Abstract
Mirror visual feedback (MVF) is potentially a powerful tool to facilitate recovery of disordered movement and stimulate activation of under-active brain areas due to stroke. The neural mechanisms underlying MVF have therefore been a focus of recent inquiry. Although it is known that sensorimotor areas can be activated via mirror feedback, the network interactions driving this effect remain unknown. The aim of the current study was to fill this gap by using dynamic causal modeling to test the interactions between regions in the frontal and parietal lobes that may be important for modulating the activation of the ipsilesional motor cortex during mirror visual feedback of unaffected hand movement in stroke patients. Our intent was to distinguish between two theoretical neural mechanisms that might mediate ipsilateral activation in response to mirror-feedback: transfer of information between bilateral motor cortices versus recruitment of regions comprising an action observation network which in turn modulate the motor cortex. In an event-related fMRI design, fourteen chronic stroke subjects performed goal-directed finger flexion movements with their unaffected hand while observing real-time visual feedback of the corresponding (veridical) or opposite (mirror) hand in virtual reality. Among 30 plausible network models that were tested, the winning model revealed significant mirror feedback-based modulation of the ipsilesional motor cortex arising from the contralesional parietal cortex, in a region along the rostral extent of the intraparietal sulcus. No winning model was identified for the veridical feedback condition. We discuss our findings in the context of supporting the latter hypothesis, that mirror feedback-based activation of motor cortex may be attributed to engagement of a contralateral (contralesional) action observation network. These findings may have important implications for identifying putative cortical areas, which may be targeted with non-invasive brain stimulation as a means of potentiating the effects of mirror training.
Keywords: Mirror feedback, DCM, Motor control, fMRI, Virtual reality, Visuomotor integration
1. Introduction
The use of mirror visual feedback (MVF) for neurorehabilitation of stroke impairment has grown in the past 20 years, however, little is known about the underlying neurophysiological mechanisms by which MVF may modulate activity in the ipsilesional sensorimotor cortex, and hence aid recovery (Deconinck et al., 2015). We have recently shown that virtual MVF of motion of the non-affected hand can elicit significant activation of the ipsilesional sensorimotor cortex in the absence of movement of the affected hand (Saleh et al., 2014). Critically, we showed that this activation overlapped with areas involved in volitional control of the affected hand. These data, therefore, provide a neural basis for virtual mirror feedback, by showing that mirror feedback can activate ipsilesional motor-related hubs that are important for the recovery process. The findings about the neural underpinnings of mirror feedback are encouraging particularly in light of recent clinical studies showing that MVF may show promise in restoring function after stroke (Yavuzer et al., 2008, Dohle et al., 2009, Thieme et al., 2012, Thieme et al., 2013). The goal of this project is to fill this gap by identifying the neural network and mechanisms by which the ipsilesional motor cortex is facilitated by MVF.
The key question we ask is, what is the source of the signal mediating MVF-elicited facilitation of ipsilesional sensorimotor cortex? Review of available literature posits two competing hypotheses that we aim to test.
The first hypothesis predicts that MVF may mediate the interhemispheric interactions between the motor cortices. Support for this prediction is rooted in a magnetoencephalography (MEG) study or chronic stroke patients that found movement-related beta desynchronization between motor cortices to be less lateralized during bilateral hand movement performed with MVF than when performed without MVF (Rossiter et al., 2015). Additional support for this hypothesis arises from literature on the neural basis of cross-activation, a phenomenon akin to overflow of activation from one hemisphere to the other during vigorous movement (Lee et al., 2010, Sehm et al., 2010, Reissig et al., 2014). In apparent contradiction, studies using TMS to directly measure changes in interhemispheric inhibitory (IHI) balance resulting from MVF have indicated either a reduction (Carson and Ruddy, 2012, Avanzino et al., 2014), or no change in IHI (Lappchen et al., 2012, Nojima et al., 2012, Lappchen et al., 2015). Therefore, it remains unclear if it is indeed the contralesional motor cortex that modulates the ipsilesional motor cortex to mediate the MVF facilitation. Here, we directly investigate this prediction by using a unilateral movement with and without MVF, to test if the source of MVF-elicited facilitation of the inactive (ipsilesional) M1 arises from the active (contralesional) motor cortex.
The second hypothesis predicts that MVF may activate a bilateral action observation network, which in turn modulates the inactive motor cortex. Here, we operationally define the action observation network (AON), according to published work, as a bilateral fronto-parietal network that is activated when primates or humans observe biological actions (Buccino et al., 2001, Howatson et al., 2013) such as the focused observation of real or virtual hand motion (Perani et al., 2001, Suchan et al., 2007, Chong et al., 2008a, Chong et al., 2008b, Adamovich et al., 2009). Parietal regions comprising the AON have been shown to be involved in transcallosally communicating with frontal areas for visuomotor remapping (Blangero et al., 2011, Pisella et al., 2011, Zult et al., 2014), and to modulate activation of M1 (Koch et al., 2009, Grefkes and Fink, 2011). Thus, it is possible that MVF-mediated facilitation of ipsilateral M1 may arise from selective regions comprising the AON. In support of this prediction is recent fMRI evidence that parts of the AON network, including inferior and superior parietal lobules, superior temporal gyrus, and sensorimotor areas, are recruited in MVF paradigms (Michielsen et al., 2011a, Hamzei et al., 2012, Saleh et al., 2014). Given the known parietal cortex involvement in movement observation and visuomotor integration, it is possible that MVF-mediated changes in motor cortex excitability arise from the AON network, perhaps via parietal-M1 modulation.
The above two hypotheses bear significant importance for stroke patients who have persistent undesirable increases in IHI from contralesional to ipsilesional M1 during hand movement (Murase et al., 2004) and weakened parietal-M1 interactions (Grefkes and Fink, 2011, Takeuchi et al., 2012). Empirical evidence suggests that the activation of these regions (Grefkes and Fink, 2011, Rehme et al., 2011, Rehme et al., 2012), and restored interactions between these regions measured as functional and effective connectivity are important predictors of recovery (He et al., 2007, Carter et al., 2010, Wang et al., 2010, van Meer et al., 2012, De Vico Fallani et al., 2016). Therefore, understanding the MVF network interactions may unveil if mirror feedback has the potential to engage circuits in a manner that may be favorable for recovery.
The focus of the current investigation was to build on our understanding of the neural mechanisms underlying virtual MVF, by analyzing the effective connectivity in our previously published dataset (Saleh et al., 2014). We used Dynamic Causal Modeling (DCM) to model interactions among activated brain regions and draw inferences on the connectivity strength within this neural network (Friston et al., 2003). Classical deterministic bilinear DCM allows testing the changes in a neural state of a brain region in terms of changes in intrinsic neurophysiological interactions among brain regions independent of the experimental stimulus (input), extrinsic interactions between brain regions modulated by the input, and the direct influence of the input on each region's activity.
2. Materials and methods
2.1. Participants
This study included fifteen right-handed (Oldfield, 1971) subjects, with hemiparesis due to stroke (5 right-hemiplegics, 5 females, mean age 54 ± 12 years, range: 37–74 years old). The subjects participated after signing informed consent approved by the institutional review board. Two subjects were excluded from analysis. One subject was excluded for excessive head motion and another because the brain lesion encompassed the sensorimotor cortex (see Table 1 for clinical information).
Table 1.
