Abstract
Background:
High cost of imported pacemakers is a main obstacle for Chinese patients suffering from bradyarrhythmia, and a domestically developed pacemaker will help lower the burden. This study aimed to evaluate the safety and efficacy of Qinming8631 DR (Qinming Medical, Baoji, China), the first domestically developed dual-chamber pacemaker of China, compared with a commercially available pacemaker Talos DR (Biotronik, Berlin, Germany) in Chinese patients.
Methods:
A prospective randomized trial was conducted at 14 centers in China. Participants were randomized into trial (Qinming8631 DR) and control (Talos DR) groups. Parameters of the pacing systems were collected immediately after device implantation and during follow-ups. The effective pacing rate at 6-month follow-up was recorded as the primary end point. Electrical properties, magnet response, single- and double-pole polarity conversion, rate response function, and adverse events of the pacing system were analyzed. The Cochran-Mantel-Haenszel Chi-square test, paired t-test, and Wilcoxon signed-rank test were used for measuring primary qualitative outcomes and comparing normally and abnormally distributed measurement data.
Results:
A total of 225 patients with a diagnosis of bradyarrhythmia and eligible for this study were randomly enrolled into the trial (n = 113) and control (n = 112) groups. They underwent successful pacemaker implantation with acceptable postoperative pacing threshold and sensitivity. Effective pacing rates of trial and control groups were comparable both in the full analysis set and the per protocol set (81.4% vs. 79.5%, P = 0.712 and 95.4% vs. 89.5%, P = 0.143, respectively). In both data sets, noninferiority of the trial group was above the predefined noninferiority limit (−9.5%).
Conclusions:
This study established the noninferiority of Qinming8631 DR to Talos DR. The safety and efficacy of Qinming8631 DR pacemaker were comparable to those of Talos DR in treating patients with cardiac bradyarrhythmia.
Keywords: Bradyarrhythmia, Cardiac Pacemaker, Qinming8631, Safety and Efficacy
Introduction
Cardiac arrhythmia is a major clinical issue leading to considerable morbidity and mortality.[1] According to the statistics of American Heart Association, the incidence of bradyarrhythmia was reported to be 4%. It is estimated to affect 5.6–12.0 million people in 2050 and will lead to more than 400,000 annual sudden cardiac deaths in the United States.[2] Implanting a cardiac pacemaker is the most effective way for treating patients with bradyarrhythmia.[3,4,5,6] In the past decades, cardiac pacemakers has saved millions of patients suffering from cardiac bradyarrhythmia and has improved the quality of life of patients.[7]
An estimated 750,000 patients with cardiac bradyarrhythmia undergo cardiac pacemaker implantation every year all over the world.[8] Although the clinical application of pacemakers grew rapidly in China, the implantation cases was 35/1 million in 2012, which was significantly lower than the numbers in Europe (951/1 million in 2010).[9] Although great progression has been made in China at the field of cardiac device implantation,[10,11,12,13] we have to recognize that only 3% patients with symptomatic cardiac bradyarrhythmia undergo pacemaker implantation. The high cost of imported pacemakers was found to be the major reason holding Chinese people from pacemaker implantation. A domestically manufactured pacemaker with much lower cost would help more Chinese patients benefit from cardiac pacing.[14]
The Qinming8631 DR implantable cardiac pacemaker (Qinming Medical, Baoji, China) is the first domestically developed dual-chamber rate-responsive pacemaker in China. It has been qualified by the General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China.
This study aimed to evaluate the safety and efficacy of the Qinming8631 DR implantable cardiac pacemaker. The commercially available Talos DR (Biotronik, Berlin, Germany) pacemaker was used as control, and noninferiority of the Qinming8631 DR to Talos DR pacemaker in Chinese patients was tested.
Methods
Study design
This prospective, multicenter, randomized controlled, single-blind, noninferiority study was conducted at 14 clinical sites in China between September 2013 and December 2014. Through a central stratified random sampling, enrolled patients were assigned into trial (Qinming8631 DR) or control (Talos DR) groups. Clinical follow-up visits were conducted at 1, 3, and 6 months after pacemaker implantation in all patients. The protocol was conducted in accordance with the Declaration of Helsinki (2013 No. 007) and approved by the Institutional Review Board at the Second Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, China) and the institutes that participated in the clinical trial. Every patient signed written informed consent. Data were collected at each clinical site by a third-party analyzer who was responsible for data processing and statistical analysis.
