiotherapy in prostate cancer: analysis of prognostic factors in a series of 282 patients. Rep Pract Oncol Radiother. 2014;20(2):113–122. doi: 10.1016/j.rpor.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldin G.H., Sheets N.C., Meyer A.M. Comparative effectiveness of intensity-modulated radiotherapy and conventional conformal radiotherapy in the treatment of prostate cancer after radical prostatectomy. JAMA Intern Med. 2013;173(12):1136–1143. doi: 10.1001/jamainternmed.2013.1020. [DOI] [PubMed] [Google Scholar]
- 17.De Meerleer G., Fonteyne V., Meersschout S. Salvage intensity-modulated radiotherapy for rising PSA after radical prostatectomy. Radiother Oncol. 2008;89(2):205–213. doi: 10.1016/j.radonc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Massaccesi M., Cilla S., Deodato F. Hypofractionated intensity-modulated radiotherapy with simultaneous integrated boost after radical prostatectomy: preliminary results of a phase II trial. Anticancer Res. 2013;33(6):2785–2789. [PubMed] [Google Scholar]
- 19.Cozzarini C., Fiorino C., Di Muzio N. Hypofractionated adjuvant radiotherapy with helical tomotherapy after radical prostatectomy: planning data and toxicity results of a Phase I–II study. Radiother Oncol. 2008;88(1):26–33. doi: 10.1016/j.radonc.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Katayama S., Striecker T., Kessel K. Hypofractionated IMRT of the prostate bed after radical prostatectomy: acute toxicity in the PRIAMOS-1 trial. Int J Radiat Oncol Biol Phys. 2014;90(4):926–933. doi: 10.1016/j.ijrobp.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Carosi A., Ingrosso G., Ponti E., Lancia A., Santoni R. Intensity-modulated and 3D-conformal radiotherapy in hypofractionated prostate cancer treatment using Elekta Beam Modulator(TM) micro-MLC: a dosimetric analysis. Acta Oncol. 2016;55(1):116–121. doi: 10.3109/0284186X.2015.1046559. [DOI] [PubMed] [Google Scholar]
- 22.Donovan E.M., James H., Bonora M., Yarnold J.R., Evans P.M. Second cancer incidence risk estimates using BEIR VII models for standard and complex external beam radiotherapy for early breast cancer. Med Phys. 2012;39(10):5814–5824. doi: 10.1118/1.4748332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alaei P., Spezi E. Imaging dose from cone beam computed tomography in radiation therapy. Phys Med. 2015;31(7):647–658. doi: 10.1016/j.ejmp.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Spezi E., Downes P., Jarvis R., Radu E., Staffurth J. Patient-specific three-dimensional concomitant dose from cone beam computed tomography exposure in image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(1):419–426. doi: 10.1016/j.ijrobp.2011.06.1972. [DOI] [PubMed] [Google Scholar]
- 25.Malone S., Croke J., Roustan-Delatour N. Postoperative radiotherapy for prostate cancer: a comparison of four consensus guidelines and dosimetric evaluation of 3D-CRT versus tomotherapy IMRT. Int J Radiat Oncol Biol Phys. 2012;84(3):725–732. doi: 10.1016/j.ijrobp.2011.12.081. [DOI] [PubMed] [Google Scholar]
- 26.Sefrova J., Odrazka K., Paluska P. Magnetic resonance imaging in postprostatectomy radiotherapy planning. Int J Radiat Oncol Biol Phys. 2012;82(2):911–918. doi: 10.1016/j.ijrobp.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Croke J., Malone S., RoustanDelatour N. Postoperative radiotherapy in prostate cancer: the case of the missing target. Int J Radiat Oncol Biol Phys. 2012;83(4):1160–1168. doi: 10.1016/j.ijrobp.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 28.Valdagni R., Rancati T., Fiorino C. Development of a set of nomograms to predict acute lower gastrointestinal toxicity for prostate cancer 3D-CRT. Int J Radiat Oncol Biol Phys. 2008;71(4):1065–1073. doi: 10.1016/j.ijrobp.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 29.Choe K.S., Jani A.B., Liauw S.L. External beam radiotherapy for prostate cancer patients on anticoagulation therapy: how significant is the bleeding toxicity? Int J Radiat Oncol Biol Phys. 2010;76(3):755–760. doi: 10.1016/j.ijrobp.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 30.Mangoni M., Sottili M., Gerini C. Protective effect of leuprorelin on radiation-induced intestinal toxicity. Anticancer Res. 2015;35(7):3875–3884. [PubMed] [Google Scholar]
- 31.Fellin G., Rancati T., Fiorino C. Long term rectal function after high-dose prostate cancer radiotherapy: results from a prospective cohort study. Radiother Oncol. 2014;110(2):272–277. doi: 10.1016/j.radonc.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Takeda K., Ogawa Y., Ariga H. Clinical correlations between treatment with anticoagulants/antiaggregants and late rectal toxicity after radiotherapy for prostate cancer. Anticancer Res. 2009;29(5):1831–1834. [PubMed] [Google Scholar]
- 33.Bolla M., van Poppel H., Collette L. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366(9485):572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 34.Thompson I.M., Jr., Tangen C.M., Paradelo J. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296(19):2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]