Subjects'clinical information.
| Subject | Age | Gender | Months | CMA/CMH | Lesion |
|---|---|---|---|---|---|
| S1 | 63 | F | 53 | 6/4 | L cortical |
| S2 | 55 | M | 41 | 5/4 | L subcortical |
| S3⁎ | 49 | M | 144 | 5/4 | L subcortical |
| S4 | 74 | M | 9 | 6/6 | R cortical |
| S5 | 70 | F | 96 | 7/5 | R subcortical |
| S6 | 58 | M | 132 | 5/4 | R cortical |
| S7 | 37 | M | 92 | 4/3 | R subcortical |
| S8 | 69 | F | 18 | 7/7 | R subcortical |
| S9 | 68 | M | 78 | 6/6 | R cortical |
| S10 | 48 | F | 148 | 4/3 | R cortical |
| S11⁎ | 41 | F | 70 | 6/6 | R cortical |
| S12 | 43 | M | 11 | 4/4 | L subcortical |
| S13 | 41 | M | 158 | 6/6 | L cortical |
| S14 | 53 | M | 156 | 6/6 | R subcortical |
| S15 | 39 | F | 14 | 4/3 | R cortical |
CVA, cerebrovascular accident; CMA, Chedokee-McMaster Motor AssessmentArm Scale; CMH, Chedokee-McMaster Motor Assessment Hand Scale; dWMFT, Distal Wolf Motor Function Test; L, left; R, right; Months, time since CVA in months. Asterisks highlight the subjects excluded from the analysis.
2.2. Experiment task and visual feedback
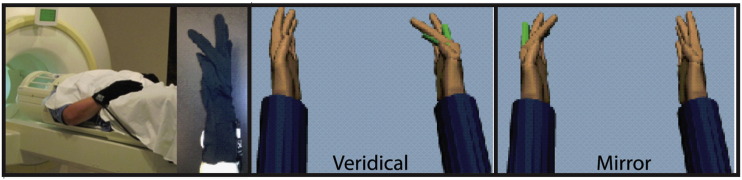
During the experiment, subjects lay in the scanner and wore an MRI-compatible instrumented glove recording 14 joint angles of the hand in real time. Subjects viewed back-projected visual stimuli reflected in a mirror within the scanner bore. In four consecutive scanning runs, subjects moved the non-paretic hand and watched the feedback in the VR environment. Movement in each trial was cued by a text prompt “move”, cuing the subject to perform an out-and-back finger movement with a short pause at the target location, followed by a text prompt “rest”, cuing the subset to rest at the start position and await the next trial. The “move” prompt was displayed for the duration of the trial event (5 s), and the “rest” prompt was displayed for the duration of the rest period (random 4–7-sec jittered). Subjects were instructed to complete the movement within the “move” epoch. Each scanning run included eight repetitions of four randomly interleaved visual feedback conditions: 1) movement of the ipsilateral VR hand model (veridical-feedback condition), 2) movement of the contralateral VR hand model (mirror-feedback condition), 3) rotation of an ellipsoidal object ipsilateral to the non-paretic moving hand (CTRL, veridical-feedback condition), and 4) rotation of an ellipsoidal object contralateral to the moving hand (CTRL, mirror-feedback condition). The hardware and experiment setup are explained in more detail in our previous publication (Saleh et al., 2014). In this study, we investigated the effect of conditions 1 and 2 on the effective connectivity within the sensorimotor network (Fig. 1).
Fig. 1.
Subjects wore MRI compatible instrumented data gloves that recorded finger movement in real-time. Finger motion was back-projected onto a screen, showing two virtual hand models. On a given trial, motion of the unaffected hand actuated one of the VR hands, located on the same (Veridical) or opposite (Mirror) side relative to the actual hand. In separate, randomly interleaved, control conditions the VR hands were replaced with ellipsoids that rotated about an oblique axis to rule out visual confounds.
2.3. fMRI acquisition and analysis
fMRI data were acquired using a 3-T Siemens Allegra head-only scanner with a Siemens standard 8-channel head coil. Functional images parameters were: (TR = 2000 ms, TE = 30 ms, FOV = 100 mm, voxel size = 3 × 3 × 3 mm, number of slices = 32, inter-slice time = 62 ms, number of volumes = 175). A high-resolution T1-weighted structural image using an MPRAGE sequence was also acquired for each subject (TR = 2000 ms, TE = 4.38 ms, voxel size = 0.938 × 0.938 × 1 mm, number of slices = 176, slice thickness = 1 mm). Imaging data were analyzed using Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm) Matlab (Matlab R2012a) toolbox.
fMRI preprocessing included alignment of the functional volumes to the first volume, co-registering with the structural image, and correcting for slice timing. Normalization of the data was optimized by creating a binary mask of each patient's lesion using DARTEL toolbox (Ashburner, 2007), consistent with established approaches (Brett et al., 2001, Ashburner and Friston, 2005, Ashburner, 2007, Andersen et al., 2010). Data were smoothed using an 8-mm full width at half maximum Gaussian kernel.
The raw data of the 5 subjects with left hemisphere lesions were flipped to the right so that all subjects had a “virtual” right hemisphere lesion in order to analyze the results in random-effects GLM model at the group level (Crinion et al., 2007). In the GLM, the four experimental sessions were concatenated into one session.
2.4. Dynamic causal modeling (DCM)
2.4.1. Selection of regions of interest
The anatomical nodes used for DCM analysis were derived from a previous analysis performed on the same data in which subjects were exposed to mirror and veridical feedback of non-paretic hand movement (Saleh et al., 2014). The main effect contrast of mirror feedback vs. control, and analysis of functional network interactions using the psychophysiological interaction (PPI), identified four regions which were significantly recruited for mirror feedback: contra- and ipsilesional parietal cortex along the intraparietal sulcus (cPar and iPar), and contra- and ipsilesional primary motor cortices (cM1 and iM1). The subject-specific coordinates of these regions were identified based on the location of the nodes in the group maxima (from the group level analysis of the main effect contrast). Thus, we selected the subject-specific maxima in regions that were within 8 mm of the group maxima and in the same gyrus. This approach, in defining the center coordinates of each region of interest (ROI, 8 mm radius), has been shown to reduce the undesired effects of inter-subject variability in ROI location (Gandolla et al., 2014). These subject-specific ROI coordinates were averaged to obtain group level ROI center coordinates (see Table 2).
Table 2.
ROI mean MNI coordinates in mm.
| Region | X (std) | Y (std) | Z (std) |
|---|---|---|---|
| iPar | 41 (6.6) | − 26 (3.9) | 50 (3.5) |
| cPar | − 30 (0) | − 54 (3.8) | 51 (0) |
| cM1 | − 41 (1.1) | − 6 (2.1) | 51 (4.3) |
| iM1 | 39 (3.2) | − 6 (1.7) | 48 (2.9) |
Std: standard deviation, i: ipilesional, c: contralesional.
2.4.2. Model selection and family analysis
Depending on the number of nodes, the number of possible models can be impossible to explore exhaustively (Stephan et al., 2010, Friston et al., 2011a). Thus, the number of plausible models was reduced based on prior knowledge of structural and functional connectivity between these regions. To limit the number of possible models to estimate, we used a step-wise procedure that first identified the architecture of interactions between cM1, iM1, cPar, iPar and the site of the driving input, and secondarily explored the possible models of extrinsic connectivity in each visual feedback condition, per established methods (Nagy et al., 2012, Vossel et al., 2012).