Bipolar passive and active fixation pacing leads were used in this study. In the trial group, QM7222 (Qinming Medical, Baoji, China) was used as the atrial electrode lead and QM7211 (Qinming Medical, Baoji, China) active lead as the ventricular electrode lead. In the control group, Selox JT (Biotronik, Berlin, Germany) was used as the atrial electrode lead and Setrox S (Biotronik, Berlin, Germany) as the ventricular electrode lead. The effective pacing rate, defined as acceptable pacing capture threshold and sensing amplitude, at the 6-month follow-up was recorded as the primary end point. Electrical properties, magnet response, single- and double-pole polarity conversion, rate response function, and adverse events of the pacing system after implantation were collected and analyzed at the end of the study. All-cause mortality, cardiovascular death, and pacemaker-related adverse events were reported by each center and analyzed at the end of the study.
Study population
Inclusion criteria
Patients were deemed eligible for the enrollment in the trial if they (1) suffered from bradyarrhythmia due to sick sinus syndrome (SSS) or atrioventricular (AV) block and indicated for dual-chamber pacemaker implantation; (2) were 18–85 years old; (3) signed the informed written consent; and (4) were capable of finishing 6 months of follow-up.
Exclusion criteria
Patients were excluded from the study if (1) they had tricuspid atresia; (2) the tricuspid valve was replaced with a mechanical valve; (3) auricular appendix was absent; (4) they had persistent atrial fibrillation; (5) they had indications for implantable cardioverter defibrillator; (6) they were contraindicated to 1.0 mg dexamethasone; (7) they were pregnant or lactating; (8) they had a life expectancy of <1 year; (9) they had coagulation dysfunction; (10) they had high-sensitivity carotid sinus syndrome and neurocardiogenic syncope; (11) they had intracardiac mural thrombus or suffered from ventriculotomy or atriotomy in the last 4 weeks; (12) they had heart transplantation, neuromuscular diseases, sleep apnea syndrome, and cardiac sarcoidosis; (13) they had acute or severe infection, malignant tumor, or end-stage diseases; (14) they used other medical devices that might interfere with the pacemaker; or (15) they had any other conditions unsuitable for the trial as considered by researchers.
Efficacy assessments
Primary end points
The effective pacing rate at the 6-month follow-up was used to be the primary end point. It mainly comprised the following parameters:
Acceptable pacing threshold (acute): With 0.5 ms of the pulse width, the pacing threshold of the right atrium was ≤1.5 V and the threshold of the right ventricle was ≤1.0 V. When the voltage outputs were set to two times the pulse amplitude of the measured pacing threshold, the target atrium or ventricle was continuously captured and observed on a surface electrocardiogram (ECG)
Acceptable long-term threshold: When the pulse width was 0.5 ms, the pacing threshold of the right atrium was ≤1.5 V (fluctuated upward ≤20%) and the threshold of the right ventricle was ≤1.0 V (fluctuated upward ≤20%). When the voltage outputs were set to two times the pulse amplitude of the measured pacing threshold, the target atrium or the ventricle was continuously captured and observed on a surface ECG at 1, 3, and 6 months after the procedure
Postoperative sensing: The sensitivity threshold of right atrium was ≥2.0 mV and the threshold of the right ventricle was ≥4.0 mV. When the sensitivity was set at 1/3–1/4 of the sensitivity threshold (should not exceed the setting range advised by the manufacturer), an electrocardio signal could be effectively perceived
Magnet response: Magnet response of the pacemaker at 3 months after the procedure was evaluated
Polarity conversion (unipolar and bipolar): Unipolar and Bipolar conversion of the pacemaker at 3 months after the procedure was evaluated
Rate response: Rate response of the pacemaker at 6 months after the procedure was evaluated.
Secondary end points
Failure to pace as detected by the 24-h ambulatory ECG at 6 months
Failure to sense as detected by the 24-h ambulatory ECG at 6 months.
Safety assessments
Safety assessments were conducted by recording all-cause death, cardiovascular death, and pacemaker-related adverse events within 6 months after the procedure. Moreover, the clinical symptoms, vital signs (blood pressure and heart rate), and laboratory parameters were monitored. If abnormal changes were noted, their correlation with the pacemaker was analyzed. Laboratory parameters included complete blood count, liver function, renal function, and blood clotting tests.
Statistical analysis
Sample size estimation
On the basis of clinical experience, this study hypothesized that the effective pacing rate in the control group was 95% (taking into account the incidence of lead-related complications), and the noninferiority margin was 9.5%. The sample size in each group would have a power of 0.80 with a level of significance of 0.05; if the expected dropout rate was 10%, the final sample size was 100 in each group.
Analysis of population
The full analysis set (FAS) comprised cases in accordance to the principle of “intention to treat,” including all the enrolled patients. For patients without the primary effect assessment, the last observation carried forward (LOCF) was conducted to carry forward the missing data. The per protocol set (PPS) was a subgroup of enrolled patients completing the trial. The safety analysis set (SAS) included cases with at least one result of safety evaluation during the follow-up.
Statistical analysis method
All continuous values were presented as mean ± standard deviation (SD), and all categorical variables were presented as number of patients (percentages). Group comparisons of categorical data were conducted by Cochran-Mantel-Haenszel Chi-square test or Fisher's exact test (when 25% of the cells have expected frequencies of <5); group comparisons of normally distributed measurement data were analyzed by student t-test; and group comparisons of abnormally distributed measurement data were analyzed by Wilcoxon signed-rank test. Data management was conducted by the EpiData 3.0 software (The EpiData Association, Odense, Denmark). Data analysis was done using the SAS 9.13 software (SAS Institute Inc., Cary, USA). A two-sided P ≤ 0.05 was considered statistically significant.
Analysis of efficacy
For primary quantitative outcome measurements, group comparisons were conducted by analysis of covariance. After testing the homogeneity of variance of each center, the least mean square (LMS), LMS error between groups, and 95% confidence interval (CI) of dependent variable were calculated. For primary qualitative outcome measurements, the Cochran-Mantel-Haenszel Chi-square test, adjusting the influence of center, was conducted and differences in the event rate between groups and the 95% CI were calculated. To evaluate the proposed noninferiority of the trial instrument, 95% CI of group variances of outcome measurements was compared with the critical value, which was clinically meaningful and specified preliminary in the trial. For other outcome measurements, comparison of the normally distributed measurement data in a group was conducted by the student t-test and that of abnormally distributed measurement data was conducted by the Wilcoxon signed-rank test.
Analysis of safety
Normal cases and proportions before the treatment and abnormal cases and proportions after the treatment were calculated in the two groups, respectively. Cases and proportions of adverse events were calculated and analyzed by continuous correction U-test or Fisher's exact test. The specific appearance, degree, and relationship with the instrument in each group were recorded.
Results
Study population
A total of 225 patients from 14 centers all over China were enrolled in this study and randomized into two groups based on a third-party random assignment: Qinming8631 DR pacemaker was implanted in 113 patients (trial group) and Talos DR pacemaker was implanted in 112 patients (control group). Comparisons of baseline characteristics, such as demographics and comorbidities, are shown in Table 1. No significant difference was found between the two groups, except for the history of syncope, which was higher in the trial group than in the control group. Patients with a diagnosis of SSS, second-degree AV block, and complete AV block were 62 versus 58 (χ2 = 0.215, P = 0.643), 10 versus 11 (χ2 = 0.063, P = 0.802), and 41 versus 43 (χ2 = 0.107, P = 0.744) in the trial versus control group, respectively. No statistical significance of diagnosis was found in these two groups [Table 1]. Study population of FAS and PPS are shown in Table 2. Finally, 87 (77.0%) patients in the trial group and 86 (76.8%) patients in the control group completed the 6-month follow-up (PPS). Reasons for discontinuation are provided in Supplementary Table 1.
Table 1.