2.4.3. DCM models
2.4.3.1. Endogenous models
We modeled four possible structural connections between cM1, iM1, cPar, and iPar, based on established anatomy (Ferbert et al., 1992, Di Lazzaro et al., 1999): 1) bidirectional between cM1-iM1 and cPar-iPar and unidirectional from cPar-cM1 and iPar-iM1, 2) bidirectional between cM1-iM1 and unidirectional from cPar-cM1 and iPar-iM1, 3) bidirectional between cM1-iM1, cPar-iPar, and iPar-iM1, and unidirectional from cPar-cM1, 4) bidirectional between cM1-iM1 and iPar-iM1, and unidirectional from cPar-cM1.
2.4.3.2. Driving inputs
The driving inputs to the models were: 1) through cPar and iPar in both conditions, 2) through iPar in the mirror feedback condition and cPar in the veridical feedback condition, or 3) through cPar in both feedback conditions and through iPar in the mirror feedback condition.
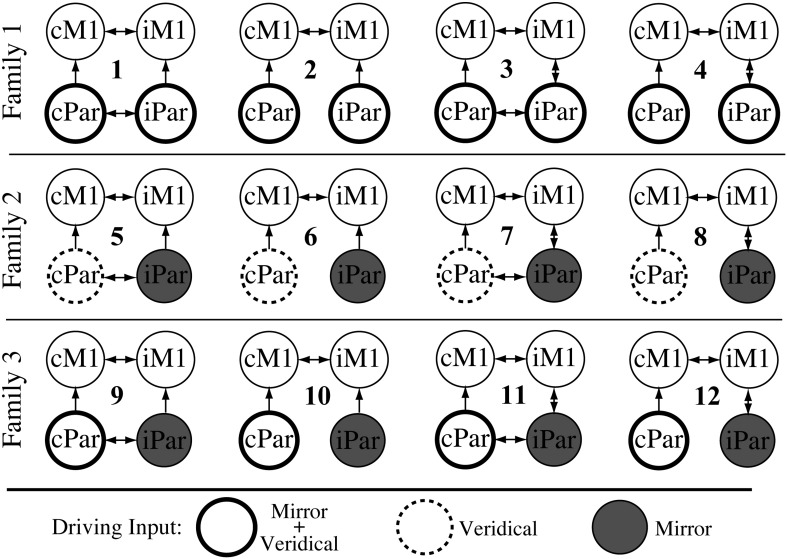
This led to 12 DCM models of possible endogenous connections and driving inputs (4 × 3). We used Bayesian model selection to compare these models in order to find the optimal driving input and the optimal representative intrinsic interactions between the four regions (Fig. 2).
Fig. 2.
Model space of endogenous connectivity for Family 1, Family 2, and Family 3. Each family has four different A matrix structures. The families differ based on the site of the driving input in each condition: In Family 1, the driving input is into cPar and iPar for the Mirror and Veridical conditions (thick circles). In Family 2, the driving input into cPar is for Veridical feedback (dashed circles) and into iPar for Mirror feedback (gray circles). In Family 3, the driving input into cPar is for Mirror and Veridical feedback (thick circles) and into iPar for Mirror feedback (gray circles).
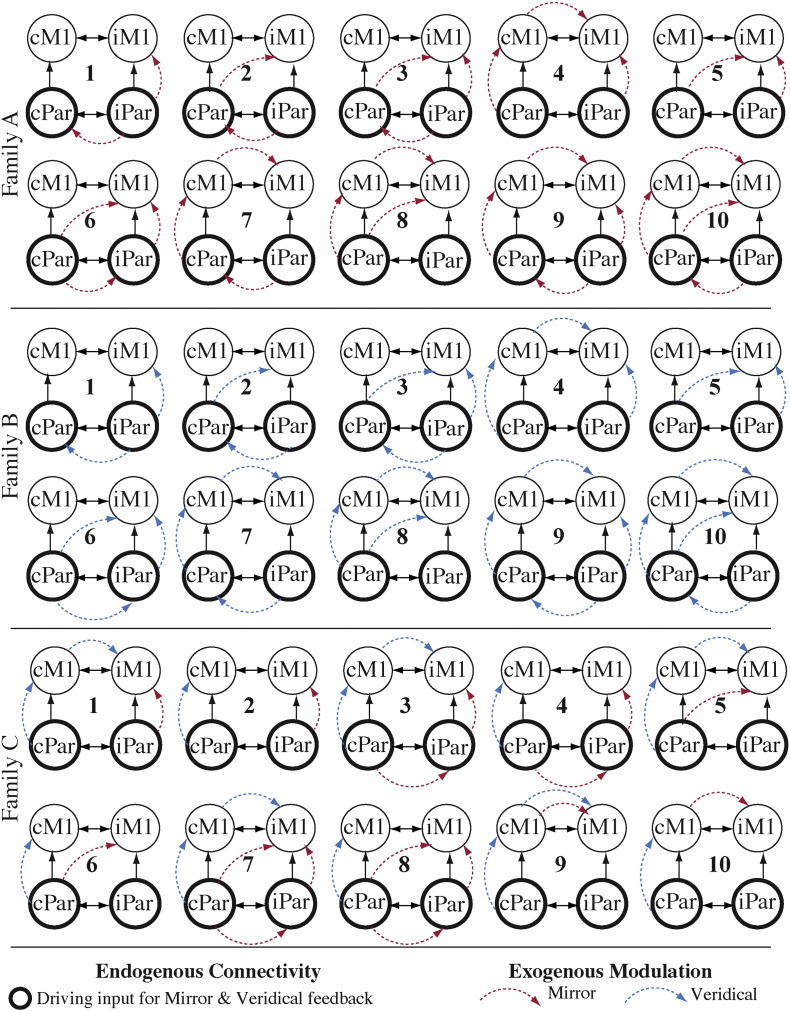
The winning model was investigated further for extrinsic connectivity by creating every plausible model that represents modulatory interactions based on our main hypotheses that: (a) iM1 activity is modulated directly by cPar or iPar, or (b) iM1 activity is modulated indirectly by cPar through a waypoint in cM1 or iPar. Ten models of possible modulatory interactions between nodes were estimated assuming modulation is solely during the mirror feedback condition (Fig. 3, Family A). Another 10 models were estimated assuming modulation of the network is solely during the veridical feedback condition (Fig. 3, Family B). The last set of 10 models (Fig. 3, Family C) was estimated assuming that cPar-iM1 and iPar-iM1 interactions are modulated by mirror feedback, while cPar-cM1 and cM1-iM1 interactions are modulated by veridical feedback.
Fig. 3.
Model space of extrinsic connectivity analysis. All models have the same DCM.A and DCM.C structure (identical with model 1 in Family 1, see Results section), but the DCM.B structure differs from model to model. Families A, B, and C are different based on the role of each condition in modulating the extrinsic connectivity.
2.4.4. Bayesian model selection (BMS)
Assuming homogeneity in model structure and driving input, a fixed effect model selection analysis (FFX) was used to compare models with different architecture. Assuming heterogeneity across subjects in terms of the modulatory effect on extrinsic connectivity (Kasess et al., 2010, Stephan et al., 2010), a random effects (RFX) analysis was used to compare the extrinsic connectivity between models. Inferences on extrinsic connectivity parameters of an optimal model were derived using one sample t-tests on the B parameters of the optimal models in the group of subjects.