Baseline characteristics of patients with bradyarrhythmia implanted cardiac pacemakers
| Variables | Qinming8631 DR group (n = 113) | Talos DR group (n = 112) | Statistical values | P |
|---|---|---|---|---|
| Age (years) | 65.6 ± 11.0 | 65.3 ± 11.6 | 0.207 | 0.837 |
| Female | 57 (50.4) | 50 (44.6) | 0.759 | 0.384 |
| SBP (mmHg) | 137.3 ± 22.5 | 139.1 ± 21.8 | −0.613 | 0.541 |
| DBP (mmHg) | 73.9 ± 12.1 | 74.8 ± 12.2 | −0.562 | 0.575 |
| Heart rate (beats/min) | 50 ± 14 | 53 ± 17 | −1.659 | 0.099 |
| Coronary heart disease | 6 (5.3) | 7 (6.3) | 0.091 | 0.762 |
| Hypertension | 47 (41.6) | 53 (47.3) | 0.748 | 0.387 |
| Diabetes | 9 (8.0) | 13 (11.6) | 0.850 | 0.357 |
| Stroke | 3 (2.7) | 6 (5.4) | Fisher | 0.333 |
| Dilated cardiomyopathy | 1 (0.9) | 0 (0) | Fisher | 1.000 |
| Valvular disease | 1 (0.9) | 2 (1.8) | Fisher | 0.622 |
| MI | 0 (0) | 2 (1.8) | Fisher | 0.247 |
| Paroxysmal atrial fibrillation | 3 (2.7) | 8 (7.1) | 2.522 | 0.112 |
| History of syncope | 10 (8.9) | 3 (2.7) | 4.209 | 0.040 |
| Diagnosis | ||||
| Sick sinus syndrome | 62 (54.9) | 58 (51.8) | 0.215 | 0.643 |
| Second degree AV block | 10 (8.8) | 11 (9.8) | 0.063 | 0.802 |
| Complete AV block | 41 (36.3) | 43 (38.4) | 0.107 | 0.744 |
Fisher: No available statistical value according to Fisher exact test. Data were expressed as n (%) or mean ± SD. SD: Standard deviation; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; AV: Atrioventricular; MI: Myocardial infarction; 1 mmgHg = 0.133 kPa.
Table 2.
Study deviation of bradyarrhythmia patients underwent pacemaker implantation during 6 months of follow-up
| Population | Qinming8631 DR group (n = 113) | Talos DR group (n = 112) |
|---|---|---|
| Cancellation of informed consent, n | 0 | 0 |
| FAS, n | 113 | 112 |
| Deviation from the protocol, n (%) | ||
| Absence of primary end points | 22 (19.5) | 23 (20.5) |
| Loss of follow-up | 4 (3.5) | 3 (2.7) |
| PPS, n (%) | 87 (77.0) | 86 (76.8) |
FAS = Number of randomized patients − number of patients withdrawing informed consent; Deviation from the protocol = Patients absence of primary end points + patients loss of follow-up; PPS = FAS − deviation from the protocol. FAS: Full analysis set; PPS: Per protocol set.
Supplementary Table 1.
Patients deviating from the protocol
| Center | Random number | Group | Gender | Age | Type | Detailed description |
|---|---|---|---|---|---|---|
| 01 | 053 | Trial | Male | 77 | Absence of primary end points | Death at the 70th day |
| 01 | 054 | Trial | Female | 71 | Absence of primary end points | No assessment of frequency response at 6-month |
| 01 | 068 | Trial | Male | 57 | Absence of primary end points | No assessment of frequency response at 6-month |
| 01 | 069 | Control | Male | 64 | Absence of primary end points | No assessment of frequency response at 6-month |
| 01 | 101 | Trial | Female | 64 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 01 | 122 | Control | Male | 55 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 01 | 129 | Trial | Male | 76 | Absence of primary end points | Death at the 68th day |
| 02 | 179 | Control | Female | 74 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 02 | 191 | Trial | Female | 60 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 02 | 192 | Control | Female | 54 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 03 | 004 | Control | Female | 73 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 04 | 002 | Trial | Male | 60 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 04 | 015 | Trial | Male | 71 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 04 | 048 | Trial | Male | 79 | Absence of primary end points | Death at the 162nd day |
| 04 | 049 | Control | Male | 77 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 04 | 056 | Trial | Male | 51 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 04 | 100 | Trial | Male | 75 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 04 | 107 | Control | Male | 66 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 04 | 138 | Trial | Male | 71 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 05 | 021 | Trial | Male | 78 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 05 | 025 | Control | Male | 70 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 05 | 043 | Trial | Male | 74 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 05 | 052 | Control | Male | 81 | Absence of primary end points | Death at the 62nd day |