3. Results
3.1. DCM model structure
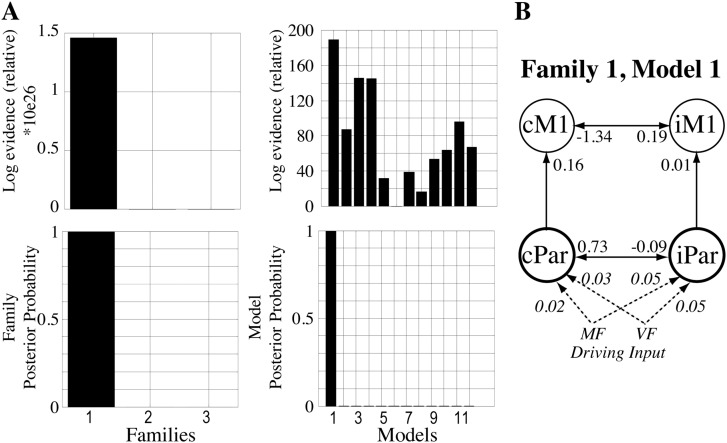
Fig. 4B (left) illustrates that BMS identified family 1 to be the optimal family, with a posterior probability of 1 and log-evidence of 1.46 × 1026. The driving input in family 1 was through the contralesional and ipsilesional parietal sites (cPar, iPar), for veridical and mirror feedback conditions. In family 1, the winning model was model 1 (Fig. 4B, right), with a posterior probability of 1, and log-evidence of 189. Fig. 4B illustrates that the structure of the intrinsic connectivity in model 1 included a bi-directional connection between bilateral motor cortices (cM1-iM1), a bidirectional connection between cPar-iPar, and unidirectional connections from cPar-to-cM1 and from iPar-to-iM1.
Fig. 4.
Results of the BMS FFX analysis for endogenous connectivity. (A) The relative log-evidence and posterior probabilities of family-based comparison are shown, as is the relative log-evidence and posterior probability of each model in the 3 families. (B) The structure of the winning model. White arrows show inter-regional connections, with the DCM.A values listed at each arrow-head. The mirror (MF) and veridical (VF) feedback driving input to the model is shown as dashed lines, with the DCM.C values listed in italics at each arrow-head.
3.2. Activation elicited by mirror feedback
Regions significantly activated by mirror-feedback are reported in our previous publication (Saleh et al., 2014) and shown as a blue-colored overlay in Fig. 5A. Significant mirror feedback-based activation was noted in the ipsilesional postcentral gyrus, corresponding to Brodmann Area 1 (BA1), extended rostrally to the primary motor cortex (BA4) and caudally along the intraparietal sulcus, and in the precuneus. Significant mirror-feedback based activation was also noted in the contralesional pre- and post-central gyri (BA1–4), and in the superior-inferior parietal lobules mostly along the intraparietal sulcus (see also figures and tables in (Saleh et al., 2014) for specific loci).
Fig. 5.
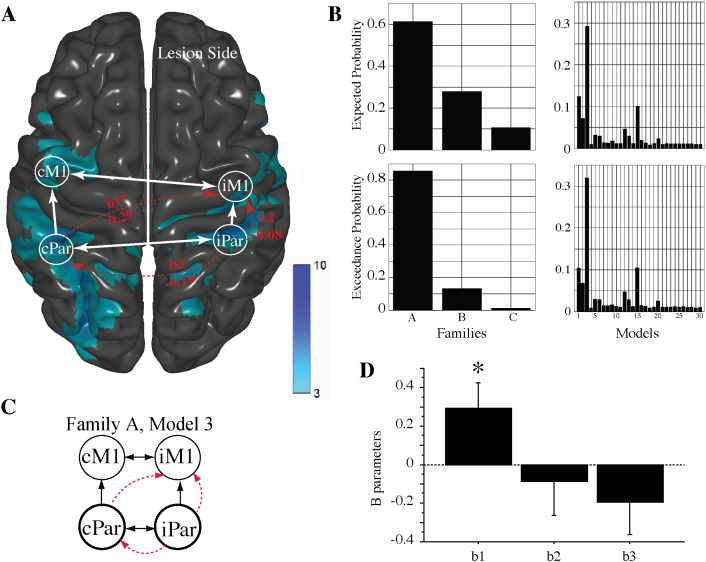
Results of the BMS RFX analysis for extrinsic connectivity. (A) The activation in the mirror feedback condition shown as a blue overlay map. Regions of interest that were used in the DCM analysis are marked as circles (iM1, cM1, iPar, cPar). White arrows show inter-regional connections of the winning model of endogenous connectivity (DCM.A, see also Fig. 4). The results for the winning model for extrinsic connectivity are shown as curved red arrows, with the DCM.B values listed for each modulatory connection (b1, b2, b3). (B) The exceedance and expected probabilities of family- and model-based comparisons are shown. (C) The winning model (Family A, Model 3). (D) Bar plot showing the results of one-sample t-tests for each ‘B’ parameter. Only the cPar-to-iM1 modulation (b1) reached statistical significance.
3.3. Extrinsic connectivity model selection
Fig. 5B shows the BMS for the 30 possible models across the three families for modulation of extrinsic connectivity. BMS analysis identified family ‘A’ as the family of models with the highest exceedance probability (0.87) and expected probability (0.62). The models comprising family ‘A’ had modulation of extrinsic connectivity by mirror feedback and none by veridical feedback (see also Fig. 3). Of the 10 models in family ‘A’, model 3 had the highest exceedance probability (0.49) and expected probability (0.29) (Fig. 5B). Model 3 of family ‘A’ (Fig. 5A and C) included modulation of extrinsic connectivity from cPar-to-iM1 (b1), from iPar-to-iM1 (b2), and from iPar-to-cPar (b3) during the mirror-feedback condition. One sample t-tests on the ‘B’ parameters revealed that only the b1 parameter was significant within this model (Fig. 5D; t12 = 2.3, p = 0.041; mean ‘b1’ parameter = 0.295). The modulation of the remaining two extrinsic connectivity parameters in model 3 (iPar-to-cPar and iPar-to-iM1) was not statistically significant.
4. Discussion
The aim of this study was to define the modulatory network dynamics mediating the activation of the ipsilesional sensorimotor cortex as a result of engaging in a mirror-feedback training session. In our prior event-related fMRI study (Saleh et al., 2014), we demonstrated in chronic stroke subjects that virtual reality-based mirror feedback of hand movements elicits significant activation in bilateral sensorimotor networks; activation that is attributed to mirror-feedback rather than motor production or other non-specific effects. In the current study, we have re-analyzed that data using dynamic causal modeling to test which nodes within the activated network exert a modulatory, task-based, influence over the ipsilesional motor cortex. We report that the mirror-feedback effect may depend on the contralesional parietal cortex which, according to our findings, exerts a significant modulatory drive onto ipsilesional M1. Importantly, no significant modulation within the a-priori defined network was noted in the control condition involving identical movement with veridical visual feedback. Accordingly, although the winning DCM model included three modulatory inputs to iM1 (from ipsilesional parietal cortex (iPar), contralesional parietal cortex (cPar), and contralesional M1 (cM1), we focus the discussion only on the cPar region as extrinsic modulation (the ‘B’ parameter) was the only one to reach statistical significance on post-hoc testing.
4.1. Action observation network and mirror feedback
To-date, eight fMRI studies have been conducted to study the neural patterns of activation attributed to mirror feedback. Of these, four are in chronic stroke patients (Michielsen et al., 2011a, Michielsen et al., 2011b, Bhasin et al., 2012, Saleh et al., 2014) and generally involve a multi-week bout of mirror training accompanied by pre/post fMRI measures while subjects move the affected hand. These studies in stroke have shown a widespread network of MVF induced activation including areas such as M1, SMC, premotor cortex, precuneus, posterior cingulate cortex and parietal areas. Associations between neurophysiological findings and clinical outcomes are discussed further in Section 4.4.