| 05 | 116 | Trial | Male | 76 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 06 | 203 | Trial | Female | 76 | Loss of follow-up | 6-month |
| 06 | 223 | Control | Female | 82 | Loss of follow-up | 1-month |
| 07 | 144 | Control | Male | 26 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 08 | 026 | Trial | Male | 26 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 08 | 040 | Control | Male | 81 | Absence of primary end points | No perception threshold immediately after operation |
| 08 | 135 | Control | Male | 33 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 08 | 178 | Control | Male | 56 | Loss of follow-up | 1-, 3-, and 6-month |
| 08 | 189 | Trial | Male | 80 | Loss of follow-up | 6-month |
| 09 | 038 | Trial | Male | 63 | Absence of primary end points | Death at the 95th day |
| 09 | 074 | Control | Male | 78 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 09 | 088 | Trial | Male | 50 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 09 | 098 | Control | Male | 56 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 09 | 175 | Trial | Female | 64 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 09 | 188 | Trial | Male | 84 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 09 | 195 | Control | Male | 69 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 09 | 089 | Trial | Female | 77 | Loss of follow-up | 3- and 6-month |
| 09 | 168 | Control | Female | 82 | Loss of follow-up | 6-month |
| 10 | 117 | Trial | Male | 43 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 10 | 133 | Trial | Male | 76 | Absence of primary end points | Death at the 126th day |
| 10 | 153 | Control | Male | 76 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 13 | 029 | Control | Male | 53 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 13 | 046 | Trial | Male | 67 | Absence of primary end points | No perception threshold immediately after operation |
| 13 | 104 | Control | Female | 52 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 13 | 216 | Trial | Female | 59 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 14 | 091 | Trial | Female | 64 | Loss of follow-up | 1-month |
| 14 | 090 | Control | Male | 56 | Absence of primary end points | No assessment of perception threshold immediately after operation |
| 14 | 115 | Control | Male | 76 | Absence of primary end points | Death at the 75th day |
| 14 | 197 | Control | Female | 73 | Absence of primary end points | No pace-making threshold at 6-month after operation; no assessment of frequency response at 6-month |
Center - 01: The Second Affiliated Hospital of Zhejiang University School of Medicine; 02: The Affiliated Hospital of Qingdao University Medical College; 03: The First People’s Hospital of Hangzhou; 04: The First Affiliated Hospital of Lanzhou University; 05: The First Affiliated Hospital of Xi’an Jiaotong University; 06: Northern Jiangsu People’s Hospital; 07: The First Affiliated Hospital of Suzhou University; 08: The Second Affiliated Hospital of Nanchang University; 09: Bethune International Peace Hospital of the Chinese PLA; 10: The First Affiliated Hospital of Kunming Medical University; 13: Jilin Province People’s Hospital; 14: Tianjin Chest Hospital.
Procedure characteristics
All the patients underwent successful pacemaker implantation. More than 75% patients underwent electrode lead implantation through the subclavian vein (around 20% was through the axillary vein) and very few through the cephalic vein, in both groups. About 70% pacemakers were implanted into the left side of the chest, and no difference was found between the two groups (69.9% vs. 70.5%, χ2 = 0.011, P = 0.919). The atrial lead was fixed in the right atrial (RA) appendage in all patients. In 39.8% of the trial group and 40.2% of the control group, the ventricular lead was fixed in the right ventricular (RV) apex, while in others, it was fixed in the ventricular septum. The RA pacing threshold was 0.63 ± 0.28 V vs. 0.65 ± 0.27 V (t = −0.643, P = 0.521) in trail and control groups while RV pacing threshold was 0.67 ± 0.27 V vs. 0.58 ± 0.22 V (t = 2.628, P = 0.009). RA and RV sensing threshold were similar between the two groups (6.09 ± 5.30 mV vs. 5.18 ± 4.36 mV, t = 1.377 P = 0.170; 11.03 ± 5.76 mV vs. 11.62 ± 6.00 mV, t = −0.751 P = 0.453, respectively).