The remaining four studies are single session designs in healthy individuals (Matthys et al., 2009, Hamzei et al., 2012, Wang et al., 2013, Fritzsch et al., 2014). Collectively, the studies of healthy individuals have found significant MVF related activation in M1, SMC, premotor, parietal, V5, STG and superior occipital areas with several noting that the prominent effect of mirrored feedback (compared to direct visual feedback, which we term ‘veridical feedback’ in our study) is reflected by more bilateral activation of sensorimotor areas (Diers et al., 2010, Fritzsch et al., 2014). The sole investigation of MVF using DCM (Hamzei et al., 2012) to probe the network interactions indicated an MVF-specific increase of effective connectivity between each premotor region and the contralateral supplementary motor area, which caused an increased functional coupling with the ipsilateral SMC. The exact underlying mechanism of this reduced lateralization remains difficult to explain because, for instance, some studies have used unimanual while others used bimanual movements, hence making it hard to tease apart whether it is related to a transcallosal transfer of information or a cross-activation (overflow) effect.
However, bilateral activation of M1 does not necessarily imply transcallosal communication between sensorimotor cortices. Indeed, other studies have reported activation of cortical areas comprising the action observation network (see discussion below), which may contribute to the bilateral activation noted above (Deconinck et al., 2015). Our data favor the hypothesis that mirror feedback-based modulation of iM1 arises from the contralesional parietal cortex, rather than contralesional M1.
The modulatory node in the parietal cortex noted in our study, the rostral portion of the inferior parietal cortex, is often ascribed to part of the action observation network (AON), dubbed as a set of regions activated by observation of biological motion (Nelissen et al., 2011, Thompson and Parasuraman, 2012, Rizzolatti et al., 2014). This body of work reveals the involvement of the intraparietal sulcus, and the adjacent convexity on the inferior bank (supramarginal gyrus) and superior bank (BA5), in hand-oriented actions. The above-mentioned parietal regions receive rich visual and somatosensory input about action goals and hand shaping, have neurons with receptive fields pertaining to the hand, and make strong connections with (pre)motor areas (Mountcastle et al., 1975, Strick and Kim, 1978, Zarzecki et al., 1978, Kalaska et al., 1983, McGuire et al., 1989, Rozzi et al., 2006, Borra et al., 2008, Gerbella et al., 2011). Retrograde tracer injections into the lateral funiculus of the cervical spinal cord of non-human primates reveal labeling of presumptive corticospinal neurons in the inferior parietal lobule convexity, area PFG (Miller, 1987, Rozzi et al., 2006), suggesting that some of these areas may even have a role in motor execution of grasp.
4.2. Transcallosal modulation from parietal to motor cortex
Although it bears relatively little surprise that the rostral intraparietal sulcus and the inferior-superior parietal convexities (what we collectively refer to as the cPar node) should be activated for hand-based actions with mirror feedback, it is striking that the modulation from cPar to iM1 is transcallosal. Indeed, traditionally most tracing studies have focused on intra-hemispheric connections between parietal and frontal lobes, or homotopic interhemispheric connections (e.g. parietal-to-parietal or frontal-to-frontal). Also present are interhemispheric projections connecting heterotopic regions, both within a lobe, as well as across lobes (for review, (Schulte and Muller-Oehring, 2010)), though they are admittedly sparser than homotopic connections. An extreme example of this pertains to the robust interhemispheric projections from visual cortical areas to contralateral speech centers in the dominant hemisphere (Di Virgilio and Clarke, 1997). Akin to this, regions of the intraparietal sulcus and inferior-superior convexities make connections with heterotopic areas of the parietal and frontal lobes in the opposite hemisphere (Matsumura and Kubota, 1979, Hedreen and Yin, 1981, Caminiti and Sbriccoli, 1985, Jarbo et al., 2012). It is therefore plausible that there exists an underlying anatomical architecture fostering interhemispheric modulation from the parietal to the motor cortex.
Support for this also stems from elegant human neurophysiology conducted by Rothwell and colleagues. In a series of experiments, the authors used a twin-coil Transcranial Magnetic Stimulation (TMS) paradigm to study the modulation that sub-regions in the parietal cortex have over M1, intra- and inter-hemispherically. The main findings were that conditioning TMS pulses applied over the rostral and caudal portions of the intraparietal sulcus led to inhibitory and facility modulation of M1 respectively, whether the effects were measured intra- or interhemispherically (Koch et al., 2007, Koch et al., 2009). It is noteworthy to point out that the parietal-to-M1 modulation (at least within a hemisphere) seems to be strongest for hand-arm actions executed to the contralateral workspace (Koch et al., 2008). In light of the above anatomic-functional interactions between cPar and iM1, we suggest that mirror feedback may be mediated by a broad interhemispheric network that integrates hand grasping and representation of contralateral peri-personal workspace. It is important to stress that no significant network modulation was noted for the veridical condition, suggesting that the cPar-M1 modulation was specific to the feedback, rather than the motor task, which was identical in both conditions.
4.3. Mirror feedback modulation does not arise from the contralesional M1
DCM analysis revealed that the effective connectivity from cM1 to iM1 was not significant in either the mirror or veridical feedback conditions, suggesting that cM1 is an unlikely source of modulation for the mirror task. This finding is in agreement with twin-coil TMS studies that did not note interhemispheric inhibition to be a potential mediator of mirror feedback (Nojima et al., 2012, Nojima et al., 2013, Avanzino et al., 2014, Lappchen et al., 2015). The Nojima group found that activation was directed to the viewed rather than the active hand (Nojima et al., 2012), and that inter-manual transfer during MVF training could still be possible despite callosotomy (Nojima et al., 2013). Our data, and the above-mentioned neurophysiology work, fit well with the above literature, suggesting that the modulatory signal in the mirror condition should not be presumed to arise from the ‘active’ M1.
4.4. Potential clinical relevance of parietal-to-M1 modulation underlying mirror training
To date, the most extensive clinical investigation of MVF which also explored neural mechanisms found that mirror therapy in chronic stroke helps attain greater improvements than control intervention, though improvements were small, lost at six months, and did not show transfer to ADLs (Michielsen et al., 2011b). The associated neurophysiological finding was a shift of activation towards the lesioned M1 after mirror therapy (change in laterality index) (Michielsen et al., 2011b, Bhasin et al., 2012). However, the findings in the above-mentioned RCT (Michielsen et al., 2011b) and another MVF investigational study from the same group (Michielsen et al., 2011a) reveal conflicting results with regard to areas activated by MVF and the loci of MVF related cortical reorganization. Better understanding of the MVF network interactions may unveil if mirror feedback has the potential to engage circuits in a manner that may favor recovery. Our investigation revealed MVF-related parietal-to-M1 coupling. Weakened parietal-to-M1 interactions (Grefkes and Fink, 2011, Takeuchi et al., 2012) have been previously identified in stroke, and restoring functional interactions among this network has been positively correlated with good recovery (Carter et al., 2010, van Meer et al., 2010, Wang et al., 2010, Grefkes and Fink, 2011, Rehme et al., 2011, Rehme et al., 2012, van Meer et al., 2012, De Vico Fallani et al., 2016). Mirror feedback, therefore, may be a useful clinical tool, as it has been shown to improve some outcomes in moderately to severely impaired patients (Thieme et al., 2012, Thieme et al., 2013, Pollock et al., 2014), and to activate specific networks that may favor recovery, particularly in patients who cannot otherwise engage their paretic hand in exercise. It remains unknown if similar networks could be activated in acutely impaired stroke patients; this is a focus of ongoing investigations in our lab. In light of the recent gain in popularity of non-invasive cortical stimulation as a therapeutic tool to boost activation of cortical areas to aide recovery, our data suggest that the contralesional parietal cortex, in addition to the motor cortex which is typically targeted, may be a viable locus to target if the stimulation is to be combined with mirror training. This too remains to be tested directly.
4.5. Study limitations
The small sample size has a potential effect of inflating the effect size. It also prevents us from analyzing the relationship between effects size and lesion location. A bigger sample size and a more homogeneous sample would be needed in future studies to establish if certain patient populations may have stronger responses to MVF. Given that we used a deterministic model with 4 nodes, our results can only be interpreted in the hypothesis-driven model space, and does not necessarily mean that the optimal model identified in our study is the absolute true model if more regions were to be tested (Friston et al., 2011b, Lohmann et al., 2012). However, as mentioned in the introduction and the methods sections, our a-priori decision to include the four regions of interest was based on previously published investigations showing, in a whole-brain analysis, those to be the most representative nodes responding to mirror feedback. Hence, inclusion of non a-priori defined visuomotor processing areas would lack a clear hypothesis, add challenges to model selection and model validation, and complicate interpretation of the results. Another limitation to the study is the absence of a healthy, age-matched, control group which could delineate whether we identified canonical network interactions or if these interactions are compensatory in nature. Although the additional group would add to our understanding of these mechanisms, and is currently under investigation, our findings nevertheless point to the importance of parietal-M1 interactions for mirror feedback in chronic stroke.
5. Conclusion
In conclusion, our data show that mirror feedback performed by chronic stroke patients is mediated by contralesional parietal cortex modulation over the ipsilesional M1. This modulation is not present in the veridical feedback condition suggesting that it is the feedback, rather than the motor output, that drives the network interaction. Our results indicate that mirror feedback may engage networks important for recovery, and that the contralesional parietal lobe may be a putative region that should be considered for non-invasive cortical stimulation if it is to be combined with mirror training.
Funding sources
This work was supported by the National Institutes of Health grant # R01NS085122 (ET) and grant # R01HD53801 (SA), and by Rehabilitation Engineering Research Center (NIDILRR # 90RE5021) (SA).
References
- Adamovich S.V., August K., Merians A., Tunik E. A virtual reality-based system integrated with fmri to study neural mechanisms of action observation-execution: a proof of concept study. Restor. Neurol. Neurosci. 2009;27(3):209–223. doi: 10.3233/RNN-2009-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.M., Rapcsak S.Z., Beeson P.M. Cost function masking during normalization of brains with focal lesions: still a necessity? NeuroImage. 2010;53(1):78–84. doi: 10.1016/j.neuroimage.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Avanzino L., Raffo A., Pelosin E., Ogliastro C., Marchese R., Ruggeri P., Abbruzzese G. Training based on mirror visual feedback influences transcallosal communication. Eur. J. Neurosci. 2014;40(3):2581–2588. doi: 10.1111/ejn.12615. [DOI] [PubMed] [Google Scholar]
- Bhasin A., Padma Srivastava M.V., Kumaran S.S., Bhatia R., Mohanty S. Neural interface of mirror therapy in chronic stroke patients: a functional magnetic resonance imaging study. Neurol. India. 2012;60(6):570–576. doi: 10.4103/0028-3886.105188. [DOI] [PubMed] [Google Scholar]
- Blangero A., Khan A., Rode G., Rossetti Y., Pisella L. Dissociation between intentional and automatic remapping: different levels of inter-hemispheric transfer. Vis. Res. 2011;51(8):932–939. doi: 10.1016/j.visres.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Borra E., Belmalih A., Calzavara R., Gerbella M., Murata A., Rozzi S., Luppino G. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb. Cortex. 2008;18(5):1094–1111. doi: 10.1093/cercor/bhm146. [DOI] [PubMed] [Google Scholar]
- Brett M., Leff A.P., Rorden C., Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage. 2001;14(2):486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Buccino G., Binkofski F., Fink G.R., Fadiga L., Fogassi L., Gallese V., Seitz R.J., Zilles K., Rizzolatti G., Freund H.J. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 2001;13(2):400–404. [PubMed] [Google Scholar]
- Caminiti R., Sbriccoli A. The callosal system of the superior parietal lobule in the monkey. J. Comp. Neurol. 1985;237(1):85–99. doi: 10.1002/cne.902370107. [DOI] [PubMed] [Google Scholar]
- Carson R.G., Ruddy K.L. Vision modulates corticospinal suppression in a functionally specific manner during movement of the opposite limb. J. Neurosci. 2012;32(2):646–652. doi: 10.1523/JNEUROSCI.4435-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A.R., Astafiev S.V., Lang C.E., Connor L.T., Rengachary J., Strube M.J., Pope D.L., Shulman G.L., Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann. Neurol. 2010;67(3):365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong T.T., Cunnington R., Williams M.A., Kanwisher N., Mattingley J.B. fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Curr. Biol. 2008;18(20):1576–1580. doi: 10.1016/j.cub.2008.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong T.T., Williams M.A., Cunnington R., Mattingley J.B. Selective attention modulates inferior frontal gyrus activity during action observation. NeuroImage. 2008;40(1):298–307. doi: 10.1016/j.neuroimage.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Crinion J., Ashburner J., Leff A., Brett M., Price C., Friston K. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. NeuroImage. 2007;37(3):866–875. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vico Fallani F., Clausi S., Leggio M., Chavez M., Valencia M., Maglione A.G., Babiloni F., Cincotti F., Mattia D., Molinari M. Interhemispheric connectivity characterizes cortical reorganization in motor-related networks after cerebellar lesions. Cerebellum. 2016 doi: 10.1007/s12311-016-0811-z. [DOI] [PubMed] [Google Scholar]
- Deconinck F.J., Smorenburg A.R., Benham A., Ledebt A., Feltham M.G., Savelsbergh G.J. Reflections on mirror therapy: a systematic review of the effect of mirror visual feedback on the brain. Neurorehabil. Neural Repair. 2015;29(4):349–361. doi: 10.1177/1545968314546134. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Oliviero A., Profice P., Insola A., Mazzone P., Tonali P., Rothwell J.C. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Experimentelle Hirnforschung. Experimentation cerebraleExp. Brain Res. 1999;124(4):520–524. doi: 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- Di Virgilio G., Clarke S. Direct interhemispheric visual input to human speech areas. Hum. Brain Mapp. 1997;5(5):347–354. doi: 10.1002/(SICI)1097-0193(1997)5:5<347::AID-HBM3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Diers M., Christmann C., Koeppe C., Ruf M., Flor H. Mirrored, imagined and executed movements differentially activate sensorimotor cortex in amputees with and without phantom limb pain. Pain. 2010;149(2):296–304. doi: 10.1016/j.pain.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Dohle C., Pullen J., Nakaten A., Kust J., Rietz C., Karbe H. Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabil. Neural Repair. 2009;23(3):209–217. doi: 10.1177/1545968308324786. [DOI] [PubMed] [Google Scholar]