Efficacy assessment
Primary end points
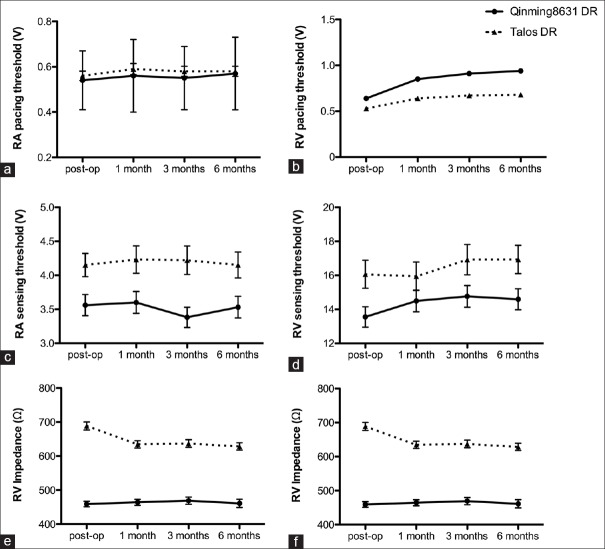
On the basis of comparability of the two groups, the primary end points in FAS and PPS were evaluated [Table 3]. The results showed that in FAS, the effective pacing rate was 81.4% in the trial group and 79.5% in the control group. The difference was 1.3% (95% CI: −7.0%–10.6%), and thus noninferiority of the trial group compared with the control group with respect to the effective pacing rate was shown with a margin of −7.0%, which was well above the predefined noninferiority limit (−9.5%). In PPS, the effective pacing rate was 95.4% in the trial group and 89.5% in the control group. The difference was 11.8% (95% CI: 0.3%–24.9%), and thus noninferiority of the trial group compared with the control group with respect to the effective pacing rate was shown with a margin of 0.3%, which was also well above the predefined noninferiority limit (−9.5%). The effective pacing rate immediately after the procedure was no significant difference in the two groups (trial: 100% vs. control: 99.1%, P = 1.000). The effective sensitivity rate immediately after the procedure was 94.8% and 93.6% in the trial and control groups, respectively, with no significant difference (P = 0.716). The stability of pacing during follow-up was very high (99.0% in trial and 98.1% in control groups), and the difference was not significant (P = 0.621). Magnet response, single- and double-pole polarity conversion at 3 months, and rate response at 6 months were 100% in both groups. Pacing threshold, sensing threshold, and lead impedance of RA and RV in both groups are shown in Figure 1.
Table 3.
Primary endpoint after 6 months of follow-up in the full analysis set and per protocol set
| Variables | Qinming8631 DR group (n = 113) | Talos DR group (n = 112) | Difference | 95% CI | P |
|---|---|---|---|---|---|
| FAS*, n (%) | |||||
| Valid | 92 (81.4) | 89 (79.5) | 1.3 | −7.0–10.6 | 0.712 |
| Invalid | 21 (18.6) | 23 (20.5) | |||
| PPS, n (%) | |||||
| Valid | 83 (95.4) | 77 (89.5) | 1.8 | 0.3–24.9 | 0.143 |
| Invalid | 4 (4.6) | 9 (10.5) |
*The first choice for dealing with missing value is LOCF. When there is still missing values after the data was added according to LOCF, the missing value would be regard as non-effective pacing. 95% CI was obtained through CMH Chi-square test adjusting center effect. FAS: Full analysis set; PPS: Per protocol set; CI: Confidence interval; LOCF: Last observation carried forward; CMH: Cochran-Mantel-Haenszel.
Figure 1.
Parameter of RA and RV leads at post-op and during follow-ups. (a and b) Pacing threshold at 0.4 ms pulse width; (c and d) sensing threshold; (e and f) impedance. Post-op: Postoperation; RA: Right atrium; RV: Right ventricle.
Secondary end points
At 6-month follow-up, the 24-h ambulatory ECG monitoring did not detect any failure of pacing or sensing in both groups.
Safety assessment
Analysis of safety parameters was based on SAS. The rate of adverse event was 3.53% in the trial group and 2.67% in the control group, with no significant difference (P = 1.000). The four cases of adverse events in the trial group (three noncardiovascular and one cardiovascular) and three cases of adverse events in the control group (zero noncardiovascular and three cardiovascular) were severe. However, the adverse events were not associated with the pacing system [Table 4 and Supplementary Table 2].
Table 4.
Adverse events in bradyarrhythmia patients underwent pacemaker implantation
| Adverse events | Qinming8631 DR group (n = 113) | Talos DR group (n = 112) | Statistical values | P |
|---|---|---|---|---|
| Noncardiac death, n (%) | ||||
| Nasopharyngeal carcinoma | 1 (0.88) | 0 | Fisher | 1.000 |
| Hepatocellular carcinoma | 1 (0.88) | 0 | Fisher | 1.000 |
| Intestinal obstruction (postcolectomy) | 1 (0.88) | 0 | Fisher | 1.000 |
| Cardiac death, n (%) | ||||
| MI | 0 | 2 (1.79) | Fisher | 0.247 |
| HF and acute renal insufficiency | 1 (0.88) | 0 | Fisher | 1.000 |
| HF | 0 | 1 (0.89) | Fisher | 0.498 |
| All-cause death | 4 (3.53) | 3 (2.67) | Fisher | 1.000 |
Fisher: No available statistical value according to Fisher’s exact test. MI: Myocardial infarction; HF: Heart failure.
Supplementary Table 2.