- Ferbert A., Priori A., Rothwell J.C., Day B.L., Colebatch J.G., Marsden C.D. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic causal modelling. NeuroImage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston K., Daunizeau J., Stephan K.E. Model selection and gobbledygook: response to Lohmann et al. NeuroImage. 2011;75:275–278. doi: 10.1016/j.neuroimage.2011.11.064. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Li B., Daunizeau J., Stephan K.E. Network discovery with DCM. NeuroImage. 2011;56(3):1202–1221. doi: 10.1016/j.neuroimage.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch C., Wang J., Dos Santos L.F., Mauritz K.H., Brunetti M., Dohle C. Different effects of the mirror illusion on motor and somatosensory processing. Restor. Neurol. Neurosci. 2014;32(2):269–280. doi: 10.3233/RNN-130343. [DOI] [PubMed] [Google Scholar]
- Gandolla M., Ferrante S., Molteni F., Guanziroli E., Frattini T., Martegani A., Ferrigno G., Friston K., Pedrocchi A., Ward N.S. Re-thinking the role of motor cortex: context-sensitive motor outputs? NeuroImage. 2014;91:366–374. doi: 10.1016/j.neuroimage.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbella M., Belmalih A., Borra E., Rozzi S., Luppino G. Cortical connections of the anterior (F5a) subdivision of the macaque ventral premotor area F5. Brain Struct. Funct. 2011;216(1):43–65. doi: 10.1007/s00429-010-0293-6. [DOI] [PubMed] [Google Scholar]
- Grefkes C., Fink G.R. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain. 2011;134(Pt 5):1264–1276. doi: 10.1093/brain/awr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei F., Lappchen C.H., Glauche V., Mader I., Rijntjes M., Weiller C. Functional plasticity induced by mirror training: the mirror as the element connecting both hands to one hemisphere. Neurorehabil. Neural Repair. 2012;26(5):484–496. doi: 10.1177/1545968311427917. [DOI] [PubMed] [Google Scholar]
- He B.J., Snyder A.Z., Vincent J.L., Epstein A., Shulman G.L., Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53(6):905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hedreen J.C., Yin T.C. Homotopic and heterotopic callosal afferents of caudal inferior parietal lobule in Macaca mulatta. J. Comp. Neurol. 1981;197(4):605–621. doi: 10.1002/cne.901970405. [DOI] [PubMed] [Google Scholar]
- Howatson G., Zult T., Farthing J.P., Zijdewind I., Hortobagyi T. Mirror training to augment cross-education during resistance training: a hypothesis. Front. Hum. Neurosci. 2013;7:396. doi: 10.3389/fnhum.2013.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbo K., Verstynen T., Schneider W. In vivo quantification of global connectivity in the human corpus callosum. NeuroImage. 2012;59(3):1988–1996. doi: 10.1016/j.neuroimage.2011.09.056. [DOI] [PubMed] [Google Scholar]
- Kalaska J.F., Caminiti R., Georgopoulos A.P. Cortical mechanisms related to the direction of two-dimensional arm movements: relations in parietal area 5 and comparison with motor cortex. Exp. Brain Res. 1983;51(2):247–260. doi: 10.1007/BF00237200. [DOI] [PubMed] [Google Scholar]
- Kasess C.H., Stephan K.E., Weissenbacher A., Pezawas L., Moser E., Windischberger C. Multi-subject analyses with dynamic causal modeling. NeuroImage. 2010;49(4):3065–3074. doi: 10.1016/j.neuroimage.2009.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Fernandez Del Olmo M., Cheeran B., Ruge D., Schippling S., Caltagirone C., Rothwell J.C. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J. Neurosci. 2007;27(25):6815–6822. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Fernandez Del Olmo M., Cheeran B., Schippling S., Caltagirone C., Driver J., Rothwell J.C. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. J. Neurosci. 2008;28(23):5944–5953. doi: 10.1523/JNEUROSCI.0957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Ruge D., Cheeran B., Fernandez Del Olmo M., Pecchioli C., Marconi B., Versace V., Lo Gerfo E., Torriero S., Oliveri M., Caltagirone C., Rothwell J.C. TMS activation of interhemispheric pathways between the posterior parietal cortex and the contralateral motor cortex. J. Physiol. 2009;587(Pt 17):4281–4292. doi: 10.1113/jphysiol.2009.174086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappchen C.H., Ringer T., Blessin J., Seidel G., Grieshammer S., Lange R., Hamzei F. Optical illusion alters M1 excitability after mirror therapy: a TMS study. J. Neurophysiol. 2012;108(10):2857–2861. doi: 10.1152/jn.00321.2012. [DOI] [PubMed] [Google Scholar]
- Lappchen C.H., Ringer T., Blessin J., Schulz K., Seidel G., Lange R., Hamzei F. Daily iTBS worsens hand motor training—a combined TMS, fMRI and mirror training study. NeuroImage. 2015;107:257–265. doi: 10.1016/j.neuroimage.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Lee M., Hinder M.R., Gandevia S.C., Carroll T.J. The ipsilateral motor cortex contributes to cross-limb transfer of performance gains after ballistic motor practice. J. Physiol. 2010;588(Pt 1):201–212. doi: 10.1113/jphysiol.2009.183855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G., Erfurth K., Muller K., Turner R. Critical comments on dynamic causal modelling. NeuroImage. 2012;59(3):2322–2329. doi: 10.1016/j.neuroimage.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Kubota K. Cortical projection to hand-arm motor area from post-arcuate area in macaque monkeys: a histological study of retrograde transport of horseradish peroxidase. Neurosci. Lett. 1979;11(3):241–246. doi: 10.1016/0304-3940(79)90001-6. [DOI] [PubMed] [Google Scholar]
- Matthys K., Smits M., Van der Geest J.N., Van der Lugt A., Seurinck R., Stam H.J., Selles R.W. Mirror-induced visual illusion of hand movements: a functional magnetic resonance imaging study. Arch. Phys. Med. Rehabil. 2009;90(4):675–681. doi: 10.1016/j.apmr.2008.09.571. [DOI] [PubMed] [Google Scholar]
- McGuire P.K., Hockfield S., Goldman-Rakic P.S. Distribution of cat-301 immunoreactivity in the frontal and parietal lobes of the macaque monkey. J. Comp. Neurol. 1989;288(2):280–296. doi: 10.1002/cne.902880207. [DOI] [PubMed] [Google Scholar]
- Michielsen M.E., Smits M., Ribbers G.M., Stam H.J., van der Geest J.N., Bussmann J.B., Selles R.W. The neuronal correlates of mirror therapy: an fMRI study on mirror induced visual illusions in patients with stroke. J. Neurol. Neurosurg. Psychiatry. 2011;82(4):393–398. doi: 10.1136/jnnp.2009.194134. [DOI] [PubMed] [Google Scholar]
- Michielsen M.E., Selles R.W., van der Geest J.N., Eckhardt M., Yavuzer G., Stam H.J., Smits M., Ribbers G.M., Bussmann J.B. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabil. Neural Repair. 2011;25(3):223–233. doi: 10.1177/1545968310385127. [DOI] [PubMed] [Google Scholar]
- Miller M.W. The origin of corticospinal projection neurons in rat. Exp. Brain Res. 1987;67(2):339–351. doi: 10.1007/BF00248554. [DOI] [PubMed] [Google Scholar]
- Mountcastle V.B., Lynch J.C., Georgopoulos A., Sakata H., Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J. Neurophysiol. 1975;38(4):871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Murase N., Duque J., Mazzocchio R., Cohen L.G. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 2004;55(3):400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nagy K., Greenlee M.W., Kovacs G. The lateral occipital cortex in the face perception network: an effective connectivity study. Front. Psychol. 2012;3:141. doi: 10.3389/fpsyg.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen K., Borra E., Gerbella M., Rozzi S., Luppino G., Vanduffel W., Rizzolatti G., Orban G.A. Action observation circuits in the macaque monkey cortex. J. Neurosci. 2011;31(10):3743–3756. doi: 10.1523/JNEUROSCI.4803-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima I., Mima T., Koganemaru S., Thabit M.N., Fukuyama H., Kawamata T. Human motor plasticity induced by mirror visual feedback. J. Neurosci. 2012;32(4):1293–1300. doi: 10.1523/JNEUROSCI.5364-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima I., Oga T., Fukuyama H., Kawamata T., Mima T. Mirror visual feedback can induce motor learning in patients with callosal disconnection. Exp. Brain Res. 2013;227(1):79–83. doi: 10.1007/s00221-013-3486-4. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Perani D., Fazio F., Borghese N.A., Tettamanti M., Ferrari S., Decety J., Gilardi M.C. Different brain correlates for watching real and virtual hand actions. NeuroImage. 2001;14(3):749–758. doi: 10.1006/nimg.2001.0872. [DOI] [PubMed] [Google Scholar]
- Pisella L., Alahyane N., Blangero A., Thery F., Blanc S., Pelisson D. Right-hemispheric dominance for visual remapping in humans. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2011;366(1564):572–585. doi: 10.1098/rstb.2010.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock A., Baer G., Campbell P., Choo P.L., Forster A., Morris J., Pomeroy V.M., L. P. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst. Rev. 2014;22(4) doi: 10.1002/14651858.CD001920.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme A.K., Eickhoff S.B., Wang L.E., Fink G.R., Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. NeuroImage. 2011;55(3):1147–1158. doi: 10.1016/j.neuroimage.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme A.K., Eickhoff S.B., Rottschy C., Fink G.R., Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage. 2012;59(3):2771–2782. doi: 10.1016/j.neuroimage.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Reissig P., Garry M.I., Summers J.J., Hinder M.R. Visual feedback-related changes in ipsilateral cortical excitability during unimanual movement: implications for mirror therapy. Neuropsychol. Rehabil. 2014;24(6):936–957. doi: 10.1080/09602011.2014.922889. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Cattaneo L., Fabbri-Destro M., Rozzi S. Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol. Rev. 2014;94(2):655–706. doi: 10.1152/physrev.00009.2013. [DOI] [PubMed] [Google Scholar]
- Rossiter H.E., Borrelli M.R., Borchert R.J., Bradbury D., Ward N.S. Cortical mechanisms of mirror therapy after stroke. Neurorehabil. Neural Repair. 2015;29(5):444–452. doi: 10.1177/1545968314554622. [DOI] [PubMed] [Google Scholar]
- Rozzi S., Calzavara R., Belmalih A., Borra E., Gregoriou G.G., Matelli M., Luppino G. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb. Cortex. 2006;16(10):1389–1417. doi: 10.1093/cercor/bhj076. [DOI] [PubMed] [Google Scholar]
- Saleh S., Adamovich S.V., Tunik E. Mirrored feedback in chronic stroke: recruitment and effective connectivity of ipsilesional sensorimotor networks. Neurorehabil. Neural Repair. 2014;28(4):344–354. doi: 10.1177/1545968313513074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T., Muller-Oehring E.M. Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. Neuropsychol. Rev. 2010;20(2):174–190. doi: 10.1007/s11065-010-9130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehm B., Perez M.A., Xu B., Hidler J., Cohen L.G. Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb. Cortex. 2010;20(1):34–45. doi: 10.1093/cercor/bhp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Penny W.D., Moran R.J., den Ouden H.E., Daunizeau J., Friston K.J. Ten simple rules for dynamic causal modeling. NeuroImage. 2010;49(4):3099–3109. doi: 10.1016/j.neuroimage.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick P.L., Kim C.C. Input to primate motor cortex from posterior parietal cortex (area 5). I. Demonstration by retrograde transport. Brain Res. 1978;157(2):325–330. doi: 10.1016/0006-8993(78)90035-5. [DOI] [PubMed] [Google Scholar]
- Suchan B., Melde C., Herzog H., Homberg V., Seitz R.J. Activation differences in observation of hand movements for imitation or velocity judgement. Behav. Brain Res. 2007;188(1):78–83. doi: 10.1016/j.bbr.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Takeuchi N., Oouchida Y., Izumi S. Motor control and neural plasticity through interhemispheric interactions. Neural Plast. 2012;2012:823285. doi: 10.1155/2012/823285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme H., Mehrholz J., Pohl M., Behrens J., Dohle C. Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 2012;3 doi: 10.1002/14651858.CD008449.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme H., Bayn M., Wurg M., Zange C., Pohl M., Behrens J. Mirror therapy for patients with severe arm paresis after stroke—a randomized controlled trial. Clin. Rehabil. 2013;27(4):314–324. doi: 10.1177/0269215512455651. [DOI] [PubMed] [Google Scholar]
- Thompson J., Parasuraman R. Attention, biological motion, and action recognition. NeuroImage. 2012;59(1):4–13. doi: 10.1016/j.neuroimage.2011.05.044. [DOI] [PubMed] [Google Scholar]
- van Meer M.P., van der Marel K., Wang K., Otte W.M., El Bouazati S., Roeling T.A., Viergever M.A., Berkelbach van der Sprenkel J.W., Dijkhuizen R.M. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J. Neurosci. 2010;30(11):3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer M.P., Otte W.M., van der Marel K., Nijboer C.H., Kavelaars A., van der Sprenkel J.W., Viergever M.A., Dijkhuizen R.M. Extent of bilateral neuronal network reorganization and functional recovery in relation to stroke severity. J. Neurosci. 2012;32(13):4495–4507. doi: 10.1523/JNEUROSCI.3662-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S., Weidner R., Driver J., Friston K.J., Fink G.R. Deconstructing the architecture of dorsal and ventral attention systems with dynamic causal modeling. J. Neurosci. 2012;32(31):10637–10648. doi: 10.1523/JNEUROSCI.0414-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Yu C., Chen H., Qin W., He Y., Fan F., Zhang Y., Wang M., Li K., Zang Y., Woodward T.S., Zhu C. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133(Pt 4):1224–1238. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- Wang J., Fritzsch C., Bernarding J., Krause T., Mauritz K.H., Brunetti M., Dohle C. Cerebral activation evoked by the mirror illusion of the hand in stroke patients compared to normal subjects. NeuroRehabilitation. 2013;33(4):593–603. doi: 10.3233/NRE-130999. [DOI] [PubMed] [Google Scholar]
- Yavuzer G., Selles R., Sezer N., Sutbeyaz S., Bussmann J.B., Koseoglu F., Atay M.B., Stam H.J. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2008;89(3):393–398. doi: 10.1016/j.apmr.2007.08.162. [DOI] [PubMed] [Google Scholar]
- Zarzecki P., Strick P.L., Asanuma H. Input to primate motor cortex from posterior parietal cortex (area 5). II. Identification by antidromic activation. Brain Res. 1978;157(2):331–335. doi: 10.1016/0006-8993(78)90036-7. [DOI] [PubMed] [Google Scholar]
- Zult T., Howatson G., Kadar E.E., Farthing J.P., Hortobagyi T. Role of the mirror-neuron system in cross-education. Sports Med. 2014;44(2):159–178. doi: 10.1007/s40279-013-0105-2. [DOI] [PubMed] [Google Scholar]