Description of patients with adverse events
| Center | Random number | Group | Age | Gender | Adverse event | Day after operation | Severity | Outcome | Instruments treatments | Quit the test |
|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 053 | Control | 77 | Male | MI | 70 | Severe | Death | No | Yes |
| 01 | 129 | Trial | 76 | Male | Intestinal obstruction (postcolon cancer operation) | 68 | Severe | Death | No | Yes |
| 04 | 048 | Trial | 79 | Male | Hepatocellular carcinoma | 162 | Severe | Death | No | Yes |
| 05 | 052 | Control | 81 | Male | MI | 60 | Severe | Death | No | Yes |
| 09 | 038 | Trial | 63 | Male | HF and acute renal insufficiency | 95 | severe | Death | No | Yes |
| 10 | 133 | Trial | 76 | Male | Nasopharyngeal carcinoma | 126 | Severe | Death | No | Yes |
| 14 | 115 | Control | 76 | Male | HF | 75 | Severe | Death | No | Yes |
Center - 01: The Second Affiliated Hospital of Zhejiang University School of Medicine; 04: The First Affiliated Hospital of Lanzhou University; 05: The First Affiliated Hospital of Xi’an Jiaotong University; 10: The First Affiliated hospital of Kunming Medical University; 14: Tianjin Chest Hospital. MI: Myocardial infarction; HF: Heart failure.
Discussion
This study demonstrated that the first domestically developed dual-chamber rate-responsive pacemaker of China was noninferior with respect to safety or efficacy compared with Talos DR at 6-month follow-up. Qinming8631 DR pacing system showed acceptable pacing and sensing threshold at both acute phase and long-term follow-up and good magnetic response, polarity conversion (unipolar and bipolar), and rate response function. No failure of pacing or sensing was found by 24-h ambulatory ECG. Most importantly, the prevalence of severe adverse events was low and similar in the two groups, and the adverse events were not associated with the pacing system.
In China, Qinming2312 implantable cardiac pacemaker, developed by Qinming Corporation in 2008, was the first domestic single-chamber cardiac pacemaker registered by the China Food and Drug Administration. In the past years, Qinming2312 implantable cardiac pacemaker was clinically used in 150 hospitals and spread over 26 provinces in China. The clinical effects obtained from different hospitals demonstrated that Qinming2312 implantable cardiac pacemaker was comparable with imported cardiac pacemaker and was, especially, inexpensive, which could be afforded by the majority of Chinese patients.[15,16] However, dual-chamber pacemaker accounted for 60% of all pacemakers in China,[9] which brought an urgent need of domestically developed dual-chamber pacemaker. On the basis of successful clinical application of Qinming2312 single-chamber cardiac pacemaker, Qinming Corporation developed Qinming8631 DR implantable cardiac pacemaker, which would be the first generation of domestically developed dual-chamber cardiac pacemaker of China.
This prospective, randomized, controlled, and single-blind study was conducted at 14 clinical centers in China. The Talos DR implantable cardiac pacemaker, which is widely distributed all over the world as the basic model of dual-chamber pacemaker, was selected as the control device. Rigorous inclusion and exclusion criteria were used while enrolling patients. The loss of follow-up rate was 2% at 1 month, 3% at 3 months, and 5% at 6 months. Good pacing performance and reliable safety shown in this study helped improve the confidence when using Qinming8631 DR implantable cardiac pacemaker in clinical practice.
Clinical significance
As a result of the continuous development in economy and improvement in people's living standards, the aging trend has increased in China, leading to a dramatically increased number of patients relying on the treatment with pacemakers. So far, Qinming8631 DR implantable cardiac pacemaker is the first dual-chamber pacemaker developed in China with independent intellectual property, possessing efficacy and safety comparable to those of Talos DR pacemaker, is much cheaper than the imported pacemaker, and has huge potential for alleviating the financial burden of Chinese patients and providing more opportunities to those who are indicated for pacemaker implantation.
Limitations
The follow-up period in this study was relatively short according to the battery expectancy of a dual-chamber pacemaker. A long-term outcome evaluation is warranted for both Qinming8631 DR and Talos DR pacemakers.
In conclusion, the present study demonstrated, through a 6-month follow-up trial, that Qinming8631 DR implantable cardiac pacemaker was feasible for treating patients with cardiac bradyarrhythmia with good safety and reliable pacing performance.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Ning-Ning Wang
References
- 1.Nattel S, Andrade J, Macle L, Rivard L, Dyrda K, Mondesert B, et al. New directions in cardiac arrhythmia management: Present challenges and future solutions. Can J Cardiol. 2014;30(12 Suppl):S420–30. doi: 10.1016/j.cjca.2014.09.027. doi: 10.1016/j.cjca.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics-2014 update: A report from the American Heart Association. Circulation. 2014;129:e28–92. doi: 10.1161/01.cir.0000441139.02102.80. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barros RT, Carvalho SM, Silva MA, Borges JB. Evaluation of patients’ quality of life aspects after cardiac pacemaker implantation. Rev Bras Cir Cardiovasc. 2014;29:37–44. doi: 10.5935/1678-9741.20140009. doi: 10.5935/1678-9741.20140009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradshaw PJ, Stobie P, Knuiman MW, Briffa TG, Hobbs MS. Trends in the incidence and prevalence of cardiac pacemaker insertions in an ageing population. Open Heart. 2014;1:000177. doi: 10.1136/openhrt-2014-000177. doi: 10.1136/openhrt-2014-000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mond HG, Crozier I. The Australian and New Zealand cardiac pacemaker and implantable cardioverter-defibrillator survey: Calendar year 2013. Heart Lung Circ. 2015;24:291–7. doi: 10.1016/j.hlc.2014.09.017. doi: 10.1016/j.hlc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Semelka M, Gera J, Usman S. Sick sinus syndrome: A review. Am Fam Physician. 2013;87:691–6. [PubMed] [Google Scholar]
- 7.Debski M, Ulman M, Zabek A, Haberka K, Lelakowski J, Malecka B. Gender differences in dual-chamber pacemaker implantation indications and long-term outcomes. Acta Cardiol. 2016;71:41–5. doi: 10.2143/AC.71.1.3132096. doi: 10.2143/AC.71.1.3132096. [DOI] [PubMed] [Google Scholar]
- 8.Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: Calendar year 2009 –A World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011;34:1013–27. doi: 10.1111/j.1540-8159.2011.03150.x. doi: 10.1111/j.1540-8159.2011.03150.x. [DOI] [PubMed] [Google Scholar]
- 9.Shu Z, Xiaohan F, Xiaohui N. on Behalf of the Writing Committee of Annual Report on Cardiovascular Disease in China. Cardiac arrhythmia in China. Chin Med J. 2014;127:71–5. [Google Scholar]
- 10.Xiao CH, Zhang XW, Wang RX, Cheng YL, Zhang CY, Wang LB, et al. Effects of different pacing algorithms on cumulative ventricular pacing proportion in patients with pacemakers. Chin Med J. 2011;124:2937–42. [PubMed] [Google Scholar]
- 11.Chen KP, Dai Y, Hua W, Yang JF, Li K, Liang ZG, et al. Reduction of atrial fibrillation in remotely monitored pacemaker patients: Results from a Chinese multicentre registry. Chin Med J. 2013;126:4216–21. [PubMed] [Google Scholar]
- 12.Cao Y, Zhang Y, Su Y, Bai J, Wang W, Ge J. Assessment of adaptive rate response provided by accelerometer, minute ventilation and dual sensor compared with normal sinus rhythm during exercise: A self-controlled study in chronotropically competent subjects. Chin Med J. 2015;128:25–31. doi: 10.4103/0366-6999.147798. doi: 10.4103/0366-6999.147798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua W, Ding LG, Fan XH, Liu ZM, Jiang CL, Qu FJ, et al. Initial experience with multipoint pacing cardiac resynchronization therapy in China. Chin Med J. 2016;129:1241–3. doi: 10.4103/0366-6999.181966. doi: 10.4103/0366-6999.181966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boriani G, Maniadakis N, Auricchio A, Vardas P. Health economics and outcomes research: A new challenge and field of action for the European Heart Rhythm Association. Europace. 2010;12:601–3. doi: 10.1093/europace/euq028. doi: 10.1093/europace/euq028. [DOI] [PubMed] [Google Scholar]
- 15.Tianyu M, Yong Z, Jixing Y. Study of clinical effects of domestic cardiac pacemaker Qinming 2312S/M and imported pacemakers (In Chinese) China Med Pharm. 2014;2014:183–5. [Google Scholar]
- 16.Xiao-Yan L, Hong T, Wei LK, Ai-Guo L, Yu-Qi G, Qun J, et al. The study of the clinical application of domestic pacemaker Qinming2312S/M (In Chinese) Chin J Card Pacing Electrophysiol. 2013;2013:11–2. [Google Scholar]